Abstract
Humans are known to have energetically optimal walking and running speeds at which the cost to travel a given distance is minimized. We hypothesized that “optimal” walking and running speeds would also exist at the level of individual locomotor muscles. Additionally, because humans are 60–70% more economical when they walk than when they run, we predicted that the different muscles would exhibit a greater degree of tuning to the energetically optimal speed during walking than during running. To test these hypotheses, we used electromyography to measure the activity of 13 muscles of the back and legs over a range of walking and running speeds in human subjects and calculated the cumulative activity required from each muscle to traverse a kilometer. We found that activity of each of these muscles was minimized at specific walking and running speeds but the different muscles were not tuned to a particular speed in either gait. Although humans are clearly highly specialized for terrestrial locomotion compared with other great apes, the results of this study indicate that our locomotor muscles are not tuned to specific walking or running speeds and, therefore, do not maximize the economy of locomotion. This pattern may have evolved in response to selection to broaden the range of sustainable running speeds, to improve performance in motor behaviors not related to endurance locomotion, or in response to selection for both.
Keywords: cost of transport, human evolution, persistence hunting, locomotor energetics, electromyography
The energetic cost to travel a given distance, the cost of transport (COT), has long been known to strongly depend on walking speed in humans (1, 2). The cost is minimized at intermediate walking speeds of 4.5–5.4 km⋅h−1 (1.25–1.5 m⋅s−1) and rises rapidly as speed increases above or decreases below this optimum. In contrast, the metabolic cost to run a given distance is generally recognized to be independent of speed in humans (2–6). Recently, however, a reevaluation of the COT in running humans has shown that humans also have energetically optimal speeds when running (7). This study relied on repeated measures from individual subjects, an approach that had the potential to identify the subtle relationship observed. There are at least three reasons to expect the COT to depend on locomotor speed. First, the force that a muscle generates decreases as its shortening velocity increases in a hyperbolic relationship. As a consequence of this relationship, a muscle's capacity to perform work and its energetic efficiency are highest at intermediate shortening velocities (8). Thus, if there is a relationship between locomotor speed and muscle shortening velocity, the force–velocity relationship may account for the COT being lowest at intermediate walking or running speeds. Note that this relationship is unlikely to explain energetically optimal speeds in both walking and running gaits because the shortening velocity of muscles in these two gaits will most often be very different. Second, the external mechanical work done on the center of mass to run a unit distance decreases with speed, whereas the work done to oscillate the segments of the body relative to each other (i.e., internal work) increases with speed (9–11). The high external work at low running speeds and the high internal work at high running speeds might produce a U-shaped COT relationship. Third, during walking, the pendular transfer of kinetic and potential energy is greatest at intermediate speeds (9), reducing the total mechanical work that must be performed by muscles at these speeds. Thus, the observed energetically optimal walking and running speeds are consistent with the contractile physiology of skeletal muscle and biomechanics of terrestrial locomotion.
Ultimately, the COT is primarily a function of the cumulative metabolic rates of the muscles that produce locomotion. For the reasons explained above, we expect metabolism per distance traveled to be minimized at intermediate walking or running speeds for individual muscles. As is true for the metabolism of the whole organism, individual muscles likely have metabolically optimal walking and running speeds. If muscles do have optimal speeds, the extent to which different muscles share similar optimal speeds may distinguish species that are locomotor specialists from those that are locomotor generalists. Among primates and compared with most species of mammals, humans are recognized as being anatomically and physiologically specialized for economical walking (6, 12–18) and, perhaps, endurance running (4, 5, 19). If humans underwent selection for long distance walking and/or running, evolution of improved locomotor economy would likely have occurred. Humans are exceptionally economical walkers (2, 6, 20) and although our locomotor economy during running is only average for mammals of our body size (20–22), our long legs and capacity to store and recover elastic strain energy (5) make it likely that we are economical runners compared with other great apes. If activity of individual muscles is minimized at intermediate walking or running speeds, as suggested above, we would expect the various muscles of the human locomotor system to all be tuned to the same optimal speed to reduce the energetic cost of locomotion. In contrast, if other functions of the limbs, such as accelerating, climbing or fighting, were as important to survival and fitness of early humans as was economical transport, or if there was selection to broaden the range of sustainable running speeds, then tuning of the various muscles to the same optimal walking and running speeds would presumably not have occurred.
Unfortunately, current methods make these hypotheses difficult to test. Measuring blood flow to individual muscles with colored microspheres is an approach that has been used to quantify metabolism of individual muscles during exercise (23, 24); however, it cannot be used in humans. Although computational modeling of muscle energy consumption is an approach that could be used to address the hypotheses of this study (24, 25), the current lack of running models make this approach unfeasible and results from a modeling analysis would ultimately need to be verified with in vivo recordings. Other methods of quantifying muscle metabolism are also not possible (e.g., blood gas and flow measurements) or impractical (e.g., repeated muscle biopsies) in exercising humans. An indirect approach that could falsify the hypotheses is electromyography (EMG). Although EMG cannot provide direct measures of muscle force, work, or metabolism, increases in amplitude and/or duration of EMG indicate increases in activity of muscle fibers and, therefore, provide a correlative indication of muscle metabolism within the recording field of the electrode (26).
