Abstract
Urocortin 3 (Ucn 3), member of the corticotropin-releasing factor (CRF) family of peptide hormones, is released from β-cells to potentiate insulin secretion. Ucn 3 activates the CRF type-2 receptor (CRFR2) but does not activate the type-1 receptor (CRFR1), which was recently demonstrated on β-cells. While the direct actions of Ucn 3 on insulin secretion suggest the presence of cognate receptors within the islet microenvironment, this has not been established. Here we demonstrate that CRFR2α is expressed by MIN6 insulinoma cells and by primary mouse and human islets, with no detectable expression of CRFR2β. Furthermore, stimulation of MIN6 cells or primary mouse islets in vitro or in vivo with glucocorticoids (GCs) robustly and dose-dependently increases the expression of CRFR2α, while simultaneously inhibiting the expression of CRFR1 and incretin receptors. Luciferase reporters driven by the mouse CRFR1 or CRFR2α promoter in MIN6 cells confirm these differential effects of GCs. In contrast, GCs inhibit CRFR2α promoter activity in HEK293 cells and inhibit the expression of CRFR2β in A7r5 rat aortic smooth muscle cells and differentiated C2C12 myotubes. These findings suggest that the GC-mediated increase of CRFR2α depends on the cellular context of the islet and deviates from the GC-mediated suppression of CRFR1 and incretin receptors. Furthermore, GC-induced increases in CRFR2α expression coincide with increased Ucn 3-dependent activation of cAMP and MAPK pathways. We postulate that differential effect of GCs on the expression of CRFR1 and CRFR2α in the endocrine pancreas represent a mechanism to shift sensitivity from CRFR1 to CRFR2 ligands.
CRFR2α is expressed in pancreatic islets; glucocorticoids robustly increase expression of CRFR2α, in contrast to the expression of CRFR1 and incretin receptors in β-cells.
Secretion of insulin and glucagon from pancreatic islets depends on circulating glucose levels but can be substantially modulated by an intricate network of extracellular signals whose coordinated efforts dictate the endocrine output of the pancreas. This network includes local signals that act in a paracrine fashion, such as somatostatin (Sst), which, when released from δ-cells, inhibits the release of both insulin and glucagon from β- and α-cells, respectively (1). The islets are also well-equipped to integrate information from other parts of the body, most notably the gastro-intestinal tract and the central nervous system (CNS), to fine-tune the timing and amplitude of insulin and glucagon secretion in relation to metabolic cues. Chief among these endocrine signals are the incretins, secreted from specialized cells within the gastro-intestinal tract, which potentiate glucose-stimulated insulin secretion (GSIS) and facilitate rapid normalization of post-prandially elevated plasma glucose (2). The incretins are glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP), which activate the GLP-1 receptor (GLP-1R) and GIP receptor (GIPR), respectively (3). Additional peptide hormones including pituitary adenylate cyclase-activating polypeptide, vasoactive intestinal peptide and others modulate insulin secretion (4).
Recently, members of the corticotropin releasing factor (CRF) family of neuropeptides, which includes urocortin 1 (Ucn 1), Ucn 2, and Ucn 3, were demonstrated to promote GSIS in an incretin-like manner (5,6,7,8). CRF and Ucn 1 can signal via CRFR1 and CRFR2, while Ucn 2 and Ucn 3 are selective agonists for CRFR2 (9,10). CRFR1 is expressed on primary β-cells and the mouse insulinoma cell line MIN6, potentiates GSIS in vitro and in vivo, and can induce proliferation of neonatal rat β-cells (5). The appreciation of the pancreatic β-cell as a major site of Ucn 3 expression (7), along with a series of observations that established inhibition of endogenous Ucn 3 to impair glucose- and incretin-induced insulin secretion (6), suggested the presence of local receptors for Ucn 3 within the islet micro-environment. Because Ucn 3 is known to selectively activate CRFR2, local presence of CRFR2 within the islet would be the most parsimonious explanation for the observed Ucn 3–mediated effects, although this has not been directly demonstrated to date. Furthermore, CRF receptors are known targets of glucocorticoid (GC) regulation, with GC-induced inhibition of CRFR1 expression in the anterior pituitary a part of the negative feedback exerted by GCs on the activated hypothalamus-pituitary-adrenal (HPA)–axis (11,12).
Here we establish the expression of CRFR2 in the MIN6 insulinoma cell line as well as in primary mouse and human islets, providing conclusive evidence that β-cell–derived Ucn 3 can act on cognate receptors locally expressed within the islet microenvironment. While two isoforms, α and β, can be transcribed from the CRFR2 gene using alternative transcription start sites (13,14,15,16), MIN6 cells and rodent and human islets express CRFR2α. We demonstrate that GCs increase the expression of CRFR2α in the MIN6 cell line in a glucocorticoid receptor (GR)-dependent manner, as well as in rodent islets in vitro and in vivo. The GC-induced elevation of CRFR2 is remarkable as the expression of CRFR2 in most other tissues and cell types and the expression of CRFR1, GLP-1R, and GIPR within MIN6 cells and the endocrine pancreas is subject to GC-induced suppression. Dexamethasone-induced up-regulation of CRFR2 potentiates the response of MIN6 cells to the CRFR2-selective agonist Ucn 3, suggesting that GCs increase the sensitivity of the endocrine pancreas for β-cell–derived Ucn 3 by increasing the expression of its cognate receptor.
Materials and Methods
Animals and procedures
Animals were maintained on 12-h light (0600 h to 1800 h), 12-h dark (1800 h to 0600 h) cycle with free access to water and standard rodent chow. Corticosterone pellets (12.5 mg/pellet, calculated release rate 0.595 mg/d; Innovative Research of America, Sarasota, FL) were implanted in the afternoon under the dorsal skin in male 6-month old C57/Bl6 mice. Animals received ibuprofen in the drinking water post surgery. Animals were euthanized the following morning by decapitation, trunk blood was collected for the determination of corticosterone levels by RIA (MP Biomedicals, Inc. Orangeburg, NY). Retired age-matched male C57/Bl6 breeders (Harlan Laboratories, Indianapolis, IN) were used for acute restraint experiments, and to isolate primary mouse islets for culture in vitro. Primary rat islets were isolated for culture in vitro from male Sprague Dawley, 180–200 g (Harlan Laboratories, Indianapolis, IN). All experiments and procedures were approved by the Salk Institutional Animal Care and Use Committee.
Islet isolation
Primary mouse and rat islets were isolated as previously described (5) and were hand-picked several times under a dissecting scope before flash freezing in lysis buffer for expression analysis or transfer to a cell culture dish containing RPMI with 10% FBS and penicillin/streptomycin for overnight culture before GC stimulation.
Human islets
Human islets were obtained from the ICR Basic Science Islet Distribution Program. Approximately 2000 islet equivalents were collected by centrifugation from 22 specimens that were of >65% purity and stored at −80 C until use. Mean donor age was 44 ± 17 yr, and 60% were male. Donors were of European (75%), Hispanic/Latino (15%) or African-American ancestry (10%) and had a mean body mass index of 29.0 ± 6.9 kg/m2. None of the donors had documented type 1 or type 2 diabetes. The Salk Institute for Biological Studies Institutional Review Board declared the human islet material used in this study exempt on April 16, 2008.
Expression analysis
MIN6 cells (passage 21 to 35) were cultured in DMEM containing 11 mm glucose, 10% FBS, 2 mm glutaMAX-I (Invitrogen, Carlsbad, CA), and 10 μm β-mercaptoethanol. A7r5 cells (ATCC) (17) and undifferentiated C2C12 cells (ATCC) (18,19) were cultured in DMEM, supplemented with 25 mm glucose, 10% FBS, and 2 mm glutaMAX-I. For differentiation into myotubes, C2C12 cells were plated at 150,000 cells per well in 24-well plates. The following day, media were changed to differentiation media (DMEM, 2% horse serum, 2 mm glutaMAX-I) supplemented with 10 nm acylated human ghrelin to promote differentiation (20,21). Cells were stimulated overnight with GCs at day 8 of differentiation, when fused, multinucleated and contractile myotubes were readily observed. MIN6 and A7r5 cells were plated at 100,000 cells per well in 24-well plates. All cells were stimulated for 12 h with steroid hormones (Sigma, St. Louis MO; 20 mm stocks in ethanol) and antagonists, unless otherwise indicated. Total RNA from cell lines and rodent islets was isolated with the GenElute Mammalian Total RNA Miniprep kit (Sigma, St. Louis, MO). Total RNA from human islets was purified with RNeasy mini columns (Qiagen, Valencia, CA). RNA samples were digested with DNase I (Invitrogen, Carlsbad, CA) and converted into cDNA with the High-Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA). Gene expression was quantified by qRT-PCR using SYBR chemistry on a Lightcycler 480 platform (Roche, Indianapolis, IN). Relative expression levels (ΔΔCt) were normalized to hypoxanthine guanine phosphoribosyl transferase (HPRT); normalization to 18S ribosomal RNA and β-actin gave very similar results. The strategy to discriminate mouse CRFR2α and CRFR2β is detailed in Fig. 1. Identity of CRFR2α transcripts in MIN6 cells and islets, and CRFR2α and CRFR2β transcripts in undifferentiated and differentiated C2C12 cells, respectively, was confirmed by direct sequencing of purified qRT-PCR products. Primers (Supplemental Table 1 published on The Endocrine Society’s Journals Online web site at http://endo.endojournals.org) were designed using the universalprobelibrary online primer design feature (Roche, Indianapolis, IN), unless indicated otherwise.
