Abstract
In the United States, school-located influenza vaccination (SLIV) programs have increased significantly in recent years. In June 2010, the Office of Inspector General issued a report regarding 38 elementary school H1N1 SLIV programs conducted in 6 localities in November/December 2009. By locality, there was a mean of 14 to 46 first doses of vaccine administered per 100 students. The locality that conducted programs in early November had a higher uptake rate than localities with later programs (46 vs. 21 per 100 students; p < 0.01). Among localities with programs in mid- to late-November, the locality with programs after school hours had a lower uptake rate than the two localities with programs during school hours (16 vs. 28, p = 0.05 and 16 vs. 30, p < 0.01, respectively). These data suggest that future SLIV programs may achieve higher uptake rates if conducted during school hours with advance parental consent and when parental demand is highest.
Keywords: H1N1, influenza, school, pediatric, vaccination
Introduction
In the United States, school-located influenza vaccination (SLIV) programs have increased significantly in recent years due to expanding recommendations for the annual vaccination of children. SLIV programs have been identified as an efficient means of vaccinating large numbers of children in a short period of time and have been adopted in many areas to help increase pediatric influenza vaccination rates.1–13 The US response to the 2009 H1N1 pandemic led to further implementation of SLIV programs, with approximately 40 states using SLIV programs to some degree to distribute H1N1 vaccine to targeted populations.14
In June 2010, the Office of Inspector General (OIG) issued a public report on 38 single-day urban elementary H1N1 SLIV programs conducted from November to December 2009 in six localities in Arizona, Maryland, Minnesota, Missouri, New York and Virginia.15 According to the report, the data were collected in response to statements by the Centers for Disease Control and Prevention that “data about local implementation of SLV programs have been limited, especially during influenza pandemics.” Data were collected via onsite interviews and observations, follow-up email surveys and reviews of program documentation.
To the best of our knowledge, the data contained in the OIG report represent the only real-time, quantitative assessment of multiple concurrent and geographically diverse SLIV programs in the United States. The purpose of the current analysis was to use the data collected by the OIG to identify factors associated with higher uptake rates in SLIV programs.
Results
The 38 SLIV programs occurred between November 4 and December 15, 2009. Each locality had between six and eight schools surveyed and program implementation characteristics were generally similar in each locality (Table 1). In each locality, SLIV programs occurred within a 2-d span. The mean number of enrolled students per school ranged from 394 to 763 across localities. The mean number of first doses administered in the SLIV programs varied by locality, ranging from 16 to 46 doses administered per 100 students. Schools in localities A–C administered on average significantly more doses per 100 students than localities E and F, which reported the lowest mean doses (p < 0.01, p < 0.01, p = 0.03 for A–C vs. E, respectively; p < 0.01, p < 0.01 and p < 0.05 for A–C vs. F, respectively; Table 1).
Table 1.
Locality characteristics
| Characteristic | Locality | |||||
| A | B | C | D | E | F | |
| SLIV programs surveyed, n | 8 | 6 | 6 | 6 | 6 | 6 |
| Days after November 1, mean | 4 | 23 | 11 | 44 | 18 | 33 |
| Programs during school hours, % | 50 | 100 | 100 | 100 | 0 | 100 |
| Staff at SLIV site, mean | 10 | 12 | 13 | 6 | 31 | 7 |
| Staff per 100 students, mean | 1.5 | 2.3 | 2.8 | 1.6 | 4.5 | 1.9 |
| Days to provide consent, mean | 10 | 35 | 25 | 27 | 0 | 44 |
| Programs with consent forms available online, % | 100 | 0 | 33 | 0 | 0 | 0 |
| Programs vaccinating children who were not students, % | 25 | 0 | 0 | 0 | 100 | 0 |
| Students enrolled, mean | 689 | 572 | 495 | 407 | 763 | 394 |
| Doses of H1N1 vaccine administered, mean | 308 | 169 | 139 | 83 | 134 | 57 |
| Doses of H1N1 vaccine administered per 100 students, mean | 46 | 30 | 28 | 20 | 16 | 16 |
SLIV, school-located influenza vaccination.