This investigation had two goals. First, to determine whether individual muscles exhibit optimal locomotor speeds, we measured the electrical activity of 13 muscles of the back and legs over a range of walking and running speeds in human subjects and calculated the cumulative muscle activity per distance traveled (CMAPD). We predicted that the CMAPD for each muscle would be lowest at intermediate walking or running speeds. Second, we tested if these 13 muscles were all tuned to the same optimal walking or running speed, as might be expected to enhance economy in a species that was adapted for endurance locomotion. Lastly, because humans are much more economical at walking than running (2, 6) and tuning is one of many factors (10, 11, 27) that might explain the remarkable economy of walking humans, we hypothesized that the greater economy of walking might be due, in part, to tighter tuning of the locomotor muscles during walking than running.
Results
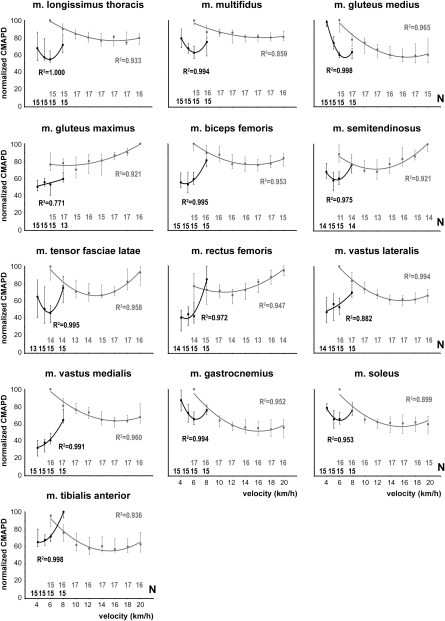
The integrated muscle activity required to walk or run a kilometer (i.e., CMAPD) exhibited a curvilinear and, in most cases, a U-shaped, relationship with locomotor speed (Fig. 1 and Fig. S1). The greatest activity occurred at the lowest and/or highest speeds, and activity was usually minimal at intermediate walking and running speeds (Fig. 1). During walking, in 5 of the 13 muscles, the lowest CMAPD occurred at the lowest speed studied (4 km⋅h−1). During running, the median CMAPD was low and largely independent of speed at the highest speeds for the multifidus, gastrocnemius, and soleus muscles. Activity per distance at the optimal walking and running speeds was generally 20–40% lower than the peak activity recorded. For most of the muscles, the U-shaped relationship was more compressed during walking than during running; exhibiting steeper increases in activity as speed changed above or below the minimum. These patterns were present when the CMAPD was plotted against absolute walking and running speeds (Fig. 1) or plotted against speed normalized for limb length (Fig. S1).
Fig. 1.
Median values of normalized CMAPD versus walking (black) and running (gray) speeds (km⋅h−1) for each of the 13 muscles. Lines fitted to the data were derived from second-order polynomial least-squares regressions. Note that these regressions were fit to the median values of all of the subjects. Error bars represent the upper and lower quartiles. Sample sizes at each speed are listed along the x axes. Squared R values (coefficients of determination) are given in each case.
For most of the muscles, there was a correspondence in the walking and running speeds at which activity was minimized. Muscles that had a high optimal walking speed tended to have a high optimal running speed and muscles that had a low optimal walking speed generally also had a low optimal running speed. This pattern was not the case, however, for the vastus lateralis, vastus medialis, and the tibialis anterior muscles. These three muscles exhibited low optimal walking speeds but high optimal running speeds.
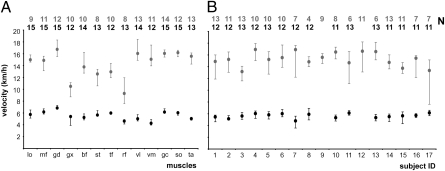
The walking and running speeds at which CMAPD was minimized in individual subjects varied dramatically among the 13 muscles investigated (Fig. 2A and Fig. S2). The highest optimal speeds were 61% greater in the walk and 65% greater in the run than the lowest optimal speeds (Table 1). Furthermore, after normalizing for leg length, there was a twofold difference in the highest and lowest optimal walking and running speeds. The coefficients of variation of the optimal speeds for individual muscles were also high: 18% for walking and 21% for running. Although the average optimal speed of the 13 muscles varied among the 17 subjects (Fig. 2B), the coefficients of variation among the subjects (10% for walking and 12% for running) were approximately one-half the coefficients of variation among the different muscles. Thus, the optimal speeds of individual muscles varied more than the overall EMG optimal speeds of individual subjects (i.e., speed at which the activity of all of the muscles was minimized).
Fig. 2.
