Abstract
Background
A life-attenuated vaccine aimed at preventing herpes zoster (HZ) and its main complication, post-herpetic neuralgia (PHN), will soon be available in Europe. The study's objective was to assess the clinical and economic impact of a vaccination program for adults aged 70–79 years in Switzerland.
Results
A vaccination strategy compared to a no-vaccination resulted in lifetime incremental cost-effectiveness ratios (ICER s) of 25,538 CHF (23,646 USD) per QALY gained, 6,625 CHF (6,134 USD) per HZ case avoided,and 15,487 CHF (14,340 USD) per PHN3 case avoided under the third-party payer perspective. Sensitivity analyses showed that the model was most sensitive to the discount rates, HZ epidemiological data and vaccine price used.
Methods
A Markov model, simulating the natural history of HZ and PHN and the lifetime effects of vaccination, previously developed for the UK was adapted to the Swiss context. The model includes several health states including good health, HZ, PHN and death. HZ and PHN states reflected pain severity.
Conclusion
The model predicts clinical and economic benefits of vaccination in the form of fewer HZ and PHN cases and reductions in healthcare resource use. ICERs were within the commonly accepted thresholds in Switzerland, indicating that a HZ vaccination program would be considered a cost-effective strategy in the Swiss setting.
Key words: cost-effectiveness analysis, herpes zoster, post-herpetic neuralgia, vaccination, health policy
Introduction
Age-related diseases are becoming a growing burden on developed societies presenting a major health challenge with significant socio-economic consequences. Herpes zoster (HZ) affects more than 500,000 older adults in the US1 and approximately 290,000 adults aged over 50 years in Germany every year.2 In Switzerland, data from the national sentinel surveillance network indicate that the annual incidence of HZ among the 50+ population ranges between 0.31% and 0.82%, resulting in approximately 11,000 new Swiss HZ patients aged 50+ every year.3
HZ results in major morbidity for a substantial number of individuals through its most severe and painful complication, post-herpetic neuralgia (PHN). HZ is caused by reactivation of the varicella zoster virus (VZV) in the dorsal root ganglia of individuals having had a primary VZV infection and is associated with age-related normal decrease in varicella zoster-specific cell-mediated immunity.
The total lifetime risk of HZ is 25%4 with the incidence almost doubling with every decade after the age of 50.5 HZ symptoms include numbness, itching and pain during the prodromal phase, followed by painful unilateral vesicular eruptions on the skin that last for approximately 3–4 weeks.6 However, painful symptoms can persist for months or years after the cutaneous eruption has healed in about 20% to 25% of HZ cases,7 i.e., developing the chronic pain syndrome PHN. PHN is clinically defined as pain occurring or persisting at least 1 or 3 months after rash onset. While there is no international consensus on PHN definition, the most commonly accepted one is that of 3 months.6,8,9 PHN is associated with considerable reduction in quality of life with many patients developing severe physical, occupational and social disabilities as a consequence of the enduring pain.7,10
The efficacy of a new live attenuated vaccine, Zostavax®, has been tested in a large randomized, double-blind, placebo-controlled trial (Shingles Prevention Study, SPS).9 In Europe, Zostavax® is indicated for the prevention of HZ and PHN in adults aged over 50 years.
The objective of this study is to explore the cost-effectiveness of a VZV universal vaccination strategy of adults aged 70–79 years in Switzerland, considered as an important piece of information for successful market access.
Results
Base case.
The model predicts that vaccinating 20% of the Swiss population aged 70–79 years would result in 3,412 fewer cases of HZ, 1,460 fewer PHN3 cases and 885 additional QALYs over the lifetime of this population, compared to the current policy of no vaccination. Additional costs of a vaccination strategy equal 22.6 million CHF and 25.3 million CHF, under a TPP and a societal perspective respectively. On average, a patient in the vaccination policy is associated with a lifetime cost of 108.84 CHF under a TPP perspective and 99.78 CHF under a societal perspective versus 61.30 CHF and 57.25 CHF in the non-vaccination policy.
Resulting ICERs are 25,538 CHF (23,646 USD) per QALY gained, 6,625 CHF (6,134 USD) per HZ avoided and 15,487 CHF (14,340 USD) per PHN3 case avoided. From a societal perspective, results are 28,544 CHF (26,430 USD) per QALY gained 7,405 CHF (6,856 USD) per HZ case avoided and 17,310 CHF (16,028 USD) per PHN3 case avoided.
31 and 73 people would need to be vaccinated to prevent one case of HZ and PHN, respectively.
Sub-group analyses.
