Abstract
We tested the working hypothesis that Fos will identify the critical population of kisspeptin neurons that accompanies the LHRH surge using a synchronized follicular phase model in intact cycling ewes. The model generates an LH surge that starts within a defined 2-h window in a 20-d synchronized cycle. With a modified push-pull cannula in vivo LHRH release from the median eminence was sampled in luteal phase ewes, ewes undergoing an LH surge for 2–4 h, and postsurge animals whose LH surge peaked 10–12 h earlier. In vivo release of LHRH was lower in the luteal and follicular phases than in animals undergoing an LH surge (P < 0.01); it fell to presurge levels after the LH surge. Ewes killed 2–4 h after the surge started, expressed Fos in a large portion of preoptic area (POA) kisspeptin (53.90 ± 4.69%, P < 0.01) and LHRH neurons (48.20 ± 4.49%, P < 0.0001) compared with animals euthanized at any of the other times tested (under <5% of the cells activated). Little Fos activation (under 5%) was observed during any of the times sampled in arcuate (Arc) kisspeptin neurons. The relationship between the number of LHRH neurons and the POA kisspeptin neurons stimulated showed a striking positive correlation with r2 = 0.68, P = 0.0003, reinforcing the evidence that POA kisspeptin neurons actively participate in the stimulation of LHRH surges.
Preoptic area, but not arcuate, kisspeptin neurons are synchronously activated with LHRH neurons during LH/LHRH surges in ewes.
The discovery that kisspeptins are essential for reproductive function and are found in two discrete populations of neurons, one in the preoptic area (POA) and a second in the hypothalamic arcuate nucleus (Arc), prompted speculation over the role each population plays in LH secretion. In rats and mice, growing evidence identifies the POA kisspeptin population in estrogen’s positive feedback effects at the time of the preovulatory LH surge, whereas the Arc kisspeptin cells are thought to stimulate LHRH neurons after removal of negative feedback (1). These conclusions are derived from multiple lines of evidence from studies in rodents: 1) the expression of KiSS-1 mRNA is elevated by estrogen in the anteroventral periventricular preoptic nucleus (AVPV) but suppressed by the same treatment in the Arc (2,3); 2) the stimulation of Fos in LHRH neurons at the time of a preovulatory LH surge is accompanied by stimulated Fos expression in AVPV but not in Arc kisspeptin neurons (4); 3) without the AVPV there is no positive feedback by estrogen on gonadotropin secretion, even though the LHRH system and Arc are both intact (5,6,7,8); 4) excitotoxic lesions of the Arc affect negative, but not positive, estrogen regulation of gonadotropins (9,10,11); and lastly, 5) the greatest expression of estrogen receptor-α is in the female rodent’s AVPV, and administration of estrogen antagonists into the POA blocks positive feedback (12).
In ewes, activation of Fos in LHRH neurons at the time of the LH surge presents a pattern similar to that in rodents (13). Synchrony of LHRH release into portal blood, LH release into the plasma, and Fos activation in LHRH neurons verifies LHRH activity is stimulated at the time of the LH surge as in other species. The ewe also has kisspeptin neurons in the POA and Arc (14). However, data question the POA as the primary site of estrogen’s action for stimulating the LHRH system. For example, whereas the POA sends a projection to LHRH neurons in ewes (15) just as it does in the rodent species (16), placement of estradiol implants to the POA does not evoke positive feedback release of gonadotropins in sheep (17). The mediobasal hypothalamus appears most sensitive for stimulating LH surges by estrogen, but it is unclear whether the kisspeptin neurons at that site show activity changes at the time of the surge. One recent report suggested that Arc kisspeptin neurons were activated shortly after estrogen exposure (18), but those studies did not link the activation of these neurons to the LH or LHRH surge. Thus, we tested the working hypothesis that Fos will identify the critical population of kisspeptin neurons that accompanies the LHRH surge using a synchronized follicular phase model in intact cycling ewes capable of generating an LH surge that begins in a defined 2-h window (19,20,21).
Materials and Methods
Adult cycling Dorset ewes raised at the Cook College Ovine Reproductive Facility were used (n = 53). All animals were between 2 and 4 yr of age with an average body weight of 50–70 kg. Ewes were fed a diet recommended by the National Research Council (22). Protocols were approved by the Animal Care and Use Committee at Rutger’s University according to National Institutes of Health (NIH) guidelines.
