Abstract
The breakdown of triglycerides, or lipolysis, is a tightly controlled process that regulates fat mobilization in accord with an animal's energy needs. It is well established that lipolysis is stimulated by hormones that signal energy demand and is suppressed by the antilipolytic hormone insulin. However, much still remains to be learned about regulation of lipolysis by intracellular signaling pathways in adipocytes. Here we show that galectin-12, a member of a β-galactoside–binding lectin family preferentially expressed by adipocytes, functions as an intrinsic negative regulator of lipolysis. Galectin-12 is primarily localized on lipid droplets and regulates lipolytic protein kinase A signaling by acting upstream of phosphodiesterase activity to control cAMP levels. Ablation of galectin-12 in mice results in increased adipocyte mitochondrial respiration, reduced adiposity, and ameliorated insulin resistance/glucose intolerance. This study identifies unique properties of this intracellular galectin that is localized to an organelle and performs a critical function in lipid metabolism. These findings add to the significant functions exhibited by intracellular galectins, and have important therapeutic implications for human metabolic disorders.
Keywords: obesity, energy metabolism, diabetes
The galectin family of animal lectins encompasses 15 members in mammals with conserved carbohydrate-recognition domains (CRD) that bind β-galactoside (1). Unlike most other animal lectins that are synthesized on endoplasmic reticulum-bound ribosomes and delivered to the cell surface or secreted via the endoplasmic reticulum/Golgi pathway, galectins possess characteristics of intracellular proteins and are synthesized on free ribosomes (2). However, galectins can also be detected on the cell surface and extracellular space. Previous research describe galectins as possessing both intracellular and extracellular functions in a variety of cellular processes, including cell–cell and cell–extracellular matrix interactions, intracellular vesicle trafficking, cell growth, apoptosis, and cell activation that impact innate and adaptive immunity, as well as cancer initiation, progression, and metastasis (reviewed in refs. 2–7).
We cloned cDNA coding for a human two-CRD galectin, galectin-12 (8). The mouse gene was subsequently cloned by Hotta et al. (9), who also showed by Northern blotting its preferential expression in adipose tissue. Using the serial analysis of gene expression strategy, the gene was later found to be one of the few genes that are specifically expressed in mouse adipose tissue (10). At times of energy surplus, fatty acids are converted into triglycerides in these cells and stored in specialized lipid-droplet organelles (11). When needed, triglyceride can be hydrolyzed into fatty acids and glycerol in a tightly controlled process known as lipolysis (12). There is a delicate balance between triglyceride synthesis and lipolysis in healthy animals. Disturbance of such a balance can result in lipodystrophy or obesity. It is well established that both obesity and lipodystrophy can result in insulin resistance, type 2 diabetes, and an increased risk for cardiovascular disease (13).
Lipolysis is regulated by opposing mechanisms, largely via modulation of intracellular concentrations of cAMP. In the present studies, we have investigated the localization of galectin-12 and the role of this protein in adipocytes and adiposity by generating and studying galectin-12–deficient (Lgals12−/−) mice. Here we demonstrate that galectin-12 is a lipid-droplet protein that regulates lipolytic PKA signaling. Galectin-12 deficiency in mice results in enhanced lipolysis, reduced adiposity, and ameliorated insulin resistance.
Results
Galectin-12 Ablation Reduces Adiposity Associated with Decreased Adipocyte Triglyceride Content.
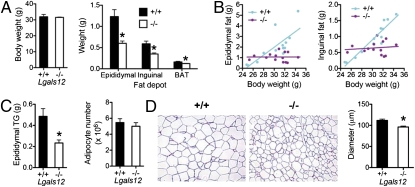
It has been previously reported that galectin-12 is preferentially expressed in adipose tissue and that its expression is regulated by hormones and cytokines that regulate insulin sensitivity (9, 14), suggesting its involvement in energy homeostasis. To study the role of galectin-12 in energy metabolism in vivo, we generated galectin-12–deficient (Lgals12−/−) mice (Fig. S1) and examined their adipose tissue phenotype. We found that Lgals12−/− animals had substantially reduced visceral (epididymal) and subcutaneous (inguinal) white adipose tissue, despite normal body weights (Fig. 1A). Reduced adiposity of Lgals12−/− mice was also supported by body composition analysis, which showed a ∼40% reduction in whole-body lipid content (Table S1). The strong positive correlation between body weights and weights of fat depots seen in Lgals12+/+ mice was not observed in Lgals12−/− mice (Fig. 1B). Epididymal fat depots of Lgals12−/− mice contained less triglyceride compared with Lgals12+/+ mice, but the number of adipocytes was not significantly altered (Fig. 1C). Consistent with this finding, the adipocytes of Lgals12−/− mice were smaller in size (Fig. 1D). These results indicate that the decrease in size of fat depots in Lgals12−/− mice is because of a reduction of triglyceride content and not tissue cellularity.