Median optimal walking (black) and running (gray) speeds for the 13 muscles (A) and the 17 subjects (B). Note that the medians were calculated from independently derived optima for each muscle (A) and the average optimal value of all of the muscles for each subject (B). Error bars represent the upper and lower quartiles of speed. Muscle name abbreviations are given in Table 2. Sample sizes at each speed are listed at the top of the graphs.
Table 1.
Median, minimum, maximum, and upper and lower quartiles of the walking and running speeds at which CMAPD was minimized for the 13 investigated muscles and the 17 subjects
| Measurement | Gait | Median | Minimum | Maximum | Upper quartile | Lower quartile | Interquartile range as CV* |
| Muscles† | |||||||
| Absolute speed, km/h | Walking | 5.65 | 4.26 | 6.84 | 6.03 | 5.03 | 17.75 |
| Running | 14.98 | 10.16 | 16.74 | 16.04 | 12.95 | 20.65 | |
| Relative speed | Walking | 0.33 | 0.21 | 0.45 | 0.36 | 0.28 | 26.74 |
| Froude number | Running | 2.53 | 1.38 | 2.78 | 2.65 | 2.08 | 22.58 |
| Subjects‡ | |||||||
| Absolute speed, km/h | Walking | 5.65 | 4.79 | 6.15 | 5.95 | 5.39 | 9.95 |
| Running | 15.24 | 13.17 | 16.92 | 16.50 | 14.73 | 11.64 | |
| Relative speed | Walking | 0.34 | 0.25 | 0.39 | 0.37 | 0.31 | 16.29 |
| Froude number | Running | 2.62 | 1.93 | 3.30 | 2.72 | 2.40 | 12.30 |
*CV, coefficient of variation.
†Muscles, the respective minimum speeds for each muscle were used to calculate the median minimum speed of all of the muscles.
‡Subjects, the respective minimum speeds of each subject (calculated as the median speed of all muscles for that subject) were used to calculate the median minimum speed for all of the subjects.
Discussion
In this study, we found that the activity of individual muscles required to walk or run a given distance is minimized at specific speeds. For most of the muscles studied, minimal activity occurs at intermediate walking and running speeds but, in some cases, it occurs at slower or faster speeds. As discussed above, minimization of activity at specific speeds may be due to the mechanical work of locomotion and/or the force–velocity relationship of striated muscle.
What is the significance of the observation that muscle activity is minimized at specific locomotor speeds? There is not a direct linear relationship between the level of muscle activity and muscle force, work, or metabolism (28–30). Nevertheless, at the level of individual muscles, greater force and work are produced by increased activity of motor units, and increased force and work production are associated with elevated muscle metabolism (26, 31). Thus, we believe we are justified in assuming that under the conditions of this study, changes in muscle activity during walking and running broadly reflect changes in the metabolism of the muscles studied. Consequently, the observation that muscle activity is minimized at specific walking and running speeds is consistent with recordings of whole body metabolism of walking and running horses (32, 33) and humans (7), which indicate that the COT is minimized at intermediate speeds. The COT has also been found to be minimized at intermediate speeds in walking but not in running birds (34).
If the musculoskeletal system of humans was specialized primarily for economical walking or running, we would expect all locomotor muscles to be tuned to approximately the same walking or running speed. The results of this study indicate that this pattern is not the case. Thus, for a given locomotor speed, the relative level of activity varies from muscle to muscle such that activity is relatively high in some muscles but relatively low in others. Importantly, the optimal speeds of the 13 muscles were much more variable than the optimal speeds of the individual subjects. These observations suggest that the locomotor muscles of humans are not solely specialized for economical transport. In addition to walking and running on level ground, the ancestors of humans needed to ascend and descend steep terrain and trees; accelerate rapidly when avoiding unexpected hazards, hunting, and competing with conspecifics; lift and carry heavy loads; and apply large forces to objects in their environment. For example, the gluteus maximus muscle has long been recognized to play an important role in acceleration (35), stair and incline climbing (36, 37), and throwing, clubbing, digging, and lifting (38). The low optimal walking and running speeds of this muscle may be indicative of low musculoskeletal gearing appropriate for its role in acceleration and climbing. Nevertheless, the results of this study are consistent with the hypothesis that the “locomotor” muscles of humans are adapted for a variety of both locomotor and nonlocomotor behaviors.
