Abstract
A member of the steroid receptor coactivator (SRC)/p160 family, SRC-3 acts as a coregulator for nuclear receptor (NR) and non-NR transcription factors. Such coregulator pleiotropy enables SRC-3 to influence a myriad of signaling networks that are essential for normal physiology and pathophysiology. Although SRC-3’s proliferative role in primary tumor formation in the mammary gland is well established, a role for this oncogenic coregulator in tumor cell motility and invasion has only recently been elucidated. In the nucleus, SRC-3 is required for the execution of the epithelial–mesenchymal transition, a programming step which endows an immotile cancer cell with motile and invasive characteristics. Nuclear SRC-3 is also essential for proteolytic breakdown of the extracellular matrix by matrix-metalloproteinases, a process which enables primary tumor cell invasion into the surrounding stroma. At the plasma membrane, however, a truncated isoform of SRC-3 (SRC-3Δ4) serves as a signaling adaptor for the epidermal growth factor→focal adhesion kinase→c-Src signal transduction pathway, a signaling cascade that is central to growth factor–induced cell migration and invasion. Together, these studies underscore a pivotal role for SRC-3 not only as a proto-oncogene but also as a prometastatic factor during the early steps in the invasion-metastasis cascade. Beyond furnishing critical mechanistic insights into SRC-3’s involvement in mammary tumor progression, these findings provide opportunities to develop new approaches for breast cancer diagnosis and intervention.
From the nucleus and plasma membrane, steroid receptor coactivator-3 projects its pro-metastatic effects through multiple signals that collectively control cell migration and invasion.
Breast cancer delivers its lethality through metastasizing to vital organs, a process which presents tremendous clinical challenges for treatment and disease management (1,2). Owing to its clinical importance, a greater understanding of the molecular mechanisms that underpin the metastatic process is an urgent priority for clinical and basic research alike. However, much of the complexity associated with this aspect of the neoplastic progression program is based on the multiple cellular and molecular steps that a primary tumor cell must take before it can form a metastatic lesion in a distant anatomic site (3).
For metastasis to manifest, the primary tumor cell must first acquire a motile and invasive phenotype that enables its invasion into the adjacent stroma and entry into the local circulation (intravasation). After dissemination through the vascular network, tumor cells must then exit microvessels of distant tissues (extravasation) to invade the tissue’s surrounding parenchyma to form micro-metastases. A subset of these micro-metastatic lesions may enlarge to macroscopic tumors [and indeed, these tumors in turn can metastasize—the so-called “metastasis of metastasis” effect (4)]. The establishment of micro-metastases and their expansion to macroscopic tumors is also termed metastatic colonization and is the least efficient stage of the invasion–metastasis cascade (3).
In this review, we describe recent advancements that have uncovered a pivotal role for steroid receptor coactivator-3 (SRC-3) during key steps in the invasion-metastasis cascade. Also known as Amplified In Breast Cancer-1 (5) [among other names (6)], SRC-3 is a member of the SRC/p160 family of coregulators which also includes SRC-1 and SRC-2 (7). As their moniker suggests, SRCs were originally identified as coregulators of steroid hormone receptors (8), such as the estrogen receptor-α (ER) and the progesterone receptor (PR), which are members of the nuclear receptor (NR) family of transcription factors. However, multiple studies since have demonstrated that SRCs also can act as coregulators for a broad-spectrum of non-NR transcription factors (7). Due to this coregulator pleiotropy, SRCs play crucial roles in many aspects of organ physiology and pathophysiology that are not necessarily dependent on steroid hormone signaling.
Rather than dwell on SRC-3’s established role in primary tumor initiation and expansion, this minireview focuses on new findings that reveal SRC-3 as a potent coregulator and signaling adapter during tumor cell motility and invasion, prerequisite steps in the invasion-metastasis cascade.
SRC-3: An Evolutionary Conserved Coregulator in Cell Motility and Invasion
An important early step in the invasion-metastasis cascade is that stationary tumor cells must acquire motility and invasive attributes to allow escape from the primary tumor site, invasion into the surrounding stroma, and entry into the vascular network (2). The first indication that SRC-3 is involved in cell migration and invasion originates from studies on oogenesis in the fruit fly ovary. Using a forward-genetic screen (9), the Montell laboratory identified Taiman, the Drosophila homolog of SRC-3, as indispensable for ovarian border cell migration and invasion during oogenesis. In the absence of SRC-3’s coregulator functions, ecdysone receptor–dependent border cell motility and invasiveness was markedly suppressed [SRC-3 is a known coactivator for the ecdysone steroid hormone receptor, which is a member of the NR superfamily (10)]. The motility/invasive phenotype displayed by border cells deficient in SRC-3 was associated with abnormal cellular build-up of E-cadherin, β-catenin, and focal adhesion complexes, which together provided the first molecular clues that the epithelial-mesenchymal transition (EMT) process is dependent on SRC-3 function (EMT is described in more detail below). Together, the insect data highlighted a novel coregulator role for SRC-3 in steroid hormone-initiated cell motility and invasion that is separate from its role in steroid hormone-dependent cellular proliferation, suggesting that SRC-3 may exert independent “mitogenic and motogenic effects” as a proto-oncogene and prometastatic coregulator in more evolved animals (9,11).