Synchronized model in intact cycling ewes
The model used has already been described (19,20,21). In short, progesterone (P4) SILASTIC packets (Dow Corning Inc., Midland, MI) were implanted on d 12 of the estrous cycle (luteal phase) and removed a week later (estrous detected by vasectomized ram is d 0). Estradiol (E2) implants were placed 16 h after P4 packets were removed and remained in the animal until 24 h after the sampling period ended. The onset of the LH surge occurred 22 (±2) h after E2 implants were introduced, with each LH surge beginning within the same 2-h period in consecutive 20-d synchronized cycles of the same ewe. Because of this precision, it was possible to position reproducible windows to sample in vivo median eminence (ME) release of LHRH and plasma LH and to euthanize ewes for the Fos experiments (Fig. 1).
Figure 1.
Diagram of the animal model and the sampling windows used in each of the experiments performed. The P4 packets were introduced on d 12 of the estrous cycle. E2 was implanted 16 h after the removal of the P4 packet and removed immediately after the LH surge ended (24 h later). The synchronized preovulatory surge of LH occurred within a 2-h window, 22 h after E2 implantation in consecutive synchronized cycles of the same ewe. For additional information on the model, see text.
In vivo release of LHRH and plasma LH
In vivo release of LHRH and LH was sampled for 2-h periods while each ewe was in the luteal phase (−20 h from E2 implantation), early follicular phase (4 h after E2 implantation), late follicular phase (12 h after E2 implantation), immediately before the LH surge (20 h after E2 implantation), during the LH surge (26 h after E2 implantation), and immediately after the LH surge (34 h after E2 implantation).
A modified push-pull cannula (PPC) repetitive sampling technique
A sterilized multiple-guide cannula assembly directed by dorsal and lateral roengentograms was attached to each skull at least 2 wk before the start of PPC sampling. The multiple-guide cannula assembly has parallel guide holes positioned 1.5 mm apart (48 holes in a 6 × 8 array) through which removable PPC probes were directed into the ME. The arrangement and specific RIAs (see below) allowed for the serial long-term assessment of in vivo LHRH release from a discrete area of the ewe ME, disturbing neither in vivo neuropeptide release from the ME nor spontaneous estrous cyclicity (19,20,21).
Jugular blood-sampling technique
All blood samples for LH were taken at 10-min intervals during PPC sampling and before euthanizing the animals used in the Fos experiment. Thus, the values represented are those of the PPC sampled ewes plus those used in the Fos experiment. Ewes were cannulated with polyethylene (PE 90; Bectin Dickenson, Sparks, MD) jugular catheters under ketamine HCl (25 mg/kg body weight) anesthesia before sampling. Blood samples (2 ml each) were drawn with sterile syringes and the catheter flushed with heparinized normal saline between blood withdrawals. Blood collected into heparinized borosilicate culture tubes for LH measurements was immediately centrifuged and the plasma frozen until assayed.
RIA for LHRH and LH
LHRH concentrations in the PPC perfusate were measured by a double-antibody RIA, using a synthetic LHRH standard (Peninsula Laboratories, Belmont, CA) and an LHRH antiserum (no. CRR 11B73, provided by Dr. V. D. Ramirez, University of Illinois, Urbana, IL). As previously reported (20), 50 μl of PPC perfusate was used for this purpose. The intraassay and interassay coefficients of variation for LHRH were 4.7 and 9.3%, respectively. Plasma sample aliquots of 200 μl in duplicate were used to measure LH concentrations (23). Antiovine LH serum (National Institutes of Health, Bethesda, MD) and ovariectomized plasma standards (calibrated based on NIH LH S12) were used for this RIA. The intra- and interassay coefficients of variation for LH were 8.2 and 10.3%, respectively. Mean values (which include the averages for all values for each 2 h sampling period of each ewe) are included for both LHRH and LH.
Immunocytochemical procedures and analyses
A parallel group of 22 ewes with jugular cannulae were euthanized during the period 20 h before E2 implantation (n = 2), immediately after 2 h of LH sampling ending at 20 h (n = 8), at 26 h (n = 9) after E2 implantation, and after the LH surge (34 h after E2, n = 2, or 6 d after the surge, n = 1). Two of these ewes had cannulae inserted into the ME and jugular vein and had been sampled for both molecules during a cycle but were euthanized in a later cycle. Sheep for Fos analyses were anesthetized and perfused transaortically with 4% paraformaldehyde containing 2% acrolein. After fixation, the brain was removed; blocked to isolate the entire diencephalon and forebrain from the cortex, temporal lobes, mesencephalon, and brainstem; sunk in 25% aqueous sucrose; and cut in serial 25-μm coronal sections with a freezing microtome. The sections were collected in serial 1-in-12 series and placed in tissue culture wells containing cryoprotectant antifreeze solution (24) and maintained at −20 C until staining was initiated. The brains from all the animals were initially used only for analysis of Fos expression in LHRH neurons and for Fos and β-endorphin analyses (now being prepared for publication). Sections remained from 15 of the ewes from this original study. Because tissue remained in storage under conditions that preserved the immunoreactivity of both Fos and neuropeptides (25), assay for kisspeptin/ Fos expression could be applied to the same animals several years later.
