Abstract
Drosophila adult midgut intestinal stem cells (ISCs) maintain tissue homeostasis by producing progeny that replace dying enterocytes and enteroendocrine cells. ISCs adjust their rates of proliferation in response to enterocyte turnover through a positive feedback loop initiated by secreted enterocyte-derived ligands. However, less is known about whether ISC proliferation is affected by growth of the progeny as they differentiate. Here we show that nutrient deprivation and reduced insulin signaling results in production of growth-delayed enterocytes and prolonged contact between ISCs and newly formed daughters. Premature disruption of cell contact between ISCs and their progeny leads to increased ISC proliferation and rescues proliferation defects in insulin receptor mutants and nutrient-deprived animals. These results suggest that ISCs can indirectly sense changes in nutrient and insulin levels through contact with their daughters and reveal a mechanism that could link physiological changes in tissue growth to stem cell proliferation.
Keywords: cadherin, cell adhesion, nutrition
Self-renewing stem cells respond to perturbations in tissue homeostasis by adjusting their rate of proliferation or by altering their total number (1). Although much is known about the mechanism of stem cell self-renewal (2), less is known about how stem cells sense and respond to these perturbations.
The Drosophila adult midgut contains multipotent intestinal stem cells (ISCs) that divide once a day and generate daughters, known as enteroblasts, which then differentiate without dividing into either enteroendocrine cells or polyploid enterocytes (Fig. S1A) (3, 4). Injury to the adult midgut results in acute loss of enterocytes and release of JAK-STAT and Egfr ligands by dying cells. These ligands directly activate the JAK-STAT and the Egfr-signaling pathways in ISCs (5–17), which results in an increased rate of ISC proliferation and enterocyte production, thereby linking the death of differentiated cells to a response that restores tissue homeostasis.
Although regulation of ISC proliferation by enterocyte turnover is well understood, it remains unclear whether growth and differentiation of stem cell daughters can regulate ISC proliferation. In Drosophila, cell growth is dependent on nutrition and insulin signaling (18, 19), and these physiological variables might also regulate ISC proliferation and growth of ISC daughters. Indeed, homeostatic mechanisms involving nutrition, insulin signaling, and stem cells have been characterized in the Drosophila ovary and testis, where germline and somatic stem cells respond to changes in the nutritional availability for gametogenesis by adjusting their rates of proliferation (20–24). Recently, it has been shown that the rate of ISC proliferation in both the Periplaneta americana and Drosophila midgut changes in response to dietary manipulations and insulin signaling (17, 25–27). However, the cell types mediating this response are not known. We show that enteroblasts nonautonomously regulate ISC proliferation in response to nutrition and insulin signaling. Thus, both enterocytes and enteroblasts contribute to the maintenance of tissue homeostasis in the Drosophila midgut.
Results
To determine the effect of diet on maintenance, growth, and differentiation of ISCs and their progeny, we made positively marked lac-Z stem cell clones (3, 28) (Fig. S1 B and C) and followed the fates of marked stem cells and daughters in females fed a protein-rich (molasses and live yeast) or a protein-poor (molasses only) diet. One-day-old flies were fed a rich diet for 1 d and then heat-shocked to induce clones. Animals were allowed to recover for 1 d on a rich diet and then were separated into two cohorts: one fed a rich diet and one fed a poor diet. Intestines were stained at various times after clone induction (ACI) to assay for the number of cells per ISC clone (β-gal) and the presence of stem cells (Delta positive), enteroendocrine cells (Prospero positive), enterocytes (Delta and Prospero minus), and enterocyte ploidy (DAPI) (3, 4, 29).
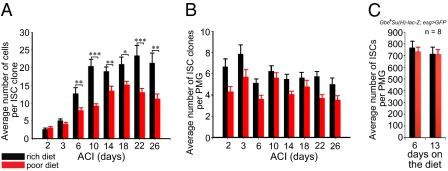
In animals fed a rich diet, the number of cells per ISC clone increased until it reached a plateau of approximately 20 cells on day 10 (Fig. 1A). In animals fed a poor diet, the number of cells per ISC clone also increased, but reached a lower plateau at a slower rate than in animals fed a rich diet (Fig. 1A). This observed decrease in cell number per clone suggests that diet can regulate ISC proliferation and is in accordance with recently published results (17). However, a decrease in ISC number was not observed as previously claimed (17), as the number of ISC clones per posterior midgut did not decrease significantly with time from animals fed either diet (Fig. 1B). Furthermore, the total number of ISCs per posterior midgut (SI Materials and Methods) from animals fed either diet did not change significantly over a 2-wk period (Fig. 1C).
Fig. 1.
Diet regulates adult posterior midgut homeostasis. (A) ISC clones from females fed a rich diet contain more cells than ISC clones from females fed a poor diet. (B) ISC clone number or (C) total posterior midgut stem cell number remains stable in females fed a rich or a poor diet. PMG, posterior midgut.
Nutrition Regulates Enterocyte Growth.