In four localities (B–D and F), all programs were conducted during school hours with parental consent obtained in advance by distributing consent forms online, by mail, or by sending the forms home with children 7 to 61 d in advance of the program. In contrast, all programs in locality E were conducted after hours with parental consent obtained on-site. In locality A, four programs were conducting during school hours and four were conducted after school hours.
All localities used the H1N1 injectable and nasal spray vaccines. Use varied by locality, with three of six localities reporting predominant use of the nasal spray vaccine (range, 59% to 74% of vaccinations) and three of six localities reporting predominant use of injectable vaccine (range, 67% to 73%). Across individual SLIV programs, 87% (n = 33) of the SLIV programs used both vaccines, while 13% (n = 5) used only the injectable vaccine. The OIG report commented that “parental and staff misconceptions about the safety of the nasal mist” affected uptake in some localities. Vaccinations were offered free-of-charge to students.
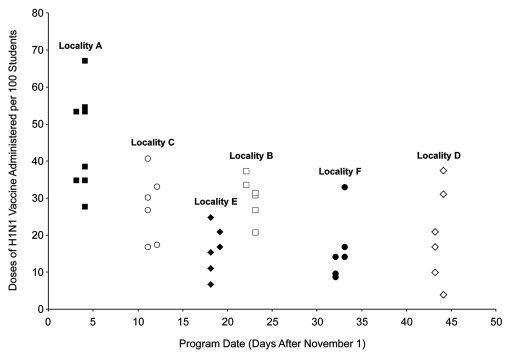
Examination of program characteristics and uptake rates at the locality level suggested that program date was the principal factor associated with increased vaccination (Fig. 1). Programs conducted during the first week of November (locality A) administered more first doses than later programs. The mean number of doses per 100 students was 46 doses for the 8 programs in locality A, compared with 21 doses for the 30 later programs in other localities (p < 0.01).
Figure 1.
Doses of H1N1 vaccine administered by program date and locality. Each school-located influenza vaccination program is represented by a single data point.
In addition to the increased uptake rate among programs conducted in early November, there appeared to be a general trend toward decreasing uptake rates across localities with increased time after November 1 (Fig. 1). The notable exception to this trend was locality E, which had a mean uptake rate that was significantly lower than that of localities C and B, whose SLIV programs also took place in mid- to late-November (means of 16 vs. 28 doses per 100 students, p = 0.05 and 16 vs. 30 doses per 100 students, p < 0.01, respectively). The most apparent difference in program characteristics between locality E and localities C and B was that all SLIV programs in locality E were conducted after school hours without advance parental consent; parental consent was obtained on-site on the day of the programs.
For other program characteristics evaluated, there were no other apparent associations in the available sample (Table 1).
Discussion
In this analysis of elementary school H1N1 SLIV programs conducted in six localities in the United States, programs conducted in early November near the peak of H1N1 activity achieved higher uptake rates, as one might expect. According to the Centers for Disease Control and Prevention, H1N1 influenza activity for the fall 2009 epidemic peaked in late October.16 Additionally, through late October, few doses of H1N1 vaccine were available.17 However, in early November, media reports of local peaks in US communities18–20 and increased availability of H1N1 vaccine17 likely decreased parental demand for H1N1 vaccination in SLIV programs. This decreased demand would explain the decreased uptake rates in programs conducted in mid-November and later.
Among these later programs, data from the current analysis suggest that SLIV programs may be able to achieve higher uptake rates if conducted during school hours with parental consent obtained in advance. Although after-hours programs may be more easily implemented due to a reduced need for advance planning, programs conducted after school hours with on-site consenting may be less successful in achieving high uptake rates. The OIG report noted that long registration lines were observed at most SLIV sites that distributed consent forms on the day of vaccination, which was attributed to the amount of time it took for a parent to complete the consent form and ask questions. Among early programs in locality A, several after-hours programs achieved uptake rates comparable with programs conducted during school hours, likely because high levels of parental demand for vaccination overcame the logistical challenges of after-hours programs. However, in situations in which there is more moderate demand, as would be expected in future nonpandemic, seasonal influenza vaccination programs, after-hours programs appear to be at a logistical disadvantage compared with programs conducted during school hours with advance parental consent.