The methods used for Fos and LHRH staining were published previously (16,26,27,28,29,30,31,32), and the same protocol was used for Fos and kisspeptin. For Fos, incubation with rabbit anti-alu-Fos (provided by Dr. Thomas Curran, Roche Institute, Nutley, NJ; 1:50,000 in PBS containing 0.4% Triton X-100) or rabbit anti-cFos (amino acids 1–17, bleed 4191; Oncogene Sciences, Boston, MA; 1:200,000–500,000) proceeded at 4 C for 48 h. After staining for Fos with nickel diaminobenzidine (DAB), the tissue was incubated with rabbit antikisspeptin [1:150,000 or 1:300,000 (14)] or rabbit anti-LHRH (LR-1 from Drs. Benoit and Guillemin, Salk Institute, La Jolla, CA at 1:150,000) for 48 h at 4 C followed by avidin biotin complex staining using DAB as the chromogen. The use of two consecutive staining series employing primary antibodies generated in the same series does not present a confound since the NiDAB deposited in the first reaction series was exclusively nuclear and masked any further reactivity of enzymes or antibodies in that compartment (33,34). After staining, the tissue was transferred to Tris-buffered saline to stop the reaction, rinsed in saline, mounted on gelatin, chrome alum-subbed glass slides, dehydrated through graded ethanol solutions, cleared in Histoclear, and coverslipped with Histomount (VWR Scientific, West Chester, PA). Controls for specificity of each of the antibodies were determined by preadsorption with purified antigen. The presence of Fos was evident as a blue-black reaction product in cell nuclei. LHRH or kisspeptin immunoreactivities within the cell cytoplasm were stained brown. Although data from all the ewes were graphed to show general patterns of activation, statistical tests were performed only on the groups with more than three ewes.
In a subset of the ewes (n = 6), triple labeling of Fos, LHRH, and kisspeptin was performed. In studies that use immunofluorescence for localization of transmitters and NiDAB for Fos, we found no difference in detection of neurons compared with detection that used only NiDAB/DAB double labeling of the cells. However, the fluorescence triple labeling enabled more parsimonious use of sections because it enabled analysis of both LHRH and kisspeptin in the same series of sections. Fos was stained with NiDAB as described above. Because this antigen is nuclear, there is no opportunity for the immunoreaction product to obscure or be confused with the cytoplasmic localization of the kisspeptin and LHRH. In so doing, we verified that the LHRH Fos patterns originally obtained in the ewes remained stable, and we could accurately document the locations of both LHRH and kisspeptin neurons that were activated in the same sections. The antikisspeptin was applied at a concentration of 1:70,000 and was reacted using tyramine signal amplified fluorescence as described previously (26) with Texas Red as the fluorophore; anti-LHRH was next applied (1:15,000) and then reacted with a Cy-2-tagged secondary antibody (Molecular Probes, Eugene, OR). The two-fluorescence approach does not produce the possibility of false-positive double labeling for reasons outlined by Shindler and Roth (35). In addition, the choice of the correct fluorescent molecules ensured that no bleed-through of the first fluorophore with the second could occur (36).
All stained LHRH or kisspeptin neurons in a 1-in-12 series of sections were analyzed for each animal, and fluorescent and immuoperoxidase double-stained material were grouped together. In this and all the other analyses, sections were coded so the person counting cells was blind to the cycle stage of the ewe. Each LHRH- or kisspeptin-immunoreactive cell with a visible nucleus was counted and scored for the presence of Fos under the microscope at a magnification of ×200. Analysis included both total numbers of cells and the percentage of cells for each population that coexpressed the Fos protein. Fos expression in LHRH or kisspeptin neurons is presented as the percentage of these neurons expressing Fos.
Statistical analysis
Analysis of temporal differences in release of LHRH during select times during the synchronized estrous cycle was determined by one-way ANOVA for repeated measurements. The multiple comparison of differences in the means was done using the least significant difference test (37). Differences in the expression of Fos in LHRH and kisspeptin neurons before and at the peak of the LH surge were determined by ANOVA; the group of two − 20-h luteal phase ewes and the three postsurge ewes in the Fos study are presented in the graph to show their levels of activation compared with the other groups but were not included for statistical analyses. A P < 0.05 was used as the level of significance.
Results and Discussion
Figure 2 presents the mean plasma levels of LH (mean ± sem) and mean ME in vivo release of LHRH at various times preceding and immediately after a synchronized LH preovulatory surge. Release of LHRH and LH was low in luteal phase animals (−20 h from E2), in early follicular ewes (4 h after E2), and after the LH surge (34 h after E2). In vivo release of LHRH rose in the late follicular phase ewes 12–20 h after E2 (P < 0.01). The peak rise in released LHRH accompanied the LH surge at 26 h after E2. Values in parentheses are the numbers of animals.
