Abstract
Successful restoration of phenylalanine (Phe) clearance following liver-directed gene therapy in murine phenylketonuria (PKU) is likely dependent upon both the number of cells successfully transduced and the amount of phenylalanine hydroxylase (PAH) activity expressed per cell. At low levels of transduction, Phe clearance could be limited by the low absolute number of PAH-expressing cells rather than the total amount of PAH activity produced in the liver. We have evaluated the interrelationship between the number of PAH positive cells, the amount of PAH activity produced and Phe clearance through experiments with hepatocyte-mediated therapeutic liver repopulation in the Pahenu2 mouse, a model of PKU. We compared the therapeutic efficacy of transplantation with either wild-type hepatocytes or hepatocytes from heterozygous Pahenu2/+ donors into PAH deficient, hyperphenylalaninemic Pahenu2/Pahenu2 mice. The recipient mice were also homozygous for fumarylacetoacetate hydrolase (FAH) deficiency. In this model system, FAH positive donor hepatocytes enjoy a selective growth advantage in the FAH-deficient recipient. If Phe clearance is governed predominantly by the total PAH activity, then more heterozygous cells, which express lower PAH activity than wild-type cells, should be required to correct Phe clearance. If the absolute donor cell number is more important, then wild-type hepatocytes should have no advantage over heterozygous cells. We successfully carried out therapeutic liver repopulation with heterozygous donor cells in fifteen mice and an additional thirteen transplants with wild-type cells. Blood Phe was successfully reduced in both transplant groups, and the relationship between the final blood Phe level and the extent of liver repopulation with donor cells did not differ between the two donor groups. Regardless of the type of donor cell, liver repopulation of approximately 3–10% was sufficient to at least partially reduce blood phenylalanine, and blood Phe levels were completely corrected in mice that had attained greater than approximately 10% liver repopulation. We conclude from our study that the absolute number of PAH-expressing cells likely governs Phe clearance at least at the levels of repopulation reported here and that the amount of PAH activity per donor cell is a less critical variable. The implication for liver-directed gene therapy of PKU is that only partial correction of cellular PAH deficiency may yet improve Phe clearance as long as a sufficient number of hepatocytes is successfully transduced.
Keywords: phenylketonuria, phenylalanine, phenylalanine hydroxylase deficiency, therapeutic liver repopulation, hepatocyte transplantation, mouse model
1. Introduction
Phenylketonuria (PKU; OMIM #261600), one of the most common inborn errors of metabolism with an incidence of approximately 1:16000 births in North America, is caused by deficiency of phenylalanine hydroxylase (PAH; EC 1.14.16.1) secondary to recessively-inherited mutations in the PAH gene. The pathophysiology of PKU is linked to effects of chronically elevated Phe concentration in blood and other tissues, most importantly the brain. Therefore, the aim of PKU treatment is the reduction of Phe concentration in the body. In some individuals with PKU, blood Phe concentration decreases following supplementation with sapropterin dihydrochloride (Kuvan™) [1], a synthetic form of the naturally-occurring and essential PAH cofactor tetrahydrobiopterin, but for the majority of patients, dietary Phe restriction remains the mainstay of treatment. Dietary therapy prevents the major manifestations of the disease (mental retardation, seizures, and growth failure), but shortcomings in this strategy exist, including lifelong commitment to an unpalatable and expensive diet, and persistent mild cognitive deficits in some treated children [2]. Enzyme replacement or substitution, cell transplantation and gene therapy are promising alternative approaches to the treatment of inborn errors of metabolism such as PKU [3, 4]. However, a detailed understanding of the physiologic requirements for inducing effective Phe clearance is critical to the successful development of these treatment strategies.
We have previously demonstrated that therapeutic liver repopulation following transplantation of wild-type hepatocytes successfully corrected hyperphenylalaninemia in Pahenu2 mice, a model of human PKU [5]. We confirmed that successful therapeutic liver repopulation can be achieved only if the donor hepatocytes have a selective growth advantage over native hepatocytes [6], and that unmanipulated PAH positive hepatocytes unfortunately do not exhibit any selective growth advantage over PAH-deficient cells. However, when a selective growth condition was experimentally achieved, we found that liver repopulation with as few as 3–5% PAH positive wild-type hepatocytes was associated with partial correction of hyperphenylalaninemia. In mice with greater than 10% liver repopulation, blood Phe concentration was corrected to normal. However, the effect of transplantation with hepatocytes expressing only partial PAH activity, such as hepatocytes from a heterozygous donor, remained to be examined. Here, we sought to explore the relationships between cellular PAH activity, degree of therapeutic liver repopulation and Phe clearance by comparing the efficacy of transplantation with hepatocytes having either full (Pah+/+) or only partial (Pah+/−) PAH activity. Our initial hypothesis was that complete correction of blood Phe concentrations in PAH-deficient mice following transplantation of hepatocytes with only partial PAH activity would require a greater extent of liver repopulation than following transplantation of hepatocytes with full PAH activity. An alternative hypothesis was that if Phe clearance is, at low levels of liver repopulation, primarily governed by a cellular factor other than total PAH activity, such as the capacity for Phe transport into hepatocytes, then transplantation of hepatocytes with either full or partial PAH activity should be equally effective at similar degrees of liver repopulation. To distinguish between these two hypotheses, we compared the efficacy of therapeutic liver repopulation in hyperphenylalaninemic Pahenu2/Pahenu2 mice following the transplantation of wild-type hepatocytes or hepatocytes isolated from a Pahenu2/+ heterozygous donor mouse with only partial PAH activity.