There are several limitations to the current analysis. The analysis is based on a limited, nonrandom sample and therefore may not be generalizable to all US 2009 H1N1 SLIV programs. Additionally, as noted by the OIG, the identified programs occurred at various stages in the localities' SLIV programs, and thus programs conducted later in a locality could have had more opportunity to improve overall program performance; this effect could not be evaluated because the program date relative to other nonsurveyed programs in the same locality was not reported. All programs were conducted in response to the H1N1 pandemic; as a result, factors affecting vaccination uptake could differ compared with nonpandemic, seasonal influenza. In particular, as noted in the OIG report: “Compared to SLV programs for seasonal influenza, the 2009 SLV programs were unique because of delays in vaccine production and delivery, compressed timelines for planning and additional concerns about vaccine safety.” Additionally, significant funding was available for 2009 H1N1 SLIV programs through Public Health Emergency Response grants, which would not be available for seasonal SLIV programs. Although parental and staff motivation and program funding would be expected to differ between pandemic and nonpandemic SLIV programs, the operational aspects are similar and thus logistical factors associated with increased uptake rates would be expected to apply in some degree to future US nonpandemic, seasonal SLIV programs. Lastly, many other variables that could affect vaccination uptake, including parental and staff knowledge/attitudes, community demographics, local media coverage and clarity of program communications, were not evaluated and were not available for the present analysis.
Methods
The available data for each of the 38 schools in the six localities were extracted directly from the OIG report for analysis. There was no data collection beyond the information available in the report. The main outcome for this analysis was the uptake rate achieved by schools in each locality during the SLIV programs. A school's uptake rate was calculated using the number of H1N1 vaccine first doses administered as the numerator and the number of students enrolled as the denominator. Additional data in the analysis included the date of the program calculated as days after November 1, the timing of the program (during or after school hours), the vaccine type (injectable vs. nasal spray), the consent process, the number of days allowed for return of parental consent, whether non-student children (e.g., siblings) were vaccinated, and the number of staff involved in the SLIV program.
Because of the similar characteristics (measured and unmeasured) of programs in each locality, an analysis was conducted at the locality level to identify potential associations between program characteristics and uptake rates. Differences between localities were examined by comparing the mean number of first doses per 100 students using the pooled t-test for schools in the localities in question. Variance equality was determined using the F statistic. A statistically significant difference in the mean number of first doses per 100 students was accepted at p ≤ 0.05. All analyses used SAS v.8 (SAS Institute, Cary, NC).
Conclusions
Despite its limitations, this analysis highlights the key roles of program design and parental demand in achieving successful SLIV programs. Results suggest that uptake rates are likely to be higher if SLIV programs are conducted during school hours with advance parental consent and when parental demand is highest.
Acknowledgments
The study was sponsored by MedImmune, LLC. Editorial support in the form of formatting the manuscript for submission was provided by John E. Fincke, Ph.D., and Gerard P. Johnson, Ph.D., of Complete Healthcare Communications, Inc., (Chadds Ford, PA) and funded by MedImmune.
Abbreviations
- OIG
Office of Inspector General
- SLIV
school-located influenza vaccination
Conflict of Interest
Both authors are employees of MedImmune, LLC.