Both cell-culture and mouse studies would quickly confirm a similar role for SRC-3 in mammalian cell motility and invasion, underscoring the evolutionary importance of this coregulator in this aspect of cell behavior. By reducing SRC-3 levels alone, human ovarian cancer cells in culture failed to exhibit cellular spreading and migration on the substratum (12), such cellular dynamics represent signature traits of a motile cancer cell in vitro. Surprisingly, SRC-3 effects on ovarian cancer cell migration were shown to be independent of steroid hormone signaling, suggesting that SRC-3 may act as a coregulator of motility signaling networks that are not necessarily under steroid hormone control.
In the case of the mammary gland, absence of SRC-3 in the MMTV-polyoma middle T antigen (PyMT) transgenic mouse sharply attenuated mammary tumor metastasis to the lung (13). While not an oncogene in human breast cancer, PyMT expressed in the mammary epithelium of the MMTV-PyMT transgenic mouse activates many of the same signaling pathways that function downstream of HER2/neu in human breast cancers; these include the Shc/ras/MAPK protein kinase and the c-Src/PI3K/Akt pathways (14). Noteworthy, expression levels of SRC-3 (and SRC-1) are significantly elevated in PyMT-induced mammary tumors (13,15), and a similar expression pattern for both coregulators is also observed in HER2/neu-positive human breast tumors (16,17,18). Structurally related to the epidermal growth factor receptor (EGFR/HER1), HER2/neu is a transmembrane tyrosine kinase receptor which is overexpressed in 20–30% of human breast cancers (19). Invariably, HER2/neu-positive tumors exhibit an aggressive-growth phenotype with enhanced metastatic potential and a propensity for recurrence after standard adjuvant endocrine therapy (20).
From a mechanistic standpoint, the positive correlation in expression levels between both SRCs and HER2/neu coupled with recent molecular studies suggested a signaling connection between these factors (17,21,22). Indeed, the observation that PEA3, which is a member of the mitogen-activated protein kinase (MAPK) activated Ets transcription factor family (23,24), is also elevated along with SRC-3, SRC-1, and HER2/neu, suggests that SRCs may potentiate the HER2/neu signal through coregulating select members of the Ets transcription factor family (17). Furthermore, recent cell-based studies have shown that SRC-3 (and SRC-1) can directly coregulate Ets transcription factor members to enhance their transactivational properties (13,25). These findings are significant because Ets transcription factors have been implicated in the execution of a wide-range of transcriptional programs involved in extracellular remodeling and in the induction of HER2/neu (26,27), molecular pathways essential not only for tumor expansion, invasion, and metastasis but also for resistance to endocrine therapy (28,29). That absence of SRC-3 blocks mammary tumorigenesis in the MMTV-HER2/neu transgenic mouse provides compelling in vivo support for the proposal that SRC-3 is a limiting factor in HER2/neu dependent mammary tumorigenesis and metastasis (30).
In cell culture experiments, SRC-3 was shown to be pivotal for the execution of the EMT program that is required for PyMT tumor cell migration and invasion (13). As an evolutionary conserved developmental program, the EMT process is an essential early step in the conversion of immotile tumor cells into mesenchymal cells with enhanced migratory potential (31). With EMT, epithelial cells lose their defining markers (i.e. E-cadherin, zonula occludens and/or claudin family members) which are required for normal baso-apical polarity, cell–cell adhesion, and cell–substratum contact. In the absence of SRC-3, PyMT tumor cells in three-dimensional culture maintain their polarity, continue to express epithelial markers, and exhibit poor motility and invasiveness compared with wild-type PyMT tumor cells (13).
By virtue of their unique endopeptidase activities, matrix metalloproteinases (MMPs) enable tumor cell populations to breach the extracellular matrix (ECM) and invade the stromal compartment (32). In both human (MDA-MB-231) and PyMT tumor cells in culture, SRC-3 was shown to act as a direct coregulator for PEA3-driven expression of MMP-2 and MMP-9 (13). Apart from MMP-2 and -9, SRC-3 also serves as a coactivator of AP-1 driven MMP-7 and MMP-10 expression in MDA-MC231 cells (33). In addition, SRC-3 was shown to be critical for the expression of MMP-2 and MMP-13 via its simultaneous coactivation of AP-1 and PEA3 during prostate cancer metastasis (34). Together, these findings indicate that SRC-3 can coregulate different sets of transcription factors to induce different combinations of MMP family members depending on the cancer cell of origin and signaling context. Of note, SRC-1 also has been shown to be an important coregulator of mammary tumor cell motility and invasion through regulation of the EMT process via direct coregulation of PEA3-induced Twist expression (25); Twist is a basic-helix-loop-helix transcription factor and master regulator of the EMT program (35). Determining whether or how the coregulator activities of SRC-1 and SRC-3 collaborate in the invasion-metastasis cascade is obviously an important goal for future studies. Of clinical significance, the expression profiles of SRC-3 and PEA3 along with their targets, MMP-2, and MMP-9, positively correlate in human breast cancer tissues (13). Together, these studies demonstrate that SRC-3 can reprogram a cancer cell to be motile and invasive while at the same time remodel the local microenvironment to facilitate tumor cell invasion into the adjacent stroma, intravasation into the vasculature, and dissemination to distant anatomic sites.