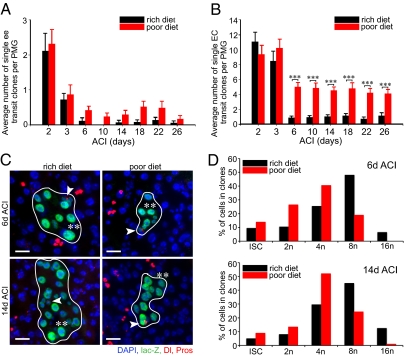
We next followed the fate of transit clones to determine the effect of a poor diet on enteroblast differentiation and enterocyte turnover (Fig. S1C). Both transit enteroendocrine clones (Fig. 2A) and transit enterocyte clones (Fig. 2B) from animals fed a rich diet turned over in a week, similar to that described previously (3). Enteroendocrine clone turnover appeared unaffected in animals raised on a poor diet (Fig. 2A). In contrast, turnover of transit enterocyte clones in animals fed a poor diet slowed during the first week ACI and then ceased (Fig. 2B), suggesting that dietary protein is required for enterocyte turnover and/or differentiation.
Fig. 2.
Diet regulates enterocyte growth. (A) Enteroendocrine transit clone turnover is unaffected in females fed a poor diet. (B) Enterocyte transit clone turnover decreases in females fed a poor diet. (C) Micrographs of ISC clones 6 d ACI and 14 d ACI from animals reared on a rich diet or a poor diet. Each clone contains a single ISC (arrowhead) and differentiating enterocytes. β-Galactosidase, green; Delta, red cytoplasmic vesicles; Prospero, red nuclear; and DAPI, blue nuclear. (Scale bar, 20 μm.) (D) Percentage of ISCs, 2n (non-ISCs), 4n, 8n, and 16n cells within ISC clones from females reared on a rich diet or a poor diet 6 and 14 d ACI. PMG, posterior midgut.
Stem cell clones from animals fed either diet contained ISCs, enteroblasts, enteroendocrine pairs (Fig. S1B), and enterocytes (Fig. 2C). However, the largest nuclei of enterocytes in animals fed a poor diet often appeared smaller than those fed a rich diet (Fig. 2C, double asterisks). To determine the effect of diet on the onset of enterocyte endoreduplication, we DAPI-quantified cell nuclei in clones 6 and 14 d ACI (Fig. 2D). At 6 d ACI, in animals fed a rich diet, 25.5% of cells were 4n (51/200), 48% were 8n (96/200), and 6.5% were 16n (13/200), whereas in animals fed a poor diet, 40.5% of cells were 4n (81/200), 19% were 8n (38/200), and 0% were 16n (0/200). At 14 d ACI, 34.5% of cells were 4n (69/200), 40% were 8n (80/200), and 12.5% were 16n (25/200) in animals fed a rich diet, whereas 52% of cells were 4n (104/200), 24.5% were 8n (49/200), and 1% were 16n (2/200) in animals fed a poor diet. Together, these data demonstrate that a protein-poor diet results in severe slowdown of enterocyte endoreduplication.
Reduction in the number of cells per ISC clone (Fig. 1A) and decreased number of phospho-histone-H3 cells (17) from animals fed a poor diet suggest that ISCs directly sense the nutritional status of the organism. However, because diet also affects growth of enteroblasts into enterocytes (Fig. 2 B–D), changes in ISC proliferation rates might be regulated indirectly by enteroblast growth. Because the insulin pathway in Drosophila is used by the ovarian germline (22), follicle stem cells (23), and testis germline stem cells (17) to sense nutritional status of the animal, we closely examined the role of insulin signaling in the posterior midgut to determine through which cells nutrition regulates ISC proliferation.
Insulin Signaling Regulates Enterocyte Growth and Endoreduplication.
To delineate the role of dInR signaling in daughter differentiation and how it might regulate ISC proliferation, we made MARCM (mosaic analysis of a repressible cell marker) clones of two mutant alleles of dInR: dInRE19 (30), a hypomorph, and dInR339, a genetic null (30), and of chico1 (Fig. S2A), the Drosophila homolog of the insulin receptor substrate protein (21), and followed the fate of stem cell and transit clones (Fig. S3A).
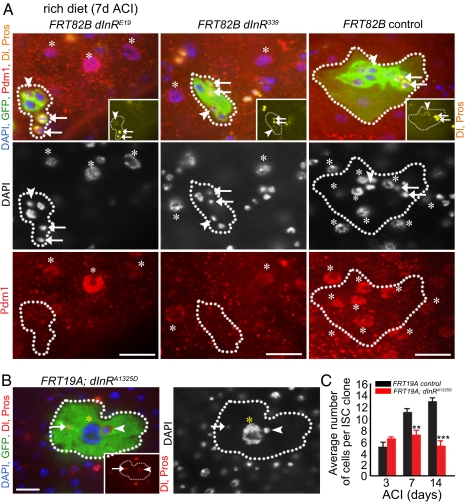
Consistent with recently published work (27), the average number of cells in mutant clones did not increase over time after 3 d ACI (Figs. S2B and S3B). Both wild-type and dInR mutant clones always contained one ISC and were capable of giving rise to pairs of enteroendocrine cells (Fig. 3A), some of which stained positive for an antibody that recognizes the FMRF-amide (Phe-Met-Arg-Phe-NH2) modification of the peptide hormone NPF (Fig. S4) (31), suggesting that insulin signaling is not necessary for enteroendocrine differentiation. In contrast, both mutant dInR and chico enterocyte nuclei appeared smaller than wild-type enterocytes (Fig. 3A and Fig. S2A) and were negative for Pdm1 (Fig. 3A and Fig. S2A), a marker of differentiated enterocytes (8), demonstrating that insulin signaling is necessary for differentiation of enteroblasts into enterocytes. We made wild-type MARCM clones overexpressing an activated form of dInR (dInRA1325D) and found that these clones contained large enterocytes that were able to reach a ploidy as high as 128n (Fig. 3B). Thus, insulin signaling is necessary and sufficient for enterocyte growth and endoreduplication.