Fig. 1.
Galectin-12 deficiency reduces adiposity in mice. (A) Comparison of weights of visceral (epididymal) and subcutaneous (inguinal) white adipose tissue in Lgals12+/+ and Lgals12−/− mice (+/+, n = 13; −/−, n = 18), as well as interscapular brown adipose tissue (BAT) and body weight. (B) Linear regression analyses show that body weight and fat depot weight are highly correlated in Lgals12+/+ mice (epididymal, R2 = 0.586, P = 0.002; inguinal, R2 = 0.887, P < 0.0001), but not in Lgals12−/− mice (epididymal, R2 < 0.00001, P = 0.992; inguinal, R2 = 0.015, P = 0.676). (C) Triglyceride contents and adipocyte numbers of epidydymal fat depots from Lgals12+/+ (n = 5) and Lgals12−/− (n = 4) mice. (D) H&E staining of paraffin sections of epididymal fat depots from Lgals12+/+ and Lgals12−/− mice (representative of four experiments). Average diameters of >200 isolated adipocytes were determined from their digital images with ImageJ software using 100-μm Polybead polystyrene microspheres (Polysciences) as references. Results are from 22- to 24-wk-old males. Asterisks denote statistical significance (*P < 0.05).
An early report suggested that galectin-12 is involved in adipogenesis in vitro (15). However, we did not observe major alterations in the expression of several adipose genes examined in Lgals12−/− mice, suggesting that adipose tissue development in these mice are largely normal (Fig. S2 and Table S2). Nevertheless, the levels of leptin expression in adipose tissue were significantly lower in these mice. This is likely the result of reduced adiposity because leptin expression is regulated by adiposity (16).
Galectin-12 Is Localized to Adipocyte Lipid Droplets.
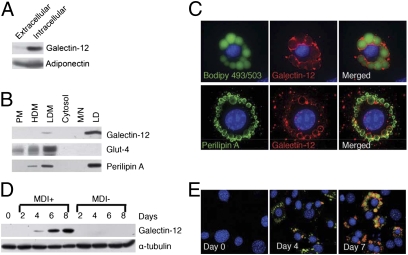
We generated mouse anti-galectin-12 antibodies and used them for cellular localization of galectin-12. The antibodies recognize a single protein band on Western blotting of protein extracts from Lgals12+/+ adipose tissue but not Lgals12−/− adipose tissue (Fig. S1C, Lower), establishing its specificity. Unlike adipokines, such as adiponectin, galectin-12 is not secreted in a significant amount under normal conditions (Fig. 2A). Using a well-established cellular fractionation method for 3T3-L1 adipocytes (17), we found that galectin-12 was detectable in the low-density microsomal fraction, but the vast majority copurified with lipid droplets (Fig. 2B), as did perilipin A, a known lipid-droplet protein (18). This finding was further confirmed by costaining with Bodipy 493/503, a fluorescent dye specific for neutral lipids in lipid droplets (Fig. 2C, Upper). Costaining with anti-perilipin A antibody revealed that although perilipin A was found on both small and large lipid droplets, galectin-12 was mainly localized on large droplets (Fig. 2C, Lower).
Fig. 2.
Galectin-12 is a lipid droplet protein. (A) 3T3-L1 adipocytes were incubated for 2 h in serum-free DMEM. Protein levels of extracellular (in conditioned medium) and intracellular (cell-associated) galectin-12 and adiponectin were determined by Western blotting using specific antibodies. (B) Lipid droplets (LD) were purified from 3T3-L1 adipocytes by density gradient centrifugation, and the remaining cellular components were separated by differential centrifugation into five fractions containing plasma membrane (PM), high-density microsomes (HDM), low-density microsomes (LDM), cytosol, and mitochondria/nuclei (M/N). Levels of galectin-12, Glut-4, and perilipin A were determined in each fraction by Western blotting with respective specific antibodies. Each lane represents samples from an equal number of cells. (C) Immunostaining of 3T3 adipocytes showing galectin-12 (red) and perilipin A (green, Lower) on lipid droplets (green, Upper). (D) 3T3-L1 cells were incubated in the presence (MDI+) or absence (MDI−) of the adipogenic hormone mixture to induce adipocyte differentiation. At indicated time points, cells were extracted for analysis of galectin-12 expression by Western blotting. (E) 3T3-L1 cells at various periods during differentiation were stained for galectin-12 (red), lipid droplets (green), and the nuclei (blue). Data are representative of three experiments with similar results.