2. Materials and Methods
2a. Animal husbandry
Animal care and experimentation were performed in accordance with the guidelines of the Dept. of Comparative Medicine, Oregon Health & Science University. Because donor PAH positive hepatocytes do not enjoy any selective growth advantage over PAH deficient cells in hepatocyte transplant recipients, we employed the 129/Sv-Pahenu2-FahΔexon5 mouse model of combined PAH and fumarylacetoacetate hydrolase (FAH; EC 3.7.1.2) deficiencies [5] in these experiments. 129/Sv- Pahenu2-FahΔexon5 mice (henceforth designated Pah/Fah mice) are homozygous for the missense Pahenu2 mutation that causes murine PKU [7] but are also homozygous for a targeted deletion of FAH exon 5 and exhibit FAH deficiency and tyrosinemia analogous to human tyrosinemia type 1 [8]. Transplantation of FAH+ hepatocytes into FAH deficient mice yields nearly complete liver repopulation with FAH+ cells due to necrosis of FAH-deficient hepatocytes and a selective growth advantage for FAH+ cells. Prior to transplantation, FAH-deficient FAH/PAH breeders and experimental mice were treated with 2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione (NTBC) at a concentration of 4 mg/ml in drinking water to prevent liver dysfunction and death. NTBC is a reversible competitive inhibitor of p-hydroxyphenylpyruvate dioxidase, an intermediate enzymatic step in tyrosine metabolism that is proximal to FAH in the metabolic pathway. The cellular toxin fumarylacetoacetate (FAA) accumulates in FAH deficiency and causes liver failure; administration of NTBC prevents FAA production and rescues FAH-deficient mice. Genotyping for the presence of the FahΔexon5 deletion [8] or the Pahenu2 mutation [9] was performed by PCR analysis of tail biopsy DNA. All animals were fed standard mouse chow ad libitum providing approximately 23% of energy as protein.
2b. Hepatocyte transplants
Pah/Fah mice were transplanted with isolated hepatocytes from congenic donor animals that were either wild-type 129/Sv mice having full FAH and PAH activity or were 129/Sv-Pahenu2/+ mice with full FAH activity but only partial PAH activity. Parenchymal hepatocytes were isolated from donor mice by two step collagenase perfusion as described [10] except that the collagenase preparation used was Liberase Blendzyme (Roche, Indianapolis, IN), 23 μg/ml. Cell number and viability were determined by trypan blue exclusion in a hemocytometer. The desired number of donor cells (1–4 × 105) were suspended in 100 μl Dulbecco’s minimal essential medium (DMEM) supplemented with 10% Fetal Bovine Serum (FBS) and injected intrasplenically into recipient animals [11]. NTBC therapy of FAH deficient recipient mice was discontinued following transplantation to allow therapeutic liver repopulation with FAH+ donor hepatocytes. The weight of transplanted animals was measured weekly.
2c. Phenotypic characterization of transplanted animals
Serum Phe concentrations were measured weekly in 10 μl serum using a modification of a fluorometric procedure [12]. Transplanted mice were euthanized at various time points after hepatocyte transplantation to develop a series of mice with between 1–80% liver repopulation. At the endpoint of the experiment, transplanted mice were euthanized by cardiac exsanguination under inhaled anesthesia. Primary hepatocytes were then isolated by collagenase perfusion. This procedure allowed biochemical and molecular evaluation of a suspension of primary hepatocytes from the entire liver, eliminated stromal cells, and avoided possible data artifact secondary to differences in extent of repopulation among different regions of the liver.