Fig. 3.
Insulin signaling regulates enterocyte differentiation. (A) dInRE19 mutant, dInR339 mutant, and control clones 7 d ACI from females reared on a rich diet. Clone, GFP green; Delta (yellow vesicular); Prospero (yellow nuclear); Pdm1 (red, nuclear) and DAPI (blue, nuclear). Asterisks mark mature enterocytes (Pdm1-positive cells). Dotted lines outline clone boundaries. (B) A clone overexpressing dInRA1325D in all cells contains a large enterocyte (yellow asterisks), an ISC (arrowhead), and an enteroendocrine cell (arrow). Clone, GFP green; Delta, red vesicular (Inset and merge); Prospero, red nuclear (Inset and merge); DAPI, blue nuclear. Arrowheads and arrows indicate, respectively, ISCs (Delta-positive) and enteroendocrine (Prospero-positive) cells in A and B. (Scale bar, 20 μm in A and B.) (C) Wild-type ISC clones contain more cells than ISC clones overexpressing an activated form of the insulin receptor (dInRA1325D).
An increase in ISC division was recently reported in animals in which dInRA1325D (32) was driven by esg-GAL4, a driver expressed in ISCs and enteroblasts (4) (Fig. S5A). These data predict that the average number of cells per ISC clone should increase in wild-type MARCM clones overexpressing dInRA1325D. However, we did not observe an increase in cell number per clone over a 2-wk period in clones overexpressing dInRA1325D (Fig. 3C). In fact, the average number of cells per ISC clone was significantly smaller than that seen in wild-type clones, raising the possibility that the enlarged dInRA1325D enterocytes might exert a nonautonomous inhibitory effect on ISC proliferation. When we used esg-GAL4 to drive expression of dInRA1325D as described previously (32), esg-GAL4 was no longer ISC- and enteroblast-specific (Fig. S5C). Furthermore, although we observed an increase in the number of phospho-histone H3-positive (PHH3+) cells as previously reported, we also detected a sixfold increase in apoptotic enterocytes compared with controls (Fig. S5 B and D), suggesting that the increase in PHH3+ number was a nonautonomous effect resulting from injury to the midgut and not an autonomous effect on insulin signaling in the ISC.
Insulin Signaling Nonautonomously Regulates ISC Proliferation.
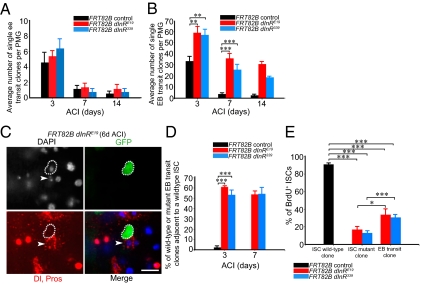
We next followed the fate of dInR and chico mutant and wild-type enterocytes and enteroendocrine cells generated as transit clones (Fig. S3A). Mutant enteroendocrine clone turnover at 1 wk ACI matched the turnover rate of control enteroendocrine clones (Fig. 4A and Fig. S2C). Control enterocyte clones also turned over in 1 wk ACI (Fig. 4B and Fig. S2D). However, mutant enteroblast clone turnover slowed for the first week ACI and then ceased thereafter (Fig. 4B and Fig. S2D). We examined dInR and chico mutant daughters that were still present 3 and 7 d after clone induction and noted that they were small and remained in contact with the wild-type ISCs that generated them (Fig. 4 C and D; Tables S1 and S2). From these observations we realized that the continued presence of mutant enteroblasts adjacent to wild-type ISCs created a unique opportunity for us to directly evaluate the role of insulin signaling in the enteroblast on ISC proliferation.
Fig. 4.
Insulin signaling nonautonomously regulates intestinal stem cell proliferation. (A) Enteroendocrine dInR mutant transit clone and wild-type enteroendocrine transit clone turnover are similar. (B) Enteroblast dInR mutant transit clone turnover is decreased compared with wild-type enterocyte transit clone turnover. (C) A dInR mutant transit clone (green) in contact with a wild-type intestinal stem cell (arrowhead). Clone, GFP green; Delta, red vesicular; Prospero, red nuclear; DAPI, blue nuclear. (Scale bar, 20 μm.) (D) Percentage of mutant (FRT82B dInRE19, FRT82B dInR339) and wild-type (FRT82B) transit enteroblast clones adjacent to a wild-type ISC at 3 and 7 d ACI. (E) Percentage of BrdU incorporation in wild-type ISCs from wild-type clones and from wild-type ISCs adjacent to mutant transit clones 6 d ACI.