Galectin-12 protein could be detected 4 d after induction of 3T3-L1 adipocyte differentiation. At this early stage, most lipid droplets were small and only a few larger droplets were coated with galectin-12. The levels of galectin-12 plateaued around 1 wk into differentiation. At this mature stage, most lipid droplets were large and positive for galectin-12, but some remained small and negative for this protein (Fig. 2 D and E).
To test whether galectin-12 association with lipid droplets is glycan-dependent, we also purified lipid droplets in the presence of lactose. Lactose at a concentration of 25 mM, which completely inhibited the binding of galectin-12 to fetuin-agarose (Fig. S3A), did not affect galectin-12 association with lipid droplets (Fig. S3B), suggesting that the association is not likely to be mediated by glycans.
Galectin-12 Deficiency Enhances Lipolysis.
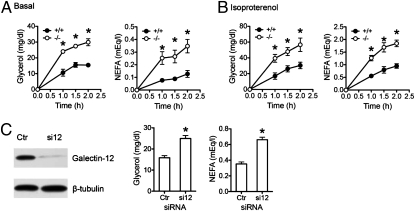
The lipid-droplet protein perilipin A plays an important role in lipolysis. Similarly, we found that lipolysis in Lgals12−/− adipocytes was approximately twofold higher compared with equal numbers of Lgals12+/+ cells, both under basal conditions (Fig. 3A) and when stimulated with the β-adrenergic receptor agonist isoproterenol (Fig. 3B). Even greater differences were observed when equal volumes of packed cells were compared (Fig. S4). We also used RNA interference to further confirm the effects of galectin-12 on lipolysis. Transfection of 3T3-L1 adipocytes with galectin-12 siRNAs significantly suppressed galectin-12 expression and enhanced isoproterenol-stimulated lipolysis (Fig. 3C). Negative control of lipolysis by galectin-12 is consistent with its specific localization to large lipid droplets in mature adipocytes (Fig. 2C), as centrally located, large lipid droplets are known to be less sensitive to lipolytic stimulation than those small, peripheral droplets (19).
Fig. 3.
Galectin-12 deficiency results in elevated lipolysis in adipocytes. (A and B) Adipocytes were isolated from epididymal fat depots of Lgals12+/+ and Lgals12−/− mice (n = 3–6) on a regular diet ad libitum. Equal numbers of cells were incubated in the absence (A) or presence (B) of 0.1 μM of isoproterenol. Glycerol and nonesterified fatty acid (NEFA) released into the medium were measured at different time points of incubation at 37 °C. (C) siRNA-mediated knockdown was performed by electroporating 3T3-L1 adipocytes with either control siRNA (Ctrl) or a combination of two galectin-12–specific siRNAs (si12). Three days later, galectin-12 levels were analyzed by Western blotting, and lipolysis was determined by monitoring the release of fatty acids and glycerol 1.5 h after isopreterenol stimulation. Asterisks denote statistical significance (*P < 0.05). Similar results were observed in four (A and B) or three (C) experiments.