2d. Estimation of % liver repopulation by measurement of PAH activity
PAH activity was measured in duplicate in hepatocytes using a radiochemical technique [13] modified as previously described [9] using 6-methyltetrahydropteridine (6MPH4) as cofactor. Total protein was measured using a bicinchonic acid procedure (BCA Protein Assay, Pierce, Rockford, IL). Primary hepatocytes isolated from wild-type mice were used as positive controls. The % liver repopulation was calculated by measuring PAH activity in hepatocytes isolated from transplant recipients and expressing the result as a percentage of the PAH activity measured in wild-type hepatocytes in that day’s assay. Because of a dominant negative effect of the Pahenu2 mutation (Pahenu2 monomers complex with and inhibit wild type PAH monomers in the formation of PAH tetramers), Pahenu2/+ hepatocytes express approximately 25% wild type PAH activity {Charron, 2004 #65} rather than 50% activity as would be typically predicted for a recessively-inherited disorder. Therefore, for recipient mice that had received Pahenu2/+ hepatocytes, the % liver repopulation calculated from the measured PAH activity was corrected upward four fold to account for partial PAH activity in donor cells; for example, 10% wild type PAH activity measured in recipient mice corresponded to 40% repopulation with Pahenu2/+ hepatocytes.
2e. Estimation of % liver repopulation by PCR-HRM analysis
The percent liver repopulation was also estimated using polymerase chain reaction-high resolution melt analysis (PCR-HRM) on hepatocyte genomic DNA to measure the proportion of wild-type FAH genomes among a predominantly FahΔexon5/FahΔexon5 background. In this assay, 100 ng hepatocyte genomic DNA isolated by standard proteinase digestion and phenol/chloroform extraction was subjected to PCR amplification of the FAH gene in the region spanning the 5′ junction of the FahΔexon5 insertion (Figure 1). The forward primer used (FAH576F – 5′-ACGGACTTCTACCCTTTTCGG-3′) is complementary to both wild-type and FahΔexon5 genomes; two different reverse primers, one complementary to the wild-type FAH gene (FAH661R - 5′-ATCCAATTTGGCAACAGCG-3′) and the other to the FahΔexon5 insertion (FAH640R – 5′-GCCTACACGGAGCGCG-3′) were included in the PCR reaction. The reactions were catalyzed using standard conditions with HotStar Taq Plus™ DNA polymerase in the presence of EvaGreen™ fluorescent dye (Type-It HRM PCR kit™, Qiagen Inc., Valencia, CA) in a Rotorgene Q™ real-time thermocycler with built in high resolution melt analysis capability according to the manufacturer’s instructions. The thermocycler conditions were 95°C for 10 minutes to activate the HotStar Taq enzyme followed by 40 cycles of 95°C × 10 seconds, 55°C × 30 seconds. This multiplex PCR reaction yielded amplicons of 85 bp and 64 bp from the wild-type and FahΔexon5 genomes respectively. High resolution melt analysis ramping from 75°C to 90°C in 0.1°C increments was automatically carried out in the Rotorgene Q following completion of the PCR amplification. The melting temperatures of the wild-type and FahΔexon5 PCR products were approximately 82.8°C and 79.8°C respectively. This difference in melting temperature was exploited to accurately measure the fluorescence intensity of the wild-type PCR product relative to the fluorescence of the FahΔexon5 PCR product. Software provided with the Rotorgene Q was used to calculate the % wild-type PCR product. Analysis of a standard series of wild-type 129/Sv genomic DNA mixed into FahΔexon5 genomic DNA yielded a linear curve in relative fluorescence intensity over a range from 0.78% to 50% wild-type FAH genomic DNA.
Figure 1. PCR reaction to detect wild-type FAH and FahΔexon5 genomes.
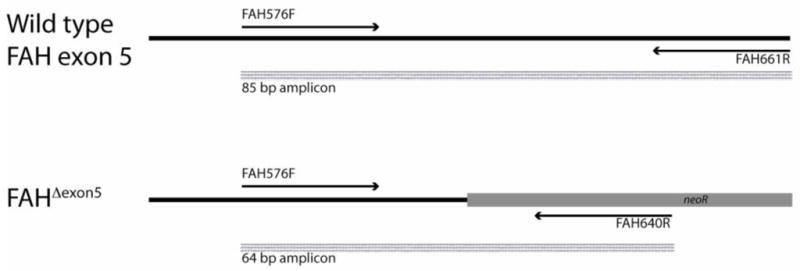
A duplex PCR reaction was designed to simultaneously detect wild-type mouse FAH exon 5 and FahΔexon5 mutant genomes in total hepatocyte genomic DNA isolated from transplanted mice. The 5′ primer FAH576F is complementary to both wild-type and FahΔexon5 genomes while the 3′ primers are complementary to their individual respective targets. PCR reactions were carried out in triplicate in the presence of EvaGreen™ fluorescent dye. The relative abundance of the wild-type amplicon (85 bp) among a predominantly mutant amplicon (64 bp) population was measured by post-PCR high resolution melt analysis and comparison to a standard series of FahΔexon5 genomic liver DNA spiked with known amounts of wild-type DNA.