To this point, we have reviewed SRC-3’s role in the invasion-metastasis cascade based on its coregulator activities in the nucleus. However, new research findings unexpectedly reveal that SRC-3 can “morph” into a truncated isoform which, at the plasma membrane, exerts strong potentiating effects on cancer cell motility and invasion.
A Second Act for SRC-3 in Its Role as a Prometastatic Factor
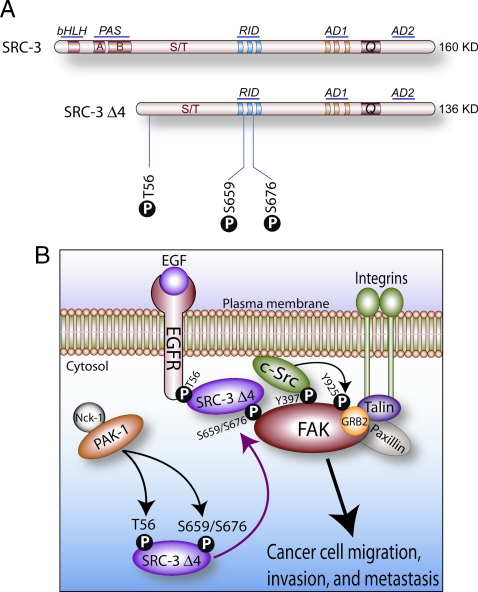
The epidermal growth factor (EGF) is a pivotal promoter of cancer cell migration and metastasis (36). Through its transmembrane receptor (EGFR), extracellular EGF activates numerous intracellular protein kinases which include p21-activated kinase 1 (PAK1) (37), focal adhesion kinase (FAK) (38), and c-Src (39). In response to the EGF signal, PAK1 (a cytoskeletal-associated kinase) is activated by complexing with the EGFR via the adaptor protein Nck1 (40) (Figure 1). Next, FAK (a cell motility–associated nonreceptor tyrosine kinase) is activated and autophosphorylated at tyrosine 397 (Y397) after interactions with members of the integrin receptor matrix complex (i.e. paxillin and talin) at cell adhesion sites (41). With FAK activation, c-Src kinase (through its SH2 domain) is then recruited to the FAK complex through interaction with the FAK phosphorylated residue, Y397 (42). As a result of this interaction, c-Src kinase phosphorylates FAK at numerous tyrosine residues, including Y925 (43). The phosphorylation of Y925 in particular enhances the interaction of FAK with its associating partners, such as Grb2 and paxillin, an association which is crucial for FAK promotion of cell migration (44,45), tumor angiogenesis (46), and tumor metastasis (47). Although FAK represents a central integrator linking transmembrane EGFR and integrin mediated signaling to the cell motility apparatus, evidence supporting a direct interaction between EGFR and FAK has remained elusive—until now.
Figure 1.
The SRC-3Δ4 isoform acts a signaling adaptor for EGF-dependent cancer cell migration and invasion. A, Comparative structures of full-length SRC-3 and the SRC-3Δ4 isoform. The basic-helix-loop-helix, Per/ARNT/Sim, receptor interaction, and activation domains are denoted by bHLH, PAS, RID, and AD, respectively; Q denotes the glutamine-rich region (7). The three phosphorylation sites in the SRC-3Δ4 isoform that are essential for its signal adaptor function in growth factor signaling are indicated. B, Phosphorylated SRC-3Δ4 acts as a signaling adaptor for EGF→FAK→c-Src transduction pathway that leads to cancer cell migration, invasion, and metastasis (see text for details).