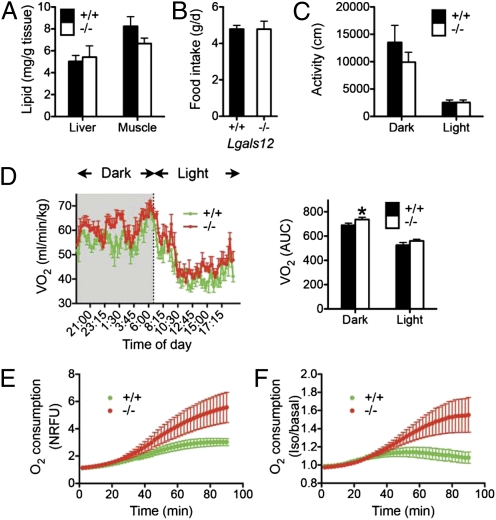
Despite increased lipolysis, serum glycerol and fatty acid levels were not increased in Lgals12−/− mice (Table S3). Lipid contents of liver and muscle were also comparable between Lgals12+/+ and Lgals12−/− mice (Fig. 4A). There were no significant differences in food intake (Fig. 4B) or ambulatory activity (Fig. 4C) between the two genotypes, yet we observed increased oxygen consumption by Lgals12−/− animals indicative of increased energy expenditure (Fig. 4D). In a similar fashion to white adipocytes deficient in the lipid-droplet protein FSP27 (fat-specific protein of 27 kDa) (20, 21) or the critical macroautophage gene Atg7 (autophagy-related 7) (22), Lgals12−/− white adipocytes exhibited increased oxygen consumption (Fig. 4E). Induction of lipolysis with isoproterenol resulted in greater stimulation of oxygen consumption in Lgals12−/− adipocytes compared with Lgals12+/+ cells (Fig. 4F). The results indicate that enhanced mitochondrial respiration in white adipocytes contributes to increased energy expenditure in Lgals12−/− mice.
Fig. 4.
Tissue lipid content, total ambulatory activity, energy expenditure, and food intake in Lgals12+/+ and Lgals12−/− mice. (A) Lipid contents of liver and muscle were determined for Lgals12+/+ and Lgals12−/− male mice (n = 5 for each genotype), by the ethanolic-KOH saponification method. (B) Food intake of Lgals12+/+ (n = 5) and Lgals12−/− (n = 6). (C and D) Total ambulatory activity (distance of movement) and oxygen consumption rates (VO2) were determined by indirect calorimetry during the dark (7:00 PM to 7:00 AM) and light (7:00 AM to 7:00 PM) periods in Lgals12+/+ and Lgals12−/− male mice (n = 5 for each genotype) on standard diet. (E and F) Basal (E) or isoproterenol-stimulated (F) oxygen consumption of isolated epididymal white adipocytes from Lgals12+/+ and Lgals12−/− mice (n = 5–7) measured with the BD Oxygen Biosensor System. Oxygen consumption in E is expressed as normalized relative fluorescence unit (NRFU). Results in F are the ratios of oxygen consumption by adipocytes stimulated with isoproterenol to that by adipocytes under basal conditions. All animals were studied at 22 to 26 wk of age. Asterisks denote statistical significance (*P < 0.05).
Lgals12−/− Adipocytes Show Elevated PKA Phosphorylation of Hormone-Sensitive Lipase and Association of Adipocyte Triglyceride Lipase with Lipid Droplets.
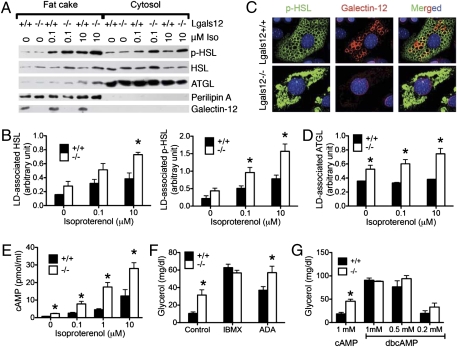
PKA phosphorylation is a critical event for the activation and recruitment of hormone-sensitive lipase (HSL) to lipid droplets (23, 24), where it operates in concert with adipocyte triglyceride lipase (ATGL) to hydrolyze stored lipids (25–27). We treated primary adipocytes from Lgals12+/+ and Lgals12−/− mice with isoproterenol, separated the cytosolic and fat cake proteins, and then analyzed them by Western blotting. Total HSL tended to be higher in Lgals12−/− adipocytes (Fig. 5A), which could be partially responsible for the increased lipolysis. Stimulation with isoproterenol resulted in approximately twofold higher phospho-HSL levels in the fat cake of Lgals−/− adipocytes compared with Lgals12+/+ adipocytes (Fig. 5 A and B). Consistent with enhanced translocation of PKA-phosphorylated HSL, the total HSL levels in lipid droplets of Lgals−/− adipocytes were also higher than those in lipid droplets of Lgals12+/+ adipocytes after isoproterenol stimulation (Fig. 5 A and B).
Fig. 5.