To determine the effect of mutant dInR daughters on proliferation of wild-type ISCs, we assayed for BrdU incorporation (Materials and Methods), a marker of progression through S phase of the cell cycle, in wild-type ISCs from wild-type stem cell clones, mutant ISCs from mutant dInR stem cell clones, and wild-type ISCs adjacent to mutant dInR transit clones (Fig. S6 A and B) from animals continuously fed BrdU for 3 d on days 4, 5, and 6 ACI. In wild-type ISC clones, 91% of ISCs were BrdU positive (Fig. 4E and Table S3). In contrast, only 35 and 31% of wild-type ISCs adjacent to mutant dInRE19 and dInR339 transit daughters, respectively, incorporated BrdU (Fig. 4E), demonstrating a nonautonomous role for insulin signaling in regulation of ISC proliferation. To further verify the necessity for dInR in the enteroblast for ISC proliferation, we drove RNAi knockdown of dInR using the enteroblast-specific driver Gbe+-Su(H)-GAL4 (33). In accordance with clonal analysis, RNAi knockdown of dInR specifically in the enteroblast caused a significant reduction in ISC proliferation (Fig. S6C). Removing dInR function from ISCs and enteroblasts simultaneously led to a further reduction in BrdU incorporation in ISCs (Fig. 4E), suggesting an autonomous role for insulin signaling in regulation of ISC proliferation as well (Fig. 4E).
Insulin Signaling Regulates Adherens Junction Stability.
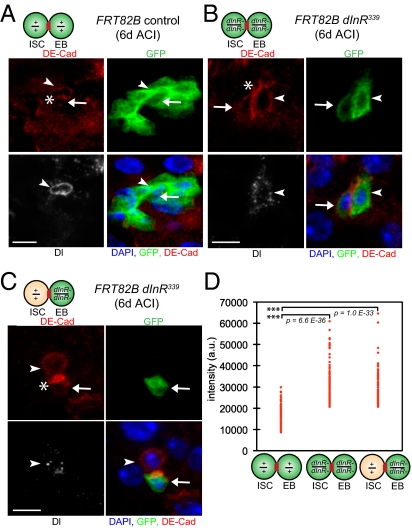
In the adult Drosophila midgut, the adherens junction components Armadillo (Drosophila β-catenin) and DE–cadherin (Drosophila E-cadherin) are found between ISCs and their recent daughters (3, 34). We therefore hypothesized that mutant dInR enteroblasts remain in prolonged contact with wild-type ISCs because they fail to break down these junctions. We measured DE–cadherin levels between ISCs and enteroblasts in wild-type ISC clones (Fig. 5A), in dInR339 mutant ISC clones (Fig. 5B), and in dInR339 mutant enteroblasts adjacent to wild-type ISCs (Fig. 5C) 6 d ACI. DE–cadherin levels between mutant ISCs and mutant enteroblasts or levels between wild-type ISCs and mutant enteroblasts were significantly higher on average than DE–cadherin levels between wild-type ISCs and enteroblasts (Fig. 5D). Therefore, insulin signaling regulates adherens junction stability between ISCs and enteroblasts.
Fig. 5.
The insulin-signaling pathway regulates the stability of the adherens junction between ISCs and enteroblasts. (A) An FRT82B control ISC clone (green) 6 d ACI containing an ISC (arrowhead) and an enteroblast (arrow) adjacent (asterisk) to one another, as well as enterocytes. (B) A dInR339 mutant ISC clone (green) containing an ISC (arrowhead) and an enteroblast (arrow) in contact (asterisk). (C) A dInR339 mutant transit enteroblast clone (green, arrow) in contact (asterisk) with a wild-type intestinal stem cell (arrowhead). (A–C) Clone, GFP green; Delta, white vesicular; DE–cadherin, red; DAPI, blue nuclear. (Scale bar, 10 μm.) (D) DE–cadherin levels measured at the junction of the ISC and the enteroblast are significantly higher in dInR339 mutant clones than in FRT82B clones.
We also measured DE-cadherin levels in mutant ISC clones consisting of only one cell. Eleven percent of all mutant ISC clones (n = 454) were single cell (n = 51), and approximately half of single-cell mutant ISC clones were adjacent to wild-type enteroblasts (n = 25). DE–cadherin levels between mutant ISC and wild-type enteroblasts were comparable to that between ISCs and enteroblasts from wild-type clones (Fig. S3C), suggesting that the autonomous effect by insulin signaling on ISC proliferation is not due to an effect on adherens junction stability.
Stem Cell–Enteroblast Contact Regulates ISC Proliferation.
dInR mutant enteroblasts remain in prolonged contact with ISCs (Fig. 4 C and D), stabilize their adherens junction (Fig. 5D), and nonautonomously inhibit ISC proliferation (Fig. 4E). We therefore hypothesized that premature disruption of ISC and enteroblast contact might lead to increases in ISC proliferation, even in ISCs and enteroblasts with depressed levels of insulin signaling or in animals fed a protein-poor diet.
RNAi knockdown of the Drosophila homolog of DE–cadherin (35), known as shotgun (shg), in ISCs and enteroblasts using esg-GAL4 leads to a dramatic increase in the number of ISCs and enteroblasts that are no longer in contact with one another (36). However, the effect of shg-RNAi knockdown on ISC proliferation was not determined.