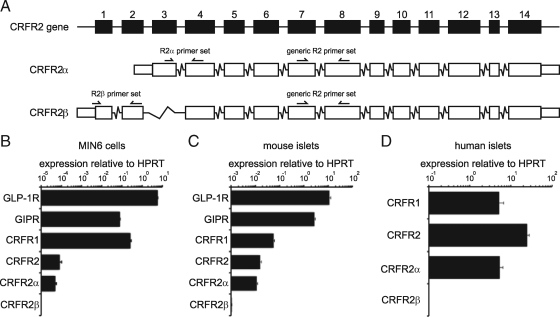
Figure 1.
The α isoform of CRFR2 is expressed in the MIN6 insulinoma cell line and in primary mouse and human islets. The use of primers designed to discriminate between mouse CRFR2α and CRFRβ isoforms (A) reveals that CRFR2α is the major CRFR2 isoform expressed in MIN6 cells (B). MIN6 cells express CRFR2 at relatively low abundance compared with the expression of incretin receptors and CRFR1. Primary mouse islets also express the CRFR2α isoform at levels that approach CRFR1 in abundance (C). A similar strategy for the design of isoform-specific primers revealed that CRFR2α is the major isoform of CRFR2 that is expressed in human islets as well (D). Boxes in A represent exons and are drawn to scale. Data are expressed relative to HPRT. Error bars in B and C indicate the SE of quadruplicate treatments, error bars in D reflect the SE across 20 individual donors.
CRFR promoter-driven luciferase assay
Reporter constructs of the CRFR1, CRFR2α, and CRFR2β promoters were constructed with restriction enzymes or by PCR from mouse BAC clones (bacpac.chori.org) (22). Promoters were subcloned into MluI/XhoI (CRFR1) and XhoI/HindIII (CRFR2) sites of pGL3 basic vector containing the luciferase reporter gene (Promega, Madison, WI). Analysis of glucocorticoid response element (GRE) binding sites was carried out using gene2promoter (genomatrix.de). Reporter constructs (2.4 mg per well) were transiently transfected into MIN6 cells (5 × 105 cells per well in 12-well plates) with Lipofectamine 2000 (Qiagen, Santa Clarita, CA). The following day, dexamethasone (Sigma, St. Louis, MO) was applied to the cells. After 36 h, cells were washed once and lysed as described in detail (23). Luciferase activity was measured in triplicate and normalized to β-galactosidase activity.
cAMP assay
MIN6 cells were plated into 48-well plates at 50,000 cells per well. The following day cells were washed 3× with DMEM containing 5.5 mm glucose, 2% FBS, glutamax, and 10 μm β-mercaptoethanol. After 24 h, the cells were washed once and preincubated with 0.1 mm 3-isobutyl-1-methylxanthine (IBMX; AG Scientific Inc., San Diego, CA) for 20 min to inhibit phosphodiesterase and enable measurement of cumulative cAMP levels over time. The cells were treated with mUcn 3 for 30 min. Intracellular cAMP was measured from triplicate wells using an RIA kit (Biomedical Technologies, Stoughton, MA) as previously described (24).
Western immunoblotting
MIN6 cells were seeded at 100,000 cells per well in 24-well plates and grown for 2 d to approximately 60% confluency before stimulation with GCs as indicated for 12 h. Cells were then starved for 1 h in Krebs Ringer buffer supplemented with 0.1% BSA and 2.8 mmd-glucose supplemented with the same GCs before treatment with mUcn 3 for 5 min. Cells were processed for Western immunoblotting as previously described (5). Rabbit anti–phospho-ERK (1:1000) and total ERK antibodies (1:1000) were from Cell Signaling Technology (Beverly, MA). Secondary antibody (1:5000) was donkey antirabbit/HRP (GE Healthcare UK Ltd, UK).
Statistics
Results were evaluated for statistical significance by two-sided t test, assuming equal variance between groups.
Results
The insulinoma cell line MIN6 and primary mouse and human islets express the α-isoform of CRFR2
We demonstrated the expression of CRFR2 in MIN6 insulinoma cells at levels that are lower than the expression of CRFR1 and incretin receptors, GLP-1R and GIPR. Using primers designed to discriminate between CRFR2α and CRFR2β isoforms (Fig. 1A), we demonstrated that the CRFR2 expression levels detected with generic CRFR2 primers is matched by the expression level of CRFR2α, with no detectable levels of CRFR2β (Fig. 1B). In primary mouse and human islets expression levels of CRFR2 were similar to CRFR1 (Fig. 1, C and D), with CRFR2α the main isoform detected.
Glucocorticoids differentially regulate the expression of CRFR1 and CRFR2 in MIN6 cells
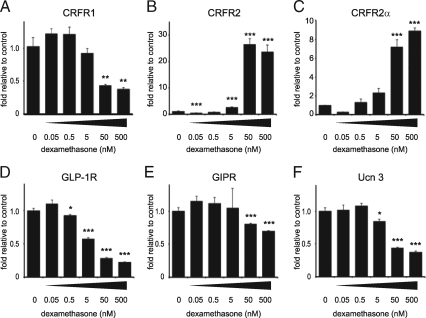
The inhibitory feedback exerted by GCs on the expression of CRF receptors in the CNS and the pituitary gland, although not uniform across the brain and subject to overriding inputs by other factors including CRF, is well documented (11,12). To assess the effects of GCs on pancreatic CRFR expression, we stimulated MIN6 insulinoma cells for 12 h with increasing doses of the synthetic GC dexamethasone. Dexamethasone dose-dependently inhibited the expression of CRFR1 (Fig. 2A) and simultaneously increased the expression of the α-isoform of CRFR2 (Fig. 2, B and C). The expression of the incretin receptors is dose-dependently inhibited by dexamethasone, with GLP-1R robustly inhibited similar to CRFR1 (Fig. 2D), while inhibition of GIPR expression is modest in comparison (Fig. 2E). The expression of Ucn 3, the endogenous ligand for pancreatic CRFR2, was also inhibited by dexamethasone to approximately 50% of original levels (Fig. 2F).
Figure 2.
