Abstract
Changes in physical activities and feeding habits have transformed the historically rare disease of obesity into a modern metabolic pandemic. Obesity occurs when energy intake exceeds energy expenditure over time. This energy imbalance significantly increases the risk for cardiovascular disease and type 2 diabetes mellitus and as such represents an enormous socioeconomic burden and health threat. To combat obesity, a better understanding of the molecular mechanisms and neurocircuitries underlying normal body weight homeostasis is required. In the 1940s, pioneering lesion experiments unveiled the importance of medial and lateral hypothalamic structures. In the 1980s and 1990s, several neuropeptides and peripheral hormones critical for appropriate feeding behavior, energy expenditure, and hence body weight homeostasis were identified. In the 2000s, results from metabolic analyses of genetically engineered mice bearing mutations only in selected neuronal groups greatly advanced our knowledge of the peripheral/brain feedback-loop modalities by which central neurons control energy balance. In this review, we will summarize these recent progresses with particular emphasis on the biochemical identities of hypothalamic neurons and molecular components underlying normal appetite, energy expenditure, and body weight homeostasis. We will also parse which of those neurons and molecules are critical components of homeostatic adaptive pathways against obesity induced by hypercaloric feeding.
Metabolic-sensing mechanisms in hypothalamic neurons are critical for maintaining normal body weight homeostasis and thus protect against obesity.
Brain-mediated control of energy intake and expenditure keeps body weight within normal values. Even a small change in this homeostatic balance, as, for example, a slight daily increase in food intake and/or reduction in energy expenditure in the long run leads to increased body weight (1). There are different degrees of increased body weight in humans; the World Health Organization defines adults with a body mass index (BMI; body weight in kilograms divided by the square of the height in meters) between 25 and 29.9 kg/m2 as overweight and those with higher BMIs as obese. Frighteningly, in 2005 approximately 400 million adults were classed as obese. Because of its serious comorbidities (e.g. heart disease, diabetes), obesity is therefore a huge threat to human health. Unfortunately, primary defects underlying obesity are still poorly understood, except for very rare monogenic forms that have been well-characterized in rodents and humans (2). Due to the rapid surge in the incidence of obesity seen in industrialized and rapidly developing countries in which consumption of calorie-rich food is also a common habit, it has been postulated that chronic feeding on these diets is a critical factor in the pathogenesis of obesity (3). This contention is experimentally supported because chronic hypercaloric feeding causes energy imbalance in mammalian organisms (including nonhuman primates) (4). However, some individuals appear to be very resistant from, whereas others very sensitive to, dietary obesity, suggesting a large variability in the robustness of homeostatic mechanisms against energy imbalance brought about by overnutrition (5).
The contention that mammals (including humans) have evolved mechanisms geared for positive energy balance as a means to better cope with periods in which food may be scarce or even not available has been put forth. In modern obesogenic environments the aforementioned anabolic pathways have been suggested to underlie the excessive body weight gain seen in many obese subjects, an idea known as the thrifty gene hypothesis (6). However, this hypothesis seems to be at odds with the fact that mammals allowed to eat ad libitum do not normally develop obesity and that even when fed on hypercaloric diets, mammals attempt to resist obesity by triggering adaptive metabolic responses (e.g. reduced food intake and increased energy expenditure) (7,8,9). These results would suggest that mammals have a body weight set point and that mechanisms to defend it are activated when the brain detects changes in body energy status. Why then do so many people develop obesity? To address this question, we will need to better understand the following factors: 1) the signals underlying body weight homeostasis, 2) the identities of the neuronal populations that translate these signals into coordinated food intake and energy expenditure, and 3) what causes these homeostatic mechanisms to malfunction in obesogenic environments. To date, the answers to the aforementioned questions exist but are yet to be satisfactory. In this review, we will summarize this knowledge and discuss how this information may be instrumental for developing novel and more effective antiobesity strategies.
Peripheral signals underlying body weight homeostasis
The activity of metabolically relevant brain neurons is affected by several hormones, the circulating amounts of which reflect the status of body energy stores. These peptides are secreted by specialized endocrine cells mainly found in adipose tissue, pancreas, stomach, and small intestine and include the following. Leptin is secreted by adipocytes (fat cells) proportionally to the body fat mass, and its adipostatic actions are chiefly mediated by hypothalamic, midbrain, and caudal brain stem neurons (10). This hormone exerts a pivotal role for body weight homeostasis as demonstrated by the fact that deficiency in leptin receptors (LEPRs) signaling causes severe obesity, owing to reduced energy expenditure and augmented food intake (hyperphagia) (11). Another hormonal signal contributing to body energy balance is insulin, a peptide secreted from pancreatic β-cells that also circulates in proportion to adipose mass (12). Interestingly, insulin’s peripheral and brain actions on energy balance appear to be diametrically opposite from each other. In fact, insulin’s effect on liver, skeletal muscle, and adipose tissue is anabolic as insulin promotes the storage of chemical energy in these tissues (13). However, when insulin is delivered intracerebroventricularly it exerts catabolic effects (12).