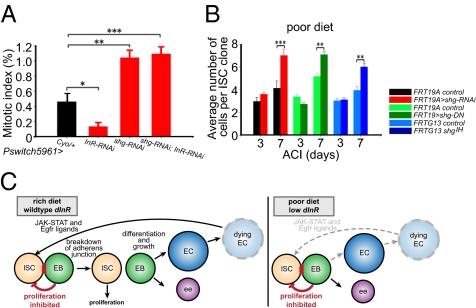
We induced shg-RNAi in flies using a drug-inducible GAL4 driver (P-switch5961) (37) expressed in ISCs and enteroblasts (27), which also contained an enteroblast marker (Gbe+Su(H)lac-Z) (4, 29, 38) (Fig. S7). RNAi knockdown of shg in ISCs and enteroblasts for 6 d resulted in an approximately twofold increase in the percent mitotic index (SI Materials and Methods) compared with controls (Fig. 6A, Table S4, Fig. S2E). This increase in the percent mitotic index was presumably not due to an increased stress response or an increase in apoptosis (Fig. S8). Knockdown of dInR or chico in ISCs and enteroblasts for 6 d led to an almost fourfold reduction in the percent mitotic index (Fig. 6A, Table S4, Fig. S2E), which is consistent with results obtained with mutant dInR and chico MARCM clones (Figs. 3, 4, and Fig. S7). Knockdown of dInR or chico and shg simultaneously from ISCs and enteroblasts for 6 d resulted in a mitotic index percentage similar to that seen with knockdown of shg alone (Fig. 6A, Table S4, Fig S2E), demonstrating that reduction in DE–cadherin can alleviate the decrease in ISC proliferation caused by reduction in insulin signaling. To determine if reduction of DE–cadherin in the enteroblast alone also behaves similarly, we used Gbe+-Su(H)-GAL4 to drive RNAi knockdown of shg. Upon RNAi knockdown of shg in the enteroblast, wild-type ISCs and mutant enteroblasts separate (Fig. S7) and the percent mitotic index increases (Fig. S6C). Furthermore, knockdown of dInR and shg simultaneously in the enteroblast resulted in a percent mitotic index similar to that seen with knockdown of shg alone (Fig. S6C), demonstrating that reduction in DE–cadherin in the enteroblast alone can alleviate the decrease in ISC proliferation caused by reduction in insulin signaling.
Fig. 6.
Cell contact regulates ISC proliferation. (A) Expression of shg-RNAi in ISCs and enteroblasts suppresses proliferation defects caused by insulin receptor knockdown (dInR-RNAi). (B) shgIH MARCM clones, MARCM clones overexpressing shg-RNAi, or overexpressing shg-DN containing one stem cell from females reared on a poor diet grow larger than wild-type controls. (Scale bar, 20 μm.) (C) Model describing the nonautonomous role of nutrition and insulin signaling on ISC proliferation.
To determine the effect of shg loss on differentiation of daughter cells, we made MARCM clones overexpressing shg-RNAi (Figs. S9A and S10B), shgDN (Figs. S9B and S10C), or MARCM clones homozygous null for shg1H (Fig. S9C) and found that they contained enterocytes and enteroendocrine cells (Fig. S9), demonstrating that, in contrast to previous claims (36), Drosophila DE–cadherin is not required for enterocyte or enteroendocrine differentiation. However, some clones contained more than one Delta-positive cell in separate regions of the clone (Fig. S9E), suggesting that loss of shg results in an increase in ISC number.
Finally, to determine whether loss of shg could suppress the decrease in the rate of ISC proliferation seen in animals reared on a poor diet (Fig. 1A), we made MARCM clones overexpressing shg-RNAi, shgDN, or null shg1H MARCM clones from animals reared on a poor diet and found that mutant clones, which contained only one ISC, contained significantly more cells than wild-type controls by 1 wk ACI (Fig. 6B). These data demonstrate that reduction in DE–cadherin can alleviate both the decrease in the rate of ISC proliferation seen with reduced insulin signaling and a protein-poor diet.
Discussion
Previous studies have focused on responses of ISC proliferation to enterocyte death, delineating a positive feedback mechanism by which ligands secreted from dying enterocytes activate ISC proliferation. Our data propose a model of additional regulation where cell contact between ISCs and newly formed enteroblasts acts to inhibit ISC proliferation through a negative feedback loop (Fig. 6C).
Nutrient deprivation leads to decreased ISC proliferation rates and clones containing fewer cells than clones made in animals fed a rich diet. However, it is unclear why these clones fail to eventually reach the same size as wild-type clones. One possibility is that nutrient-deprived midguts contain fewer cells. Therefore, the number of cells that each ISC needs to generate to maintain tissue homeostasis would be smaller. A second possibility is built on our observation that turnover and production of 8n and 16n enterocytes is reduced in animals fed a poor diet, and this could result in the depletion of a source of promitotic ligands, thereby decreasing the need for a stem cell to divide.
Protein deprivation and reduced insulin signaling leads to an increase in the number of lower ploidy enterocyte daughters per midgut, suggesting that endoreduplication in the midgut is regulated by nutrition. Because enterocyte turnover is reduced in nutrition-deprived animals, it raises the intriguing possibility that 8n cells act to inhibit the growth and endoreduplication of 4n cells into mature enterocytes through an as-yet-unidentified signal. These similarities between nutrient-deprived clones and dInR mutant clones suggest that the effects of nutrition may be mediated in part through the insulin-signaling pathway. Consistent with a role for nutrition and the insulin-signaling pathway in growth and endoreduplication, constitutive activation of dInR in ISC clones led to enterocytes with significantly higher ploidy than normal. Interestingly, these clones were smaller than wild-type, suggesting that excessive or prolonged contact between enterocytes and ISCs may also play a role in the regulation of ISC proliferation.