GCs dose-dependently inhibit CRFR1 and increase CRFR2 expression in MIN6 cells. Stimulation of MIN6 cells for 12 h with the synthetic GC dexamethasone dose-dependently inhibits the expression of CRFR1 (A). In contrast, CRFR2α expression is robustly and dose-dependently increased by dexamethasone, as measured with generic (B) or CRFR2α-selective (C) qRT-PCR primers. The expression of GLP-1R (D), GIPR (E), and Ucn 3 (F) are inhibited by dexamethasone in a pattern similar to CRFR1. Data are normalized to HPRT expression and expressed relative to untreated controls. Error bars indicate the SE of quadruplicate treatments, asterisks indicate statistically significant differences with controls (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
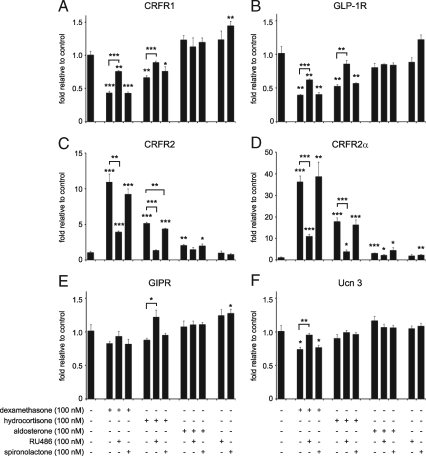
To elucidate the mechanism underlying the GC-mediated changes in GPCR gene expression, we compared the effects of several GR and mineralocorticoid receptor (MR) selective agonists and antagonists. Incubation of MIN6 cells for 12 h with dexamethasone inhibited the expression of CRFR1 (Fig. 3A). CRFR1 expression was partially restored by co-administration of equimolar amounts of the GR-selective antagonist RU486, but not the MR-selective antagonist spironolactone. Hydrocortisone, a less potent GC agonist, also reduced CRFR1 expression in MIN6 cells, preventable by RU486 but not spironolactone. The MR-selective agonist aldosterone did not reduce the expression of CRFR1. Stimulation of MIN6 cells with RU486 alone did not affect CRFR1 expression levels, while spironolactone caused a modest increase in CRFR1 expression. The expression pattern of GLP-1R closely matched that of CRFR1, with robust inhibition by GR-, but not MR-, agonists, partially negated by equimolar amounts of GR-, but not MR-, antagonists (Fig. 3B). In sharp contrast, the GC-mediated transcriptional changes of CRFR2 and CRFR2α were opposite to the effects of GCs on CRFR1 and GLP-1R. Dexamethasone and, to a lesser extent hydrocortisone, robustly elevated the expression of CRFR2 and CRFR2α, preventable by co-administration of RU486 but not spironolactone (Fig. 3, C and D). The MR-selective agonist aldosterone marginally elevated the expression of CRFR2, but with a modest magnitude compared with the increase observed following dexamethasone stimulation. The expression of GIPR and the CRFR2-selective ligand Ucn 3 showed a similar pattern of expression to CRFR1 and GLP-1R, with the distinction that the amplitude of the GC-mediated inhibition was considerably more modest (Fig. 3, E and F). Collectively, these results indicate that the dexamethasone-induced transcriptional changes are selectively mediated by GR.
Figure 3.
The GC-mediated transcriptional changes in MIN6 cells are GR-mediated. MIN6 cells were stimulated for 12 h with the GR-selective agonist dexamethasone, the MR-selective agonist aldosterone, or the general GR/MR agonist hydrocortisone alone or in combination with RU486 (GR antagonist) or spironolactone (MR antagonist) as indicated. Dexamethasone and hydrocortisone robustly inhibit the expression of CRFR1 (A) and GLP-1R (B), which is negated by co-administration of RU486, but not spironolactone, both at equimolar levels. Expression of neither gene is affected by aldosterone. In contrast, the expression of CRFR2, as detected by generic (C) or CRFR2α-specific (D) primers, is potently increased by dexamethasone and hydrocortisone. These increases are partially prevented by RU486, but not spironolactone, indicating that these changes are GR-dependent. The expression of GIPR (E) and Ucn 3 (F) is inhibited in a pattern that reminisces the expression of CRFR1 and GLP-1R, although this inhibition is less robust. Data are normalized to HPRT expression and expressed relative to untreated controls. Error bars indicate the SE of quadruplicate treatments, asterisks indicate statistically significant differences with controls unless indicated otherwise (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
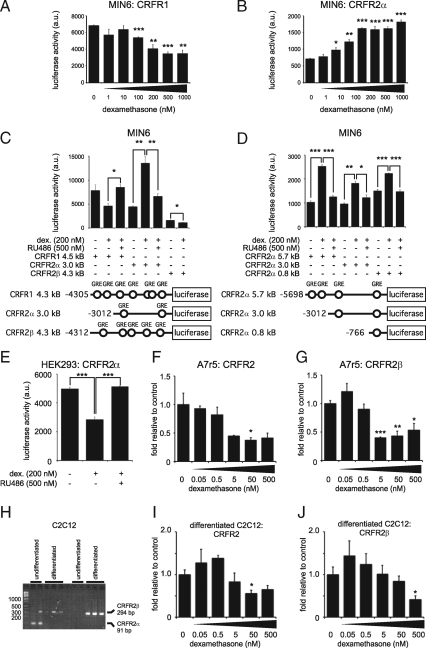
Additional experiments, measuring CRFR promoter-driven luciferase activity, confirmed the opposing effects of dexamethasone on the transcription of CRFR1 and CRFR2α in MIN6 cells. MIN6 cells transfected with luciferase reporter constructs under control of the putative CRFR1 promoter displayed a dose-dependent decrease in basal luciferase activity after stimulation with increasing doses of dexamethasone (Fig. 4A). In contrast, MIN6 cells transfected with CRFR2α promoter-driven luciferase reporter responded to dexamethasone with a dose-dependent increase in luciferase activity (Fig. 4B). The basal activity of the mouse CRFR1 promoter was higher than the basal luciferase activity driven by the mouse CRFR2α promoter, in agreement with the differences in basal CRFR1 and CRFR2α gene expression in MIN6 cells (Fig. 1A). We next compared the effects of GCs on the activity of CRFR1, CRFR2α, and CRFR2β promoters by transfecting MIN6 cells with luciferase reporters driven by each of these promoters. Dexamethasone reduced the luciferase activity under control of the CRFR1 promoter (1-sided P = 0.038), which was significantly inhibited by the GR-selective antagonist RU486 (Fig. 4C). CRFR2α promoter activity was significantly enhanced by dexamethasone in a GR-dependent manner. In contrast to CRFR2α, CRFR2β promoter activity, already low under basal conditions, was significantly inhibited by dexamethasone. To identify the region of CRFR2α that controls transcriptional activation in response to GCs, we transfected MIN6 cells with a series of three progressively shorter fragments of the putative CRFR2α promoter (5.7 kb, 3.0 kb, 0.8 kb) driving the expression of a luciferase reporter (Fig. 4D). For each of these constructs, luciferase activity was increased after stimulation of MIN6 cells with 200 nm dexamethasone, suggesting that the most proximal GRE in the CRFR2α promoter is sufficient to up-regulate CRFR2α expression in response to GCs (Fig. 4D). Furthermore, the dexamethasone-induced increase in CRFR2 expression was inhibited fully by co-administration of the GR-selective antagonist RU486.
Figure 4.
CRFR1- and CRFR2α promoter-driven luciferase activity is regulated oppositely in MIN6 cells and GC-mediated expression of CRFR2 is dependent on cellular context. MIN6 cells transfected with a luciferase reporter construct under control of the putative CRFR1 (A) or CRFR2α (B) promoters display opposite, dose-dependent effects on luciferase activity after 12-hour stimulation with increasing doses of dexamethasone. The opposite effects of dexamethasone on CRFR1 and CRFR2α promoter activity are fully inhibited by the GR-selective antagonist RU486 (C). CRFR2β promoter activity in MIN6 cells is relatively low, and is inhibited by dexamethasone (C). MIN6 cells transfected with one of three successively shorter fragments of the putative CRFR2α promoter consistently demonstrate dexamethasone-dependent increases in luciferase activity that are fully inhibited by co-administration of the GR-selective antagonist RU486 (D). Predicted GREs consisting of two palindromic half sites are represented by open circles. By contrast, CRFR2α promoter-driven luciferase activity in HEK293 cells is inhibited dose-dependently by dexamethasone, which is prevented by co-administration of RU486 (E). Stimulation of rat A7r5 aortic smooth muscle cells with dexamethasone for 12 h dose-dependently inhibits the expression of CRFR2β, as measured with generic CRFR2 (F) and CRFR2β-specific (G) primers. CRFR2α was not detected in A7r5 cells. Undifferentiated mouse C2C12 cells express low levels of CRFR2α (91 bp) but switch to express CRFR2β (294 bp) when differentiated (H). The approximately 300-bp species that appears in some CRFR2α lanes is an artifact resulting from the mis-priming of the mouse CRFR2α primers to laminin 5a cDNA, and has so far only been observed in C2C12 cells. Expression of CRFR2β in differentiated C2C12 myotubes is inhibited dose-dependently after stimulation for 12 h with increasing doses of dexamethasone, as measured with generic CRFR2 (I) and CRFR2β-specific (J) primers. Data in F, G, I, and J are normalized to HPRT expression and expressed relative to untreated controls. Error bars indicate the SE of triplicate (A–G) or quadruplicate (I and J) treatments, asterisks indicate statistically significant differences with controls unless indicated otherwise (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
The effect of glucocorticoids on CRFR2 expression is dependent on receptor isoform and cell type
To assess the extent to which these GR-mediated transcriptional changes are dependent on cellular context, we transfected the CRFR2α promoter construct in HEK293 cells. CRFR2α promoter-driven luciferase activity was inhibited by dexamethasone, which was fully reversible upon co-administration of the GR-selective antagonist RU486 (Fig. 4E). This contrasts to the dexamethasone-mediated increase in CRFR2α promoter activity observed in MIN6 cells (Fig. 4, B–D) and suggests the effects of GCs on CRFR2α expression depend on cellular context. To explore this further, we assessed the effect of dexamethasone on cell lines known to express CRFR2. Exposure of the rat aortic smooth muscle cell line A7r5 with increasing doses of dexamethasone for 12 h inhibited the expression of CRFR2 and CRFR2β in a dose-dependent manner (Fig. 4, F and G).