Further genetic evidences support this bifurcation of central vs. peripheral insulin’s actions on energy balance as brain-specific deletion of insulin receptor (IR) has been shown to engender increased adiposity (14). Other hormonal signals include peptide YY, glucagon-like peptide 1, and oxyntomodulin (produced by endocrine L cells in the gut), the secretion of which augments postprandially (15) and ghrelin (secreted by gastrointestinal endocrine cells lining the stomach and proximal small intestine), the circulating amounts of which rise before and fall after meals (16). In addition to hormones, the brain also directly responds to fluctuations in circulating metabolites levels (e.g. fatty acids and glucose). For example, an increase in long-chain fatty acyl-CoAs in the cytoplasm of brain cells causes inhibition of food intake, suggesting that neuronal lipid-sensing mechanisms also contribute to safeguarding normal energy balance (17). Changes in blood glucose levels affect the activity of hypothalamic neurons governing body weight, as for example the leptin- and insulin-responsive proopiomelanocortin (POMC) neurons (18). Nevertheless, despite glucostatic and lipostatic theories have been put forth, strong genetic evidences bolstering the contention that changes in blood lipids and/or glucose levels underlie long-term energy balance are still lacking.
Lastly, the brain (and in particular the caudal brain stem neurons) also receives neural afferent inputs regarding the status of energy balance (19). Below we will mainly focus on genetic evidences supporting that hormonal-sensing mechanisms in hypothalamic neurons are key for coordinated energy balance. We thus suggest other manuscripts that have dealt with roles on eating exerted by hedonic and visceral afferents systems, both of which are also key components of the circuitry underlying body weight homeostasis (2,10,19,20,21).
Hypothalamic neurons underlying body weight homeostasis
The hypothalamus is a tiny brain structure that occupies the ventral portion of the diencephalon, the borders of which are the optic chiasm (rostrally), the optic tracts and cerebral peduncles (laterally), and the mammillary bodies (caudally). Several nuclei are found within the hypothalamus. Here we touch on those that have been shown to be important for coordinated body energy balance. The first important clues about the physiological relevance of hypothalamic neurons on energy balance were obtained in the 1940s by Hetherington and Ranson (22). They showed that destruction of ventromedial hypothalamic areas including the ventromedial (VMH) and arcuate (ARH) nuclei leads to hyperphagia and obesity. Conversely, lesion of lateral hypothalamus causes reduced food intake and weight loss. The dual centers concept originated from these very important findings indicates the ventromedial hypothalamus as the satiety and the lateral hypothalamus as the phagic center. In light of recent findings, this concept needs slight revision. Indeed, it is now known that neurons within the same hypothalamic structure may serve opposite metabolic functions. One example of the complexity of these hypothalamic circuitries is the central melanocortin system.
Two distinct populations of neurons that belong to this system are POMC and agouti-related peptide (AgRP) neurons. Despite that these cells are located next to each other in the ARH, POMC, and AgRP neurons have opposite effects on energy balance (23). Interestingly, α-MSH (secreted from POMC neurons) activates, whereas AgRP inhibits melanocortin-3 and -4 receptors (MC3R and MC4R). As a consequence, α-MSH suppresses feeding and body weight gain, whereas AgRP promotes positive energy balance (24,25,26,27). Noteworthy, POMC and AgRP neurons are diversely regulated by leptin as this hormone stimulates POMC neurons at the action potential (e.g. leptin depolarizes and hence increases POMC neurons firing rate) (28) and the transcriptional level (e.g. LEPR signaling induces Pomc gene expression) (29,30,31,32). In contrast, leptin inhibits AgRP neurons as lack of LEPR signaling or fasting-induced hypoleptinemia leads to increased hypothalamic Agrp mRNA levels, an effect that is reversed by exogenous leptin administration (33,34,35). Whether leptin directly hyperpolarizes and decreases AgRP neurons firing is yet to be established. Of note, ablation of AgRP neurons in adult mice leads to anorexia (36,37,38). Thus, AgRP neurons are metabolically relevant phagic neurons residing in a previously considered satiety center.
The lateral hypothalamus also contains neurons, the effects of which appear to be opposite to what Hetherington and Ranson’s results would have suggested. For example, orexins (also known as hypocretins) are neuropeptides secreted by a group of neurons only found in the lateral hypothalamic area (LHA) (39). The orexin system is made of two neuropeptides (orexin-A and orexin-B that are the products of the same preproorexin gene) and their cognate receptors (orexin receptor types 1 and 2) (39). Although intracranial orexin injection acutely stimulates appetite, a null mutation in preproorexin gene leads to obesity (40,41). This obesity is likely not the consequence of narcolepsy (a major phenotype caused by orexin deficiency) (40,41) as suggested by the fact that narcoleptic humans lacking orexin have higher BMIs compared with orexin-intact narcoleptics (42). Moreover, enhanced orexigenic signaling suppresses food intake and body weight gain in part by augmenting leptin sensitivity (43). Thus, orexin-expressing neurons exert actions that are typical of satiety neurons, even though they are located in the LHA, a previously considered phagic center (22). Collectively these results indicate that distinct neuronal groups exerting opposite actions on energy balance coexist within a specific hypothalamic nucleus. Therefore, from a pharmacological prospective, the metabolic outcomes of stimulation (or inhibition) of neurons within a hypothalamic structure are likely the summation of events triggered by the simultaneous stimulation (or inhibition) of phagic and satiety neurons.