There is at least one ecological and evolutionary context in which having locomotor muscles with a wide range of running speeds at which CMAPD is minimized might be advantageous. A range of optimal running speeds makes the COT relatively independent of running speed, and that may have helped early human hunters capture large prey by running them to exhaustion (4, 19, 39). Steep U-shaped relationships between the COT and speed have been observed in walking and trotting horses (32, 33). This relationship allows horses to travel very economically at their optimal speeds but gives them poor economy when they walk and run at speeds substantially slower or faster than their optimal speeds. In contrast, although humans appear to have optimal running speeds (7), the COT is relatively independent of running speed compared with horses. This independence results in humans being not as economical at their optimal running speed, as would be the case if all muscles were tightly tuned to that speed. However, it also means that we have relatively good economy when running at both slow and fast speeds, presumably broadening the range of sustainable speeds. The context in which a broad range of sustainable running speeds has been suggested to be advantageous is persistence hunting (4, 19, 39). Before early humans were equipped with projectile weapons that can disable prey from a distance, our ancestors are hypothesized to have captured large prey species by running them to exhaustion during the hottest part of the day. A number of indigenous hunter-gatherer populations have been observed to use this hunting strategy (see refs. 4 and 39), and Liebenberg has documented it in modern San Bushmen of the Kalahari (39). Successful persistence hunts cover 25–35 km and last from 2 to 5 h. During this period, the hunter must, at times, run slowly, for example while he struggles to follow the prey's tracks among the tracks of other individuals or when the tracks are faint. At other times, the hunter must run rapidly to minimize the time the prey is able to rest in the shade of a bush or tree. Thus, the wide range of running speeds at which CMAPD is minimized in our locomotor muscles may have enhanced success in persistence hunting by allowing our ancestors to run with reasonable economy over a wide range of speeds.
The persistence hunting hypothesis of human evolution raises the possibility that the wide range of optimal running speeds of our locomotor muscles evolved in response to selection for a broad range of sustainable running speeds. This possibility, however, is entirely compatible with selection for motor behaviors not related to endurance locomotion. For example, if the gluteus maximus muscle has evolved to play an important role in acceleration, as a number of studies indicate (35–37), its musculoskeletal gearing may require that it shortens at relatively high velocities when humans run fast, limiting its potential to produce force and requiring increasing activity per distance as running speed increases. Thus, selection for rapid acceleration could result in some muscles having low optimal CMADP running speeds, indirectly reducing the COT at low running speeds below what would be the case if all or most locomotor muscles were tuned to a single running speed. Nevertheless, whether the broad range of running speeds at which CMAPD is minimized is the result of selection for persistence hunting, motor behaviors not related to endurance locomotion, or some combination of both, we can be confident that our musculoskeletal system is not tuned to maximize the economy of locomotion.
When we initiated this study, we hypothesized that the optimal speeds of the 13 muscles would be less variable during walking than running, because humans are more economical walkers than runners. We found, however, that the speed at which activity was minimized for individual muscles was equally variable for walking and running, suggesting that the locomotor muscles of humans are not more specialized for walking than for running. This result is consistent with a set of traits that distinguish humans from other great apes. In terms of locomotor specializations, humans appear to have a composite phenotype. Some traits make more sense for economical walking, whereas others are appropriate for endurance running. The most dramatic example suggesting that we are more specialized for walking than running is that the COT is 60–70% less for walking than running (2, 6, 20). This difference is at least partially the consequence of our erect limb posture, which gives extensor muscles of the limb joints high mechanical advantage during walking (27) and our plantigrade feet that enhance the economy of walking but not the economy of running (6). In contrast, some human characters appear more consistent with specialization for endurance running performance. Our remarkable capacity to dissipate metabolic heat loads, as a result of hairlessness and sweating, makes more sense for the substantially higher metabolic rates of running than walking (4, 40). Our ability to store and recover elastic strain energy in the Achilles tendon of the ankle joint and plantar fascia of the foot is much greater in running than in walking (5, 41–44). Additionally, a suite of traits that enhance counter rotation of the trunk versus the hips and head (5) and a significant shortening of the toes relative to other apes (45) appear to enhance the economy of running.
Although humans are clearly highly specialized for terrestrial locomotion compared with other great apes, have exceptional energetic economy when walking, and rank among the best long distance running species on the planet, the results of this study indicate that we are not solely specialized for terrestrial locomotion. Our locomotor muscles are tuned to a variety of walking and running speeds, and this pattern is inconsistent with the evolution of an optimal phenotype to maximize the economy of long distance travel. Instead, the locomotor muscles of the human body appear to have been selected for a variety of yet-undetermined functions, and some of these functions likely entailed limits on specialization for locomotor economy.
Methods
We investigated 17 healthy males [age: 31.8 ± 8 y (mean ± SD); height: 1.79 ± 0.05 m; weight: 73.7 ± 7.2 kg; body-mass index: 22.9 ± 1.5 kg⋅m−2] with no history or presence of orthopedic and cardiovascular diseases. Because males and females differ in the maximum speeds they can run and exhibit subtle differences in EMG patterns and locomotor kinematics, we chose to limit our sample to males to maximize the signal-to-noise ratio in our data. All gave their informed consent to voluntarily participate in this study, which was part of a larger study that was approved by the ethics committee of the University Hospital Jena, Germany (0558-11/00).
Data were collected as the subjects walked and ran on a horizontal motorized treadmill (Quasar.med; HP cosmos). All subjects had previous experience with treadmills and were given an adequate habituation period to become familiar with the study treadmill and the experimental situation. Walking speeds were 4, 6, and 8 km⋅h−1 (i.e., 1.11, 1.67, and 2.22 m⋅s−1), and the individual preferred walking speed, which was determined by the subject walking on the treadmill, was on average 5.1 ± 0.49 km⋅h−1 (1.42 ± 0.14 m⋅s−1). The subjects' preferred speeds were within the range previously reported in the literature (2, 46). Running speeds ranged from 6 to 20 km⋅h−1 (1.67–5.56 m⋅s−1) in 2 km⋅h−1 (0.56 m⋅s−1) increments. For the collection of walking data, subjects walked for ≈40 s at constant speed, resulting in at least 30 strides to be analyzed. To avoid artifacts due to fatigue when running at the higher speeds, only 20 strides were recorded for each running speed.