Undifferentiated mouse C2C12 cells express low levels of CRFR2α, which is lost upon differentiation into skeletal muscle myotubes, while differentiation of C2C12 cells into myotubes coincides with the appearance of CRFR2β expression (Fig. 4H), in agreement with findings that CRFR2β is the major CRFR2 isoform expressed in rodent skeletal muscle tissue (25,26,27). Stimulation of differentiated C2C12 cells for 12 h with dexamethasone dose-dependently inhibited CRFR2β gene expression, as measured with generic (Fig. 4I) and CRFR2β-specific (Fig. 4J) CRFR2 primers.
Glucocorticoids differentially regulate expression of CRFR1 and CRFR2 in islets
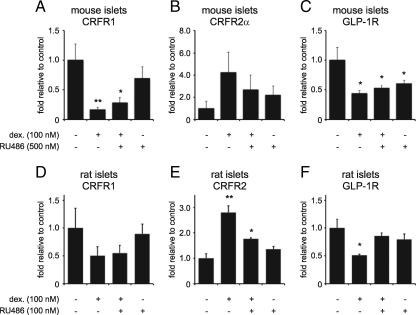
Next, we assessed the transcriptional effects of GCs on CRFR1 and CRFR2 and related transcripts in primary mouse and rat islets. Stimulation of mouse islets (Fig. 5, A–C) with 100 nm dexamethasone for 12 h inhibited the expression of CRFR1 and GLP-1R, while the expression of CRFR2α was elevated (1-sided P = 0.051). These effects were partially negated by the simultaneous application of a fivefold molar excess of RU486. Similarly, in rat islets (Fig. 5, D–F), stimulation with 100 nm dexamethasone for 12 h inhibited the expression of CRFR1 and GLP-1R (although the former effect was not statistically significant) and increased the expression of CRFR2. Co-administration of an equimolar dose of RU486 inhibited the dexamethasone-mediated transcriptional changes. These studies validate in rodent islets our observations in MIN6 cells of GC-mediated GR-dependent inhibition of CRFR1 and GLP-1R expression, while the expression of CRFR2α responds to dexamethasone in an opposite manner.
Figure 5.
GCs differentially regulate the expression of CRFR2 and CRFR1 and GLP-1R in primary mouse and rat islets. Primary mouse (A–C) or rat (D–F) islets were stimulated for 12 h with dexamethasone and/or the GR-selective antagonist RU486 as indicated. Dexamethasone inhibited the expression of CRFR1 in mouse islets (A) and tended to inhibit CRFR1 expression in rat islets (D). In contrast, CRFR2 expression was increased in both mouse (B) and rat (E) islets, although the GC-induced increase of CRFR2α in mouse islets narrowly failed to reach statistical significance (one-sided P = 0.051). The expression of GLP-1R is inhibited by dexamethasone in both mouse (C) and rat (F) islets, although RU486 fails to fully revert the GC-mediated suppression of mouse GLP-1R and even slightly inhibits the expression of GLP-1R itself for reasons not entirely understood. Data are normalized to HPRT expression and expressed relative to untreated controls. Error bars indicate the SE of quadruplicate (A–C) or triplicate (D–F) treatments, asterisks indicate statistically significant differences with controls (*, P < 0.05; **, P < 0.01).
Increased glucocorticoid levels acutely alter islet CRFR expression in vivo
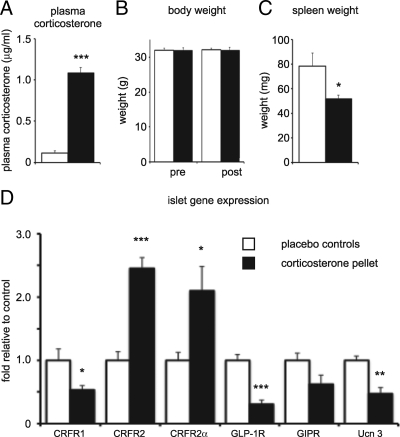
To assess the extent to which GCs affect islet cell expression of CRFR1 and CRFR2 in vivo, we implanted corticosterone pellets subcutaneously in mice, while control animals received placebo pellets. Overnight exposure to corticosterone pellets resulted in a robust increase in plasma corticosterone (Fig. 6A). Total body weight was not different between animals receiving corticosterone or placebo pellets and was unaffected by the pellet implantation (Fig. 6B). Spleen weight, a sensitive marker for GC exposure, was significantly reduced after overnight exposure to corticosterone, likely resulting from the combined effects of GC-induced leukocyte redistribution and apoptosis (Fig. 6C). Acute elevation of plasma corticosterone significantly altered the islet gene expression profile, with expression of CRFR1, GLP-1R, GIPR, and Ucn 3 reduced, and CRFR2 and CRFR2α expression robustly elevated, compared with placebo controls (Fig. 6D).
Figure 6.
GCs differentially affect islet CRFR1 and CRFR2 expression in vivo. Corticosterone pellets were implanted subcutaneously in wild-type mice, leading to significantly elevated plasma corticosterone levels (A). Total body weight was not affected by pellet implantation (B), but spleen weight was significantly reduced (C). The islet gene expression profile reveals GC-induced inhibitions of CRFR1, GLP-1R, GIPR, and Ucn 3 expression, while the expression of CRFR2α is increased (D). Gene expression data are normalized to HPRT expression and expressed relative to placebo controls. Error bars indicate the SE of five (placebo group) or six (corticosterone group) animals, asterisks indicate statistically significant differences with placebo controls (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
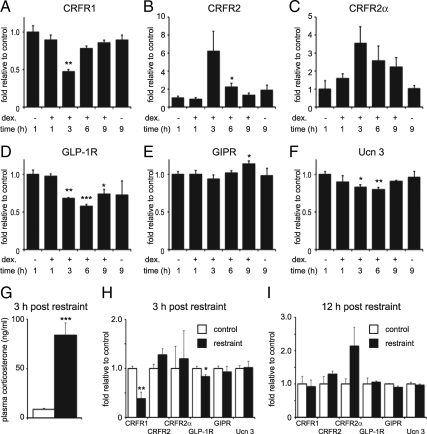
We next addressed the question of whether islet expression of CRFR1 and CRFR2 was affected by acute HPA-axis activity in vivo. To assess the kinetics of GC-mediated transcriptional changes, we stimulated MIN6 cells with a 1-h pulse of dexamethasone, to mimic the transient rise in plasma GCs elicited by an acute stressor. This short exposure to dexamethasone led to a rapid and transient inhibition of CRFR1 expression (Fig. 7A), accompanied by increased expression of CRFR2α (Fig. 7, B and C). The expression of GLP-1R, and to a lesser extent Ucn 3, was also transiently inhibited by dexamethasone, whereas GIPR expression was unaffected (Fig. 7, D–F). All GC-mediated changes normalized 9 h after the initiation of dexamethasone treatment. As GC-mediated changes in expression reached maximal amplitude between 3 and 6 h after the administration of dexamethasone, we assessed the effects of 30 min of acute restraint on the expression of islet CRFR1 and CRFR2 3 h later. This model relies on the transient elevations in plasma GCs that follow acute HPA axis activity to elicit changes in islet gene expression, and constitutes a physiological model of GC-mediated changes in islet CRF receptors compared with subcutaneously implanted corticosterone pellets. Three hours after the start of restraint, plasma corticosterone remained significantly elevated compared with controls (Fig. 7G) and islet expression of CRFR1 and GLP-1R was significantly reduced (Fig. 7H). In contrast, the expression of CRFR2 trended toward an increase (1-sided P = 0.0492). Twelve hours after acute restraint, these transient changes in islet gene expression had subsided (Fig. 7I).
Figure 7.