In addition to ARH and LHA, VMH neurons have also been shown to be of particular importance. For example, aberrant VMH development causes obesity (44). Also, loss of LEPR signaling only in neurons expressing steroidogenic factor 1 (a transcription factor expressed only by VMH neurons within the brain) (45,46) causes mild obesity (7). Because VMH neurons also project to ARH POMC neurons (47), it is very likely that VMH and ARH neurons communicate (directly and/or indirectly) to each other as part of the intricate hypothalamic neuronal web underlying normal energy balance. Neurons in the paraventricular nucleus (PVH) are also indispensable for normal energy balance because hyperphagia occurs after the PVH is damaged (48). Moreover, obesity develops in mice (and humans) heterozygous for a null mutation in single-minded 1 (Sim1), a gene encoding for a transcription factor required for PVH development (49,50). Furthermore, POMC and AgRP neurons project to the PVH, a site of abundant MC4R expression (51,52,53).
Interestingly, neurons coexpressing MC4R and SIM1 have been shown to mediate the anorectic effects induced by central melanocortin signals (51). The dorsomedial hypothalamic nucleus (DMH) is another site of importance because energy imbalance is caused by DMH lesion (54). Similarly to the PVH, ARH, LHA, and VMH, the DMH also contains leptin-responsive neurons (55). The biochemical signature(s) of DMH neurons has been elusive, and this lack of knowledge has greatly impaired the ability to genetically dissect the physiological relevance of specific genes in specialized DMH neurons. However, due to the fact that DMH neurons are metabolic-sensing cells projecting to metabolically relevant sites (e.g. PVH and ARH) (56), it is tantalizing to suggest that metabolic-sensing mechanisms in DMH neurons are also important for body weight homeostasis.
The use of the Cre/LoxP technology has been instrumental in assigning metabolic roles to specific genes in biochemically defined neuronal groups. For example, several POMC neuron- and/or AgRP neuron-specific genetic mutations affecting body weight homeostasis have been reported. In 2004 the group of Lowell and colleagues has shown that LEPR signaling in POMC neurons is required for normal energy balance because its absence causes mild obesity (29) owing to reduced energy expenditure (57) (Table 1). A similar energy imbalance occurs when domain-containing protein tyrosine phosphatase-2 (SHP2; a positive regulator of LEPR signaling) is knocked out only in POMC neurons (58) (Table 1). Also, deletion of the protein deacetylase sirtuin 1 (SIRT1) only from POMC neurons leads to leptin resistance and consequentially to hypersensitivity to dietary obesity (9) (Table 1). Conversely, enhanced leptin sensitivity only in POMC neurons protects against diet-induced obesity. For example, high-calorie-fed mice bearing POMC-neuron-specific ablation of suppressor of cytokine signaling-3 (SOCS-3; a negative regulator of LEPR signaling) display a lean phenotype owing to increased energy expenditure (59) (Table 1).
Table 1.
ARH neuron-specific mutations affecting body weight homeostasis
| Neuron | Mutation | Diet | Food intake | Energy expenditure | Adiposity | Reference(s) |
|---|---|---|---|---|---|---|
| POMC | Lepr KO | SC and HC | – | ▾ | ▴ | (29,57) |
| POMC | SIRT1 KO | HC | – | ▾ | ▴ | (9) |
| POMC | Shp2 KO | SC and HC | – | ▾ | ▴ | (58) |
| POMC | Lepr and IR KO | SC | – | ▾ | ▴ | (66) |
| POMC | Socs3 KO | HC | – | ▴ | ▾ | (59) |
| POMC | PTP1B KO | HC | – | ▴ | ▾ | (58) |
| POMC | 5-HT2CR React | SC and HC | ▾ | – | ▾ | (69) |
| POMC | FoxO1 KO | SC and HC | ▾ | – | ▾ | (70) |
| POMC | IR KO | SC | – | – | – | (57,66) |
| AgRP | Lepr KO | SC | ? | ? | ▴ | (62) |
| AgRP | STAT3 CA | SC and HC | – | ▴ | ▾ | (76) |
| AgRP | IKKβ KO | HC | ▾ | ? | ▾ | (78) |
| AgRP | Vgat KO | SC and HC | – | ▴ | ▾ | (78) |
–, Unchanged; ▴, increased; ▾, decreased; ?, not measured; SC, standard diet; HC, hypercaloric diet; KO, knockout; React, reactivated; CA, constitutively active; STAT3, signal transducer and activator of transcription 3; Vgat, vesicular GABA transporter.
Another negative regulator of leptin signaling is the protein tyrosine phosphatase 1B (PTP1B); similarly to the metabolic outcomes of POMC neuron-specific SOCS-3 deletion, high-calorie-fed mice lacking PTP1B only in POMC cells are also more resistant to developing obesity via a mechanism that includes increased energy expenditure (58) (Table 1). The result that both abolished/impaired or enhanced LEPR signaling in POMC neurons does not cause altered food intake is somewhat unexpected, mainly because previous results from nonneuron-specific experiments strongly suggested that leptin’s anorectic effects were mediated by POMC neurons (60,61). Results from studies using mice lacking LEPR in other biochemically defined neurons have now led to the well-accepted contention that leptin’s actions on body weight are not mediated by a single neuronal population but by multiple hypothalamic and extrahypothalamic neuronal groups (7,29,62,63). On the other hand and somewhat unexpected, reactivation of LEPR in ARH POMC neurons completely rescues the hyperglycemia phenotype caused by LEPR signaling deficiency (64,65). Altogether these results led to a paradigm shift: LEPR in ARH POMC neurons play only marginal roles on body weight homeostasis (contrary to what was previously suggested) (60,61) but are key for mediating the antidiabetic actions of leptin (64,65).