Subgroup analyses were conducted for the 60–69, 65+ and 70+ age-groups showing that vaccination is most cost-effective in the younger 60–69 group and least cost-effective in the older 70+ group, mainly due to decreased vaccine protection and increased mortality in this age group. In particular, ICERs are 18,089 CHF per QALY gained in the 60–69 group, 26,083 CHF per QALY gained in the 65+ group and 30,934 CHF per QALY gained in the 70+ group, from a TPP perspective. From the societal perspective the corresponding results for the three age-groups are 19,998 CHF per QALY gained 29,104 CHF, per QALY gained and 34,543 CHF per QALY gained respectively.
Sensitivity analyses.
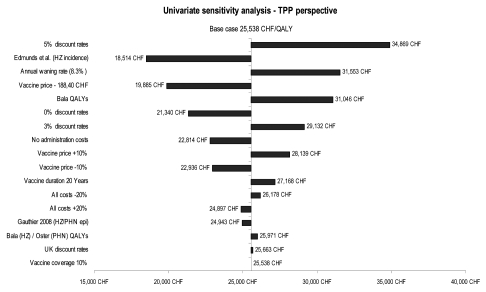
As shown in Figure 2, ICERs are most sensitive to discount rates, HZ incidence data and vaccine price. Discount rates of 5% for both costs and benefits will result in the highest ICERs, while the use of an alternative source for HZ incidence will produce the lowest ICERs. A change in the resource utilization cost has a marginal effect, while a change in the vaccine coverage rate does not have any effect on the cost-effectiveness results, as outcomes and costs increase at a proportional rate depending on the coverage rates selected.
Figure 2.
Univariate sensitivity analyses: tornado diagram.
Methods
A health-economic model was originally11 developed for the UK to assess the lifetime clinical and economic impact of the vaccine. The model, validated successfully by comparison of its results to the SPS trial data, demonstrated that a vaccination strategy for those aged 50+ would result in reduced numbers of HZ and PHN cases and substantial economic benefits, thus being cost-effective. It was adapted to the Swiss situation in order to compare the adoption of a vaccination policy targeting the immunocompetent population aged 70–79 years to the current policy of no vaccination, applying a realistically achievable coverage rate. Additional subgroup analyses were conducted for other age-groups of interest, i.e., the 60–69, 65+ and 75+ age-groups. Model outcomes, assessed over the lifetime of the study population, include total costs, quality-adjusted life-years (QALYs) gained, as well as HZ cases and PHN cases avoided (see Appendix). In addition the number needed to vaccinate (NNV) quantifying the number of people that need to be vaccinated to prevent one case of HZ or PHN has been estimated. Incremental cost-effectiveness ratios (ICERs) are calculated by dividing the difference in total costs between the two policies by the difference in effectiveness. Cost per QALY gained, cost per HZ case avoided, and cost per PHN case avoided are calculated under both a third-party payer (TPP) and a societal perspective. The TPP perspective includes all health care related expenses, while the societal perspective includes also productivity losses due to work absenteeism and co-payments.
The model takes into account ageing of the population over the duration of the analysis, adjusting for changes in mortality, the likelihood of contracting HZ and PHN and vaccine efficacy, depending on age.
Model structure.
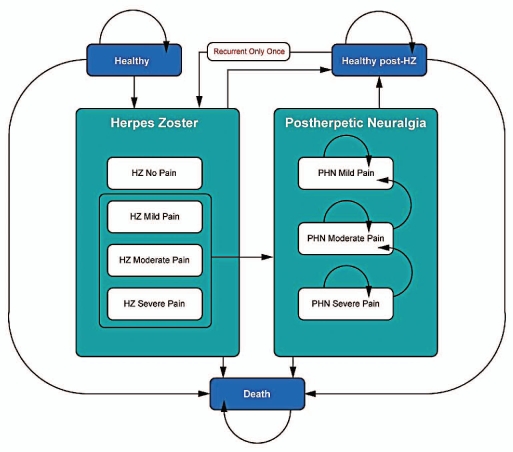
The model11 uses a Markov process to simulate the lifetime incidence and consequences of HZ among the Swiss population aged 50+. The base case analysis is focusing on the population aged 70–79. The cohort is analyzed in separate 5-year age-groups, by applying a matrix of transition probabilities to the different health states (Fig. 1). The lifetime of the cohort is divided into monthly Markov cycles. Within each cycle, cohort members may remain in their current health state or transition to one of the allowable states: “Healthy” (i.e., no HZ symptoms), “HZ”, “PHN” and “Death”. “HZ” and “PHN” health states are further divided into different pain severity levels (i.e., mild, moderate or severe). “Recurrent HZ” and subsequent PHN states are also included but are constrained to single recurrent episodes. Cohort cycle's members through the model eventually reaching death, based on Swiss age-specific mortality rates.