References
- 1.Tran CH, McElrath J, Hughes P, Ryan K, Munden J, Castleman JB, et al. Implementing a community-supported school-based influenza immunization program. Biosecur Bioterror. 2010;8:331–341. doi: 10.1089/bsp.2010.0029. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter LR, Lott J, Lawson BM, Hall S, Craig AS, Schaffner W, et al. Mass distribution of free, intranasally administered influenza vaccine in a public school system. Pediatrics. 2007;120:172–178. doi: 10.1542/peds.2006-603. [DOI] [PubMed] [Google Scholar]
- 3.Cawley J, Hull HF, Rousculp MD. Strategies for implementing school-located influenza vaccination of children: a systematic literature review. J Sch Health. 2010;80:167–175. doi: 10.1111/j.1746-561.2009.00482.x. [DOI] [PubMed] [Google Scholar]
- 4.Davis MM, King JC, Jr, Moag L, Cummings G, Magder LS. Countywide school-based influenza immunization: direct and indirect impact on student absenteeism. Pediatrics. 2008;122:260–265. doi: 10.1542/peds.2007-963. [DOI] [PubMed] [Google Scholar]
- 5.Effler PV, Chu C, He H, Gaynor K, Sakamoto S, Nagao M, et al. Statewide school-located influenza vaccination program for children 5–13 years of age, Hawaii USA. Emerg Infect Dis. 2010;16:244–250. doi: 10.3201/eid1602.091375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glezen WP, Gaglani MJ, Kozinetz CA, Piedra PA. Direct and indirect effectiveness of influenza vaccination delivered to children at school preceding an epidemic caused by 3 new influenza virus variants. J Infect Dis. 2010;202:1626–1633. doi: 10.1086/657089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grijalva CG, Zhu Y, Griffin MR. Evidence of effectiveness from a large county-wide school-based influenza immunization campaign. Vaccine. 2009;27:2633–2636. doi: 10.1016/j.vaccine.2009.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hull HF. A survey of physician-led influenza immunization programs in schools. Clin Pediatr (Phila) 2010;49:439–442. doi: 10.1177/0009922809346573. [DOI] [PubMed] [Google Scholar]
- 9.Hull HF, Frauendienst RS, Gundersen ML, Monsen SM, Fishbein DB. School-based influenza immunization. Vaccine. 2008;26:4312–4313. doi: 10.1016/j.vaccine.2008.06.015. [DOI] [PubMed] [Google Scholar]
- 10.Mears CJ, Lawler EN, Sanders LD, 3rd, Katz BZ. Efficacy of LAIV-T on absentee rates in a school-based health center sample. J Adolesc Health. 2009;45:91–94. doi: 10.1016/j.jado-health.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Painter JE, Sales JM, Pazol K, Grimes T, Wingood GM, DiClemente RJ. Development, theoretical framework and lessons learned from implementation of a school-based influenza vaccination intervention. Health Promot Pract. 2010;11:42–52. doi: 10.1177/1524839909360171. [DOI] [PubMed] [Google Scholar]
- 12.Ransom J. School-located influenza vaccination clinics: local health department perspectives. J Sch Nurs. 2009;25:13–17. doi: 10.1177/1059840508330068. [DOI] [PubMed] [Google Scholar]
- 13.Wiggs-Stayner KS, Purdy TR, Go GN, McLaughlin NC, Tryzynka PS, Sines JR, et al. The impact of mass school immunization on school attendance. J Sch Nurs. 2006;22:219–222. doi: 10.1177/10598405050220040601. [DOI] [PubMed] [Google Scholar]
- 14.Center for Infectious Disease Research and Policy, author. H1N1 Lessons Learned: Vaccination campaign weathered rough road, paid dividends. [January 3, 2011]. Available at: http://www.cidrap.umn.edu/cidrap/content/influenza/swineflu/news/apr3010campaign.html.
- 15.Wright S. Memorandum Report: 2009 H1N1 School-Located Vaccination Program Implementation, OEI-04-10-00020. [January 3, 2011]. Available at: http://oig.hhs.gov/oei/reports/oei-04-10-00020.pdf.
- 16.Centers for Disease Control and Prevention, author. Weekly 2009 H1N1 Flu Media Briefing—October 27, 2009. [January 3, 2011]. Available at: http://www.cdc.gov/media/transcripts/2009/t091027.htm.
- 17.Centers for Disease Control and Prevention, author. Weekly 2009 H1N1 Flu Media Briefing—November 6, 2009. [January 3, 2011]. Available at: http://www.cdc.gov/media/transcripts/2009/t091106.htm.
- 18.Andersen M. Flu activity in Lincoln declining after peak. [January 3, 2011]. Available at: http://journalstar.com/news/local/article_33401dda-cb2a-11de-92e4-001cc4c03286.html.
- 19.Quick D. Doctor: Pandemic leveling—Director warns to remain guarded. [January 3, 2011]. Available at: http://www.postandcourier.com/news/2009/nov/09/doctor-pandemicleveling/
- 20.Cohen J. H1N1: Has the Second Wave Peaked in US and UK? [January 3, 2011]. Available at: http://news.sciencemag.org/scienceinsider/2009/11/h1n1-has-the-se.html.