The idea that POMC neurons are also important for controlling glucose balance is further bolstered by other findings. For example, glucose sensing in POMC neurons is dispensable for body weight balance but is required for normal glucose tolerance (18). Moreover, deletion of both LEPR and IR in POMC neurons causes overt hepatic insulin resistance, whereas IR deletion alone has no effects on glucose/body weight homeostasis (57,66). However, because POMC neurons are established regulators of food intake as demonstrated by the fact that Pomc gene deletion causes hyperphagia and obesity, whereas intracerebroventricular delivery of α-MSH potently suppresses food intake (67,68), other inputs to POMC neurons must underlie coordinated feeding. One of these inputs is serotonin because reactivation of 5-hydroxytryptamine 2C serotonin receptor (5-HT2CR) only in POMC neurons normalizes the hyperphagia and obesity phenotypes displayed by 5-HT2CR-null mice (69) (Table 1). Forkhead box O (FoxO)-1 transcription factor in POMC neurons is also indispensable for appropriate food intake because POMC neuron-specific deletion of FoxO1 leads to increased α-MSH levels and an anorectic phenotype (70) (Table 1).
AgRP neurons also express neuropeptide Y (NPY) (71). Ablation of the Npy and/or AgRP gene does not result in altered body weight and food intake (72) despite the fact that intracranial injection of either one of these neuropeptides potently stimulates hyperphagia and body weight gain (73,74). However, the consequence of AgRP/NPY neuron ablation in adulthood includes hypophagia and reduced body weight (36,37,38,75). These results indicate that, in addition to AgRP and NPY, other molecules secreted by these neurons must be critical for normal energy balance. Recently one of these molecules has been identified: the neurotransmitter γ-aminobutyric acid. Indeed, mutant mice unable to secrete γ-aminobutyric acid from AgRP/NPY neurons display reduced body weigh due to increased energy expenditure without changes in food intake (8) (Table 1). Because these mutants eat normally (8), other yet-to-be-identified factor(s) produced by AgRP/NPY neurons must exert potent orexigenic actions. Further strengthening an important role for AgRP neurons on body weight regulation are results from mice in which a constitutively active signal transducer and activator of transcription 3 (a downstream signaling molecule of LEPR signaling) is selectively expressed in AgRP/NPY neurons, a mutation that causes resistance to dietary obesity (76). On the other hand, ablation of LEPR only in these neurons leads to mild obesity (62) (Table 1).
What causes energy imbalance in obesogenic environments?
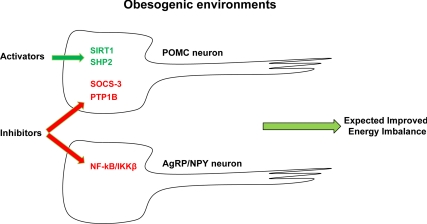
Little is known about the molecular mechanisms by which hypercaloric feeding causes energy imbalance. However, impaired responsiveness to changes in circulating hormonal and substrate levels in hypothalamic neurons is a hallmark defect brought on by hypercaloric feeding (18,77), a defect that likely contributes to the pathogenesis of dietary obesity. For example, obese subjects are hyperleptinemic, suggesting that leptin resistance underlies, at least in part, their metabolic imbalance. At the molecular level, both, impaired leptin transport from blood to brain and altered LEPR signaling underlie leptin resistance (77). Of note, chronic hypercaloric feeding stimulates nuclear factor-κB (NF-κB)/inhibitory-κB kinase (IKK)-β inflammatory pathways in hypothalamus, an effect that impairs normal metabolic sensing in hypothalamic neurons (78). Interestingly, mice lacking IKKβ only in AgRP/NPY neurons appear to be protected from diet-induced leptin resistance and hence obesity (Table 1), suggesting that endogenously and/or exogenously mediated NF-κB/IKKβ inhibition in these cells may be an effective antiobesity strategy (78) (Fig. 1).
Figure 1.
Potential antiobesity molecular targets in metabolically relevant hypothalamic neurons. POMC and AgRP/NPY neurons are critical for coordinated energy balance. In green are depicted molecules that positively regulate, whereas in red are the ones that negatively affect metabolic sensing in these neurons. Pharmacologically mediated activation of SIRT1 and SHP2 in POMC neurons combined with the inhibition of SOCS3 and PTP1B in POMC and NF-κB/IKKβ in AgRP neurons may be an effective approach to reverse energy imbalance brought about by hypercaloric feeding.
Conclusions
We have come a long way from Hetherington and Ranson’s findings suggesting that medial and lateral hypothalamic neurons are critical regulators of appetite, metabolic rate, and body weight homeostasis (22). Indeed, we now know the biochemical identities of some of these neurons (e.g. POMC, AgRP/NPY, SIM1, steroidogenic factor 1, orexin expressing neurons) and the nature of some of the peripheral signals that regulate the activity of these specialized cells (e.g. leptin, insulin, ghrelin, glucose, fatty acids, etc.). Also, some of the intracellular molecular components required for translating these peripheral signals into normal body weight have been identified. These molecules enhance (e.g. SIRT1, SHP2) whereas other antagonize (e.g. SOCS-3, PTP1B) metabolic sensing in POMC neurons (Fig. 1). From a pharmacological perspective, activation of SIRT1 and SHP2 and inhibition of SOCS-3 and PTP1B selectively in POMC neurons combined with suppression of NF-κB/IKKβ in AgRP neurons may therefore be an effective approach that in obesogenic environment could reestablish normal body weight (Fig. 1). It must be noted, however, that two major problems need to be overcome if antiobesity therapies are to be effective. First, due to significant overlaps between brain circuitries controlling feeding and hedonic/reward pathways, ideal antiobesity drugs must target the former but not the latter. One example of the limitation of the use of drugs targeting both pathways is represented by the inverse agonists at the cannabinoid receptor 1. Clinical trials at final phases were recently halted because of psychotropic side effects elicited by these drugs (79,80,81). Second, to target only specific molecules in specific neurons, ways to deliver drugs in a neuron-selective manner must be developed; these approaches are theoretically feasible yet still beyond our capabilities. In summary, there is urgency for developing effective antiobesity therapies. Our progresses in understanding mechanisms underlying body weight homeostasis have already provided potential molecular targets in defined neuronal groups. Harnessing these molecules in these neurons could be an effective and long-lasting antiobesity approach.