Figure 1.
Model Health States.
Using a monthly cycle length requires that all model inputs reflect their corresponding monthly values. Therefore, the 1-month definition of PHN (PHN1) has been employed in the modeling process. However, given that the 3-month definition of PHN (PHN3) is most commonly accepted, the model has been adjusted to produce results according to PHN3.
Model inputs.
Input parameters used in the model (Tables 1 and 2) are derived from a range of sources, such as the Swiss sentinel surveillance network,3,12 national statistics,13 the SPS trial,9 published literature and a Swiss HZ and PHN burden of illness study.14
Table 1.
Input data: epidemiology, utilities and vaccine characteristics
| Base case | Sensitivity analysis | ||||
| HZ | PHN | HZ | PHN | ||
| Epidemiology | |||||
| Annual HZ Incidence (per 1,000 people)/PHN1 Proportion per HZ case | Swiss sentinel surveillance12 | Gauthier 200815 | Gauthier 200815/Edmunds 200120 | Edmunds 200120 | |
| Age 50–54 | 3.06 | 10.3% | 3.44/4.61 | 7.40% | |
| Age 55–59 | 3.06 | 13.7% | 4.08/5.21 | 7.40% | |
| Age 60–64 | 4.14 | 15.7% | 4.9/5.92 | 21.20% | |
| Age 65–69 | 4.14 | 18.7% | 5.96/6.70 | 21.20% | |
| Age 70–74 | 5.99 | 22.5% | 6.34/7.53 | 28.60% | |
| Age 75–79 | 5.99 | 26.6% | 7.09/8.42 | 28.60% | |
| Age 80–84 | 7.48 | 28.9% | 7.29/9.37 | 34.40% | |
| Age 85–89 | 7.48 | 25.9% | 6.22/11.58 | 34.40% | |
| Age 90+ | 8.17 | 25.9% | 6.22/11.58 | 34.40% | |
| Mean Duration (in months) | Oxman 20059 | Gauthier 200815 | |||
| Age ≤ 69 | 1.0 | 8.3 | - | 10.9 | |
| Age ≥ 70 | 1.0 | 10.9 | - | 11.0 | |
| Gender split | Gauthier 200815 | ||||
| % female | 61% | 65% | |||
| Pain severity split at diagnosis | Oxman 20059 | Gauthier 200815 | |||
| Age ≤ 69 | |||||
| No pain | 27% | - | 65% | - | |
| Mild pain | 41% | 42% | 24% | 47% | |
| Moderate pain | 18% | 9% | 4% | 42% | |
| Severe pain | 14% | 49% | 8% | 11% | |
| Age ≥ 70 | |||||
| No pain | 26% | - | 45% | - | |
| Mild pain | 32% | 17% | 41% | 34% | |
| Moderate pain | 23% | 16% | 5% | 54% | |
| Severe pain | 19% | 67% | 3% | 12% | |
| Quality of life | |||||
| Utility Decrements | Oster 200517 | Bala 199822 | Bala 199822 | ||
| No pain | 0.00 | - | 0.00 | 0.00 | |
| Mild pain | 0.31 | 0.31 | 0.27 | 0.27 | |
| Moderate pain | 0.42 | 0.42 | 0.40 | 0.40 | |
| Severe pain | 0.75 | 0.75 | 0.53 | 0.53 | |
| Vaccine characteristics | Oxman 20059 | ||||
| Efficacy: total % reduction in cases (PHN direct effect) | |||||
| Age ≤69 | 63.90% | 65.70% | |||
| Age ≥70 | 37.60% | 66.85% | |||
| Efficacy: number of months reduction of PHN pain | |||||
| Age ≤69 | - | −2.2 | |||
| Age ≥70 | - | −3.3 | |||
Table 2.