Figure 2.
Mean in vivo ME release of LHRH (A) and plasma LH (B) at specified times before and after implantation of E2.
Figure 3 shows plots of the location of LHRH and kisspeptin neurons to illustrate the relative positions of the LHRH cells in relation to the two kisspeptin populations. Figure 3, A–G, represents a series through the rostral forebrain and POA. Figure 3, H and I, represents more caudal regions that include the Arc and dorsomedial nuclei. LHRH neurons were located throughout the forebrain and extended into the anterior hypothalamus; occasional cells were found in the medial basal hypothalamus. The majority of LHRH neurons were located within the regions immediately rostral to and surrounding the organum vasculosum of the lamina terminalis (OVLT) (levels in Fig. 3, C–F). The number of LHRH neurons detected (overall mean 194.44 for every 12th section) remained unchanged over the course of the cycle. Kisspeptin neurons of the POA lie immediately lateral to the OVLT, rostral to the third ventricle (VIII, Fig. 3, E and F); fewer kisspeptin neurons were seen once the third ventricle (VIII) was present (Fig. 3G); these were mainly positioned dorsally within the periventricular POA. Within the medial basal hypothalamus (Fig. 3, H and I), the second population of kisspeptin neurons was located; these were most numerous at the more caudal pole of the Arc (Fig. 3I). No kisspeptin neurons were noted in the dorsomedial nucleus, nor were kisspeptin cells found elsewhere in the hypothalamus. As was noted for LHRH, numbers of kisspeptin neurons did not vary with cycle stage in either the POA or Arc (POA mean 126.07 for every 12th section; arcuate 285.89). In sections from animals that were double labeled for kisspeptin and LHRH using immunofluorescence, none of the LHRH neurons showed coexpression with kisspeptin. An example of this is shown in Fig. 4A. In addition, the ewes displayed a distinct innervation of the median eminence by kisspeptin, likely arising from the Arc kisspeptin neurons. None of the LHRH axons in the ME (Fig. 4B) or elsewhere in the brain were double labeled for kisspeptin.
Figure 3.
Graphic representation of the location of LHRH (red) and kisspeptin neurons (blue) in the ewe. A–G levels represent a series of section space 350 μm apart extending from the rostal forebrain to the junction between the POA and the anterior hypothalamus. H and I levels depict the regions of the rostral and caudal median eminence. AC, Anterior commissure; fx, fornix; LV, lateral ventricle; mPOA, medial preoptic area; OC, optic chiasm; VIII, third ventricle.
Figure 4.
A, Double labeling for kisspeptin (red) and LHRH (green) indicates that the LHRH neurons do not coexpress kisspeptin. Bar, 25 μm. B, In the ME (external zone), in which the LHRH neurons terminate, although numerous kisspeptin axons are found (red), those kisspeptin terminals are distinct from the LHRH terminals (green), supporting the notion that the LHRH neurons do not coexpress kisspeptin. Bar, 25 μm.
The percentages of activated POA LHRH neurons, POA kisspeptin, and Arc kisspeptin neurons are summarized in Fig. 5. POA LHRH neurons showed little activation (<1% overall) in animals killed before the peak of the LH surge or after the surge ended (Fig. 5A). The degree of LHRH Fos activation increased markedly at the time of the surge peak, reaching mean levels just under 50% just after the peak of the LH surge (26 h after E2). Fos activation in LHRH neurons returned to baseline presurge levels after the LH surge had subsided. There is a lag in the time that it takes for Fos to be induced by neuronal stimulation, and thus, not surprisingly, LHRH and LH are elevated before Fos in the LHRH and kisspeptin neurons. It should be noted that even in animals exhibiting the highest level of LHRH activation, not all LHRH neurons expressed Fos. Specifically, LHRH neurons in the most rostral part of the forebrain, rostral to the OVLT (levels shown in Fig. 3, A and B), did not show Fos activation under any condition. Cells in the remaining portions of the LHRH population (from level 3C extending to the level of the rostral ME) showed a surge-related change in Fos expression.
Figure 5.
Bar graphs showing the Fos activation in LHRH (A) POA kisspeptin (KP) neurons (B) and Arc kisspeptin neurons (C) before, during, and after the LH surge. Bars with different letters are significant at P < 0.05.