3. Theory and calculations
Our initial hypothesis was that phenylalanine clearance is primarily governed by the total liver PAH activity achieved after therapeutic liver repopulation of Pahenu2 mice. If this hypothesis is true, then in comparison to transplantation with wild-type hepatocytes expressing 100% normal PAH activity, greater numbers of Pahenu2/+ hepatocytes with only partial PAH activity will be required to correct serum phenylalanine levels in Pah/Fah mice. An alternative hypothesis was that if at low liver repopulation frequency, phenylalanine clearance is limited primarily by the absolute number of PAH-expressing cells regardless of the amount of PAH activity per cell, then Pahenu2/+ hepatocytes would be equally effective as wild-type hepatocytes in correcting hyperphenylalaninemia. A model for these two hypotheses is presented in Figure 2.
Figure 2. Physiologic model.
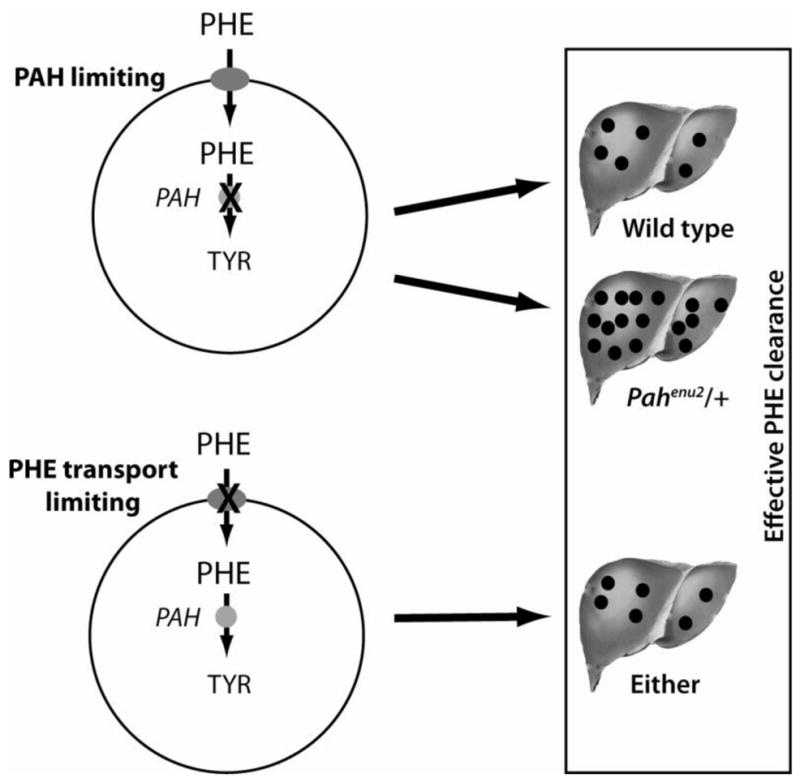
Phenylalanine clearance may be limited either by the rate of phenylalanine transport into PAH-expressing hepatocytes or by total PAH activity in liver. If phenylalanine clearance is primarily limited by PAH activity, then fewer wild-type hepatocytes with 100% PAH activity will be required to correct hyperphenylalaninemia than Pahenu2/+ hepatocytes with only partial PAH activity. If phenylalanine clearance is primarily related to transmembrane phenylalanine transport, then wild-type and Pahenu2/+ hepatocytes would be equally effective in restoring phenylalanine clearance.
To evaluate our hypotheses, the final blood Phe concentration at euthanasia of transplanted mice was plotted vs. the % liver repopulation as determined by two independent methods: measurement of PAH activity and PCR-HRM analysis. Inspection of these plots suggested that the decrease in blood Phe with increasing liver repopulation fit a one-phase decay model. Therefore, non-linear regression analysis was employed to find the best exponential curve fit using GraphPad Prism 5.0™ software. This analysis yielded equations of the form y = [y0 − plateau] e−kx + plateau where x = % liver repopulation, y and y0 equal the blood Phe at % liver repopulation x and the initial blood Phe prior to transplant respectively, plateau = blood Phe achieved after complete liver repopulation, and k is a constant analogous to a decay rate constant. The results obtained from transplants with either wild-type or Pahenu2/+ hepatocytes were plotted separately and the decay constants k of the resulting exponential equations were compared using an extra sum of squares F test. For the purpose of statistical analysis, the null hypothesis was that the k values for wild-type and Pahenu2/+ transplants were not different, and this hypothesis was rejected only if the F test yielded p < 0.05. If the number of hepatocytes required to achieve therapeutic liver repopulation differed substantially between the wild-type and Pahenu2/+ transplant groups, then the k values of the two analyses would be significantly different.
4. Results
Successful therapeutic liver repopulation was achieved in thirteen Pah/Fah mice following transplantation with wild-type hepatocytes and in fifteen Pah/Fah mice following transplantation with Pahenu2/+ hepatocytes. Data from nine mice that had received wild-type hepatocytes were previously reported [5] including the effect of therapeutic liver repopulation upon blood Phe and % liver repopulation as calculated from the measurement of liver PAH activity. However, the estimation of % liver repopulation by PCR-HRM analysis in these mice is new and had not been presented previously. Four additional wild-type hepatocyte transplantations were performed contemporaneously to the fifteen transplants with Pahenu2/+ hepatocytes.