Input data: management costs, productivity costs and vaccination costs
| Base case | HZ | PHN | |||||
| Management costs—TPP perspective | Michel 200614 | ||||||
| No pain | Mild | Moderate | Severe | Mild | Moderate | Severe | |
| Outpatient visits | CHF 127 | CHF 127 | CHF 127 | CHF 289 | CHF 117 | CHF 117 | CHF 373 |
| Diagnostic tests | CHF 44 | CHF 53 | CHF 53 | CHF 53 | CHF 2 | CHF 2 | CHF 2 |
| Medications | CHF 191 | CHF 191 | CHF 206 | CHF 256 | CHF 8 | CHF 15 | CHF 91 |
| Non pharmacologic treatments | - | - | - | CHF 81 | - | CHF 95 | CHF 324 |
| Hospitalizations | - | - | - | CHF 548 | - | CHF 371 | CHF 742 |
| Working days loss (WDL) | - | - | - | - | - | - | - |
| Total | CHF 362 | CHF 371 | CHF 386 | CHF 1,227 | CHF 127 | CHF 600 | CHF 1,532 |
| Range for sensitivity analysis: ±20% | |||||||
| Management costs—societal perspective | Michel 200614 | ||||||
| No pain | Mild | Moderate | Severe | Mild | Moderate | Severe | |
| Outpatient visits | CHF 142 | CHF 142 | CHF 142 | CHF 322 | CHF 130 | CHF 130 | CHF 414 |
| Diagnostic tests | CHF 49 | CHF 59 | CHF 59 | CHF 59 | CHF 2 | CHF 2 | CHF 2 |
| Medications | CHF 212 | CHF 212 | CHF 229 | CHF 285 | CHF 15 | CHF 27 | CHF 128 |
| Non pharmacologic treatments | - | - | - | CHF 90 | - | CHF 105 | CHF 360 |
| Hospitalisations | - | - | - | CHF 609 | - | CHF 412 | CHF 825 |
| Working days loss (WDL) | - | - | - | CHF 509 | CHF 242 | CHF 364 | CHF 764 |
| Total | CHF 403 | CHF 413 | CHF 430 | CHF 1,874 | CHF 389 | CHF 1,040 | CHF 2,493 |
| Range for sensitivity analysis: ±20% | |||||||
| Employment | Males | Females | |||||
| Age 60–64 | 74.90% | 56.60% | Federal statistics office of Switzerland13 | ||||
| Age 65–69 | 38.41% | 21.64% | Federal statistics office of Switzerland13 | ||||
| Age 70+ | 0% | 0% | Assumption | ||||
| Productivity costs | Federal statistics office of Switzerland13 | ||||||
| Average daily wage rate | CHF 302.7 | ||||||
| Vaccination costs | Sensitivity Analysis | ||||||
| Unit cost | Assumption | ||||||
| One dose of vaccine | CHF 240.7 | −10% | +10% | CHF 188.4 | - | ||
| Administration cost | Assumption | ||||||
| CHF 25.2 | - | - | - | CHF 0.0 | |||
Management of HZ/PHN.
All data related to HZ and PHN management in Switzerland were sourced from a Swiss burden of illness study,14 commissioned to inform the present analysis. It was based on two expert's opinion, both experienced in HZ and PHN management in Switzerland.
The objective of the study was to investigate the health care-related resource use due to HZ and PHN, by pain severity level and economic perspective, i.e., societal and TPP, using both definitions of PHN (1- and 3-months). In particular the study reported detailed information on outpatient and inpatient care, pharmacological and non-pharmacological treatments, diagnostic tests, as well as number of work-days lost due to HZ and PHN. In this context, it was estimated that approximately 20% of HZ patients with severe pain miss 21 days of work while 20–30% of PHN patients miss 10–21 days of work depending on pain severity.14
In Switzerland, health care costs are partially reimbursed. There is an annual minimum deductible of 300 CHF, i.e., the “franchise” and a 10% charge for all outpatient medical costs exceeding the “franchise”. As the model population is relatively old and, therefore, more frequent consumers of health care, it was assumed that the “franchise” has already been reached, regardless of whether the population will have HZ or PHN. Outpatient visit costs were estimated using expert opinion. Drug costs were obtained from the Swiss “Compendium”, diagnostic test costs from TARMED, while n°272 and n°18 DRG costs were used to account for HZ and PHN hospitalization costs. Productivity losses were estimated using the human capital approach and applying data from the Swiss Federal Statistics Office.13 Hence, the average overall cost of an HZ episode ranged (no and severe pain respectively) between 362 CHF-1,227 CHF from the TPP perspective and 403 CHF-1,874 CHF from a societal perspective, while the average monthly cost of a PHN episode ranged (mild, severe pain respectively) between 127 CHF-1,532 CHF and 389 CHF-2,493 CHF under each perspective, respectively.14
Epidemiology.