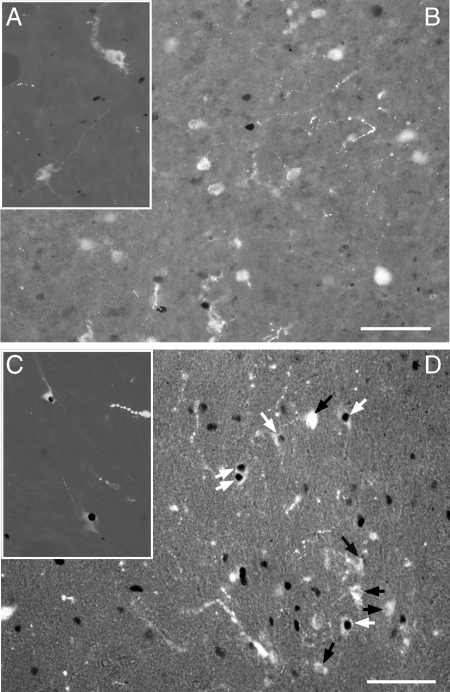
POA kisspeptin neurons showed high levels of activation at the time of the LH surge as well (Fig. 5B). A significant positive correlation between the proportion of POA kisspeptin neurons expressing Fos and the percentage of LHRH neurons that were activated was noted (r2 = 0.676, P = 0.0003). In contrast, Arc kisspeptin neurons showed little Fos activation at any time (Fig. 5C). Small numbers of Arc kisspeptin neurons expressed Fos before E2 administration in some ewes (two of the presurge ewes showed 3–4% activation; the other two showed either no Fos activation or only one activated kisspeptin neuron). Figure 6 shows micrographs from two ewes: one euthanized before the LH surge (Fig. 6, A and B) and the other at the time of the LH surge (Fig. 6, C and D), showing LHRH neurons near the OVLT (Fig. 6, A and C) and POA kisspeptin neurons (Fig. 6, B and D). Note that when POA kisspeptin neurons were activated, LHRH neurons were also stimulated. In Figure 7, the kisspeptin neurons of the Arc show little Fos activation either before (Fig. 7A) or during the LH surge (Fig. 7B). This feature was true for the entirety of the Arc.
Figure 6.
Micrographs of localization of POA Fos (black)/LHRH (white) (A and C) and POA Fos/kisspeptin (B and D) from a representative ewe euthanized before the ascending phase of the LH surge 20 h after E2 implantation (A and B) and one ewe euthanized during the LH surge 26 h after E2 implantation (which was 210 min after the onset of the surge) in C and D. The sections shown were derived from material from triple-labeled sections usually from a level between that illustrated in Fig. 4, E and F; results were identical to those in double-labeled sections. Before the surge, few kisspeptin neurons or LHRH neurons showed Fos immunoreactivity, whereas at the time of the surge, numerous kisspeptin and LHRH neurons were Fos+. White arrows, Fos+ kisspeptin cells; black arrows, Fos-kisspeptin cells. Bars, 25 μm. Postsurge animals and animals killed during the luteal phase were similar to the presurge animals.
Figure 7.
Micrographs of localization of Fos (black) and kisspeptin (white) in the Arc (at the level illustrated in Fig. 5I) from a ewe euthanized before the ascending phase of the LH surge (A, 20 h after E2 implantation) and a ewe euthanized during the LH surge (B, 26 h after E2 implantation). At either time, few kisspeptin neurons expressed Fos (white arrows). Bars, 100 μm.
Activation of Fos in LHRH neurons after synchronization of the preovulatory surge of LH and LHRH in cycling ewes is quite similar to that observed after the induction of a preovulatory-like LH surge in the ovariectomized ewe treated with exogenous hormones (13). Our data confirm the association of Fos in the majority of LHRH neurons as a marker for stimulated release of LHRH. We did, however, find that the most rostral portion of the LHRH population did not display Fos during LH surges, unlike the earlier report (13). A possible reason for this is that our sections may have encompassed regions positioned more rostrally than were assessed in the study by Moenter et al. (13). The presence of anterior LHRH cells that fail to show Fos activation at the time of the LH surge in our animals appears quite similar to the rat (28).