Acknowledgments
We thank Giorgio Ramadori, Teppei Fujikawa, and Jason Anderson (all members of the Coppari laboratory) for helpful discussions and critical reading of the manuscript.
Footnotes
This work was supported by American Heart Association (Scientist Development Grant to R.C.) and National Institutes of Health Grant DK080836 (to R.C.).
Disclosure Summary: The authors have nothing to disclose.
First Published Online November 10, 2010
Abbreviations: AgRP, Agouti-related peptide; ARH, arcuate nucleus; BMI, body mass index; DMH, dorsomedial hypothalamic nucleus; FoxO, Forkhead box O; HT2CR, 5-hydroxytryptamine 2C serotonin receptor; IR, insulin receptor; LEPR, leptin receptor; LHA, lateral hypothalamic area; MC3R, melanocortin-3 receptor; MC4R, melanocortin-4 receptor; NF-κB, nuclear factor-κB; IKK, inhibitory-κB kinase; NPY, neuropeptide Y; POMC, proopiomelanocortin; PTP1B, protein tyrosine phosphatase 1B; PVH, paraventricular nucleus; SIM1, single-minded 1; VMH, ventromedial nucleus; SHP2, tyrosine phosphatase-2; SIRT1, sirtuin 1; SOCS-3, suppressor of cytokine signaling-3.
References
- Spiegelman BM, Flier JS 2001 Obesity and the regulation of energy balance. Cell 104:531–543 [DOI] [PubMed] [Google Scholar]
- O'Rahilly S 2009 Human genetics illuminates the paths to metabolic disease. Nature 462:307–314 [DOI] [PubMed] [Google Scholar]
- Obici S 2009 Minireview: molecular targets for obesity therapy in the brain. Endocrinology 150:2512–2517 [DOI] [PubMed] [Google Scholar]
- McCurdy CE, Bishop JM, Williams SM, Grayson BE, Smith MS, Friedman JE, Grove KL 2009 Maternal high-fat diet triggers lipotoxicity in the fetal livers of nonhuman primates. J Clin Invest 119:323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA 2007 Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab 5:181–194 [DOI] [PubMed] [Google Scholar]
- Neel JV 1999 The “thrifty genotype” in 1998. Nutr Rev 57:S2–S9 [DOI] [PubMed] [Google Scholar]
- Dhillon H, Zigman JM, Ye C, Lee CE, McGovern RA, Tang V, Kenny CD, Christiansen LM, White RD, Edelstein EA, Coppari R, Balthasar N, Cowley MA, Chua Jr S, Elmquist JK, Lowell BB 2006 Leptin directly activates SF1 neurons in the VMH, and this action by leptin is required for normal body-weight homeostasis. Neuron 49:191–203 [DOI] [PubMed] [Google Scholar]
- Tong Q, Ye CP, Jones JE, Elmquist JK, Lowell BB 2008 Synaptic release of GABA by AgRP neurons is required for normal regulation of energy balance. Nat Neurosci 11:998–1000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramadori G, Fujikawa T, Fukuda M, Anderson J, Morgan DA, Mostoslavsky R, Stuart RC, Perello M, Vianna CR, Nillni EA, Rahmouni K, Coppari R 2010 SIRT1 deacetylase in POMC neurons is required for homeostatic defenses against diet-induced obesity. Cell Metab 12:78–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saper CB, Chou TC, Elmquist JK 2002 The need to feed: homeostatic and hedonic control of eating. Neuron 36:199–211 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM 1994 Positional cloning of the mouse obese gene and its human homologue. Nature 372:425–432 [DOI] [PubMed] [Google Scholar]
- Woods SC, Lotter EC, McKay LD, Porte Jr D 1979 Chronic intracerebroventricular infusion of insulin reduces food intake and body weight of baboons. Nature 282:503–505 [DOI] [PubMed] [Google Scholar]
- Unger RH, Orci L 2010 Paracrinology of islets and the paracrinopathy of diabetes. Proc Natl Acad Sci USA 107:16009–16012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brüning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Müller-Wieland D, Kahn CR 2000 Role of brain insulin receptor in control of body weight and reproduction. Science (New York, NY) 289:2122–2125 [DOI] [PubMed] [Google Scholar]
- Stanley S, Wynne K, Bloom S 2004 Gastrointestinal satiety signals III. Glucagon-like peptide 1, oxyntomodulin, peptide YY, and pancreatic polypeptide. Am J Physiol Gastrointest Liver Physiol 286:G693–G697 [DOI] [PubMed] [Google Scholar]
- Nogueiras R, Tschöp MH, Zigman JM 2008 Central nervous system regulation of energy metabolism: ghrelin versus leptin. Ann NY Acad Sci 1126:14–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obici S, Feng Z, Arduini A, Conti R, Rossetti L 2003 Inhibition of hypothalamic carnitine palmitoyltransferase-1 decreases food intake and glucose production. Nat Med 9:756–761 [DOI] [PubMed] [Google Scholar]
- Parton LE, Ye CP, Coppari R, Enriori PJ, Choi B, Zhang CY, Xu C, Vianna CR, Balthasar N, Lee CE, Elmquist JK, Cowley MA, Lowell BB 2007 Glucose sensing by POMC neurons regulates glucose homeostasis and is impaired in obesity. Nature 449:228–232 [DOI] [PubMed] [Google Scholar]