Data on HZ incidence was obtained from the Swiss Sentinel Surveillance Network which is a cooperative surveillance project of the Federal Office of Public Health and the participating Swiss physicians, consisting of a convenience sample of 150 to 250 general practitioners, internists and pediatricians.3,12
In the absence of suitable data on PHN proportion and HZ/PHN gender split, the model employed information from the UK GPRD study15 which was developed to populate the UK analysis.11 The GPRD study estimated HZ and PHN data by age-group, based on a sample of 27,225 immunocompetent UK patients. GPRD provided data using both 1-month and 3-months definition of PHN and calibration was required before entering these data in the model. However, as this concerned non-Swiss data, impact of using UK data was explored in sensitivity analyses. In addition, the GPRD study indicated no mortality directly linked to HZ or PHN, further confirmed by the lack of literature on the subject, as well as expert opinion. As no information was available for Switzerland, the same assumption was applied in the Swiss analysis.
Moreover, information from the SPS trial9 and literature16 was used to populate selected input parameters such as pain split, PHN duration and HZ recurrence rates.
Vaccination data.
Vaccine efficacy, obtained from the SPS trial,9 is expected to offer lifetime protection against HZ and PHN. However, vaccine duration of protection is not known yet. While vaccine efficacy may diminish over time, long-term trial results are not yet sufficient to accurately document this, though more reliable data may become available in the future. Vaccination is assumed to have both direct and indirect effects on HZ and PHN. The direct effect is obtained through the reduction in the number of HZ and PHN cases. The indirect effect, relates only to PHN, and refers to the further reduction of PHN cases obtained through the reduced number of HZ cases.
Zostavax®'s efficacy has been studied in the pivotal SPS trial.9 Compared to placebo, Zostavax® significantly reduces by 61% [95% CI: 51–69%] the HZ burden of illness, by 67% PHN incidence [95% CI: 48–79%] and by 51% [95% CI: 44–58%] the HZ incidence. Zostavax® also prevents 73% [95% CI: 46–87%] of zoster cases with severe and long-lasting pain.
Efficacy in reducing the burden of illness and PHN incidence has been demonstrated to remain stable regardless of age, whereas reduction of HZ incidence was shown to be significantly higher in the younger age-group (i.e., 60–69).
A 20% coverage rate was assumed in the absence of relevant data. Vaccine administration costs were included, assuming that all patients would be vaccinated during a routine visit. In addition, it was assumed that 30% of patients would receive Zostavax® and the influenza vaccine at the same visit, and therefore cost-sharing of administration costs was applied.
Other inputs.
Demographic and general mortality data were obtained from national statistics13 by 5-year age group and gender.
For the calculation of QALYs, utility weights associated with the different HZ and PHN pain states were obtained from literature.17 In lieu of country-specific data, UK age-specific utilities were used to adjust for the fact that healthy and subsequent states would be affected by the age of the population under consideration.
Discount rates of 3.5% for costs and 1.5% for outcomes (QALYs) were applied in the model.18,19
Sensitivity analyses.
The base case results variability to alternative values of key input parameters was explored by varying these within feasible ranges in one-way sensitivity analyses. Thus, the effects of alternative values of discount rates, HZ incidence,2,20 PHN proportion,2 vaccine coverage rate, waning immunity,21 duration of vaccine efficacy, vaccine price, health care costs and utility decrements,9,17,22 on results were explored.
Conclusion
The model predicts that from both TPP and societal perspective, the adoption of a vaccination policy in Switzerland for adults aged 70–79 years would result in a reduction of HZ and PHN cases and an improvement of health-related quality of life in the form of increased number of QALYs. Sensitivity analyses showed that although results are sensitive to certain parameters, such as discount rates and the choice of epidemiological data, the model is robust to change, providing reliable estimates of the QALYs and costs associated with HZ and PHN. In addition, ICERs remained below commonly accepted thresholds for all deterministic sensitivity analyses.
Resulting ICERs are comparable to those of other vaccination strategies implemented in Switzerland and are within the commonly accepted cost-effectiveness thresholds in Europe, suggesting that such a vaccination strategy would be cost-effective in the Swiss setting.23,24
Strengths and limitations of the model have been previously described in the original discussion of the UK analysis.11 However, there are some limitations specific to this Swiss analysis, which are mainly related to the availability of country-specific data. No accurate Swiss information was available for the proportion of PHN and therefore the model applied UK data. As the PHN proportion together with HZ incidence represent the main drivers of the model results, the use of values specific to the Swiss population are essential. Effect of using UK parameters was tested in sensitivity analysis. Furthermore, disease-specific utility decrements were obtained from a US population.17 However, according to expert opinion, (Professor Mike Drummond: personal communication) while age-specific utilities may vary between countries, disease-specific decrements can be considered transferable, thus the applied decrements17 are suitable for this analysis. Again, effect of using alternative decrements was tested in sensitivity analyses, showing a moderate impact on results. Finally, resource use and cost data were obtained from a study based on Swiss expert opinion14,25 rather than on actual patient-level data, limiting to some extent the predictive model strength and the results' generalization to the whole population. Nevertheless, a sensitivity analysis which varied all health care costs 20% above and below their base case value demonstrated that this parameter had only a marginal effect on results (2.5% decrease and increase compared to base case, respectively).