Remarkably, a splice variant of SRC-3 (SRC-3Δ4) was revealed to be the elusive signal adaptor that acts as a molecular bridge between EGFR and FAK at the cell-membrane (48). Lacking the first six coding exons, the alternatively spliced SRC-3Δ4 transcript does not express the N-terminal basic-helix loop-helix-Per/ARNT/Sim (bHLH-PAS) region which also contains a nuclear localization sequence (NLS) motif (Figure 1A). Lacking the NLS, SRC-3Δ4 is primarily sequestered in the cytosol, unlike full-length SRC-3 which is predominantly located in the nucleus. After EGF exposure, PAK-1 phosphorylates SRC-3Δ4 on three discrete serine/threonine residues: threonine 56 (T56) located in the amino-terminal domain and serines 659 and 676 present in the centrally located receptor interacting domain (RID) of SRC-3Δ4 (Figure 1A). These specific posttranslational modifications trigger translocation of SRC-3Δ4 to the plasma membrane where it binds the EGFR and FAK at the leading edge of the cell (lamellipodia). At this cellular location, EGFR engages T56 of SRC-3Δ4 whereas the FERM domain of FAK interacts with residues S659 and S676 located in the RID of SRC-3Δ4 (Figure 1B). Within this signaling complex, therefore, SRC-3Δ4 serves as a critical bridge that spans the molecular divide between EGFR and FAK to allow full activation of the EGF-FAK-cSrc signal transduction axis, a signaling cascade which is essential for cancer cell invasiveness and metastasis (46). As a testament to its pivotal adapter role in this signaling complex, knockdown of only SRC-3Δ4 in MDA-MB231 cancer cells significantly curtails EGF-induced c-Src activation, FAK phosphorylation at Y925, and cell motility in culture (48). Moreover, when injected into the cleared mammary fat pad of immunocompromised mice, MDA-MB-231 cells (previously engineered to overexpress SRC-3Δ4) contribute to marked increases in mammary tumor metastasis to thoracic lymph nodes and pulmonary tissue without altering primary tumor incidence or latency (48). From a clinical perspective, these observations are made all the more significant because SRC-3Δ4 isoform expression levels are elevated in human breast tumors and preneoplastic tissue (49). It should be noted that the Riegel group recently reported that forced overexpression of SRC-3Δ4 (formally known as SRC-3Δ3) can potently coactivate ER mediated transactivation in cell culture (49,50). The nuclear activity of SRC-3Δ4 under these circumstances is most likely due to direct binding of SRC-3Δ4 to nuclear ER through its RID; whether this response occurs in vivo is unclear. Irrespective of these findings, the fact that advanced malignancies lose much of their ER positivity indicates that tumor cell motility and invasiveness driven by cytosolic SRC-3Δ4 is the predominant activity of this isoform during mammary tumor invasion.
Although disclosing SRC-3Δ4 as the missing signaling adapter for EGFR and FAK constitutes a seminal conceptual advance in our understanding of coregulator involvement in breast cancer metastasis, these studies pose a number of tantalizing questions to be addressed by future experiments: First, apart from EGFR, can SRC-3Δ4 link other transmembrane growth factor receptors to FAK? For example, it’s known that FAK is also activated by multiple growth factors (including: platelet derived growth factor, heregulin, vascular endothelial growth factor, and basic fibroblast growth factor) via mechanisms similar to EGF (41). Second, can full-length SRC-3 contribute to FAK-mediated cancer cell motility from the nucleus? Intriguingly, suppression of full-length SRC-3 expression in ovarian cancer cells markedly impairs correct FAK cellular localization which in turn blocks ovarian carcinoma cell motility, suggesting a signaling connection between nuclear localized full-length SRC-3 and FAK activity at the plasma membrane (12). In separate studies, elevated expression of full-length SRC-3 positively correlates with increased prostate cancer cell migration and invasiveness in which overexpression of SRC-3 is linked to focal adhesion turnover and FAK activation (34). Indeed, earlier insect studies predicted a possible link between SRC-3 and focal adhesion turnover (9), although a mechanism has yet to be defined. Finally, are SRC-3Δ4 expression levels elevated in other cancer cell-types? This would demonstrate a more universal tumorigenic role for SRC-3Δ4 as has been demonstrated for SRC-3 (7).
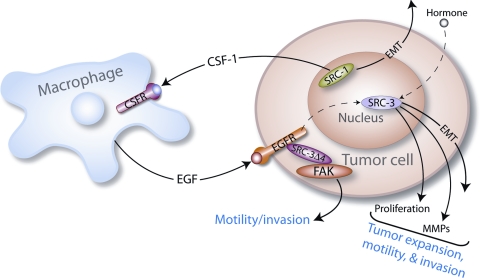
Based on recent studies reviewed here, a speculative model can be proposed in which nuclear confined SRC-3 along with SRC-1 cooperate with cytosolic SRC-3Δ4 to enhance cancer cell proliferation, migration, and invasion (Figure 2). Along with promoting unfettered cellular proliferation and EMT progression, full-length SRC-3 induces MMP family members in its role as a transcriptional coactivator in the nucleus whereas nuclear SRC-1 elicits the EMT process through up-regulation of Twist. At the plasma membrane, phosphorylated SRC-3Δ4 directly transduces the EGF signal (and possibly other growth factor and angiogenic signals) to FAK and c-SRC to license cell motility and invasion. These intracellular effects could conceivably be augmented by extracellular signaling scenarios. For example, SRC-1 has been shown to induce colony stimulating factor-1 (CSF-1) (15), which is a potent chemokine that can attract tumor-associated macrophages (TAMs) to the tumor site (51). In turn, TAMs are known to secrete a spectrum of growth factors (including EGF) that are essential for cancer cell proliferation, migration, and invasion (51). Within this hypothetical paracrine signaling scenario, macrophage–derived growth factors—acting on their cognate receptors on tumor cells—could further enhance SRC-dependent mammary tumor cell proliferation, migration, and invasion (Figure 2).
Figure 2.
A hypothetical model of SRC-3, SRC-3Δ4, and SRC-1 acting as a deadly triad in promoting tumor cell proliferation, migration, invasion, and metastasis (see text for further details).