- Powley TL, Chi MM, Schier LA, Phillips RJ 2005 Obesity: should treatments target visceral afferents? Physiol Behav 86:698–708 [DOI] [PubMed] [Google Scholar]
- Myers Jr MG, Münzberg H, Leinninger GM, Leshan RL 2009 The geometry of leptin action in the brain: more complicated than a simple ARC. Cell Metab 9:117–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coll AP, Farooqi IS, O'Rahilly S 2007 The hormonal control of food intake. Cell 129:251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hetherington AW, Ranson SW 1940 Hypothalamic lesions and adiposity in the rat. Anat Rec 78:149–172 [Google Scholar]
- Cone RD 2005 Anatomy and regulation of the central melanocortin system. Nat Neurosci 8:571–578 [DOI] [PubMed] [Google Scholar]
- Coll AP, Farooqi IS, Challis BG, Yeo GS, O'Rahilly S 2004 Proopiomelanocortin and energy balance: insights from human and murine genetics. J Clin Endocrinol Metab 89:2557–2562 [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD 1997 Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385:165–168 [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS 1997 Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science (New York, NY) 278:135–138 [DOI] [PubMed] [Google Scholar]
- Cone RD, Lu D, Koppula S, Vage DI, Klungland H, Boston B, Chen W, Orth DN, Pouton C, Kesterson RA 1996 The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Rec Prog Horm Res 51:287–317; discussion 318 [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ 2001 Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature 411:480–484 [DOI] [PubMed] [Google Scholar]
- Balthasar N, Coppari R, McMinn J, Liu SM, Lee CE, Tang V, Kenny CD, McGovern RA, Chua Jr SC, Elmquist JK, Lowell BB 2004 Leptin receptor signaling in POMC neurons is required for normal body weight homeostasis. Neuron 42:983–991 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG 1997 Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 46:2119–2123 [DOI] [PubMed] [Google Scholar]
- Thornton JE, Cheung CC, Clifton DK, Steiner RA 1997 Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology 138:5063–5066 [DOI] [PubMed] [Google Scholar]
- Mizuno TM, Kleopoulos SP, Bergen HT, Roberts JL, Priest CA, Mobbs CV 1998 Hypothalamic pro-opiomelanocortin mRNA is reduced by fasting and [corrected] in ob/ob and db/db mice, but is stimulated by leptin. Diabetes 47:294–297 [DOI] [PubMed] [Google Scholar]
- Ahima RS, Prabakaran D, Mantzoros C, Qu D, Lowell B, Maratos-Flier E, Flier JS 1996 Role of leptin in the neuroendocrine response to fasting. Nature 382:250–252 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Baskin DG, Bukowski TR, Kuijper JL, Foster D, Lasser G, Prunkard DE, Porte Jr D, Woods SC, Seeley RJ, Weigle DS 1996 Specificity of leptin action on elevated blood glucose levels and hypothalamic neuropeptide Y gene expression in ob/ob mice. Diabetes 45:531–535 [DOI] [PubMed] [Google Scholar]
- Stephens TW, Basinski M, Bristow PK, Bue-Valleskey JM, Burgett SG, Craft L, Hale J, Hoffmann J, Hsiung HM, Kriauciunas A, MacKellar W, Rosteck Jr PR, Schoner B, Smith D, Tinsley FC, Zhang XY, Heiman M 1995 The role of neuropeptide Y in the antiobesity action of the obese gene product. Nature 377:530–532 [DOI] [PubMed] [Google Scholar]
- Gropp E, Shanabrough M, Borok E, Xu AW, Janoschek R, Buch T, Plum L, Balthasar N, Hampel B, Waisman A, Barsh GS, Horvath TL, Brüning JC 2005 Agouti-related peptide-expressing neurons are mandatory for feeding. Nature neuroscience 8:1289–1291 [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD 2005 NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science (New York, NY) 310:683–685 [DOI] [PubMed] [Google Scholar]
- Bewick GA, Gardiner JV, Dhillo WS, Kent AS, White NE, Webster Z, Ghatei MA, Bloom SR 2005 Post-embryonic ablation of AgRP neurons in mice leads to a lean, hypophagic phenotype. FASEB J 19:1680–1682 [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M 1998 Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585 [DOI] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T 2001 Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron 30:345–354 [DOI] [PubMed] [Google Scholar]
- Hara J, Yanagisawa M, Sakurai T 2005 Difference in obesity phenotype between orexin-knockout mice and orexin neuron-deficient mice with same genetic background and environmental conditions. Neurosci Lett 380:239–242 [DOI] [PubMed] [Google Scholar]
- Nishino S, Ripley B, Overeem S, Nevsimalova S, Lammers GJ, Vankova J, Okun M, Rogers W, Brooks S, Mignot E 2001 Low cerebrospinal fluid hypocretin (orexin) and altered energy homeostasis in human narcolepsy. Ann Neurol 50:381–388 [DOI] [PubMed] [Google Scholar]