Decrease in blood phenylalanine vs. time following hepatocyte transplantation
Transplantation of either wild-type or Pahenu2/+ hepatocytes yielded correction of blood Phe in Pah/Fah mice (Figure 3). Blood Phe decreased over time following transplantation as the % liver repopulation increased. The time to achieve correction of blood Phe to below 800 μM ranged from 22–70 days for mice receiving wild-type hepatocytes transplants and from 57–117 days after transplantation with Pahenu2/+ hepatocytes. Treated mice had received 1–4 × 105 hepatocytes depending upon the yields of the hepatocyte isolation procedures; differences in cell dose between animals contributes to the difference in the time course of the experiment. Linear regression analysis of the two groups detected no significant difference between the slopes of the best-fit curves (p = 0.433) indicating no difference in the rate of decrease in blood Phe following transplantation with either wild type or Pahenu2/+ hepatocytes.
Figure 3. Serum Phenylalanine vs. Time.
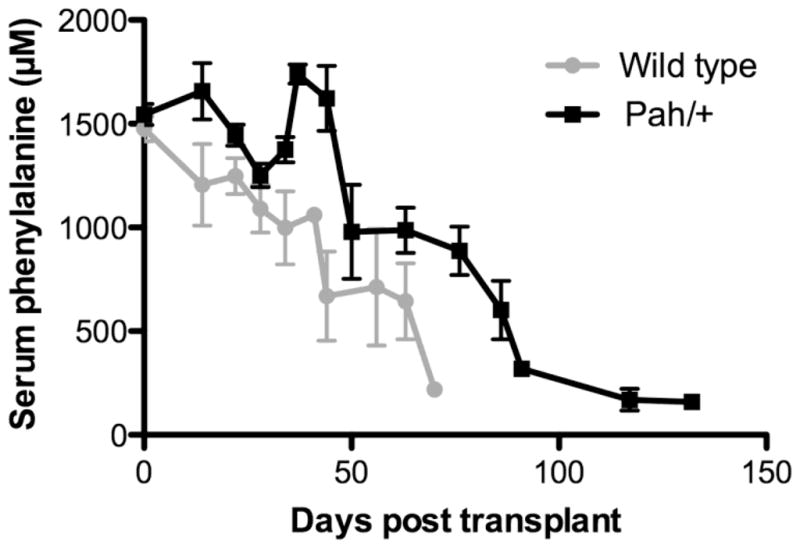
Serum phenylalanine (μM) of transplanted mice vs. time (days) after transplant. Recipient mice had received either wild-type (gray circles) or Pahenu2/+ (black squares) hepatocytes.
Relationship between blood phenylalanine and % liver repopulation following hepatocyte transplantation
Transplanted Pah/Fah mice were euthanized at different time points following transplantation to develop a series of animals with a range of % liver repopulation. Because accurate measurement of liver repopulation is technically difficult across a wide range of % repopulation, two different methods, measurement of PAH activity or detection of wild type genomic DNA by PCR-HRM, were used. Comparison of the two methods demonstrated that % liver repopulation was generally underestimated by PAH activity measurement, and therefore the sensitivity of the PAH activity to detect low levels of liver repopulation is decreased in comparison to the PCR-HRM method (data not shown); this could be due to instability of PAH activity during collagenase-mediated isolation of hepatocytes. On the other hand, the PCR-HRM method was associated with relatively larger standard errors in animals with extensive liver repopulation. For these reasons, further analyses of the relationship between serum Phe and % liver repopulation were carried out independently for the two methods.