HZ and PHN can be extremely painful conditions affecting patients' quality of life over an extended period of time and resulting in considerable costs from both a TPP and a societal perspective. This study attempts to estimate the health-economic impact of vaccinating older adults against these conditions, indicating that a vaccine which is able to prevent HZ and PHN and reduce their severity can be considered a cost-effective investment of health care resources in Switzerland.
Abbreviations
- CHF
Swiss franc
- DRG
diagnostic-related groups
- GPRD
general practice research database
- HZ
herpes zoster
- ICER
incremental cost-effectiveness ratio
- NNV
number needed to vaccinate
- PHN
post-herpetic neuralgia
- PHN1
1-month definition of post-herpetic neuralgia
- PHN3
3-month definition of post-herpetic neuralgia
- QALY
quality adjusted life years
- UK
United Kingdom
- SPS
shingles prevention study
- TPP
third party payer
- VZV
varicella zoster virus
Appendix—Health Economic Analysis
Clinical and economic outcomes.
In the current analysis clinical and economic results are presented over the lifetime of the population following the introduction of the vaccine.
Results are reported for total costs, Quality Adjusted Life Years (QALYs), HZ cases and PHN cases (3-month definition) under a vaccination policy and a no-vaccination policy. In addition, Number Needed to Vaccinate (NNV) results have been estimated.
QALYs or Quality Adjusted Life Years are often used in health economic analyses. QALYs are life years weighted by factors representing the utility or preference of individuals for health outcomes. These preferences are measured on a scale from 0 (death) to 1 (perfect health), so QALYs are derived by multiplying the years of survival in a given health state by the preference weight for that state. Where progression through a series of health states occurs, such as the HZ and PHN states, the total QALYs accrued in a lifetime are the sums over time spent in each state multiplied by the preference weight for each state. There are several instruments for measuring health state preferences and utilities (such as SF-36 etc.). Among the generic instruments for measuring health-related quality of life, EQ-5D is one of the candidate preference-based instruments for use as a measure of health outcome. The EQ-5D is a health profile describing five domains of health each at three possible levels. A tariff of preference values for the 243 individual health states which can be described by the EQ-5D has been obtained for several countries.1
To further illustrate the potential benefit of VZV vaccination, the NNV or Number Needed to Vaccinate results are calculated, quantifying the number of people that need to be vaccinated to prevent one case of HZ or PHN.
Cost-effectiveness measures.
A major constraint faced by all national healthcare systems is the limited amount of resource available. Therefore it is important that budgets are spent on programmes that represent value-for-money. Health economics involves the study of the efficient allocation of scare health care resources.
In cost-effectiveness analysis (CEA), the difference in cost (incremental cost) is compared to the difference in benefits (incremental effect) between the strategy under study and usual care from the perspective of a specific payer. That is, if the new policy costs more than the comparator policy, does it provide sufficient additional benefits (or if a new policy is less effective than the comparator, does it offer sufficient cost savings)? CEA provides a framework to assist in this decision process. In using the results of a CEA, a decision-maker may judge whether a particular cost per effect represents acceptable value for money.
Equation 1. Incremental cost-effectiveness ratio
In the present anaysis, the cost-effectiveness of the vaccination policy was assessed by comparing the total cost and effectiveness, measured either by HZ or PHN cases avoided or QALYs of both policies. In the situation where the vaccination policy is both more effective and more costly than the existing policy, the incremental cost-effectiveness ratio (ICER) is calculated by dividing the difference in total costs between the two vaccination policies by the difference in effectiveness (see Equation 1). Depending on the effectiveness measure used as the denominator, ICERs in this analysis represent either the cost per HZ/PHN case averted or the cost per QALY.
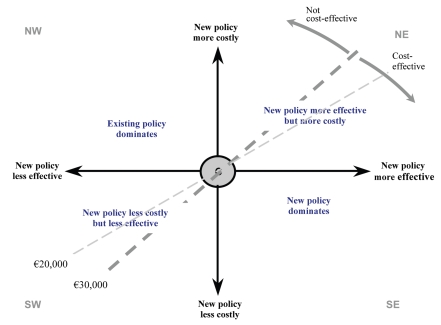
The cost-effectiveness plane is a useful means of considering the results of cost-effectiveness analyses. Plotting the incremental effect on the horizontal axis and the incremental cost on the vertical axis defines four ‘quadrants’ of the CE plane that can be related to decision-making (see Appendex Fig. 1). Frequently, a new treatment strategy falls in the north-east (NE) quadrant, where it is both more costly and more effective than the existing strategy. In this case, a trade-off between costs and effects has to be made in deciding which strategy to employ.