Galectin-12 ablation promotes PKA phosphorylation of HSL and association of phosphorylated HSL and ATGL with lipid droplets as a result of elevated cAMP levels. (A) Adipocytes from the epididymal fat depots of Lgals12+/+ and Lgals12−/− mice were incubated with indicated concentrations of isoproterenol before being separated into cytosol and fat cake. Lipolytic proteins in each fraction were analyzed by Western blotting with indicated antibodies. (B) Quantification of lipid droplet (LD)-associated p-HSL and HSL by densitometry of Western blots (n = 3 for each genotype). (C) Immunofluorescence of adipocytes differentiated from MEFs shows elevated levels of phospho-HSL associated with lipid droplets in Lgals12−/− adipocytes 15 min after treatment with 0.1 μM isoproterenol. (D) Quantification of lipid droplet-associated ATGL by densitometry of Western blots (n = 3 for each genotype). (E) Adipocytes from Lgals12+/+ (n = 3) and Lgals12−/− (n = 3) mice were incubated with indicated concentrations of isoproterenol for 5 min at 37 °C and intracellular cAMP levels were determined by ELISA. (F) Adipocytes from Lgals12+/+ and Lgals12−/− mice (n = 3–6) were treated with or without 0.1 mM IBMX or 1 U/mL ADA for 1 h at 37 °C and lipolysis was determined by measuring glycerol release. (G) cAMP/dbcAMP-stimulated lipolysis in adipocytes from Lgals12+/+ and Lgals12−/− mice (n = 4). Asterisks denote statistical significance (*P < 0.05). Results are representative of three experiments.
Enhanced PKA phosphorylation of HSL was also confirmed by immunofluorescence staining of mouse primary embryonic fibroblast (MEF)-derived adipocytes from Lgals12+/+ and Lgals−/− mice (Fig. 5C). We also observed more ATGL associated with lipid droplets in Lgals12−/− adipocytes compared with Lgals12+/+ counterparts, with or without isoproterenol stimulation (Fig. 5 A and D). There were no significant differences in total ATGL or perilipin levels between adipocytes of the two genotypes (Fig. 5A). These results suggest that increased PKA phosphorylation/activation of adipocyte lipases and their recruitment to lipid droplets account for enhanced lipolysis in Lgals12−/− adipocytes.
Defective PDE Activity Contributes to Enhanced cAMP Levels and Lipolysis in Lgals12−/− Adipocytes.
PKA activity is dynamically regulated by the second messenger cAMP. We compared the intracellular cAMP levels of Lgals12+/+ and Lgals12−/− adipocytes before and after stimulation with various concentrations of isoproterenol and found that the cAMP levels in Lgals12−/− adipocytes were significantly higher than in Lgals12+/+ adipocytes (Fig. 5E). This finding suggests that galectin-12 acts upstream of PKA to regulate lipolysis by restricting intracellular cAMP levels.
Intracellular cAMP levels are regulated by stimulatory and inhibitory signaling that regulate adenylyl cyclase activity, as well as enzymes (PDEs) that catalyze its degradation. Treatment with isobutylmethylxanthine (IBMX), which is both a broad-spectrum PDE inhibitor and an adenosine antagonist, greatly enhanced lipolysis in both Lgals12+/+ and Lgals12−/− adipocytes and eliminated the observed differences between the two genotypes (Fig. 5F). This result suggests that upstream stimulatory signaling leading to adenylyl cyclase activation does not differ between the two genotypes, and defective tonic antilipolytic mechanisms (adenosine signaling or PDE activity) are responsible for enhanced lipolysis in Lgals12−/− adipocytes.
Incubation with adenosine deaminase (ADA), which converts extracellular adenosine to inosine, failed to eliminate differential lipolysis between Lgals12+/+ and Lgals−/− cells (Fig. 5F). In the meantime, (−)-N6-(2-Phenylisopropyl)adenosine, an ADA-resistant A1 adenosine receptor agonist, suppressed lipolysis in both genotypes with similar efficiencies (Fig. S5A). The results suggest that the adenosine signaling pathway does not account for differential lipolysis in Lgals12+/+ and Lgals−/− cells. Instead, defective PDE activity is responsible for enhanced lipolysis in Lgals12−/− adipocytes. This finding was further confirmed by direct incubation of Lgals12+/+ and Lgals12−/− adipocytes with cAMP, or its PDE-resistant analog dibutyryl cAMP (dbcAMP), in the presence of the cell-permeable adenylyl cyclase inhibitor, SQ 22536. Incubation with cAMP stimulated greater lipolysis in Lgals12−/− adipocytes than in Lgals12+/+ adipocytes, whereas incubation with the PDE-resistant dbcAMP induced comparable lipolysis in adipocytes of the two genotypes (Fig. 5G). Results from experiments with inhibitors of PDE3 and PDE4 suggest that the PDE modulated by galectin-12 in lipolysis is distinct from these two families of phosphodiesterases (Fig. S5B). Consistent with our hypothesis, lipolysis in Lgals12−/− adipocytes remained sensitive to insulin (Fig. S5C), which suppresses lipolysis by activating PDE3B (28, 29).