Summary and Perspective
Since our previous SRC review in this series (52), our understanding of SRC-3 in the neoplastic progression pathway has accelerated at an exponential pace. If past is prologue, we anticipate an even greater expansion of this knowledge-base in the foreseeable future. Although significant advances have been achieved regarding SRC-3’s role in tumor cell motility and invasion, comparatively little is known concerning this coregulator’s involvement in the later stages of the invasion-metastasis cascade; these include intravasation, tumor-cell survival in the circulation, extravasation, and finally establishment/expansion of the micro-metastatic lesion in a distant target site. Because a significant percentage of patients who are diagnosed with breast cancer also present evidence of metastasis (53), targeting only tumor cell invasion and other early stages of the metastatic process is likely to be ineffective in influencing clinical outcome. In this regard, improving our understanding of SRC-3’s role during the remaining steps of the invasion-metastasis cascade represents one of the next key challenges in this field of study. Interestingly, recent reports have implicated the importance of FAK-mediated signaling in metastatic colonization of distant tissues (54,55). The proposal that SRC-3 could conceivably regulate similar adhesion-related signaling pathways during extravasation, invasion, and/or metastatic lesion formation as revealed for this coregulator in primary tumor cell migration will certainly be a focus of intensive investigation in the near future.
It is well established that SRC-3 protein levels and activity are regulated by posttranslational modifications (i.e. phosphorylation, methylation, acetylation, ubiquitination, and sumoylation) (56,57,58). Posttranslational regulation of SRC-3 protein turnover is also mediated by the cellular proteasome system in which both the pool of inactive coactivator and the concentration of “activated SRC-3” engaged in transcription are kept in-check (59,60,61). In this regard, further research into the mechanisms which underlie posttranslational control of SRC-3 levels could lead to the development of a novel class of drugs designed to abrogate or curtail oncogenic coactivator activity in neoplastic tissue. Emerging data also suggest that SRC-3 levels are controlled posttranscriptionally by microRNAs (miRNAs) (62,63). As a consequence of binding site-specific sequences within the 3′untranslated region of target mRNAs, miRNAs block protein translation (64). In addition to regulatory roles in normal development and physiology, miRNAs are implicated as tumor suppressors and oncogenes (65). Apart from representing new mechanisms that control SRC-3 availability during the neoplastic progression program, the ability of these miRNAs to restrain SRC-3’s involvement in breast cancer cell proliferation and invasion also may prove clinically useful as a therapeutic silencing tool in the future (66,67).
Another obvious area for future study is to address the role of the other SRC family members in the invasion-metastasis cascade, a field of investigation that has not proceeded apace with SRC-3 research. Although an increasing amount of data support an important role for SRC-1 in this neoplastic progression program (15,17,25), information on SRC-2 in this process is scant (68,69). Clearly, elucidating the relative contributions of each SRC family member throughout the invasion-metastasis cascade constitutes a critical underpinning for an improved understanding of whether or how these coregulators collaborate during mammary tumor progression.
In conclusion, this review showcases SRC-3’s attributes as a multifunctional prometastatic factor. Endowed with the ability to influence cellular events from both the nucleus and plasma membrane, SRC-3 projects its prometastatic effects via a multitude of signaling pathways, many of which display pleiotropic effects on cellular behavior. Beyond providing critical mechanistic insights into SRC-3’s role in mammary tumor invasion and metastasis, these studies promise to advance the efficacy of clinical management of this malignancy through formulation of new intervention strategies based on SRC-3 as a diagnostic biomarker for poor disease-free survival and/or as a direct target for antimetastatic therapy.
Footnotes
This work was supported in part by National Institutes of Health Grants RO1-CA077530 (to J.P.L.) and CPRIT and RO1-HD-07857 (to B.W.O.).
Disclosure Summary: The authors have nothing to declare.
Abbreviations: bHLH-PAS, Basic-helix loop-helix-Per/ARNT/Sim; CSF-1, colony stimulating factor-1; ECM, extracellular matrix; EGF, epidermal growth factor; EGFR, epidermal growth factor receptor; EMT, epithelial-mesenchymal transition; ER, estrogen receptor; FAK, focal adhesion kinase; MAPK, mitogen-activated protein kinase; miRNA, microRNA; MMP, matrix metalloproteinase; NLS, nuclear localization sequence; NR, nuclear receptor; PAK1, p21-activated kinase 1; PR, progesterone receptor; PyMT, polyoma middle T antigen; RID, receptor interacting domain; SRC, steroid receptor coactivator; TAM, tumor-associated macrophage.