Appendix Figure 1.
The cost-effectiveness plane.
Cost-effectiveness threshold.
The advantage of reporting a cost per QALY, as opposed to cost per HZ or PHN case averted for example, is that it allows comparisons between different types of health care programmes. In the interpretation of the incremental cost-effectiveness of different treatment strategies, there is often some threshold ratio above which a strategy would be considered not cost-effective.
The cost-effectiveness threshold is represented by the dashed line in Figure 1. In Switzerland, there is no official cost-effectiveness threshold. In this context, the current analysis has assumed that health care interventions of less than €30,000 per QALY are likely to be considered as cost-effective based on commonly accepted cost-effectiveness thresholds in Europe.
Discount rate.
Discounting allows the present value of costs and outcomes which accrued over the time horizon of the analysis to be appropriately used in calculations.3,4 Thus, as the outcomes of the analyses are assessed over the lifetime of the Swiss population, discount rates have been applied for both costs and outcomes (QALYs).
Most reimbursement agencies ask for sensitivity analyses on discount rates as, depending on the technology being assessed, a change in discount rate can have a major impact on results. This is especially the case for vaccines, where the main investment is made upfront but benefits may be in the distant future. Thus alternative discount rates were explored in sensitivity analysis.
Conflict of Interest
A. Fendl and X. Bresse are employed by SPMSD who funded the study. All other authors declare that they have no competing interests.
Financial Disclosure
This study was carried out independently by i3 Innovus and was fully funded by SPMSD.
References
- 1.Schmader KE, Sloane R, Pieper C, Coplan PM, Nikas A, Saddier P, et al. The impact of acute herpes zoster pain and discomfort on functional status and quality of life in older adults. Clin J Pain. 2007;23:490–496. doi: 10.1097/AJP.0b013e318065b6c9. [DOI] [PubMed] [Google Scholar]
- 2.Schiffner-Rohe J, Jow S, K“ ster I, Lilie M. Inzidenz von Herpes zoster HZ in Deutschland, abstract accepted by DEGAM 2009 [Google Scholar]
- 3.Richard JL, Zimmermann HP. Herpes Zoster 1998–2001. 2010 [Google Scholar]
- 4.Miller E, Marshall R, Vurdien J. Epidemiology, outcome and control of varicella-zoster infection. Rev Med Micro. 1993;4:222–230. [Google Scholar]
- 5.Donahue JG, Choo PW, Manson JE, Platt R. The incidence of herpes zoster. Arch Intern Med. 1995;155:1605–1609. [PubMed] [Google Scholar]
- 6.Opstelten W, Mauritz JW, de Wit NJ, van Wijck AJ, Stalman WA, van Essen GA. Herpes zoster and postherpetic neuralgia: incidence and risk indicators using a general practice research database. Fam Pract. 2002;19:471–475. doi: 10.1093/fampra/19.5.471. [DOI] [PubMed] [Google Scholar]
- 7.Schmader KE. Epidemiology and impact on quality of life of postherpetic neuralgia and painful diabetic neuropathy. Clin J Pain. 2002;18:350–354. doi: 10.1097/00002508-200211000-00002. [DOI] [PubMed] [Google Scholar]
- 8.Scott FT, Leedham-Green ME, Barrett-Muir WY, Hawrami K, Gallagher WJ, Johnson R, et al. A study of shingles and the development of postherpetic neuralgia in East London. J Med Virol. 2003;70:24–30. doi: 10.1002/jmv.10316. [DOI] [PubMed] [Google Scholar]
- 9.Oxman MN, Levin MJ, Johnson GR, Schmader KE, Straus SE, Gelb LD, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med. 2005;352:2271–2284. doi: 10.1056/NEJMoa051016. [DOI] [PubMed] [Google Scholar]
- 10.Dworkin RH, Portenoy RK. Pain and its persistence in herpes zoster. Pain. 1996;67:241–251. doi: 10.1016/0304-3959(96)03122-3. [DOI] [PubMed] [Google Scholar]
- 11.Moore L, Remy V, Martin M, Beillat M, McGuire A. A health economic model for evaluating a vaccine for the prevention of herpes zoster and post-herpetic neuralgia in the UK 2010. doi: 10.1186/1478-7547-8-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The Swiss Sentinel Surveillance Network. 2010. http://www.bag.admin.ch/k_m_meldesystem/00736/00817/index.html?lang=en.