Galectin-12 Deficiency Prevents the Development of Insulin Resistance and Glucose Intolerance Associated with Weight Gain.
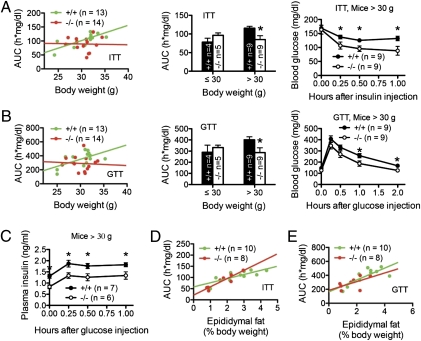
Elevated weight gain and obesity are associated with alterations in adipose tissue functions that predispose an individual to insulin resistance and glucose intolerance preceding the development of type 2 diabetes (13, 30). We compared insulin resistance (Fig. 6A) and glucose intolerance (Fig. 6B) of Lgals12+/+ and Lgals12−/− mice, as measured by integrating blood glucose levels as a function of time after intraperitoneal injection of insulin or glucose indicated by area under the curve (AUC). In Lgals12+/+ mice, insulin resistance and glucose intolerance strongly and positively correlated with body weight. In contrast, no such correlations were observed in Lgals12−/− mice (Fig. 6 A and B). Because Lgals12+/+ mice > 30 g developed insulin resistance and glucose intolerance (Fig. 6 A and B, Center), we used the 30-g cutoff to test whether galectin-12 ablation improves these parameters, a common practice used in similar studies (31, 32). Results presented in Fig. 6 A and B, Center and Right, show that galectin-12 deficiency improved insulin sensitivity and glucose tolerance in mice heavier than 30 g. In comparison, perilipin deficiency augmented insulin resistance and glucose intolerance in mice exceeding 30 g, possibly as a result of elevated blood levels of fatty acids that impair insulin sensitivity (32).
Fig. 6.
Galectin-12 deficiency reduces insulin resistance and glucose intolerance associated with weight gain. (A and B) AUC was computed from the plot of blood glucose levels as a function of time after intraperitoneal injection of Lgals12+/+ and Lgals12−/− mice with insulin (A) or glucose (B). The AUC values, which reflect insulin resistance (A) or glucose intolerance (B), were then plotted as a function of body weight. Note that insulin resistance and glucose intolerance correlate with body weight in Lgals12+/+ mice (insulin resistance vs. body weight, R2 = 0.583, P = 0.002; glucose intolerance vs. body weight, R2 = 0.353, P = 0.032). Such correlation was absent in Lgals12−/− mice (insulin resistance vs. body weight, R2 < 0.001, P = 0.933; glucose intolerance vs. body weight, R2 = 0.004, P = 0.829). (C) Changes in plasma insulin levels in mice weighing > 30 g during the first hour of the glucose tolerance test. (D and E) Plots of insulin resistance and glucose intolerance, as described in A and B, respectively, as a function of adiposity. Asterisks denote statistical significance (*P < 0.05). Results are representative of three to four experiments.
Compared with Lgals12+/+ animals, improved glucose tolerance in mice > 30 g was achieved with lower insulin levels in Lgals12−/− mice (Fig. 6C), consistent with increased insulin sensitivity in these animals. Improved insulin action and glucose homeostasis in Lgals12−/− mice could be the result of reduced adiposity in these mice, or improved adipose tissue function compared with wild-type mice of similar adiposity. Analyses of insulin resistance and glucose intolerance in relation to adiposity in mice revealed similar functional correlations between both genotypes (Fig. 6 D and E), suggesting that galectin-12 deficiency enhances insulin responses primarily as a result of reduced adiposity.