Acute GC exposure changes CRFR1 and CRFR2 expression in MIN6 cells and in islets in vivo. MIN6 cells were exposed to 100 nm dexamethasone, which was removed by triple washing after 1 h. This short exposure to dexamethasone suffices to inhibit CRFR1 expression (A) and increase CRFR2 expression (B and C). Expression of GLP-1R (D) and Ucn 3 (F) but not GIPR (E) is inhibited by dexamethasone as well. All GC-mediated effects on gene expression are transient, reach maximum amplitude between 3 h and 6 h, and normalize 9 h after the initiation of dexamethasone stimulation. To assess whether a similar acute and transient GC exposure would elicit gene expression changes in islets in vivo, we assessed islet gene expression after 30 min of restraint stress. Three hours after restraint, plasma cortisol levels are still significantly elevated in stressed animals compared with controls (G), and the islet expression of CRFR1 and GLP-1R is significantly reduced (H). However, 12 h after acute restraint, no significant changes in islet gene expression remain (I). Error bars on MIN6 data (A–F) indicate the SE of quadruplicates, whereas error bars reflect the SE of six controls and seven restrained animals (G and H) or five controls and four restrained animals (I). Asterisks indicate statistically significant differences with placebo controls (*, P < 0.05; **, P < 0.01; ***, P < 0.001).
GC-mediated up-regulation of CRFR2α increases responsiveness to Ucn 3
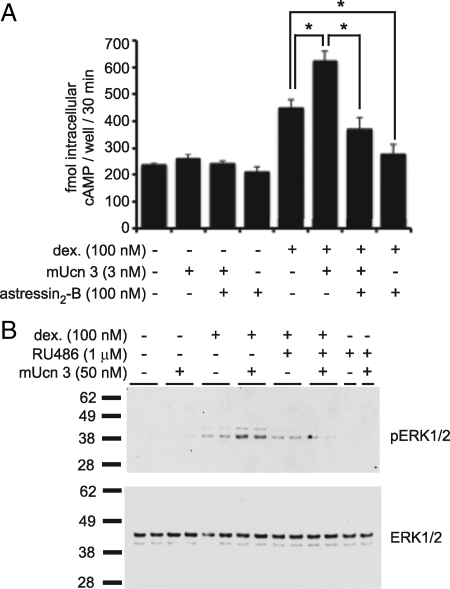
To evaluate the functional consequences of GC-mediated modulation of CRFRs, we tested the responsivity of the MIN6 insulinoma cell line to the CRFR2-selective agonist mUcn 3. MIN6 cells express CRFR2α at relatively low levels compared with other class B GPCRs (Fig. 1A) and therefore respond marginally to increasing doses of mUcn 3, as measured by the generation of intracellular cAMP (Fig. 8A). Co-stimulation with the CRFR2-selective antagonist astressin2-B had little effect on cAMP levels. Overnight pre-treatment of MIN6 cells with 100 nm dexamethasone, which robustly increases CRFR2α expression levels (Figs. 2 and 3), conferred mUcn 3 responsivity to MIN6 cells (Fig. 8A). Pretreatment with dexamethasone elevated basal cAMP levels, likely in part due to endogenous Ucn 3 expressed and secreted by MIN6 cells (6). Addition of synthetic mUcn 3 significantly elevated cAMP levels over the basal levels in the presence of dexamethasone (Fig. 8A). Moreover, the Ucn 3–mediated accumulation of intracellular cAMP levels was fully inhibited by the CRFR2-selective antagonist astressin2-B, demonstrating that the response is mediated via CRFR2. The observation that astressin2-B by itself inhibits intracellular cAMP levels to below baseline values supports the presence of Ucn 3 tone of MIN6 origin (Fig. 8A).
Figure 8.
GC-mediated up-regulation of CRFR2 increases responsiveness to Ucn 3. The relatively low expression of CRFR2α in MIN6 cells is insufficient to induce the accumulation of intracellular cAMP in response to mUcn 3 (A). However, 12-h pretreatment with 100 nm dexamethasone instills MIN6 cells with the ability to respond to mUcn 3 with increased levels of intracellular cAMP. Note that basal levels of cAMP are elevated after dexamethasone pre-treatment. This may be caused in part by endogenous Ucn 3 expressed in, and secreted from MIN6 cells, as stimulation with 100 nm of the CRFR2-selective antagonist astressin2-B inhibits cAMP levels to well below the initial baseline, suggesting the presence of Ucn 3 tone. Similarly, MIN6 cells do not respond to a short (5 min) stimulation with 50 nm mUcn 3 with phosphorylation of ERK1/2, unless pretreated overnight with 100 nm dexamethasone (B). Co-administration of the GR antagonist RU486 during dexamethasone pre-treatment interferes with the ability of MIN6 cells to gain responsivity to Ucn 3. Total ERK1/2 levels were equal among all wells. Error bars in A indicate the SE of triplicate treatments, asterisks indicate statistically significant differences compared with controls that received dexamethasone (*, P < 0.05).
We addressed the ability of mUcn 3 to activate the MAPK signaling pathway after dexamethasone pretreatment. MAPK signaling is activated downstream of CRF receptors in many cells and tissues (15), including β-cells (5). MIN6 cells were untreated or treated overnight with 100 nm dexamethasone in the presence or absence of a 10-fold molar excess of the GR-selective antagonist RU486 and tested for their acute response to mUcn 3. In the absence of dexamethasone pretreatment, MIN6 cells failed to phosphorylate ERK1/2 in response to mUcn 3 (Fig. 8B). In contrast, after dexamethasone pretreatment, MIN6 cells responded to mUcn 3 with a robust increase in pERK1/2 levels (Fig 8B). Basal pERK1/2 levels were detectable in dexamethasone-treated cells in the absence of exogenously administered mUcn 3, perhaps attributable to endogenous Ucn 3. Co-administration of a 10-fold molar excess of RU486 prevented the dexamethasone-mediated increase.
Discussion
Glucocorticoids are known for their ability to elevate circulating glucose levels by increasing hepatic gluconeogenesis and adipocyte lipolysis and inhibiting peripheral glucose disposal, thus functionally antagonizing the actions of insulin (28,29). Our findings highlight an additional mechanism by which changes in circulating GCs, such as those encountered during stress, obesity, or the metabolic syndrome, may modulate the islet’s sensitivity to inputs from peptide hormones through regulation of the expression of their cognate receptors. Specifically, we demonstrated that CRFR2α is the predominant isoform expressed within pancreatic islets and that its expression is increased by GCs. This is in contrast to the effect of GCs on the expression of CRFR1, GLP-1R, and GIPR within the islets. These findings suggest that increased GC levels resulting from GC administration or HPA activity may shift the sensitivity of the islet from inputs of CRFR1 to CRFR2-selective agonists and increase the responsiveness of the endocrine pancreas to β-cell–derived Ucn 3.
Our finding that the major isoform of CRFR2 within the endocrine pancreas is CRFR2α is in opposition to the notion that CRFR2β is the predominant receptor isoform expressed in the periphery (14,26) and that expression of CRFR2α is restricted to the central nervous system in rodents (15,16,26,30). However, expression of CRFR2α in islets should be viewed in light of the parallels that have been observed between islet endocrine cells and neurons. These parallels include similar mechanistic basis for the release of insulin and neurotransmitters (31) and the expression of neuronal markers by β-cells, such as the GABA-synthesizing enzyme glutamic acid decarboxylase 65 (GAD65) that constitutes a common auto-antigen in patients with T1D (32). Both the islet and specialized sets of neurons in the hypothalamus and the brain stem participate in glucose homeostasis and share the ability to sense and respond to changes in ambient glucose (33,34). Thus, despite their origins in different germ layers (the pancreas is of endodermal origin while the CNS is derived from neurectoderm), islet cells have converged with neurons in regards to some phenotypical characteristics, to which we can now add the expression of the α form of CRFR2.
The observation that CRFR2α is the predominant receptor isoform in the islet may hold therapeutic relevance. While CRFR2α and CRFR2β do not differ greatly in affinity for their endogenous ligands (15,35,36), the isoforms differ in their N-terminal amino acid sequence. These differences might serve as the basis for development of CRFR2α- and CRFR2β-selective peptides. Such agonists would activate pancreatic CRFR2α to potentiate glucose-stimulated insulin secretion (6,7) without engaging skeletal muscle CRFR2β, which functionally opposes the activation of islet CRFR2α by inhibiting insulin signaling and insulin-induced glucose uptake (37).