- 13.Federal statistics office of Switzerland. 2010. http://www.bfs.admin.ch/bfs/portal/en/index.html.
- 14.Michel JP, Kempf W. Data collection related to the management of herpes zoster (HZ) and post-herpetic neuralgia (PHN) and demographic parameters in Switzerland 2007 [Google Scholar]
- 15.Gauthier A, Breuer J, Carrington D, Martin M, Remy V. Epidemiology and cost of herpes zoster and postherpetic neuralgia in the United Kingdom. Epidemiol Infect. 2008:1–10. doi: 10.1017/S0950268808000678. [DOI] [PubMed] [Google Scholar]
- 16.Cunningham AL, Dworkin RH. The management of post-herpetic neuralgia. BMJ. 2000;321:778–779. doi: 10.1136/bmj.321.7264.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oster G, Harding G, Dukes E, Edelsberg J, Cleary PD. Pain, medication use and health-related quality of life in older persons with postherpetic neuralgia: results from a population-based survey. J Pain. 2005;6:356–363. doi: 10.1016/j.jpain.2005.01.359. [DOI] [PubMed] [Google Scholar]
- 18.Bonneux L, Birnie E. The discount rate in the economic evaluation of prevention: a thought experiment. J Epidemiol Community Health. 2001;55:123–125. doi: 10.1136/jech.55.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bos JM, Postma MJ, Annemans L. Discounting health effects in pharmacoeconomic evaluations: current controversies. Pharmacoeconomics. 2005;23:639–649. doi: 10.2165/00019053-200523070-00001. [DOI] [PubMed] [Google Scholar]
- 20.Edmunds WJ, Brisson M, Rose JD. The epidemiology of herpes zoster and potential cost-effectiveness of vaccination in England and Wales. Vaccine. 2001;19:3076–3090. doi: 10.1016/s0264-410x(01)00044-5. [DOI] [PubMed] [Google Scholar]
- 21.Pellissier JM. Evaluation of the Cost-Effectiveness in the United States of a Vaccine to Prevent Herpes Zoster and Postherpetic Neuralgia in Older Adults. Vaccine. 2007;25:8326–8337. doi: 10.1016/j.vaccine.2007.09.066. [DOI] [PubMed] [Google Scholar]
- 22.Bala MV, Wood LL, Zarkin GA, Norton EC, Gafni A, O'Brien B. Valuing outcomes in health care: a comparison of willingness to pay and quality-adjusted life-years. J Clin Epidemiol. 1998;51:667–676. doi: 10.1016/s0895-4356(98)00036-5. [DOI] [PubMed] [Google Scholar]
- 23.Szucs TD, Largeron N, Dedes KJ, Rafia R, Benard S. Cost-effectiveness analysis of adding a quadrivalent HPV vaccine to the cervical cancer screening programme in Switzerland. Curr Med Res Opin. 2008;24:1473–1483. doi: 10.1185/030079908x297826. [DOI] [PubMed] [Google Scholar]
- 24.Haldemann R, Luscher TF, Szucs TD. [Cost effectiveness of clopidogrel in secondary cardiovascular prevention: a cost-effectiveness analysis based on the Caprie Study] Praxis. 2001;90:539–545. [PubMed] [Google Scholar]
- 25.Michel JP, Kempf W. Data collection related to the management of herpes zoster (HZ) and post-herpetic neuralgia (PHN) and demographic parameters in Switzerland 2008 [Google Scholar]
Appendix References
- 1.Drummond M, O'Brien B, Stoddart G, Torrance G. Methods for the Economic Evaluation of Health Care Programmes. Oxford: Oxford University Press; 1997. [Google Scholar]
- 2.Briggs AH. Handling uncertainty in economic evaluation and presenting the results. In: Drummond M, McGuire AJ, editors. Economic evaluation in health care. Oxford: Oxford University Press; 2001. pp. 172–214. [Google Scholar]
- 3.Bonneux L, Birnie E. The discount rate in the economic evaluation of prevention: a thought experiment. J Epidemiol Community Health. 2001;55:123–125. doi: 10.1136/jech.55.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bos JM, Postma MJ, Annemans L. Discounting health effects in pharmacoeconomic evaluations: current controversies. Pharmacoeconomics. 2005;23:639–649. doi: 10.2165/00019053-200523070-00001. [DOI] [PubMed] [Google Scholar]