- Funato H, Tsai AL, Willie JT, Kisanuki Y, Williams SC, Sakurai T, Yanagisawa M 2009 Enhanced orexin receptor-2 signaling prevents diet-induced obesity and improves leptin sensitivity. Cell Metab 9:64–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majdic G, Young M, Gomez-Sanchez E, Anderson P, Szczepaniak LS, Dobbins RL, McGarry JD, Parker KL 2002 Knockout mice lacking steroidogenic factor 1 are a novel genetic model of hypothalamic obesity. Endocrinology 143:607–614 [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker KL 1994 A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell 77:481–490 [DOI] [PubMed] [Google Scholar]
- Ikeda Y, Lala DS, Luo X, Kim E, Moisan MP, Parker KL 1993 Characterization of the mouse FTZ-F1 gene, which encodes a key regulator of steroid hydroxylase gene expression. Mol Endocrinol (Baltimore, Md) 7:852–860 [DOI] [PubMed] [Google Scholar]
- Sternson SM, Shepherd GM, Friedman JM 2005 Topographic mapping of VMH –> arcuate nucleus microcircuits and their reorganization by fasting. Nat Neurosci 8:1356–1363 [DOI] [PubMed] [Google Scholar]
- Weingarten HP, Chang PK, McDonald TJ 1985 Comparison of the metabolic and behavioral disturbances following paraventricular- and ventromedial-hypothalamic lesions. Brain Res Bull 14:551–559 [DOI] [PubMed] [Google Scholar]
- Holder Jr JL, Butte NF, Zinn AR 2000 Profound obesity associated with a balanced translocation that disrupts the SIM1 gene. Hum Mol Genet 9:101–108 [DOI] [PubMed] [Google Scholar]
- Michaud JL, Rosenquist T, May NR, Fan CM 1998 Development of neuroendocrine lineages requires the bHLH-PAS transcription factor SIM1. Genes Dev 12:3264–3275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balthasar N, Dalgaard LT, Lee CE, Yu J, Funahashi H, Williams T, Ferreira M, Tang V, McGovern RA, Kenny CD, Christiansen LM, Edelstein E, Choi B, Boss O, Aschkenasi C, Zhang CY, Mountjoy K, Kishi T, Elmquist JK, Lowell BB 2005 Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 123:493–505 [DOI] [PubMed] [Google Scholar]
- Harris M, Aschkenasi C, Elias CF, Chandrankunnel A, Nillni EA, Bjøorbaek C, Elmquist JK, Flier JS, Hollenberg AN 2001 Transcriptional regulation of the thyrotropin-releasing hormone gene by leptin and melanocortin signaling. J Clin Invest 107:111–120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Aschkenasi CJ, Lee CE, Mountjoy KG, Saper CB, Elmquist JK 2003 Expression of melanocortin 4 receptor mRNA in the central nervous system of the rat. J Comp Neurol 457:213–235 [DOI] [PubMed] [Google Scholar]
- Bellinger LL, Bernardis LL 2002 The dorsomedial hypothalamic nucleus and its role in ingestive behavior and body weight regulation: lessons learned from lesioning studies. Physiol Behav 76:431–442 [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Ahima RS, Maratos-Flier E, Flier JS, Saper CB 1997 Leptin activates neurons in ventrobasal hypothalamus and brainstem. Endocrinology 138:839–842 [DOI] [PubMed] [Google Scholar]
- Gautron L, Lazarus M, Scott MM, Saper CB, Elmquist JK 2010 Identifying the efferent projections of leptin-responsive neurons in the dorsomedial hypothalamus using a novel conditional tracing approach. J Comp Neurol 518:2090–2108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill JW, Elias CF, Fukuda M, Williams KW, Berglund ED, Holland WL, Cho YR, Chuang JC, Xu Y, Choi M, Lauzon D, Lee CE, Coppari R, Richardson JA, Zigman JM, Chua S, Scherer PE, Lowell BB, Brüning JC, Elmquist JK 2010 Direct insulin and leptin action on pro-opiomelanocortin neurons is required for normal glucose homeostasis and fertility. Cell Metab 11:286–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banno R, Zimmer D, De Jonghe BC, Atienza M, Rak K, Yang W, Bence KK 2010 PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest 120:720–734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS 2006 Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab 4:123–132 [DOI] [PubMed] [Google Scholar]
- Schwartz MW, Woods SC, Seeley RJ, Barsh GS, Baskin DG, Leibel RL 2003 Is the energy homeostasis system inherently biased toward weight gain? Diabetes 52:232–238 [DOI] [PubMed] [Google Scholar]
- Seeley RJ, Yagaloff KA, Fisher SL, Burn P, Thiele TE, van Dijk G, Baskin DG, Schwartz MW 1997 Melanocortin receptors in leptin effects. Nature 390:349 [DOI] [PubMed] [Google Scholar]