Islet CRFR2α is distinct with regards to its transcriptional regulation by GCs. The expression of CRFR2α in the islet and in MIN6 insulinoma cells is robustly increased in response to GCs. This contrasts with other class B GPCRs in MIN6 cells and primary islets, such as CRFR1, GLP-1R, and GIPR, whose expression is inhibited by GCs in a GR-dependent manner. For GLP-1R, these findings concur with previously published observations that dexamethasone inhibits the expression of GLP-1R in rat islets (38). For CRFR1 and GIPR, these data are, to our knowledge, the first to establish inhibition of gene expression by GCs in the endocrine pancreas, although GC-mediated inhibition of CRFR1 expression in the anterior pituitary gland is an established part of the negative feedback exerted by GCs on HPA-axis activity (11,12). Down-regulation of GLP-1R, GIPR, and CRFR1 may serve to promote the hyperglycemic actions of GCs, which include increased hepatic glucose production, increased peripheral insulin resistance (5,28) and decreased insulin secretion (39,40), by reducing the islets’ sensitivity to incretins (2) and CRFR1 agonists (5,8).
Surprisingly, the GC-mediated effects on CRFR2 were isoform dependent. We demonstrated the expression of CRFR2β in the rat aortic smooth muscle cell line A7r5 and in differentiated mouse skeletal muscle C2C12 cells was inhibited by GCs. These findings are in agreement with previous studies that demonstrate that CRFR2β expression is inhibited dose-dependently by dexamethasone in A7r5 cells in vitro and reduced in rat heart and aorta in vivo by restraint stress or corticosterone injection (41,42). Collectively, these observations suggest that the CRFR2β promoter is inhibited by GCs. In contrast, for CRFR2α, cellular context determines the direction of the GC-mediated effects on expression. We demonstrated a GC-induced inhibition of CRFR2α promoter activity in HEK293 cells, in accordance with previous observations of GC-mediated inhibition of CRFR2α expression in the locus coereleus-derived CATH.a cell line (30,43,44). In another study, dexamethasone inhibited human CRFR2α promoter-driven reporter expression (45). Interestingly, human CRFR2α reporter constructs in this study were transfected into CRFR2β expressing A7r5 cells, supporting our finding that the effects of dexamethasone on CRFR2α promoter activity depend on cellular context. Differences in the repertoire of nuclear receptor co-activators or co-repressors between different cell types might account for the opposing GC-mediated effects on the transcription of CRFR2α.
The ventromedial hypothalamus (VMH) is an integral component of glucose homeostasis, and is key for the generation of the counter-regulatory response (CRR) to hypoglycemia, which involves increases in circulating GCs, catecholamines and glucagon to restore normoglycemia. Activation of CRFR2α on glucose-sensing neurons of the VMH inhibits, while activation of CRFR1 promotes, generation of a CRR (46,47). Several rodent studies indicate that CRFR2α expression was up-regulated in VMH, but not the paraventricular nucleus of rats following chronic corticosterone administration, while VMH CRFR2α expression was down-regulated after adrenalectomy or starvation (48). A more recent study confirmed the selective up-regulation of CRFR2 expression in the VMH as well as cortical and hippocampal CNS sites after a single episode of restraint (49). However, CRFR2α expression in total mouse hypothalamus was reduced after dexamethasone administration or acute or chronic restraint stress in vivo (30), and repeated restraint stress blunted CRFR2α expression in the VMH of rats (50). Nevertheless, it is intriguing that the glucose-sensing neurons of the VMH and the endocrine pancreas respond similarly, at least under some circumstances that involve acute GC exposure, with opposing effects on the expression of CRFR1 and CRFR2α. While the extent to which GC-induced changes in the expression of CRFR1 and CRFR2 in the islet affect the ability to mount CRRs is currently unknown, these findings are relevant in the context of GC-induced diabetes (51,52,53,54,55).
Our data suggest an additional mechanism by which GCs can precipitate hyperglycemia. In addition to GC-mediated increases in hepatic glucose production and peripheral insulin resistance (5,28), and the direct inhibitory effects of GCs on insulin secretion (39,40,56,57,58,59,60), GCs conceivably aggravate hyperglycemia via the inhibition of CRFR1 and incretin receptor expression on β-cells. This would limit insulin output by reducing sensitivity to incretin- and CRF-mediated augmentation of GSIS and could reduce cAMP-mediated protection from GC-induced apoptosis of β-cells (61). Addressing the functional ramifications of GC-mediated changes on islet cell function is challenging, given the wide-ranging actions of GCs on many intracellular signaling cascades. GCs have been reported to inhibit secretion of insulin from β-cell lines (39,40), isolated primary rodent islets (40,56,57,58,59), and in vivo (60). In our experiments, pretreatment with dexamethasone increased basal cAMP levels in MIN6, contradictory to the inhibitory actions of dexamethasone on insulin secretion. We used IBMX, a phosphodiesterase (PDE) inhibitor that prevents cAMP degradation, to measure cumulative cAMP levels over time. It has been shown previously that, in the presence of IBMX, cAMP levels in MIN6 increase upon dexamethasone stimulation, because dexamethasone inhibits cAMP levels by increasing PDE activity and can no longer do so in the presence of the PDE inhibitor IBMX (39). The direct effects of GC at multiple levels of cellular cascades that regulate insulin secretion are compounded in vivo by the profound effects that GCs, or the lack thereof, have on many aspects of glucose metabolism (5,28). We were able to demonstrate an acquired sensitivity of MIN6 insulinoma cells for the CRFR2-selective agonist mUcn 3, secondary to GC-mediated up-regulation of CRFR2α. The low basal levels of CRFR2 on MIN6 cells likely facilitated the detection of cAMP and pERK1/2 increases in response to Ucn 3, as it provided a more or less binary read-out. While GCs robustly inhibit, they do not completely block the transcription of CRFR1 and incretin receptors, suggesting a subtle reduction but not complete abrogation in sensitivity to their cognate ligands. Effects of GCs on the responsivity of islets to incretins and CRFR1 agonists is would therefore be challenging to detect, also because any read-out would suffer from the confounds exerted by GCs on most signaling cascades.
In summary, we demonstrated the presence of CRFR2α in MIN6 β-cells as well as in rodent and human islets. Furthermore, we have shown that acute exposure to GCs in vitro and in vivo substantially alters the transcriptional profile of incretin- and CRF-receptors within the endocrine pancreas, with the majority of class B GPCRs responding with transcriptional inhibition. We postulate that this reflects a general desensitization of the islet to inputs that would promote GSIS, although the pervasive actions of GCs present a formidable challenge to demonstrate this in an experimental setting. Finally, we have established that the GC-mediated regulation of CRFR2α expression in the islet is in contrast with the expression of CRFR2 at most central and peripheral sites, as well as with the transcriptional regulation of other class B GPCR family members that are expressed within the islet micro-environment. It will be an interesting challenge to unravel the specific aspects of the transcriptional landscape within islet cells that enables CRFR2α to respond to GCs with increased expression, in contrast to GC-mediated inhibition that is the norm for most islet class B GPCRs and for CRFR2 at most other sites in the body.
Supplementary Material
Acknowledgments
We thank Jean Rivier and Judit Erchegyi for providing the peptides used in this study and Cindy Donaldson for technical assistance with the cAMP assay.
Footnotes
This work was supported by award P01DK026741-30 from the National Institute of Diabetes and Digestive and Kidney Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Diabetes and Digestive and Kidney Diseases or the National Institutes of Health. This project was supported in part by the Clayton Medical Research Foundation, Inc. W.W.V. is a Senior Clayton Medical Research Foundation investigator. We gratefully acknowledge support by the Juvenile Diabetes Research Foundation. M.O.H. is a past recipient of a postdoctoral fellowship from the Adler Foundation and currently receives fellowship support from the Salk Center for Nutritional Genomics and the Leona M. & Harry B. Helmsley Charitable Trust.
Disclosure Summary: M.O.H., A.P.P., M.M., T.v.d.M., H.P., J.M.V., and S.L. have nothing to declare. W.V. is a co-founder, consultant, equity holder, and member of the Board of Directors and Scientific Advisory Board of Acceleron Pharma, Inc. and Neurocrine Biosciences, Inc. The following have been licensed by The Salk Institute for Biological Studies and/or The Clayton Foundation: CRF to Ferring Pharmaceuticals, CRFR1 and Ucn 2 to Neurocrine Biosciences, and Ucn 3 to Johnson & Johnson.