First Published Online November 3, 2010
References
- Gupta GP, Massague J 2006 Cancer metastasis: building a framework. Cell 127:679–695 [DOI] [PubMed] [Google Scholar]
- Talmadge JE, Fidler IJ 2010 AACR centennial series: the biology of cancer metastasis: historical perspective. Cancer Res 70:5649–5669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fidler IJ 2003 The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3:453–458 [DOI] [PubMed] [Google Scholar]
- Poste G, Fidler IJ 1980 The pathogenesis of cancer metastasis. Nature 283:139–146 [DOI] [PubMed] [Google Scholar]
- Anzick SL, Kononen J, Walker RL, Azorsa DO, Tanner MM, Guan XY, Sauter G, Kallioniemi OP, Trent JM, Meltzer PS 1997 AIB1, a steroid receptor coactivator amplified in breast and ovarian cancer. Science 277:965–968 [DOI] [PubMed] [Google Scholar]
- O'Malley BW, McKenna NJ 2008 Coactivators and corepressors: what’s in a name? Mol Endocrinol 22:2213–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Wu RC, O'Malley BW 2009 Normal and cancer-related functions of the p160 steroid receptor co-activator (SRC) family. Nat Rev Cancer 9:615–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onate SA, Tsai SY, Tsai MJ, O'Malley BW 1995 Sequence and characterization of a coactivator for the steroid hormone receptor superfamily. Science 270:1354–1357 [DOI] [PubMed] [Google Scholar]
- Bai J, Uehara Y, Montell DJ 2000 Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell 103:1047–1058 [DOI] [PubMed] [Google Scholar]
- Yao TP, Forman BM, Jiang Z, Cherbas L, Chen JD, McKeown M, Cherbas P, Evans RM 1993 Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature 366:476–479 [DOI] [PubMed] [Google Scholar]
- Montell DJ 2001 Command and control: regulatory pathways controlling invasive behavior of the border cells. Mech Dev 105:19–25 [DOI] [PubMed] [Google Scholar]
- Yoshida H, Liu J, Samuel S, Cheng W, Rosen D, Naora H 2005 Steroid receptor coactivator-3, a homolog of Taiman that controls cell migration in the Drosophila ovary, regulates migration of human ovarian cancer cells. Mol Cell Endocrinol 245:77–85 [DOI] [PubMed] [Google Scholar]
- Qin L, Liao L, Redmond A, Young L, Yuan Y, Chen H, O'Malley BW, Xu J 2008 The AIB1 oncogene promotes breast cancer metastasis by activation of PEA3-mediated matrix metalloproteinase 2 (MMP2) and MMP9 expression. Mol Cell Biol 28:5937–5950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis JW, Granovsky M, Warren CE 1999 Glycoprotein glycosylation and cancer progression. Biochim Biophys Acta 1473:21–34 [DOI] [PubMed] [Google Scholar]
- Wang S, Yuan Y, Liao L, Kuang SQ, Tien JC, O'Malley BW, Xu J 2009 Disruption of the SRC-1 gene in mice suppresses breast cancer metastasis without affecting primary tumor formation. Proc Natl Acad Sci USA 106:151–156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouras T, Southey MC, Venter DJ 2001 Overexpression of the steroid receptor coactivator AIB1 in breast cancer correlates with the absence of estrogen and progesterone receptors and positivity for p53 and HER2/neu. Cancer Res 61:903–907 [PubMed] [Google Scholar]
- Fleming FJ, Myers E, Kelly G, Crotty TB, McDermott EW, O'Higgins NJ, Hill AD, Young LS 2004 Expression of SRC-1, AIB1, and PEA3 in HER2 mediated endocrine resistant breast cancer; a predictive role for SRC-1. J Clin Pathol 57:1069–1074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborne CK, Bardou V, Hopp TA, Chamness GC, Hilsenbeck SG, Fuqua SA, Wong J, Allred DC, Clark GM, Schiff R 2003 Role of the estrogen receptor coactivator AIB1 (SRC-3) and HER-2/neu in tamoxifen resistance in breast cancer. J Natl Cancer Inst 95:353–361 [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL 1987 Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 235:177–182 [DOI] [PubMed] [Google Scholar]
- Shou J, Massarweh S, Osborne CK, Wakeling AE, Ali S, Weiss H, Schiff R 2004 Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst 96:926–935 [DOI] [PubMed] [Google Scholar]
- Lahusen T, Fereshteh M, Oh A, Wellstein A, Riegel AT 2007 Epidermal growth factor receptor tyrosine phosphorylation and signaling controlled by a nuclear receptor coactivator, amplified in breast cancer 1. Cancer Res 67:7256–7265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers E, Hill AD, Kelly G, McDermott EW, O'Higgins NJ, Buggy Y, Young LS 2005 Associations and interactions between Ets-1 and Ets-2 and coregulatory proteins, SRC-1, AIB1, and NCoR in breast cancer. Clin Cancer Res 11:2111–2122 [DOI] [PubMed] [Google Scholar]
- Galang CK, Garcia-Ramirez J, Solski PA, Westwick JK, Der CJ, Neznanov NN, Oshima RG, Hauser CA 1996 Oncogenic Neu/ErbB-2 increases ets, AP-1, and NF-kappaB-dependent gene expression, and inhibiting ets activation blocks Neu-mediated cellular transformation. J Biol Chem 271:7992–7998 [DOI] [PubMed] [Google Scholar]