Our analysis then takes the issue one step beyond the release of LHRH and activation of LHRH neurons to identify a key set of neurons that likely provide a stimulatory drive to the LHRH neurons at the time of the surge: the kisspeptin neurons. In sheep (14), as in the rat and mouse (2,3,4), kisspeptin neurons reside in two distinct locations: the Arc and the POA. In the rat, it is the POA kisspeptin population that becomes active at the time of the LH surge (4). Based on Fos activation patterns, the ewe’s POA kisspeptin cells showed striking changes coincident with the LHRH surge and the timing and patterns of Fos activation within POA kisspeptin neurons are reminiscent of those seen in rodents. In contrast, similar changes could not be detected in the Arc kisspeptin population. After estrogen administration, Fos activity in the Arc kisspeptin neurons decreased, and no change coincident with the surge was found. The observation that KiSS-1 mRNA expression was transiently elevated in the caudal Arc nucleus in ewes, in the late follicular phase (38) prompted speculation that estrogen-positive feedback was effected through those neurons and not those in the POA. A recent report that 1–2 h after administration of estradiol 12–18 h before the onset of the LH surge Arc kisspeptin neurons expressed Fos (18) prompted speculation that the Arc kisspeptin neurons contribute to estrogen-positive feedback of gonadotropins. Our data do not support a striking change in Arc kisspeptin neuron activity that actually accompanies the surge. One possible reason for this is that use of ovariectomized animals with replacement of high estrogen levels could produce different activation patterns than are seen when physiological hormone levels are used in intact animals. This feature is underscored by the studies that show LH surges in G protein-coupled receptor 54 (the kisspeptin receptor) knockout mice given high estrogen replacement (39), whereas either kisspeptin knockout mice or mice lacking G protein-coupled receptor 54 fail to cycle spontaneously (40). Nonetheless, although suggesting that the Arc nucleus kisspeptin system does not actively drive LHRH activity at the time of the surge, our study leaves open the possibility that some transient activation present in the first hours after estrogen administration plays a role in preparing the LHRH system for a later surge.
In an earlier study, kisspeptin neurons were also observed in the dorsomedial nucleus (DMN) of the hypothalamus in the ewe (14). No DMN-immunoreactive kisspeptin neurons were detected in our ewes. A likely explanation is that DMN neurons express much lower amounts of kisspeptin, and any peptide synthesized is rapidly transported from the soma to the terminals. The initial description of the DMN cells was derived from colchicine-treated ewes, and for many peptidergic systems rapid processing and movement out of the soma is found. Alternatively, the use of colchicine could provoke changes in peptide expression that are not normally found. Thorough mRNA analyses in the DMN after colchicine are needed to resolve this issue. An earlier report (41) had suggested that a few LHRH neurons coexpressed kisspeptin, but reevaluation did not confirm colocalization (42). Our data reinforce separation of the two systems.
In conclusion, results from this work are consistent with a role of POA kisspeptin neurons in actively stimulating LHRH neurons during the preovulatory surge of LH. Whereas the surge may also rely on stimulation of neurons in the medial basal hypothalamus during the period of time that estrogen levels first rise early in the follicular phase, if it does, those kisspeptin neurons do not appear to express Fos as the surge is provoked.
Footnotes
This work was supported by National Institute of Neurological Disorders and Stroke Grant NS28730 (to G.E.H.), a Bridge Grant from the Endocrine Society (to G.E.H.), the “French Kiss” Agence Nationale de la Recherche Grant (to A.C. and I.F.), the New Jersey Agricultural Experiment Station Project (New Jersey Agricultural Experiment Station-Hatch 06108) (to J.P.A.), and the U.S. Department of Agriculture (to J.P.A.).
Disclosure Summary: The authors have nothing to disclose.
Abbreviations: Arc, Arcuate nucleus; AVPV, anteroventral periventricular preoptic nucleus; DAB, diaminobenzidine; DMN, dorsomedial nucleus; E2, estradiol; ME, median eminence; OVLT, organum vasculosum of the lamina terminalis; P4, progesterone; POA, preoptic area; PPC, push-pull cannula.