We recorded surface EMG from 13 different muscles of the back, hip, and legs bilaterally (muscles and electrode positions are detailed in Table 2) in accordance with international recommendations (47). After gentle cleaning and shaving of the skin, disposable Ag-AgCl electrodes (H93SG; Arbo) with a circular electrode area of 1.6 cm diameter and an interelectrode distance of 2.5 cm were applied. All electrodes and amplifiers were carefully secured with tape and elastic net bandages to minimize movement artifacts. Nevertheless, despite these precautions, some electrodes inevitably failed during the course of the recording sessions, primarily at the higher running speeds because of movement artifacts or sweating of the subject. The left foot was equipped with an accelerometer to accurately identify the beginning of the stride, defined as the moment of contact of the left foot with the surface of the treadmill. Data were amplified (gain: 1000; biovision) and stored on computer (GJB Datentechnik; analog-to-digital conversion at 2,000 s−1; DAQCard-AI-16E-4, 12 bit; National Instruments) for analysis.
Table 2.
Investigated muscles and respective electrode locations according to Hermens (47)
| Muscle | Electrode location/orientation |
| M. longissimus thoracis (lo) | At L1 level over the palpable bulge of the muscle/vertical |
| M. multifidus (mf) | At L5 level, medial the line between L1 and PSIS/along line |
| M. gluteus medius (gd) | 50% on the line from iliac crest to the great trochanter/vertical |
| M. gluteus maximus (gx) | At greatest prominence of muscle/45° to outside |
| M. biceps femoris (bf) | 50% on the line between ischial tuberosity and lateral epicondyle of the tibia/along line |
| M. semitendinosus (st) | 50% on the line between ischial tuberosity and medial epicondyle of the tibia/along line |
| M. tensor fasciae latae (tf) | On the line from the ASIS to the lateral femoral condyle in the proximal 1/6/along line |
| M. rectus femoris (rf) | 50% on the line between ASIS and upper end of patella/along line |
| M. vastus lateralis (vl) | At 2/3 on the line from the ASIS to the lateral side of the patella/along line |
| M. vastus medialis (vm) | At 8/10 on the line from the ASIS to the medial knee joint space/perpendicular to line |
| M. gastrocnemius (medialis, gc) | At most prominent bulge of muscle/20° to the inside |
| M. soleus (so) | At 2/3 of the line between the medial femoral condyle to the medial malleolus (distal the lower end of the medial gastrocnemius)/vertical |
| M. tibialis anterior (ta) | At 1/3 on the line between the tip of the fibula and the tip of the medial malleolus/along line |
PSIS, posterior superior iliac spine; ASIS, anterior superior iliac spine.
EMG is a compound action potential composed of the summed action potentials of the muscle fibers located close to the recording electrode. Each of the fiber action potentials represents the activation and deactivation of that fiber. If muscles are not fatigued and the contractile state (e.g., concentric, isometric, or eccentric) of the fibers remains the same, as is likely within a single gait, the metabolic cost of contraction and Ca2+ pumping occur at fixed values. Therefore, the ratio of the metabolic cost of contraction versus the metabolic cost of activation/deactivation remains constant, regardless of the number of steps taken to travel 1 km or the number of fibers recruited per step. Thus, integration of the EMG recording provides a reliable correlative indication of muscle metabolism within the recording field of the electrode.
All analyses were performed by custom programs using the MATLAB (MathWorks) environment (48, 49). EMGs were sampled and analyzed on a stride-by-stride basis. EMG curves were identified with the help of a semiautomatic program including visual control. Only strides with a period within ±10% of the median of all strides per speed, for a given subject, were included in the analysis. Signals were first centered by subtracting any DC offset, high-pass filtered at 20 Hz, low-pass filtered at 300 Hz, and subsequently smoothed by a moving window of 15 ms. Finally, all valid EMG strides were time-normalized to 100% stride duration with an accuracy of 0.5%, resulting in 201 bins per sampled stride and root mean square (rms) curves were calculated. From these curves, grand averaged rms curves were calculated separately for every muscle, subject, and speed, respectively. Because of the extreme conditions, primarily during higher speeds (e.g., sweating and movement artifacts), some data had to be excluded from the final analysis. This exclusion was done by careful visual inspection of the separately calculated grand averaged curves by the same experienced investigator (C.A.) for all speeds, individuals, and muscles.
To calculate the CMAPD, the mean amplitudes were normalized to a travel distance of 1 km. This normalization was done separately for every muscle of each subject at each speed. CMAPD was calculated by using the following equation:
in which “x” are the time normalized values and “v” is locomotor speed. Because speed is specified in m/s, numbers were multiplied by 1,000 to get the distance of 1 km.