Discussion
Our work identifies galectin-12 as a negative regulator of lipolysis that is preferentially expressed in adipocytes. It is specifically localized on lipid droplets and regulates lipolytic PKA signaling. Galectin-12 deficiency reduces adiposity and prevents development of insulin resistance associated with increased body weight. Thus, we identify a unique intracellular galectin performing a critical function in lipid metabolism that is specifically localized to an organelle.
Galectin-12 ablation altered neither the expression of major adipose genes (Fig. S2) nor the number of adipocytes (Fig. 1C) in adipose tissue. This finding suggests that adipogenesis is largely normal in Lgals12−/− mice, despite previous observations that galectin-12 expression was required for the adipocyte differentiation of 3T3-L1 cells in vitro (15). The absence of an overt adipogenic phenotype in vivo may be explained by genetic robustness against null mutations in the germ line (33). Thus, absence of the adipogenesis phenotype in Lgals12−/− mice is likely the result of functional compensation by another adipogenic pathway.
Lgals12+/+ and Lgals12−/− mice exhibited similar growth curves when they were fed a high-fat diet for up to 12 wk (Fig. S6A). Their body weights and fat weights were indistinguishable after 22 wk on this diet (Fig. S6B). Similarly, galectin-12 deficiency did not significantly alter adiposity in young leptin-deficient (ob/ob) mice (Fig. S6C). This result may be explained by the fact that in both models, obesity develops mainly as a result of excessive food intake. Thus, synthesis of triglycerides in these animals greatly exceeds fat mobilization (lipolysis) and this massive synthesis is likely to marginalize the contribution of lipolysis to the development of increased adiposity. Indeed, in the diet-induced obesity model, a greater reduction of adiposity in ob/ob Lgals12−/− mice was observed after animals were fasted to eliminate the contributions of food/lipid intake and positive energy balance and to simultaneously stimulate lipolysis (Fig. S6B). Similarly, galectin-12 ablation reduced adiposity of ob/ob mice after aging (12 mo) (Fig. S6D), when hyperphagia lessens.
PKA signaling is characterized by spatiotemporal regulation of signal strength and specificity (34, 35). As an example, PKA activation can be regulated locally by compartmentalized PDE activity that degrades cAMP (35, 36). Predominant localization of galectin-12 in lipid droplets suggests that it could contribute to such spatial specificity of PKA signaling to lipolytic substrates on, or around the lipid droplet. Such localized regulation of PKA signaling by galectin-12 is supported by our observation that in wild-type adipocytes, lipid droplets with higher levels of galectin-12 are associated with lower PKA-phosphorylated HSL in response to stimulation by isoproterenol (Fig. 5C).
The mechanisms by which the perilipin family of lipid-droplet proteins are associated with lipid droplets may be diverse. However, it appears that they all involve hydrophobic interactions (37). Although it is presently unknown how galectin-12 is anchored to lipid droplets, galactosyl glycans are not likely to be involved, as the association of galectin-12 to lipid droplets was unaffected by the presence of lactose (Fig. S3). On the other hand, there are several hydrophobic regions in the galectin-12 molecule that may contribute to its localization to lipid droplets (Fig. S7). Lipid domains may also serve as a hydrophobic matrix that helps shape galectin-12 into a functional conformation.
Our results suggest that enhanced lipolysis in Lgals12−/− mice is associated with increased mitochondrial respiration in adipocytes and elevated whole-body energy expenditure. Because galectin-12 was not found in the mitochondrial fraction, it is not likely that it directly regulates mitochondrial function. Instead, enhanced lipolysis in these cells could lead to elevated levels of intracellular fatty acids that serve both as fuel for the mitochondria and ligands for the peroxisome proliferator-activated receptor family of transcription factors to activate genes that promote mitochondrial biogenesis (38). In addition, fatty acids have been shown to activate AMP-activated protein kinase (AMPK) that functions to stimulate fatty acid oxidation (39), all of which could contribute to higher rates of mitochondrial respiration in Lgals12−/− adipocytes.
Taken together, the results from these experiments suggest that enhanced lipolysis in Lgals12−/− mice is associated with increased utilization of lipolytic products as fuel for mitochondrial respiration, contributing to higher whole-body energy expenditure and lower adiposity in these mice. Such changes in energy metabolism favor enhanced insulin action in the regulation of glucose homeostasis. From a clinical viewpoint, pharmaceutical targeting of galectin-12 may prove beneficial by both reducing adiposity and improving insulin sensitivity.