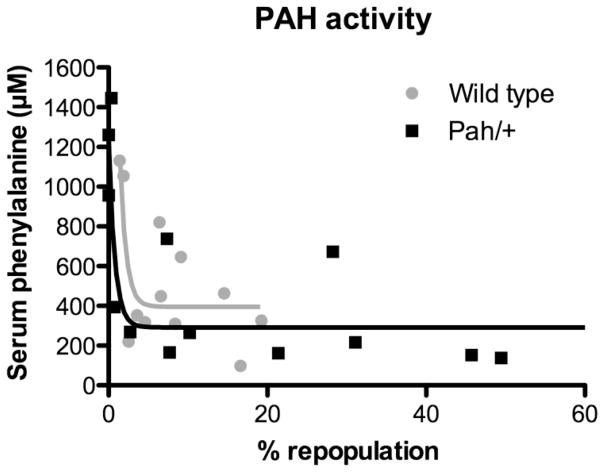
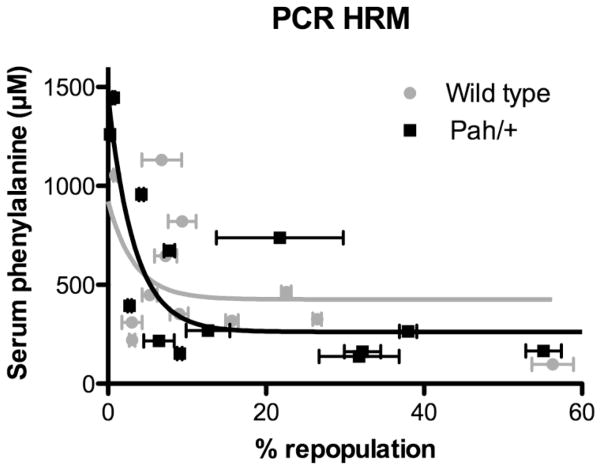
Serum Phe was plotted against % liver repopulation as estimated by either PAH activity (Figure 4) or PCR-HRM (Figure 5). Regardless of the method used to measure liver repopulation, therapeutic liver repopulation with either wild-type or Pahenu2/+ hepatocytes achieved significant reduction in blood Phe with complete correction seen only in mice achieving greater than approximately 10% liver repopulation. Application of a non-linear curve fit algorithm yielded exponential curves as the best fit for all data sets. Goodness of fit (R2) and decay constants k for these curves are displayed in Table 1. Comparison of the wild-type hepatocyte and Pahenu2/+ hepatocyte curves using an extra sum of squares F test and the null hypothesis that the constants k did not differ between data sets yielded p values of 0.6191 for the analysis of % liver repopulation measured by PAH activity and 0.2017 for PCR HRM data. The difference between the constants k from wild-type and Pahenu2/+ curves was therefore not significantly different. This result leads us to the conclusion that wild-type hepatocytes and Pahenu2/+ hepatocytes are equally effective in correcting phenylalanine clearance of Pahenu2/Pahenu2 mice despite the difference in cellular PAH activity between the two cell types.
Figure 4. Serum Phenylalanine vs. % Liver Repopulation as Measured by PAH Activity.
Serum phenylalanine (μM) is plotted vs. % liver repopulation as calculated by liver PAH activity for mice that received transplants with either wild-type (gray circles) or Pahenu2/+ (black squares) hepatocytes.
Figure 5. Serum Phenylalanine vs. % Liver Repopulation as Measured by PCR-HRM analysis.
Serum phenylalanine (μM) is plotted vs. % liver repopulation as calculated by PCR-HRM analysis that received transplants with either wild-type (gray circles) or Pahenu2/+ (black squares) hepatocytes. The PCR-HRM analysis measured the proportion of wild-type FAH genome for mice in liver genomic DNA that was predominantly FahΔexon5.
Table 1.
Non-linear curve fits
| Wild type hepatocytes | Pahenu2/+ hepatocytes | |||||
|---|---|---|---|---|---|---|
| Goodness of fit (r2) | k (SE) | Goodness of fit (r2) | k (SE) | F | p | |
| PAH activity | 0.5389 | 1.599 (1.212) | 0.6524 | 1.044 (0.8206) | 0.255 | 0.6191 |
| PCR-HRM | 0.245 | 0.009981 (0.07033) | 0.6933 | 0.3070 (0.15) | 1.743 | 0.2017 |
Serum phenylalanine (μM) was plotted versus the % liver repopulation as measured either by PAH activity or PCR-HRM analysis, and the data subject to a non-linear curve fit algorithm to yield exponential curves with a constant (k) analogous to a decay constant. The exponential curves from wild type and Pahenu2/+ hepatocytes transplants were compared using an extra sum of squares F test to calculate the probability (p) that k was the same for both data sets.
5. Conclusion
Therapeutic liver repopulation following hepatocyte transplantation requires both a growth stimulus at the time of transplant and a selective growth advantage for donor cells over the native hepatocytes [6]. In Pahenu2 mice, the required growth stimulus may be initiated by partial hepatectomy, but unfortunately PAH positive hepatocytes exhibit no growth advantage over PAH negative cells. Less than 1% liver repopulation was achieved following transplantation of PAH positive wild-type hepatocytes into hyperphenylalaninemic Pahenu2 mice [5] following partial hepatectomy. Wild-type hepatocytes do enjoy a selective growth advantage when transplanted into FAH-deficient mice [14]. We have exploited the FAH-deficient FahΔexon5 mouse model in our experiments exploring Phe clearance by the liver. Transplantation of FAH-positive hepatocytes into PAH- and FAH-deficient (PAH/FAH) mice led to successful therapeutic liver repopulation and correction of blood Phe concentration.