First Published Online November 24, 2010
Abbreviations: CNS, Central nervous system; CRF, corticotropin releasing factor; CRR, counter-regulatory response; GC, glucocorticoid; GIP, glucose-dependent insulinotropic polypeptide; GIPR, GIP receptor; GLP-1, glucagon-like peptide-1; GLP-1R, GLP-1 receptor; GR, glucocorticoid receptor; GRE, glucocorticoid response element; GSIS, glucose-stimulated insulin secretion; HPA, hypothalamus-pituitary-adrenal; HPRT, hypoxanthine guanine phosphoribosyl transferase; MR, mineralocorticoid receptor; PDE, phosphodiesterase; Sst, somatostatin; Ucn, urocortin; VMH, ventromedial hypothalamus.
References
- Strowski MZ, Parmar RM, Blake AD, Schaeffer JM 2000 Somatostatin inhibits insulin and glucagon secretion via two receptors subtypes: an in vitro study of pancreatic islets from somatostatin receptor 2 knockout mice. Endocrinology 141:111–117 [DOI] [PubMed] [Google Scholar]
- Baggio LL, Drucker DJ 2007 Biology of incretins: GLP-1 and GIP. Gastroenterology 132:2131–2157 [DOI] [PubMed] [Google Scholar]
- Hansotia T, Drucker DJ 2005 GIP and GLP-1 as incretin hormones: lessons from single and double incretin receptor knockout mice. Regul Pept 128:125–134 [DOI] [PubMed] [Google Scholar]
- Gilon P, Henquin JC 2001 Mechanisms and physiological significance of the cholinergic control of pancreatic beta-cell function. Endocr Rev 22:565–604 [DOI] [PubMed] [Google Scholar]
- Huising MO, van der Meulen T, Vaughan JM, Matsumoto M, Donaldson CJ, Park H, Billestrup N, Vale WW 2010 CRFR1 is expressed on pancreatic beta cells, promotes beta cell proliferation, and potentiates insulin secretion in a glucose-dependent manner. Proc Natl Acad Sci USA 107:912–917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen P, Vaughan J, Lee KF, Vale W 2007 Urocortin 3 regulates glucose-stimulated insulin secretion and energy homeostasis. Proc Natl Acad Sci USA 104:4206–4211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Chen P, Vaughan J, Blount A, Chen A, Jamieson PM, Rivier J, Smith MS, Vale W 2003 Urocortin III is expressed in pancreatic beta-cells and stimulates insulin and glucagon secretion. Endocrinology 144:3216–3224 [DOI] [PubMed] [Google Scholar]
- O'Carroll AM, Howell GM, Roberts EM, Lolait SJ 2008 Vasopressin potentiates corticotropin-releasing hormone-induced insulin release from mouse pancreatic beta-cells. J Endocrinol 197:231–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuperman Y, Chen A 2008 Urocortins: emerging metabolic and energy homeostasis perspectives. Trends Endocrinol Metab 19:122–129 [DOI] [PubMed] [Google Scholar]
- Fekete EM, Zorrilla EP 2007 Physiology, pharmacology, and therapeutic relevance of urocortins in mammals: ancient CRF paralogs. Front Neuroendocrinol 28:1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Spangler R, LaForge KS, Maggos CE, Ho A, Kreek MJ 1996 Modulation of CRF-R1 mRNA in rat anterior pituitary by dexamethasone: correlation with POMC mRNA. Peptides 17:435–441 [DOI] [PubMed] [Google Scholar]
- Makino S, Schulkin J, Smith MA, Pacák K, Palkovits M, Gold PW 1995 Regulation of corticotropin-releasing hormone receptor messenger ribonucleic acid in the rat brain and pituitary by glucocorticoids and stress. Endocrinology 136:4517–4525 [DOI] [PubMed] [Google Scholar]
- Catalano RD, Kyriakou T, Chen J, Easton A, Hillhouse EW 2003 Regulation of corticotropin-releasing hormone type 2 receptors by multiple promoters and alternative splicing: identification of multiple splice variants. Mol Endocrinol 17:395–410 [DOI] [PubMed] [Google Scholar]
- Perrin M, Donaldson C, Chen R, Blount A, Berggren T, Bilezikjian L, Sawchenko P, Vale W 1995 Identification of a second corticotropin-releasing factor receptor gene and characterization of a cDNA expressed in heart. Proc Natl Acad Sci USA 92:2969–2973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillhouse EW, Grammatopoulos DK 2006 The molecular mechanisms underlying the regulation of the biological activity of corticotropin-releasing hormone receptors: implications for physiology and pathophysiology. Endocr Rev 27:260–286 [DOI] [PubMed] [Google Scholar]
- Lovenberg TW, Liaw CW, Grigoriadis DE, Clevenger W, Chalmers DT, De Souza EB, Oltersdorf T 1995 Cloning and characterization of a functionally distinct corticotropin-releasing factor receptor subtype from rat brain. Proc Natl Acad Sci USA 92:836–840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimes BW, Brandt BL 1976 Characterization of two putative smooth muscle cell lines from rat thoracic aorta. Exp Cell Res 98:349–366 [DOI] [PubMed] [Google Scholar]
- Yaffe D, Saxel O 1977 Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature 270:725–727 [DOI] [PubMed] [Google Scholar]
- Blau HM, Pavlath GK, Hardeman EC, Chiu CP, Silberstein L, Webster SG, Miller SC, Webster C 1985 Plasticity of the differentiated state. Science 230:758–766 [DOI] [PubMed] [Google Scholar]
- Zhang W, Zhao L, Mulholland MW 2007 Ghrelin stimulates myocyte development. Cell Physiol Biochem 20:659–664 [DOI] [PubMed] [Google Scholar]
- Filigheddu N, Gnocchi VF, Coscia M, Cappelli M, Porporato PE, Taulli R, Traini S, Baldanzi G, Chianale F, Cutrupi S, Arnoletti E, Ghè C, Fubini A, Surico N, Sinigaglia F, Ponzetto C, Muccioli G, Crepaldi T, Graziani A 2007 Ghrelin and des-acyl ghrelin promote differentiation and fusion of C2C12 skeletal muscle cells. Mol Biol Cell 18:986–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justice NJ, Yuan ZF, Sawchenko PE, Vale W 2008 Type 1 corticotropin-releasing factor receptor expression reported in BAC transgenic mice: implications for reconciling ligand-receptor mismatch in the central corticotropin-releasing factor system. J Comp Neurol 511:479–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huising MO, Vaughan JM, Shah SH, Grillot KL, Donaldson CJ, Rivier J, Flik G, Vale WW 2008 Residues of corticotropin releasing factor-binding protein (CRF-BP) that selectively abrogate binding to CRF but not to urocortin 1. J Biol Chem 283:8902–8912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kageyama K, Gaudriault GE, Suda T, Vale WW 2003 Regulation of corticotropin-releasing factor receptor type 2beta mRNA via cyclic AMP pathway in A7r5 aortic smooth muscle cells. Cell Signal 15:17–25 [DOI] [PubMed] [Google Scholar]
- Kishimoto T, Pearse 2nd RV, Lin CR, Rosenfeld MG 1995 A sauvagine/corticotropin-releasing factor receptor expressed in heart and skeletal muscle. Proc Natl Acad Sci USA 92:1108–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovenberg TW, Chalmers DT, Liu C, De Souza EB 1995 CRF2 alpha and CRF2 beta receptor mRNAs are differentially distributed between the rat central nervous system and peripheral tissues. Endocrinology 136:4139–4142 [DOI] [PubMed] [Google Scholar]
- Coste SC, Quintos RF, Stenzel-Poore MP 2002 Corticotropin-releasing hormone-related peptides and receptors: emergent regulators of cardiovascular adaptations to stress. Trends Cardiovasc Med 12:176–182 [DOI] [PubMed] [Google Scholar]
- Chrousos GP 2000 The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int J Obes Relat Metab Disord 24(Suppl 2):S50–S55 [DOI] [PubMed] [Google Scholar]
- Strack AM, Sebastian RJ, Schwartz MW, Dallman MF 1995 Glucocorticoids and insulin: reciprocal signals for energy balance. Am J Physiol 268:R142–R149 [DOI] [PubMed] [Google Scholar]