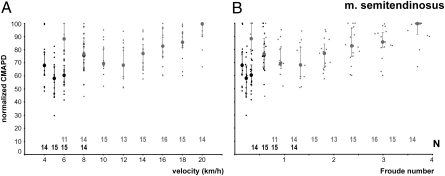
To identify curve characteristics of these CMAPD values versus locomotor speed, second-order polynomial functions were fitted to the data by using least-squares regression. Curves were calculated separately for the walking and running data. Calculations were done in two different ways to (i) provide a graphical representation of the median values of CMAPD versus locomotor speed (Fig. 1) and (ii) determine the median optimal speeds for each of the 13 muscles and the subjects (Fig. 2). The polynomial equations shown in Fig. 1 represent the median values of CMAPD versus locomotor speed for each muscle and were calculated from the median value of each subject. To provide this graphical representation, CMAPD values had to be normalized because of interindividual amplitude differences. This normalization was done separately for every subject by relating all CMAPD values to the maximum value occurring across all speeds. From these normalized values for each subject, group median and quartiles were calculated for each speed (Fig. 3A). These calculations were also performed by normalizing speeds to Froude numbers [Fr = v2/(g × l), in which “v” is velocity in meters per second, “g” is the acceleration of gravity and “l” is limb length in meters; Fig. 3B].
Fig. 3.
Example of the CMAPD to travel 1 km for one of the muscles, the m. semitendinosus. Walking and running data are shown in black and gray, respectively. Each small dot is a CMAPD value for a single subject at that walking or running speed. The large dots are the median value, and the error bars are the upper and lower quartiles. Sample sizes at each speed are listed along the x axis. (A) CMAPD data graphed against absolute walking and running speeds. (B) CMAPD data graphed against speed normalized for limb length with Froude number. Note that error bars are provided for both CMAPD and Froude number, but horizontal error bars do not extend beyond the symbol in many cases.
To calculate the median optimal speeds for each muscle, polynomial functions were fitted separately to the data from each muscle in each subject. These individually fitted polynomial functions were used to identify the walking and running speeds at which CMAPD was minimal for each muscle in each subject. These speed values were then used to calculate median optimal speeds for every muscle (Fig. 2A). To determine the optimal speed for each subject, the calculated median optima of all investigated muscles from a given subject were calculated (Fig. 2B). For this calculation, minimum values that fell outside the recorded range of locomotor speeds were excluded.
Supplementary Material
Acknowledgments
We thank the volunteers who served as subjects in this study. Elke Mey and Sabine Moritz helped us collect the data. Our interpretation of the results of this study benefited from discussions with John Hutchinson, Jim Usherwood, Tim West, and Alan Wilson. Two anonymous reviewers provided comments that improved the manuscript. This research was supported by grants from The Center of Interdisciplinary Prevention of Diseases related to Professional Activities funded by the Friedrich-Schiller University Jena and the Berufsgenossenschaft Nahrungsmittel und Gastgewerbe Erfurt and National Science Foundation Grant IOS-0817782.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105277108/-/DCSupplemental.
References
- 1.Cotes JE, Meade F. The energy expenditure and mechanical energy demand in walking. Ergonomics. 1960;3:97–120. [Google Scholar]
- 2.Margaria R, Cerretelli P, Aghemo P, Sassi G. Energy cost of running. J Appl Physiol. 1963;18:367–370. doi: 10.1152/jappl.1963.18.2.367. [DOI] [PubMed] [Google Scholar]
- 3.Menier DR, Pugh LGCE. The relation of oxygen intake and velocity of walking and running, in competition walkers. J Physiol. 1968;197:717–721. doi: 10.1113/jphysiol.1968.sp008584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carrier DR. The energetic paradox of human running and hominid evolution. Curr Anthropol. 1984;25:483–495. [Google Scholar]
- 5.Bramble DM, Lieberman DE. Endurance running and the evolution of Homo. Nature. 2004;432:345–352. doi: 10.1038/nature03052. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham CB, Schilling N, Anders C, Carrier DR. The influence of foot posture on the cost of transport in humans. J Exp Biol. 2010;213:790–797. doi: 10.1242/jeb.038984. [DOI] [PubMed] [Google Scholar]