Our study clearly demonstrates that therapeutic liver repopulation can be achieved in PAH/FAH mice following transplantation of either wild-type hepatocytes expressing full PAH activity or heterozygous Pahenu2/+ hepatocytes that express only partial PAH activity. Both donor cell populations expressed FAH activity and therefore enjoyed the required selective growth advantage over FAH-deficient hepatocytes in PAH/FAH mice. Our initial hypothesis had been that, because of partial PAH activity, a greater extent of liver repopulation with Pahenu2/+ hepatocytes would be necessary to fully correct blood phenylalanine concentration in comparison to transplantation with wild-type hepatocytes. However, the experiments reported here demonstrate that the % liver repopulation required for correction of phenylalanine clearance was no different between the two experimental groups. Measurement of % liver repopulation using either the PAH activity or PCR-HRM methods yield the same conclusion despite differences in sensitivity between the two methods. Pahenu2/+ were just as effective as Pah+/+ hepatocytes in restoring phenylalanine clearance to PAH/FAH mice. Furthermore, in both groups, complete correction of blood phenylalanine concentration was demonstrated only in mice that had achieved greater than approximately 10% liver repopulation regardless of the type of donor cell.
One conclusion from our study is that, at low liver repopulation (< 10%) at least, cellular PAH activity is not the sole contributor to the control of phenylalanine clearance by the liver. Other cellular factors must strongly influence phenylalanine clearance. This conclusion is in agreement with previously reported data suggesting that both cellular PAH activity and the rate of phenylalanine transport across the hepatocyte membrane are involved in the control of blood phenylalanine clearance [14]. In this work, extracellular and intracellular phenylalanine concentrations and conversion rates to tyrosine were measured in isolated rat hepatocytes with and without an inhibitor of phenylalanine transmembrane transport and under conditions of basal PAH activity or maximum stimulation of PAH activity by glucagon. Control Coefficients for the rate of phenylalanine flux were calculated. Under basal enzyme conditions, the Control Coefficients for phenylalanine transport and PAH activity were approximately equal, but under conditions of maximum PAH stimulation, the Control Coefficient for phenylalanine transport increased to 0.88 indicating that the measured phenylalanine flux under those conditions was primarily governed by the rate of phenylalanine transport across the hepatocyte membrane. Phenylalanine enters hepatocytes by facilitated diffusion via a high capacity sodium-independent transporter, the so-called LAT1 transporter that also transports other large neutral amino acids [15, 16]. We did not directly assess transmembrane phenylalanine transport in our experiment, but we assume that the capacity for transport did not differ between wild-type and Pahenu2/+ donor hepatocytes because all donor mice were of 129/Sv genetic background and differed only by the presence or absence of the Pahenu2 mutation. Therefore, we propose that phenylalanine clearance in transplanted animals with low liver repopulation was primarily determined by the capacity for phenylalanine transport across the cell membranes of donor hepatocytes. In this scenario, the total flux of phenylalanine to tyrosine would be directly proportional to the absolute number of PAH-expressing hepatocytes in transplanted liver and not primarily influenced by the amount of PAH activity expressed per donor cell.
Hepatocyte transplantation has been employed clinically for a number of genetic disorders with varying results [4]. This procedure is likely more successful for diseases in which wild-type hepatocytes have a natural selective growth advantage over diseased hepatocytes, such as occurs in tyrosinemia type 1 [14]. Successful therapeutic liver repopulation in individuals with PKU will require the development of treatment methods that yield a selective growth advantage for donor cells in the PAH-negative liver of the transplant recipient. If such a treatment approach can be developed, our results demonstrate that the extent of liver repopulation achieved rather than the amount of PAH activity per cell will be the most critical factor for the successful restoration of phenylalanine clearance. Our results also bear upon the potential success of liver-directed gene therapy for PKU; transduction of at least 10–20% of hepatocytes by a gene therapy vector will be necessary to attain successful correction of hyperphenylalaninemia regardless of the amount of PAH activity expressed per cell. Transplantation of hepatocytes from donors who are heterozygous for PAH deficiency will likely be just as efficacious as transplantation with wild-type hepatocytes expressing full PAH activity.
Acknowledgments
The authors thank Melanie Gillingham and Michael Liskay for critical review of the manuscript. This work was supported by NIDDK R01 grant DK059371. The authors would also like to acknowledge the support of many patients and their families affected by PKU who continue to inspire our work.
Abbreviations
- PKU
phenylketonuria
- Phe
phenylalanine
- PAH
phenylalanine hydroxylase
- Tyr
tyrosine
- FAH
fumarylacetoacetate hydrolase
- NTBC
2-(2-nitro-4-trifluoromethylbenzoyl)-1,3-cyclohexanedione
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kelly J. Hamman, Email: hammank@ohsu.edu.
Shelley Winn, Email: winns@ohsu.edu.