- Chen A, Perrin M, Brar B, Li C, Jamieson P, Digruccio M, Lewis K, Vale W 2005 Mouse corticotropin-releasing factor receptor type 2alpha gene: isolation, distribution, pharmacological characterization and regulation by stress and glucocorticoids. Mol Endocrinol 19:441–458 [DOI] [PubMed] [Google Scholar]
- Eliasson L, Abdulkader F, Braun M, Galvanovskis J, Hoppa MB, Rorsman P 2008 Novel aspects of the molecular mechanisms controlling insulin secretion. J Physiol 586:3313–3324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsirogianni A, Pipi E, Soufleros K 2009 Specificity of islet cell autoantibodies and coexistence with other organ specific autoantibodies in type 1 diabetes mellitus. Autoimmun Rev 8:687–691 [DOI] [PubMed] [Google Scholar]
- Levin BE, Routh VH, Kang L, Sanders NM, Dunn-Meynell AA 2004 Neuronal glucosensing: what do we know after 50 years? Diabetes 53:2521–2528 [DOI] [PubMed] [Google Scholar]
- Sandoval D, Cota D, Seeley RJ 2008 The integrative role of CNS fuel-sensing mechanisms in energy balance and glucose regulation. Annu Rev Physiol 70:513–535 [DOI] [PubMed] [Google Scholar]
- Kostich WA, Chen A, Sperle K, Largent BL 1998 Molecular identification and analysis of a novel human corticotropin-releasing factor (CRF) receptor: the CRF2gamma receptor. Mol Endocrinol 12:1077–1085 [DOI] [PubMed] [Google Scholar]
- Ardati A, Goetschy V, Gottowick J, Henriot S, Valdenaire O, Deuschle U, Kilpatrick GJ 1999 Human CRF2 alpha and beta splice variants: pharmacological characterization using radioligand binding and a luciferase gene expression assay. Neuropharmacology 38:441–448 [DOI] [PubMed] [Google Scholar]
- Chen A, Brar B, Choi CS, Rousso D, Vaughan J, Kuperman Y, Kim SN, Donaldson C, Smith SM, Jamieson P, Li C, Nagy TR, Shulman GI, Lee KF, Vale W 2006 Urocortin 2 modulates glucose utilization and insulin sensitivity in skeletal muscle. Proc Natl Acad Sci USA 103:16580–16585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrahamsen N, Nishimura E 1995 Regulation of glucagon and glucagon-like peptide-1 receptor messenger ribonucleic acid expression in cultured rat pancreatic islets by glucose, cyclic adenosine 3′,5′-monophosphate, and glucocorticoids. Endocrinology 136:1572–1578 [DOI] [PubMed] [Google Scholar]
- Shao J, Qiao L, Friedman JE 2004 Prolactin, progesterone, and dexamethasone coordinately and adversely regulate glucokinase and cAMP/PDE cascades in MIN6 beta-cells. Am J Physiol Endocrinol Metab 286:E304–E310 [DOI] [PubMed] [Google Scholar]
- Ullrich S, Berchtold S, Ranta F, Seebohm G, Henke G, Lupescu A, Mack AF, Chao CM, Su J, Nitschke R, Alexander D, Friedrich B, Wulff P, Kuhl D, Lang F 2005 Serum- and glucocorticoid-inducible kinase 1 (SGK1) mediates glucocorticoid-induced inhibition of insulin secretion. Diabetes 54:1090–1099 [DOI] [PubMed] [Google Scholar]
- Coste SC, Heldwein KA, Stevens SL, Tobar-Dupres E, Stenzel-Poore MP 2001 IL-1alpha and TNFalpha down-regulate CRH receptor-2 mRNA expression in the mouse heart. Endocrinology 142:3537–3545 [DOI] [PubMed] [Google Scholar]
- Kageyama K, Gaudriault GE, Bradbury MJ, Vale WW 2000 Regulation of corticotropin-releasing factor receptor type 2 beta messenger ribonucleic acid in the rat cardiovascular system by urocortin, glucocorticoids, and cytokines. Endocrinology 141:2285–2293 [DOI] [PubMed] [Google Scholar]
- Suri C, Fung BP, Tischler AS, Chikaraishi DM 1993 Catecholaminergic cell lines from the brain and adrenal glands of tyrosine hydroxylase-SV40 T antigen transgenic mice. J Neurosci 13:1280–1291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Hou LX, Aktiv A, Dahlström A 2007 Studies of the central nervous system-derived CAD cell line, a suitable model for intraneuronal transport studies? J Neurosci Res 85:2601–2609 [DOI] [PubMed] [Google Scholar]
- Nanda SA, Roseboom PH, Nash GA, Speers JM, Kalin NH 2004 Characterization of the human corticotropin-releasing factor2. (a) receptor promoter: regulation by glucocorticoids and the cyclic adenosine 5′-monophosphate pathway. Endocrinology 145:5605–5615 [DOI] [PubMed] [Google Scholar]
- Cheng H, Zhou L, Zhu W, Wang A, Tang C, Chan O, Sherwin RS, McCrimmon RJ 2007 Type 1 corticotropin-releasing factor receptors in the ventromedial hypothalamus promote hypoglycemia-induced hormonal counterregulation. Am J Physiol Endocrinol Metab 293:E705–E712 [DOI] [PubMed] [Google Scholar]
- McCrimmon RJ, Song Z, Cheng H, McNay EC, Weikart-Yeckel C, Fan X, Routh VH, Sherwin RS 2006 Corticotrophin-releasing factor receptors within the ventromedial hypothalamus regulate hypoglycemia-induced hormonal counterregulation. J Clin Invest 116:1723–1730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S, Nishiyama M, Asaba K, Gold PW, Hashimoto K 1998 Altered expression of type 2 CRH receptor mRNA in the VMH by glucocorticoids and starvation. Am J Physiol 275:R1138–R1145 [DOI] [PubMed] [Google Scholar]
- Greetfeld M, Schmidt MV, Ganea K, Sterlemann V, Liebl C, Muller MB 2009 A single episode of restraint stress regulates central CRH receptor expression and binding in specific areas of the mouse brain. J Neuroendocrinol 21:473–480 [DOI] [PubMed] [Google Scholar]
- Makino S, Asaba K, Nishiyama M, Hashimoto K 1999 Decreased type 2 corticotropin-releasing hormone receptor mRNA expression in the ventromedial hypothalamus during repeated immobilization stress. Neuroendocrinology 70:160–167 [DOI] [PubMed] [Google Scholar]
- Schäcke H, Döcke WD, Asadullah K 2002 Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther 96:23–43 [DOI] [PubMed] [Google Scholar]
- Hoogwerf B, Danese RD 1999 Drug selection and the management of corticosteroid-related diabetes mellitus. Rheum Dis Clin North Am 25:489–505 [DOI] [PubMed] [Google Scholar]
- Trence DL 2003 Management of patients on chronic glucocorticoid therapy: an endocrine perspective. Prim Care 30:593–605 [DOI] [PubMed] [Google Scholar]
- Uzu T, Harada T, Sakaguchi M, Kanasaki M, Isshiki K, Araki S, Sugiomoto T, Koya D, Haneda M, Kashiwagi A, Yamauchi A 2007 Glucocorticoid-induced diabetes mellitus: prevalence and risk factors in primary renal diseases. Nephron Clin Pract 105:c54–c57 [DOI] [PubMed] [Google Scholar]
- Clore JN, Thurby-Hay L 2009 Glucocorticoid-induced hyperglycemia. Endocr Pract 15:469–474 [DOI] [PubMed] [Google Scholar]
- Lambillotte C, Gilon P, Henquin JC 1997 Direct glucocorticoid inhibition of insulin secretion: an in vitro study of dexamethasone effects in mouse islets. J Clin Invest 99:414–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierluissi J, Navas FO, Ashcroft SJ 1986 Effect of adrenal steroids on insulin release from cultured rat islets of Langerhans. Diabetologia 29:119–121 [DOI] [PubMed] [Google Scholar]
- Billaudel B, Sutter BC 1981 Modulation of the direct effect of corticosterone upon glucose-induced insulin secretion of rat isolated islets of Langerhans. Diabete Metab 7:91–96 [PubMed] [Google Scholar]
- Billaudel B, Sutter BC 1979 Direct effect of corticosterone upon insulin secretion studied by three different techniques. Horm Metab Res 11:555–560 [DOI] [PubMed] [Google Scholar]
- Billaudel B, Sutter BC 1982 Immediate in-vivo effect of corticosterone on glucose-induced insulin secretion in the rat. J Endocrinol 95:315–320 [DOI] [PubMed] [Google Scholar]
- Ranta F, Avram D, Berchtold S, Düfer M, Drews G, Lang F, Ullrich S 2006 Dexamethasone induces cell death in insulin-secreting cells, an effect reversed by exendin-4. Diabetes 55:1380–1390 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.