First Published Online November 3, 2010
References
- Dungan HM, Clifton DK, Steiner RA 2006 Minireview: kisspeptin neurons as central processors in the regulation of gonadotropin-releasing hormone secretion. Endocrinology 147:1154–1158 [DOI] [PubMed] [Google Scholar]
- Smith JT, Cunningham MJ, Rissman EF, Clifton DK, Steiner RA 2005 Regulation of Kiss1 gene expression in the brain of the female mouse. Endocrinology 146:3686–3692 [DOI] [PubMed] [Google Scholar]
- Smith JT, Dungan HM, Stoll EA, Gottsch ML, Braun RE, Eacker SM, Clifton DK, Steiner RA 2005 Differential regulation of KiSS-1 mRNA expression by sex steroids in the brain of the male mouse. Endocrinology 146:2976–2984 [DOI] [PubMed] [Google Scholar]
- Smith JT, Popa SM, Clifton DK, Hoffman GE, Steiner RA 2006 Kiss1 neurons in the forebrain as central processors for generating the preovulatory luteinizing hormone surge. J Neurosci 26:6687–6694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnekleiv OK, Kelly MJ 1986 Luteinizing hormone-releasing hormone neuronal system during the estrous cycle of the female rat: effects of surgically induced persistent estrus. Neuroendocrinology 43:564–576 [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E 1982 Discrete lesions reveal functional heterogeneity of suprachiasmatic structures in regulation of gonadotropin secretion in the female rat. Neuroendocrinology 34:395–404 [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE 1978 Persistent estrus and blockade of progesterone-induced LH release follows lesions which do not damage the suprachiasmatic nucleus. Endocrinology 102:1645–1648 [DOI] [PubMed] [Google Scholar]
- Wiegand SJ, Terasawa E, Bridson WE, Goy RW 1980 Effects of discrete lesions of preoptic and suprachiasmatic structures in the female rat. Alterations in the feedback regulation of gonadotropin secretion. Neuroendocrinology 31:147–157 [DOI] [PubMed] [Google Scholar]
- Dyer RG, Weick RF, Mansfield S, Corbet H 1981 Secretion of luteinizing hormone in ovariectomized adult rats treated neonatally with monosodium glutamate. J Endocrinol 91:341–346 [DOI] [PubMed] [Google Scholar]
- Greeley Jr GH, Nicholson GF, Nemeroff CB, Youngblood WW, Kizer JS 1978 Direct evidence that the arcuate nucleus-median eminence tuberoinfundibular system is not of primary importance in the feedback regulation of luteinizing hormone and follicle-stimulating hormone secretion in the castrated rat. Endocrinology 103:170–175 [DOI] [PubMed] [Google Scholar]
- Inkster SE, Whitehead SA 1987 Increased responsiveness of the hypothalamic-pituitary axis to steroid feedback effects in ovariectomized rats treated neonatally with monosodium l-glutamate. Experientia 43:606–608 [DOI] [PubMed] [Google Scholar]
- Petersen SL, Barraclough CA 1989 Suppression of spontaneous LH surges in estrogen-treated ovariectomized rats by microimplants of antiestrogens into the preoptic brain. Brain Res 484:279–289 [DOI] [PubMed] [Google Scholar]
- Moenter SM, Karsch FJ, Lehman MN 1993 Fos expression during the estradiol-induced gonadotropin-releasing hormone (GnRH) surge of the ewe: induction in GnRH and other neurons. Endocrinology 133:896–903 [DOI] [PubMed] [Google Scholar]
- Franceschini I, Lomet D, Cateau M, Delsol G, Tillet Y, Caraty A 2006 Kisspeptin immunoreactive cells of the ovine preoptic area and arcuate nucleus co-express estrogen receptor α. Neurosci Lett 401:225–230 [DOI] [PubMed] [Google Scholar]
- Pompolo S, Pereira A, Scott CJ, Fujiyma F, Clarke IJ 2003 Evidence for estrogenic regulation of gonadotropin-releasing hormone neurons by glutamatergic neurons in the ewe brain: an immunohistochemical study using an antibody against vesicular glutamate transporter-2. J Comp Neurol 465:136–144 [DOI] [PubMed] [Google Scholar]
- Le WW, Berghorn KA, Rassnick S, Hoffman GE 1999 Periventricular preoptic area neurons coactivated with luteinizing hormone (LH)-releasing hormone (LHRH) neurons at the time of the LH surge are LHRH afferents. Endocrinology 140:510–519 [DOI] [PubMed] [Google Scholar]
- Caraty A, Fabre-Nys C, Delaleu B, Locatelli A, Bruneau G, Karsch FJ, Herbison A 1998 Evidence that the mediobasal hypothalamus is the primary site of action of estradiol in inducing the preovulatory gonadotropin releasing hormone surge in the ewe. Endocrinology 139:1752–1760 [DOI] [PubMed] [Google Scholar]
- Smith JT, Li Q, Pereira A, Clarke IJ 2009 Kisspeptin neurons in the ovine arcuate nucleus and preoptic area are involved in the preovulatory luteinizing hormone surge. Endocrinology 150:5530–5538 [DOI] [PubMed] [Google Scholar]
- Conover CD, Kuljis RO, Rabii J, Advis JP 1993 β-Endorphin regulation of luteinizing hormone-releasing hormone release at the median eminence in ewes: immunocytochemical and physiological evidence. Neuroendocrinology 57:1182–1195 [DOI] [PubMed] [Google Scholar]