Our findings raise the as-yet-unexplored possibility that germ-line stem cell and neuroblast stem cell daughters might also nonautonomously regulate stem cell proliferation. When both the ISC and the enteroblast were mutant for dInR, we also found a further increase in cell cycle arrest, suggesting an autonomous role for insulin signaling in the regulation of ISC proliferation.
We found significantly higher levels of DE-cadherin between both dInR mutant enteroblast and wild-type ISCs and dInR mutant enteroblasts and dInR mutant ISCs (Fig. 5D), demonstrating that the insulin-signaling pathway regulates the stability of the adherens junction. Our results are striking because, in the ovary and testis, loss of dInR signaling in the germ-line stem cell niche leads to a decrease rather than an increase in DE–cadherin at the adherens junction (17, 20).
The data presented here demonstrate that the enteroblast can nonautonomously regulate the rate of ISC proliferation. How might this be achieved? One possibility is that the enteroblast inhibits ISC proliferation by providing a short-range inhibitory signal whose effect is removed as the ISC and enteroblast separate. A second possibility is that separation of ISCs and enteroblasts leads to the release from a cellular compartment of a factor that can drive proliferation. One ideal candidate is β-catenin, which is not only a member of the adherens junction but also a transcriptional activator, which is required for ISC proliferation (39, 40).
Recently, McLeod et al. (17) examined ISCs and enteroblast number under protein-poor conditions in old animals expressing green fluorescent protein driven by the escargot promoter (esg-GFP), which is thought to be specific to ISCs and enteroblasts. A decrease in esg-GFP–positive cells was observed in 16- to 17- and 20- to 21-d-old animals fed a poor diet, leading McLeod et al. to conclude that ISC maintenance is regulated by a protein-poor diet. In contrast, we did not observe a decrease in ISC number in females fed a protein-poor diet. Presumably, the modest decrease in GFP-positive cells observed by McLeod et al. was due to loss of the excess enteroblasts seen in aging midguts (41, 42), which is consistent with recently published work showing that insulin-signaling mutants can suppress this aging phenotype (27).
Materials and Methods
Drosophila Stocks.
Drosophila stocks were maintained on standard/cornmeal molasses media, supplemented with live yeast paste, in a 23–25 °C incubator with a 12-h dark and 12-h light cycle. Information on fly lines and culture conditions used can be found in SI Materials and Methods.
lac-Z and MARCM Clonal Analysis.
Dividing intestinal cells were clonally labeled as described previously (3, 28). Guts were analyzed at various times after heat shock by staining with DAPI and the antibodies denoted throughout the text and figures. The MARCM system was used to generate both wild-type and mutant marked lineages (43, 44). Information on fly stocks and crosses can be found in SI Materials and Methods.
Immunostaining.
Female guts were dissected, fixed, and stained as previously described (3); mounted in 50% glycerol; imaged with a spinning disk confocal microscope (Olympus); and analyzed using Slidebook software (version 4.2). Images were processed using Adobe Photoshop CS3. Information on antibodies used can be found in SI Materials and Methods.
ApopTag Staining.
Programmed cell death in the posterior midgut was detected (3) using the ApopTag fluorescein in situ apoptosis detection kit (Millipore).
BrdU Labeling.
Flies were cultured on standard/corn media supplemented with wet yeast mixed with 2.5 mg/mL BrdU (Sigma-Aldrich) for 3 d. The vial and media were replaced each day. Female guts were dissected and fixed as previously described (3), incubated in DNase I buffer for 5 min, and then incubated in 20 units DNase I at 37 °C for 1 h and washed and incubated overnight with a rat anti-BrdU (Abcam) at 4 °C.
Percent Mitotic Index Determination.
Calculation of the percent mitotic index is described in SI Materials and Methods.
Quantification of Total Stem Cell Number in the Posterior Midgut.
Quantification of total stem cell number is described in SI Materials and Methods.
DAPI Quantification.
DAPI quantification is described in SI Materials and Methods.
Measuring DE–Cadherin Levels Between ISCs and Enteroblasts.
Sixteen-bit images of the clones from posterior midguts of FRT82B controls and dInR mutant animals were acquired using a Leica SP5 confocal microscope. Images were acquired at the midplane of the ISC and enteroblast using Leica Software and were analyzed using Metamorph Premiere Offline (version 7.7.1.0). A line with a line width of 3 pixels was drawn from the midpoint of the ISC nucleus to the midpoint of the enteroblast nucleus, and the maximum intensity was recorded along the line. This intensity corresponds to the highest level of DE–cadherin at the junction between the ISC and the enteroblast. One hundred measurements were taken for each condition.
Statistical Analyses.
Quantified data are expressed as the mean ± SE values. Significance testing was conducted via Student's t test. Error bars indicate SE and *P < 0.05, **P < 0.01, ***P < 0.001 in all figures except in Fig. 5D where P values are explicitly specified.
Supplementary Material
Acknowledgments
We thank Gary Struhl for use of his confocal microscope. N.H.C. was supported in part by a Korea Research Foundation Grant funded by the Korean Government (KRF-2008-357-C00105). E.L. was supported in part by Columbia University Medical Training Grant 5-T32-HD055165-01. This work was funded by National Institutes of Health Grant R01 DK082456-01 (to B.O.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109348108/-/DCSupplemental.