- 7.Steudel-Numbers KL, Wall-Scheffler CM. Optimal running speed and the evolution of hominin hunting strategies. J Hum Evol. 2009;56:355–360. doi: 10.1016/j.jhevol.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 8.Hill AV. The heat of shortening and the dynamic constants of muscle. Proc R Soc Lond B Biol Sci. 1938;126:136–195. doi: 10.1098/rspb.1949.0019. [DOI] [PubMed] [Google Scholar]
- 9.Cavagna GA, Heglund NC, Taylor CR. Mechanical work in terrestrial locomotion: two basic mechanisms for minimizing energy expenditure. Am J Physiol. 1977;233:R243–R261. doi: 10.1152/ajpregu.1977.233.5.R243. [DOI] [PubMed] [Google Scholar]
- 10.Cavagna GA, Kaneko M. Mechanical work and efficiency in level walking and running. J Physiol. 1977;268:467–481. doi: 10.1113/jphysiol.1977.sp011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Willems PA, Cavagna GA, Heglund NC. External, internal and total work in human locomotion. J Exp Biol. 1995;198:379–393. doi: 10.1242/jeb.198.2.379. [DOI] [PubMed] [Google Scholar]
- 12.Jungers WL, Stern JT. Body proportions, skeletal allometry and locomotion in the Hadar Hominids: a reply to Wolpoff. J Hum Evol. 1983;12:673–684. [Google Scholar]
- 13.Lovejoy CO. Evolution of human walking. Sci Am. 1988;259:118–125. doi: 10.1038/scientificamerican1188-118. [DOI] [PubMed] [Google Scholar]
- 14.Crompton RH, Yu L, Weijie W, Günther M, Savage R. The mechanical effectiveness of erect and “bent-hip, bent-knee” bipedal walking in Australopithecus afarensis. J Hum Evol. 1998;35:55–74. doi: 10.1006/jhev.1998.0222. [DOI] [PubMed] [Google Scholar]
- 15.Foley RA, Elton S. In: Primate Locomotion: Recent Advances. Strasser E, Fleagle J, Rosenberger A, McHenry H, editors. New York: Plenum; 1998. pp. 419–433. [Google Scholar]
- 16.Kramer PA. Modelling the locomotor energetics of extinct hominids. J Exp Biol. 1999;202:2807–2818. doi: 10.1242/jeb.202.20.2807. [DOI] [PubMed] [Google Scholar]
- 17.Kramer PA, Eck GG. Locomotor energetics and leg length in hominid bipedality. J Hum Evol. 2000;38:651–666. doi: 10.1006/jhev.1999.0375. [DOI] [PubMed] [Google Scholar]
- 18.Wall-Scheffler CM, Chumanov E, Steudel-Numbers K, Heiderscheit B. Electromyography activity across gait and incline: The impact of muscular activity on human morphology. Am J Phys Anthropol. 2010;143:601–611. doi: 10.1002/ajpa.21356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lieberman DE, Bramble DM, Raichlen DA, Shea JJ. Brains, brawn, and the evolution of human endurance running capabilities. Vert Paleobiol Paleoanthro. 2009;III:77–92. [Google Scholar]
- 20.Rubenson J, et al. Reappraisal of the comparative cost of human locomotion using gait-specific allometric analyses. J Exp Biol. 2007;210:3513–3524. doi: 10.1242/jeb.000992. [DOI] [PubMed] [Google Scholar]
- 21.Sockol MD, Raichlen DA, Pontzer H. Chimpanzee locomotor energetics and the origin of human bipedalism. Proc Natl Acad Sci USA. 2007;104:12265–12269. doi: 10.1073/pnas.0703267104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steudel-Numbers KL. The energetic cost of locomotion: Humans and primates compared to generalized endotherms. J Hum Evol. 2003;44:255–262. doi: 10.1016/s0047-2484(02)00209-9. [DOI] [PubMed] [Google Scholar]
- 23.Marsh RL, Ellerby DJ. Partitioning locomotor energy use among and within muscles. Muscle blood flow as a measure of muscle oxygen consumption. J Exp Biol. 2006;209:2385–2394. doi: 10.1242/jeb.02287. [DOI] [PubMed] [Google Scholar]
- 24.Umberger BR, Rubenson J. Understanding muscle energetics in locomotion: New modeling and experimental approaches. Exerc Sport Sci Rev. 2011;39:59–67. doi: 10.1097/JES.0b013e31820d7bc5. [DOI] [PubMed] [Google Scholar]
- 25.Sellers WI, Dennis LA, Crompton RH. Predicting the metabolic energy costs of bipedalism using evolutionary robotics. J Exp Biol. 2003;206:1127–1136. doi: 10.1242/jeb.00205. [DOI] [PubMed] [Google Scholar]
- 26.Praagman M, Veeger HE, Chadwick EK, Colier WN, van der Helm FC. Muscle oxygen consumption, determined by NIRS, in relation to external force and EMG. J Biomech. 2003;36:905–912. doi: 10.1016/s0021-9290(03)00081-2. [DOI] [PubMed] [Google Scholar]
- 27.Biewener AA, Farley CT, Roberts TJ, Temaner M. Muscle mechanical advantage of human walking and running: Implications for energy cost. J Appl Physiol. 2004;97:2266–2274. doi: 10.1152/japplphysiol.00003.2004. [DOI] [PubMed] [Google Scholar]