- Wasylyk B, Hahn SL, Giovane A 1993 The Ets family of transcription factors. Eur J Biochem 211:7–18 [DOI] [PubMed] [Google Scholar]
- Qin L, Liu Z, Chen H, Xu J 2009 The steroid receptor coactivator-1 regulates twist expression and promotes breast cancer metastasis. Cancer Res 69:3819–3827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst HC 2001 Update on HER-2 as a target for cancer therapy: the ERBB2 promoter and its exploitation for cancer treatment. Breast Cancer Res 3:395–398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd T, Hassell JA 2001 Role of Ets transcription factors in mammary gland development and oncogenesis. J Mammary Gland Biol Neoplasia 6:129–140 [DOI] [PubMed] [Google Scholar]
- Kirkegaard T, McGlynn LM, Campbell FM, Muller S, Tovey SM, Dunne B, Nielsen KV, Cooke TG, Bartlett JM 2007 Amplified in breast cancer 1 in human epidermal growth factor receptor - positive tumors of tamoxifen-treated breast cancer patients. Clin Cancer Res 13:1405–1411 [DOI] [PubMed] [Google Scholar]
- Redmond AM, Bane FT, Stafford AT, McIlroy M, Dillon MF, Crotty TB, Hill AD, Young LS 2009 Coassociation of estrogen receptor and p160 proteins predicts resistance to endocrine treatment; SRC-1 is an independent predictor of breast cancer recurrence. Clin Cancer Res 15:2098–2106 [DOI] [PubMed] [Google Scholar]
- Fereshteh MP, Tilli MT, Kim SE, Xu J, O'Malley BW, Wellstein A, Furth PA, Riegel AT 2008 The nuclear receptor coactivator amplified in breast cancer-1 is required for Neu (ErbB2/HER2) activation, signaling, and mammary tumorigenesis in mice. Cancer Res 68:3697–3706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomaskovic-Crook E, Thompson EW, Thiery JP 2009 Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res 11:213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lochter A, Sternlicht MD, Werb Z, Bissell MJ 1998 The significance of matrix metalloproteinases during early stages of tumor progression. Ann NY Acad Sci 857:180–193 [DOI] [PubMed] [Google Scholar]
- Li LB, Louie MC, Chen HW, Zou JX 2008 Proto-oncogene ACTR/AIB1 promotes cancer cell invasion by up-regulating specific matrix metalloproteinase expression. Cancer Lett 261:64–73 [DOI] [PubMed] [Google Scholar]
- Yan J, Erdem H, Li R, Cai Y, Ayala G, Ittmann M, Yu-Lee LY, Tsai SY, Tsai MJ 2008 Steroid receptor coactivator-3/AIB1 promotes cell migration and invasiveness through focal adhesion turnover and matrix metalloproteinase expression. Cancer Res 68:5460–5468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA 2004 Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell 117:927–939 [DOI] [PubMed] [Google Scholar]
- Di Fiore PP, Pierce JH, Fleming TP, Hazan R, Ullrich A, King CR, Schlessinger J, Aaronson SA 1987 Overexpression of the human EGF receptor confers an EGF-dependent transformed phenotype to NIH 3T3 cells. Cell 51:1063–1070 [DOI] [PubMed] [Google Scholar]
- Bokoch GM 2003 Biology of the p21-activated kinases. Annu Rev Biochem 72:743–781 [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD 2000 FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol 2:249–256 [DOI] [PubMed] [Google Scholar]
- Goi T, Shipitsin M, Lu Z, Foster DA, Klinz SG, Feig LA 2000 An EGF receptor/Ral-GTPase signaling cascade regulates c-Src activity and substrate specificity. EMBO J 19:623–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galisteo ML, Chernoff J, Su YC, Skolnik EY, Schlessinger J 1996 The adaptor protein Nck links receptor tyrosine kinases with the serine-threonine kinase Pak1. J Biol Chem 271:20997–21000 [DOI] [PubMed] [Google Scholar]
- Schlaepfer DD, Mitra SK 2004 Multiple connections link FAK to cell motility and invasion. Curr Opin Genet Dev 14:92–101 [DOI] [PubMed] [Google Scholar]
- Eide BL, Turck CW, Escobedo JA 1995 Identification of Tyr-397 as the primary site of tyrosine phosphorylation and pp60src association in the focal adhesion kinase, pp125FAK. Mol Cell Biol 15:2819–2827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calalb MB, Polte TR, Hanks SK 1995 Tyrosine phosphorylation of focal adhesion kinase at sites in the catalytic domain regulates kinase activity: a role for Src family kinases. Mol Cell Biol 15:954–963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlaepfer DD, Hanks SK, Hunter T, van der Geer P 1994 Integrin-mediated signal transduction linked to Ras pathway by GRB2 binding to focal adhesion kinase. Nature 372:786–791 [DOI] [PubMed] [Google Scholar]
- Westhoff MA, Serrels B, Fincham VJ, Frame MC, Carragher NO 2004 SRC-mediated phosphorylation of focal adhesion kinase couples actin and adhesion dynamics to survival signaling. Mol Cell Biol 24:8113–8133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra SK, Schlaepfer DD 2006 Integrin-regulated FAK-Src signaling in normal and cancer cells. Curr Opin Cell Biol 18:516–523 [DOI] [PubMed] [Google Scholar]