References
- 1.Voog J, Jones DL. Stem cells and the niche: A dynamic duo. Cell Stem Cell. 2010;6:103–115. doi: 10.1016/j.stem.2010.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Morrison SJ, Spradling AC. Stem cells and niches: Mechanisms that promote stem cell maintenance throughout life. Cell. 2008;132:598–611. doi: 10.1016/j.cell.2008.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ohlstein B, Spradling A. The adult Drosophila posterior midgut is maintained by pluripotent stem cells. Nature. 2006;439:470–474. doi: 10.1038/nature04333. [DOI] [PubMed] [Google Scholar]
- 4.Micchelli CA, Perrimon N. Evidence that stem cells reside in the adult Drosophila midgut epithelium. Nature. 2006;439:475–479. doi: 10.1038/nature04371. [DOI] [PubMed] [Google Scholar]
- 5.Cronin SJ, et al. Genome-wide RNAi screen identifies genes involved in intestinal pathogenic bacterial infection. Science. 2009;325:340–343. doi: 10.1126/science.1173164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang H, et al. Cytokine/Jak/Stat signaling mediates regeneration and homeostasis in the Drosophila midgut. Cell. 2009;137:1343–1355. doi: 10.1016/j.cell.2009.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lin G, Xu N, Xi R. Paracrine unpaired signaling through the JAK/STAT pathway controls self-renewal and lineage differentiation of drosophila intestinal stem cells. J Mol Cell Biol. 2010;2(1):37–49. doi: 10.1093/jmcb/mjp028. [DOI] [PubMed] [Google Scholar]
- 8.Beebe K, Lee WC, Micchelli CA. JAK/STAT signaling coordinates stem cell proliferation and multilineage differentiation in the Drosophila intestinal stem cell lineage. Dev Biol. 2010;338(1):28–37. doi: 10.1016/j.ydbio.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 9.Liu W, Singh SR, Hou SX. JAK-STAT is restrained by Notch to control cell proliferation of the Drosophila intestinal stem cells. J Cell Biochem. 2010;109:992–999. doi: 10.1002/jcb.22482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chatterjee M, Ip YT. Pathogenic stimulation of intestinal stem cell response in Drosophila. J Cell Physiol. 2009;220:664–671. doi: 10.1002/jcp.21808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jiang H, Grenley MO, Bravo MJ, Blumhagen RZ, Edgar BA. EGFR/Ras/MAPK signaling mediates adult midgut epithelial homeostasis and regeneration in Drosophila. Cell Stem Cell. 2011;8(1):84–95. doi: 10.1016/j.stem.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buchon N, Broderick NA, Kuraishi T, Lemaitre B. Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection. BMC Biol. 2010;8:152. doi: 10.1186/1741-7007-8-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karpowicz P, Perez J, Perrimon N. The Hippo tumor suppressor pathway regulates intestinal stem cell regeneration. Development. 2010;137:4135–4145. doi: 10.1242/dev.060483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw RL, et al. The Hippo pathway regulates intestinal stem cell proliferation during Drosophila adult midgut regeneration. Development. 2010;137:4147–4158. doi: 10.1242/dev.052506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren F, et al. Hippo signaling regulates Drosophila intestine stem cell proliferation through multiple pathways. Proc Natl Acad Sci USA. 2010;107:21064–21069. doi: 10.1073/pnas.1012759107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staley BK, Irvine KD. Warts and Yorkie mediate intestinal regeneration by influencing stem cell proliferation. Curr Biol. 2010;20:1580–1587. doi: 10.1016/j.cub.2010.07.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McLeod CJ, Wang L, Wong C, Jones DL. Stem cell dynamics in response to nutrient availability. Curr Biol. 2010;20:2100–2105. doi: 10.1016/j.cub.2010.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hietakangas V, Cohen SM. Regulation of tissue growth through nutrient sensing. Annu Rev Genet. 2009;43:389–410. doi: 10.1146/annurev-genet-102108-134815. [DOI] [PubMed] [Google Scholar]
- 19.Edgar BA. How flies get their size: Genetics meets physiology. Nat Rev Genet. 2006;7:907–916. doi: 10.1038/nrg1989. [DOI] [PubMed] [Google Scholar]
- 20.Hsu HJ, Drummond-Barbosa D. Insulin levels control female germline stem cell maintenance via the niche in Drosophila. Proc Natl Acad Sci USA. 2009;106:1117–1121. doi: 10.1073/pnas.0809144106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Drummond-Barbosa D, Spradling AC. Stem cells and their progeny respond to nutritional changes during Drosophila oogenesis. Dev Biol. 2001;231:265–278. doi: 10.1006/dbio.2000.0135. [DOI] [PubMed] [Google Scholar]
- 22.LaFever L, Drummond-Barbosa D. Direct control of germline stem cell division and cyst growth by neural insulin in Drosophila. Science. 2005;309:1071–1073. doi: 10.1126/science.1111410. [DOI] [PubMed] [Google Scholar]