- Conover C, Kuljis R, Rabii J, Advis JP 1993 Serial long-term assessment of in vivo LHRH release from a discrete area of the ewe median eminence using multiple guide cannula assembly and removable push-pull cannulae. Neuroendocrinology 57:1119–1132 [DOI] [PubMed] [Google Scholar]
- Advis JP, Klein J, Kuljis RO, Sarkar DK, McDonald JM, Conover CA 2003 Regulation of gonadotropin releasing hormone release by neuropeptide Y at the median eminence during the preovulatory period in ewes. Neuroendocrinology 77:246–257 [DOI] [PubMed] [Google Scholar]
- National Research Council 1985 Nutrient requirements for sheep. 6th ed. Washington, DC: National Academy Press [Google Scholar]
- Niswender G, Roche JF, Foster DL, Midgley Jr AR 1968 Radioimmunoassay of serum levels of luteinizing horomone during the cycle and early pregnancy in ewes. Proc Soc Exp Biol Med 129:901–904 [DOI] [PubMed] [Google Scholar]
- Watson RE, Wiegand SJ, Clough RW, Hoffman GE 1986 Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides 7:155–159 [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Le WW 2004 Just cool it! Cryoprotectant anti-freeze in immunocytochemistry and in situ hybridization. Peptides 25:425–431 [DOI] [PubMed] [Google Scholar]
- Berghorn KA, Bonnett JH, Hoffman GE 1994 cFos immunoreactivity is enhanced with biotin amplification. J Histochem Cytochem 42:1635–1642 [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Le WW, Schulterbrandt T, Legan SJ 2005 Estrogen and progesterone do not activate Fos in AVPV or LHRH neurons in male rats. Brain Res 1054:116–124 [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Lee WS, Attardi B, Yann V, Fitzsimmons MD 1990 Luteinizing hormone-releasing hormone neurons express c-fos antigen after steroid activation. Endocrinology 126:1736–1741 [DOI] [PubMed] [Google Scholar]
- Lee WS, Abbud R, Hoffman GE, Smith MS 1993 Effects of N-methyl-d-aspartate receptor activation on cFos expression in luteinizing hormone-releasing hormone neurons in female rats. Endocrinology 133:2248–2254 [DOI] [PubMed] [Google Scholar]
- Lee WS, Smith MS, Hoffman GE 1990 Progesterone enhances the surge of luteinizing hormone by increasing the activation of luteinizing hormone-releasing hormone neurons. Endocrinology 127:2604–2606 [DOI] [PubMed] [Google Scholar]
- Lee WS, Smith MS, Hoffman GE 1990 Luteinizing hormone-releasing hormone neurons express Fos protein during the proestrous surge of luteinizing hormone. Proc Natl Acad Sci USA 87:5163–5167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WS, Smith MS, Hoffman GE 1992 cFos activity identifies recruitment of LHRH neurons during the ascending phase of the proestrous luteinizing hormone surge. J Neuroendocrinol 4:161–166 [DOI] [PubMed] [Google Scholar]
- Joseph SA, Sternberger LA 1979 The unlabeled antibody method. Contrasting color staining of β-lipotropin and ACTH-associated hypothalamic peptides without antibody removal. J Histochem Cytochem 27:1430–1437 [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Smith MS, Fitzsimmons MD 1992 Detecting steroidal effects on immediate early gene expression in the hypothalamus. Neuroprotocols 1:52–66 [Google Scholar]
- Shindler KS, Roth KA 1996 Double immunofluorescent staining using two unconjugated primary antisera raised in the same species. J Histochem Cytochem 44:1331–1335 [DOI] [PubMed] [Google Scholar]
- Hoffman G 2009 Double your trouble, triple your fun: double labeling. Seattle, WA: Histochemical Society [Google Scholar]
- Zar J 1974 Biostatistical analysis. Englewood Cliffs, CA: Prentice Hall [Google Scholar]
- Estrada KM, Clay CM, Pompolo S, Smith JT, Clarke IJ 2006 Elevated KiSS-1 expression in the arcuate nucleus prior to the cyclic preovulatory gonadotrophin-releasing hormone/luteinising hormone surge in the ewe suggests a stimulatory role for kisspeptin in oestrogen-positive feedback. J Neuroendocrinol 18:806–809 [DOI] [PubMed] [Google Scholar]
- Dungan HM, Gottsch ML, Zeng H, Gragerov A, Bergmann JE, Vassilatis DK, Clifton DK, Steiner RA 2007 The role of kisspeptin-GPR54 signaling in the tonic regulation and surge release of gonadotropin-releasing hormone/luteinizing hormone. J Neurosci 27:12088–12095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapatto R, Pallais JC, Zhang D, Chan YM, Mahan A, Cerrato F, Le WW, Hoffman GE, Seminara SB 2007 Kiss1−/− mice exhibit more variable hypogonadism than Gpr54−/− mice. Endocrinology 148:4927–4936 [DOI] [PubMed] [Google Scholar]
- Pompolo S, Pereira A, Estrada KM, Clarke IJ 2006 Colocalization of kisspeptin and gonadotropin-releasing hormone in the ovine brain. Endocrinology 147:804–810 [DOI] [PubMed] [Google Scholar]
- Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN 2008 Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology 149:5770–5782 [DOI] [PMC free article] [PubMed] [Google Scholar]