References
- 1.Levy HL, Milanowski A, Chakrapani A, Cleary M, Lee P, Trefz FK, Whitley CB, Feillet F, Feigenbaum AS, Bebchuk JD, Christ-Schmidt H, Dorenbaum A. Efficacy of sapropterin dihydrochloride (tetrahydrobiopterin, 6R-BH4) for reduction of phenylalanine concentration in patients with phenylketonuria: a phase III randomised placebo-controlled study. Lancet. 2007;370:504–510. doi: 10.1016/S0140-6736(07)61234-3. [DOI] [PubMed] [Google Scholar]
- 2.Azen CG, Koch R, Friedman EG, Berlow S, Coldwell J, Krause W, Matalon R, McCabe E, O’Flynn M, Peterson R. Intellectual development in 12-year-old children treated for phenylketonuria. Amer J Dis Child. 1991;145:35–39. doi: 10.1001/archpedi.1991.02160010037012. [DOI] [PubMed] [Google Scholar]
- 3.Harding C. Progress toward cell-directed therapy for phenylketonuria. Clin Genet. 2008;74:97–104. doi: 10.1111/j.1399-0004.2008.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harding CO, Gibson KM. Therapeutic liver repopulation for phenylketonuria. J Inherit Metab Dis. 2010 doi: 10.1007/s10545-010-9099-1. [DOI] [PubMed] [Google Scholar]
- 5.Hamman K, Clark H, Montini E, al-Dhalimy M, Grompe M, Finegold M, Harding CO. Low therapeutic threshold for hepatocyte replacement in murine phenylketonuria. Molec Therapy. 2005;12:337–344. doi: 10.1016/j.ymthe.2005.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Laconi E, Laconi S. Principles of hepatocyte repopulation. Semin Cell Dev Biol. 2002;13:433–438. doi: 10.1016/s1084952102001313. [DOI] [PubMed] [Google Scholar]
- 7.McDonald JD, Charlton CK. Characterization of mutations at the mouse phenylalanine hydroxylase locus. Genomics. 1997;39:402–405. doi: 10.1006/geno.1996.4508. [DOI] [PubMed] [Google Scholar]
- 8.Grompe M, al-Dhalimy M, Finegold M, Ou CN, Burlingame T, Kennaway NG, Soriano P. Loss of fumarylacetoacetate hydrolase is responsible for the neonatal hepatic dysfunction phenotype of lethal albino mice. Genes and Development. 1993;7:2298–2307. doi: 10.1101/gad.7.12a.2298. [DOI] [PubMed] [Google Scholar]
- 9.Harding CO, Wild K, Chang D, Messing A, Wolff JA. Metabolic engineering as therapy for inborn errors of metabolism - development of mice with phenylalanine hydroxylase expression in muscle. Gene Therapy. 1998;5:677–683. doi: 10.1038/sj.gt.3300653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grompe M, Jones SN, Loulseged H, Caskey CT. Retroviral-mediated gene transfer of human ornithine transcarbamylase into primary hepatocytes of spf and spf-ash mice. Hum Gene Ther. 1992;3:35–44. doi: 10.1089/hum.1992.3.1-35. [DOI] [PubMed] [Google Scholar]
- 11.Ponder KP, Gupta S, Leland F, Darlington G, Finegold M, DeMayo J, Ledley FD, Chowdhury JR, Woo SL. Mouse hepatocytes migrate to liver parenchyma and function indefinitely after intrasplenic transplantation. Proc Natl Acad Sci U S A. 1991;88:1217–1221. doi: 10.1073/pnas.88.4.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McCaman MW, Robins E. Fluorimetric method for the determination of phenylalanine in serum. J Lab Clin Med. 1962;59:885–890. [Google Scholar]
- 13.Ledley FD, Hahn T, Woo SL. Selection for phenylalanine hydroxylase activity in cells transformed with recombinant retroviruses. Somat Cell Mol Genet. 1987;13:145–154. doi: 10.1007/BF01534694. [DOI] [PubMed] [Google Scholar]
- 14.Salter M, Knowles RG, Pogson CI. Quantification of the importance of individual steps in the control of aromatic amino acid metabolism. Biochem J. 1986;234:635–647. doi: 10.1042/bj2340635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kanai Y, Segawa H, Miyamoto K, Uchino H, Takeda E, Endou H. Expression cloning and characterization of a transporter for large neutral amino acids activated by the heavy chain of 4F2 antigen (CD98) J Biol Chem. 1998;273:23629–23632. doi: 10.1074/jbc.273.37.23629. [DOI] [PubMed] [Google Scholar]
- 16.Mastroberardino L, Spindler B, Pfeiffer R, Skelly PJ, Loffing J, Shoemaker CB, Verrey F. Amino-acid transport by heterodimers of 4F2hc/CD98 and members of a permease family. Nature. 1998;395:288–291. doi: 10.1038/26246. [DOI] [PubMed] [Google Scholar]