- Kaneda T, Sonoda Y, Ando K, Suzuki T, Sasaki Y, Oshio T, Tago M, Kasahara T 2008 Mutation of Y925F in focal adhesion kinase (FAK) suppresses melanoma cell proliferation and metastasis. Cancer Lett 270:354–361 [DOI] [PubMed] [Google Scholar]
- Long W, Yi P, Amazit L, LaMarca HL, Ashcroft F, Kumar R, Mancini MA, Tsai SY, Tsai MJ, O'Malley BW 2010 SRC-3Delta4 mediates the interaction of EGFR with FAK to promote cell migration. Mol Cell 37:321–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter R, Wellstein A, Riegel AT 2001 An isoform of the coactivator AIB1 that increases hormone and growth factor sensitivity is overexpressed in breast cancer. J Biol Chem 276:39736–39741 [DOI] [PubMed] [Google Scholar]
- Reiter R, Oh AS, Wellstein A, Riegel AT 2004 Impact of the nuclear receptor coactivator AIB1 isoform AIB1-Delta3 on estrogenic ligands with different intrinsic activity. Oncogene 23:403–409 [DOI] [PubMed] [Google Scholar]
- Lamagna C, Aurrand-Lions M, Imhof BA 2006 Dual role of macrophages in tumor growth and angiogenesis. J Leukoc Biol 80:705–713 [DOI] [PubMed] [Google Scholar]
- McKenna NJ, O'Malley BW 2002 Minireview: nuclear receptor coactivators–an update. Endocrinology 143:2461–2465 [DOI] [PubMed] [Google Scholar]
- Del Monte U 2009 Does the cell number 10(9) still really fit one gram of tumor tissue? Cell Cycle 8:505–506 [DOI] [PubMed] [Google Scholar]
- Luo M, Guan JL 2010 Focal adhesion kinase: a prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett 289:127–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibue T, Weinberg RA 2009 Integrin beta1-focal adhesion kinase signaling directs the proliferation of metastatic cancer cells disseminated in the lungs. Proc Natl Acad Sci USA 106:10290–10295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Lonard DM, O'Malley BW 2009 Multi-modulation of nuclear receptor coactivators through posttranslational modifications. Trends Endocrinol Metab 20:8–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Shang Y 2007 Regulation of SRC family coactivators by post-translational modifications. Cell Signal 19:1101–1112 [DOI] [PubMed] [Google Scholar]
- York B, Yu C, Sagen JV, Liu Z, Nikolai BC, Wu RC, Finegold M, Xu J, O'Malley BW 2010 Reprogramming the posttranslational code of SRC-3 confers a switch in mammalian systems biology. Proc Natl Acad Sci USA 107:11122–11127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wu RC, Amazit L, Tsai SY, Tsai MJ, O'Malley BW 2007 Specific Amino Acid Residues in the Basic Helix-Loop-Helix Domain of SRC-3 Are Essential for Its Nuclear Localization and Proteasome-Dependent Turnover. Mol Cell Biol 27:1296–1308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Amazit L, Long W, Lonard DM, Monaco JJ, O'Malley BW 2007 Ubiquitin- and ATP-independent proteolytic turnover of p21 by the REGgamma-proteasome pathway. Mol Cell 26:831–842 [DOI] [PubMed] [Google Scholar]
- Wu RC, Feng Q, Lonard DM, O'Malley BW 2007 SRC-3 coactivator functional lifetime is regulated by a phospho-dependent ubiquitin time clock. Cell 129:1125–1140 [DOI] [PubMed] [Google Scholar]
- Castellano L, Giamas G, Jacob J, Coombes RC, Lucchesi W, Thiruchelvam P, Barton G, Jiao LR, Wait R, Waxman J, Hannon GJ, Stebbing J 2009 The estrogen receptor-alpha-induced microRNA signature regulates itself and its transcriptional response. Proc Natl Acad Sci USA 106:15732–15737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain A, Kuo MT, Saunders GF 2006 Mir-17–5p regulates breast cancer cell proliferation by inhibiting translation of AIB1 mRNA. Mol Cell Biol 26:8191–8201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen BR 2004 Transcription and processing of human microRNA precursors. Mol Cell 16:861–865 [DOI] [PubMed] [Google Scholar]
- Yu Z, Baserga R, Chen L, Wang C, Lisanti MP, Pestell RG 2010 microRNA, cell cycle, and human breast cancer. Am J Pathol 176:1058–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykxhoorn DM 2010 MicroRNAs and metastasis: little RNAs go a long way. Cancer Res 70:6401–6406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szasz AM, Wang ZC, Brock JE, Richardson AL, Weinberg RA 2009 A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell 137:1032–1046 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Mukherjee A, Soyal SM, Fernandez-Valdivia R, Gehin M, Chambon P, DeMayo FJ, Lydon JP, O'Malley BW 2006 Steroid receptor coactivator 2 is critical for progesterone-dependent uterine function and mammary morphogenesis in the mouse. Mol Cell Biol 26:6571–6583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee A, Amato P, Allred DC, DeMayo FJ, Lydon JP 2007 Steroid receptor coactivator 2 is required for female fertility and mammary morphogenesis: insights from the mouse, relevance to the human. Nucl Recept Signal Nov 30; 5:e011 [DOI] [PMC free article] [PubMed] [Google Scholar]