- 28.Solomonow M, Baratta R, Shoji H, D'Ambrosia R. The EMG-force relationships of skeletal muscle; dependence on contraction rate, and motor units control strategy. Electromyogr Clin Neurophysiol. 1990;30:141–152. [PubMed] [Google Scholar]
- 29.Baratta R, Solomonow M. Dynamic performance of a load-moving skeletal muscle. J Appl Physiol. 1991;71:749–757. doi: 10.1152/jappl.1991.71.2.749. [DOI] [PubMed] [Google Scholar]
- 30.Anders C, Brose G, Hofmann GO, Scholle HC. Evaluation of the EMG-force relationship of trunk muscles during whole body tilt. J Biomech. 2008;41:333–339. doi: 10.1016/j.jbiomech.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 31.Habazettl H, et al. Near-infrared spectroscopy and indocyanine green derived blood flow index for noninvasive measurement of muscle perfusion during exercise. J Appl Physiol. 2010;108:962–967. doi: 10.1152/japplphysiol.01269.2009. [DOI] [PubMed] [Google Scholar]
- 32.Hoyt DF, Taylor CR. Gait and the energetics of locomotion in horses. Nature. 1981;292:239–240. [Google Scholar]
- 33.Wickler SJ, Hoyt DF, Cogger EA, Hirschbein MH. Preferred speed and cost of transport: The effect of incline. J Exp Biol. 2000;203:2195–2200. doi: 10.1242/jeb.203.14.2195. [DOI] [PubMed] [Google Scholar]
- 34.Watson RR, et al. Gait-specific energetics contributes to economical walking and running in emus and ostriches. Proc Biol Sci. 2011;278:2040–2046. doi: 10.1098/rspb.2010.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandy MG, Zajac FE. Optimal muscular coordination strategies for jumping. J Biomech. 1991;24:1–10. doi: 10.1016/0021-9290(91)90321-d. [DOI] [PubMed] [Google Scholar]
- 36.Sloniger MA, Cureton KJ, Prior BM, Evans EM. Lower extremity muscle activation during horizontal and uphill running. J Appl Physiol. 1997;83:2073–2079. doi: 10.1152/jappl.1997.83.6.2073. [DOI] [PubMed] [Google Scholar]
- 37.Roberts TJ, Belliveau RA. Sources of mechanical power for uphill running in humans. J Exp Biol. 2005;208:1963–1970. doi: 10.1242/jeb.01555. [DOI] [PubMed] [Google Scholar]
- 38.Marzke MW, Longhill JM, Rasmussen SA. Gluteus maximus muscle function and the origin of hominid bipedality. Am J Phys Anthropol. 1988;77:519–528. doi: 10.1002/ajpa.1330770412. [DOI] [PubMed] [Google Scholar]
- 39.Liebenberg L. Persistence hunting by modern hunter-gatherers. Curr Anthropol. 2006;47:1017–1025. [Google Scholar]
- 40.Ruxton GD, Wilkinson DM. Thermoregulation and endurance running in extinct hominins: Wheeler's models revisited. J Hum Evol. 2011;61:169–175. doi: 10.1016/j.jhevol.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 41.Ker RF, Bennett MB, Bibby SR, Kester RC, Alexander RM. The spring in the arch of the human foot. Nature. 1987;325:147–149. doi: 10.1038/325147a0. [DOI] [PubMed] [Google Scholar]
- 42.Hof AL. In vivo measurement of the series elasticity release curve of human triceps surae muscle. J Biomech. 1998;31:793–800. doi: 10.1016/s0021-9290(98)00062-1. [DOI] [PubMed] [Google Scholar]
- 43.Hof AL, Van Zandwijk JP, Bobbert MF. Mechanics of human triceps surae muscle in walking, running and jumping. Acta Physiol Scand. 2002;174:17–30. doi: 10.1046/j.1365-201x.2002.00917.x. [DOI] [PubMed] [Google Scholar]
- 44.Maganaris CN, Paul JP. Tensile properties of the in vivo human gastrocnemius tendon. J Biomech. 2002;35:1639–1646. doi: 10.1016/s0021-9290(02)00240-3. [DOI] [PubMed] [Google Scholar]
- 45.Rolian C, Lieberman DE, Hamill J, Scott JW, Werbel W. Walking, running and the evolution of short toes in humans. J Exp Biol. 2009;212:713–721. doi: 10.1242/jeb.019885. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz A, Silder A, Heiderscheit B, Mahoney J, Thelen DG. Differences in lower-extremity muscular activation during walking between healthy older and young adults. J Electromyogr Kinesiol. 2009;19:1085–1091. doi: 10.1016/j.jelekin.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hermens HJ, et al. European Recommendations for Surface ElectroMyoGraphy, Results of the SENIAM Project. The Netherlands: Roessingh Research and Development, Enschede; 1999. [Google Scholar]
- 48.Anders C, et al. Trunk muscle activation patterns during walking at different speeds. J Electromyogr Kinesiol. 2007;17:245–252. doi: 10.1016/j.jelekin.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Anders C, Wagner H, Puta C, Grassme R, Scholle HC. Healthy humans use sex-specific co-ordination patterns of trunk muscles during gait. Eur J Appl Physiol. 2009;105:585–594. doi: 10.1007/s00421-008-0938-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.