- van de Wall E, Leshan R, Xu AW, Balthasar N, Coppari R, Liu SM, Jo YH, MacKenzie RG, Allison DB, Dun NJ, Elmquist J, Lowell BB, Barsh GS, de Luca C, Myers Jr MG, Schwartz GJ, Chua Jr SC 2008 Collective and individual functions of leptin receptor modulated neurons controlling metabolism and ingestion. Endocrinology 149:1773–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes MR, Skibicka KP, Leichner TM, Guarnieri DJ, DiLeone RJ, Bence KK, Grill HJ 2010 Endogenous leptin signaling in the caudal nucleus tractus solitarius and area postrema is required for energy balance regulation. Cell Metab 11:77–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua Jr SC, Lowell BB, Elmquist JK 2005 The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab 1:63–72 [DOI] [PubMed] [Google Scholar]
- Huo L, Gamber K, Greeley S, Silva J, Huntoon N, Leng XH, Bjørbaek C 2009 Leptin-dependent control of glucose balance and locomotor activity by POMC neurons. Cell Metab 9:537–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Könner AC, Janoschek R, Plum L, Jordan SD, Rother E, Ma X, Xu C, Enriori P, Hampel B, Barsh GS, Kahn CR, Cowley MA, Ashcroft FM, Brüning JC 2007 Insulin action in AgRP-expressing neurons is required for suppression of hepatic glucose production. Cell Metab 5:438–449 [DOI] [PubMed] [Google Scholar]
- Yaswen L, Diehl N, Brennan MB, Hochgeschwender U 1999 Obesity in the mouse model of pro-opiomelanocortin deficiency responds to peripheral melanocortin. Nat Med 5:1066–1070 [DOI] [PubMed] [Google Scholar]
- Tsujii S, Bray GA 1989 Acetylation alters the feeding response to MSH and β-endorphin. Brain Res Bull 23:165–169 [DOI] [PubMed] [Google Scholar]
- Xu Y, Jones JE, Kohno D, Williams KW, Lee CE, Choi MJ, Anderson JG, Heisler LK, Zigman JM, Lowell BB, Elmquist JK 2008 5-HT2CRs expressed by pro-opiomelanocortin neurons regulate energy homeostasis. Neuron 60:582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plum L, Lin HV, Dutia R, Tanaka J, Aizawa KS, Matsumoto M, Kim AJ, Cawley NX, Paik JH, Loh YP, DePinho RA, Wardlaw SL, Accili D 2009 The obesity susceptibility gene Cpe links FoxO1 signaling in hypothalamic pro-opiomelanocortin neurons with regulation of food intake. Nat Med 15:1195–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P, Li C, Haskell-Luevano C, Cone RD, Smith MS 1999 Altered expression of agouti-related protein and its colocalization with neuropeptide Y in the arcuate nucleus of the hypothalamus during lactation. Endocrinology 140:2645–2650 [DOI] [PubMed] [Google Scholar]
- Qian S, Chen H, Weingarth D, Trumbauer ME, Novi DE, Guan X, Yu H, Shen Z, Feng Y, Frazier E, Chen A, Camacho RE, Shearman LP, Gopal-Truter S, MacNeil DJ, Van der Ploeg LH, Marsh DJ 2002 Neither agouti-related protein nor neuropeptide Y is critically required for the regulation of energy homeostasis in mice. Mol Cell Biol 22:5027–5035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JT, Kalra PS, Crowley WR, Kalra SP 1984 Neuropeptide Y and human pancreatic polypeptide stimulate feeding behavior in rats. Endocrinology 115:427–429 [DOI] [PubMed] [Google Scholar]
- Rossi M, Kim MS, Morgan DG, Small CJ, Edwards CM, Sunter D, Abusnana S, Goldstone AP, Russell SH, Stanley SA, Smith DM, Yagaloff K, Ghatei MA, Bloom SR 1998 A C-terminal fragment of Agouti-related protein increases feeding and antagonizes the effect of alpha-melanocyte stimulating hormone in vivo. Endocrinology 139:4428–4431 [DOI] [PubMed] [Google Scholar]
- Phillips CT, Palmiter RD 2008 Role of agouti-related protein-expressing neurons in lactation. Endocrinology 149:544–550 [DOI] [PubMed] [Google Scholar]
- Mesaros A, Koralov SB, Rother E, Wunderlich FT, Ernst MB, Barsh GS, Rajewsky K, Brüning JC 2008 Activation of Stat3 signaling in AgRP neurons promotes locomotor activity. Cell Metab 7:236–248 [DOI] [PubMed] [Google Scholar]
- El-Haschimi K, Pierroz DD, Hileman SM, Bjørbaek C, Flier JS 2000 Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest 105:1827–1832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D 2008 Hypothalamic IKKβ/NF-κB and ER stress link overnutrition to energy imbalance and obesity. Cell 135:61–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addy C, Wright H, Van Laere K, Gantz I, Erondu N, Musser BJ, Lu K, Yuan J, Sanabria-Bohórquez SM, Stoch A, Stevens C, Fong TM, De Lepeleire I, Cilissen C, Cote J, Rosko K, Gendrano 3rd IN, Nguyen AM, Gumbiner B, Rothenberg P, de Hoon J, Bormans G, Depré M, Eng WS, Ravussin E, Klein S, Blundell J, Herman GA, Burns HD, Hargreaves RJ, Wagner J, Gottesdiener K, Amatruda JM, Heymsfield SB 2008 The acyclic CB1R inverse agonist taranabant mediates weight loss by increasing energy expenditure and decreasing caloric intake. Cell Metab 7:68–78 [DOI] [PubMed] [Google Scholar]
- Després JP, Golay A, Sjöström L 2005 Effects of rimonabant on metabolic risk factors in overweight patients with dyslipidemia. N Engl J Med 353:2121–2134 [DOI] [PubMed] [Google Scholar]
- Di Marzo V 2008 Targeting the endocannabinoid system: to enhance or reduce? Nat Rev 7:438–455 [DOI] [PubMed] [Google Scholar]