- 23.LaFever L, Feoktistov A, Hsu HJ, Drummond-Barbosa D. Specific roles of Target of rapamycin in the control of stem cells and their progeny in the Drosophila ovary. Development. 2010;137:2117–2126. doi: 10.1242/dev.050351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu HJ, LaFever L, Drummond-Barbosa D. Diet controls normal and tumorous germline stem cells via insulin-dependent and -independent mechanisms in Drosophila. Dev Biol. 2008;313:700–712. doi: 10.1016/j.ydbio.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park MS, Takeda M. Starvation suppresses cell proliferation that rebounds after refeeding in the midgut of the American cockroach, Periplaneta americana. J Insect Physiol. 2008;54:386–392. doi: 10.1016/j.jinsphys.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Park MS, Park P, Takeda M. Starvation induces apoptosis in the midgut nidi of Periplaneta americana: A histochemical and ultrastructural study. Cell Tissue Res. 2009;335:631–638. doi: 10.1007/s00441-008-0737-y. [DOI] [PubMed] [Google Scholar]
- 27.Biteau B, et al. Lifespan extension by preserving proliferative homeostasis in Drosophila. PLoS Genet. 2010;6:e1001159. doi: 10.1371/journal.pgen.1001159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Harrison DA, Perrimon N. Simple and efficient generation of marked clones in Drosophila. Curr Biol. 1993;3:424–433. doi: 10.1016/0960-9822(93)90349-s. [DOI] [PubMed] [Google Scholar]
- 29.Ohlstein B, Spradling A. Multipotent Drosophila intestinal stem cells specify daughter cell fates by differential notch signaling. Science. 2007;315:988–992. doi: 10.1126/science.1136606. [DOI] [PubMed] [Google Scholar]
- 30.Brogiolo W, et al. An evolutionarily conserved function of the Drosophila insulin receptor and insulin-like peptides in growth control. Curr Biol. 2001;11:213–221. doi: 10.1016/s0960-9822(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 31.Veenstra JA. Peptidergic paracrine and endocrine cells in the midgut of the fruit fly maggot. Cell Tissue Res. 2009;336:309–323. doi: 10.1007/s00441-009-0769-y. [DOI] [PubMed] [Google Scholar]
- 32.Amcheslavsky A, Jiang J, Ip YT. Tissue damage-induced intestinal stem cell division in Drosophila. Cell Stem Cell. 2009;4(1):49–61. doi: 10.1016/j.stem.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zeng X, Chauhan C, Hou SX. Characterization of midgut stem cell- and enteroblast-specific Gal4 lines in Drosophila. Genesis. 2010;48:607–611. doi: 10.1002/dvg.20661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baumann O. Posterior midgut epithelial cells differ in their organization of the membrane skeleton from other Drosophila epithelia. Exp Cell Res. 2001;270:176–187. doi: 10.1006/excr.2001.5343. [DOI] [PubMed] [Google Scholar]
- 35.Oda H, Tsukita S. Nonchordate classic cadherins have a structurally and functionally unique domain that is absent from chordate classic cadherins. Dev Biol. 1999;216:406–422. doi: 10.1006/dbio.1999.9494. [DOI] [PubMed] [Google Scholar]
- 36.Maeda K, Takemura M, Umemori M, Adachi-Yamada T. E-cadherin prolongs the moment for interaction between intestinal stem cell and its progenitor cell to ensure Notch signaling in adult Drosophila midgut. Genes Cells. 2008;13:1219–1227. doi: 10.1111/j.1365-2443.2008.01239.x. [DOI] [PubMed] [Google Scholar]
- 37.Mathur D, Bost A, Driver I, Ohlstein B. A transient niche regulates the specification of Drosophila intestinal stem cells. Science. 2010;327:210–213. doi: 10.1126/science.1181958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Furriols M, Bray S. Dissecting the mechanisms of suppressor of hairless function. Dev Biol. 2000;227:520–532. doi: 10.1006/dbio.2000.9923. [DOI] [PubMed] [Google Scholar]
- 39.Lin G, Xu N, Xi R. Paracrine Wingless signalling controls self-renewal of Drosophila intestinal stem cells. Nature. 2008;455:1119–1123. doi: 10.1038/nature07329. [DOI] [PubMed] [Google Scholar]
- 40.Lee WC, Beebe K, Sudmeier L, Micchelli CA. Adenomatous polyposis coli regulates Drosophila intestinal stem cell proliferation. Development. 2009;136:2255–2264. doi: 10.1242/dev.035196. [DOI] [PubMed] [Google Scholar]
- 41.Biteau B, Hochmuth CE, Jasper H. JNK activity in somatic stem cells causes loss of tissue homeostasis in the aging Drosophila gut. Cell Stem Cell. 2008;3:442–455. doi: 10.1016/j.stem.2008.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Choi NH, Kim JG, Yang DJ, Kim YS, Yoo MA. Age-related changes in Drosophila midgut are associated with PVF2, a PDGF/VEGF-like growth factor. Aging Cell. 2008;7:318–334. doi: 10.1111/j.1474-9726.2008.00380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee T, Luo L. Mosaic analysis with a repressible cell marker (MARCM) for Drosophila neural development. Trends Neurosci. 2001;24:251–254. doi: 10.1016/s0166-2236(00)01791-4. [DOI] [PubMed] [Google Scholar]
- 44.Lee T, Luo L. Mosaic analysis with a repressible cell marker for studies of gene function in neuronal morphogenesis. Neuron. 1999;22:451–461. doi: 10.1016/s0896-6273(00)80701-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.