In conclusion, we have identified galectin-12, an intracellular galectin that is preferentially expressed in adipocytes, as a potential therapeutic target for obesity and associated metabolic conditions, such as insulin resistance and glucose intolerance, which predispose individuals to develop type 2 diabetes. The majority of previous studies of galectins have investigated their functions in the extracellular domain and generated a number of interesting findings (reviewed in ref. 4). We anticipate that this study will lead to additional investigations of the role of galectins in the regulation of intracellular signaling that controls many important cellular processes, including energy metabolism.
Materials and Methods
Isolation of Primary Mouse Adipocytes.
Adipocytes were isolated from gonadal fat depots in Krebs--Ringer Hepes (KRH) buffer by collagenase digestion and floatation (40). After the final wash, cell density and triglyceride content of the adipocyte suspension were determined as described (41).
Cellular Fractionation and Lipid Droplet Purification.
Differential centrifugation-based fractionation of 3T3-L1 adipocyte homogenates into fractions rich in plasma membranes (PM), high-density microsomes (HDM), low-density microsomes (LDM), cytosol, nuclei, and mitochondria (M/N) was performed essentially as described (17). Lipid droplets were purified from 3T3-L1 adipocytes by density gradient centrifugation (42).
Deconvolution Immunofluorescence Microscopy.
Mouse embryonic fibroblasts (MEFs) were isolated as described (43). Adipocyte differentiation of primary MEFs and 3T3-L1 fibroblasts was induced with an adipogenic hormone combination (15). Cells were precessed for immunostaining of cellular proteins as described (44). Lipid droplets and nuclei were stained with 1 μg/ml Bodipy 493/503 (Invitrogen) and 1 μg/ml Hoechst 33342 (Invitrogen), respectively. Fluorescent signals were visualized using an Olympus B×61 fluorescence microscope by capturing z-plane images at 1-μm intervals encompassing the depth of the cell. Flat-field-corrected image stacks were deconvolved by the constrained iterative method to mathematically remove out-of-focus light from the fluorescent image set using SlideBook 4.1 software (Intelligent Imaging Innovations).
Lipolysis Assay.
We monitored lipolysis in adipocytes isolated from random-fed Lgals12+/+ and Lgals12−/− mice on regular diet, by measuring fatty acid and glycerol release, as described (40), with minor modifications. Briefly, 2.5 × 105 cells were incubated in 0.3 ml KRH/3% FAA-free BSA (for basal lipolysis), or KRH/3% FAA-free BSA containing indicated concentrations of lipolysis stimulators or inhibitors (Sigma) for 0-2 h at 37 °C with shaking at 150 rpm on an Innova 4335 incubator shaker (New Brunswick Scientific Co., Inc., Edison, NJ). At the end of the incubation, glycerol and NEFA released into the infranatant was determined with the Free Glycerol Reagent (Sigma) and the Nonesterified Fatty Acids Kit (Catachem), respectively.
Generation of Lgals12−/− mice, RNA interference in 3T3-L1 adipocytes, oxygen consumption of adipocytes, assay for isoproterenol-induced protein phosphorylation and translocation in adipocytes, intraperitoneal insulin sensitivity and glucose tolerance assays, generation of galectin-12 antibodies, determination of food intake, energy expenditure, and tissue triglyceride contents, and additional details are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Hei Sook Sul and Eugene Chen for critically reading this manuscript; Craig Warden for helpful discussions; and Yiu-Fai Lee and Xin Yu for assistance in generating the galectin-12 knockout mice strain, with funding from the Dean's Office of the School of Medicine. This research is supported by National Institutes of Health Grant R01 AI020958 and R01 AR56343 (to F.-T.L.) and the Harrison Endowed Chair for Diabetes Research Award (to R.-Y.Y.); P.J.H.'s laboratory received funding from National Institutes of Health Grants R01 HL075675, R01 HL09333, AT002993, and DK087307.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. G.W.H. is a guest editor invited by the Editorial Board.
See Commentary on page 18575.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1109065108/-/DCSupplemental.
References
- 1.Cummings RD, Liu F. In: Essentials of Glycobiology. Varki A, et al., editors. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2009. pp. 475–488. [PubMed] [Google Scholar]
- 2.Wang JL, Gray RM, Haudek KC, Patterson RJ. Nucleocytoplasmic lectins. Biochim Biophys Acta. 2004;1673:75–93. doi: 10.1016/j.bbagen.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 3.Liu FT, Rabinovich GA. Galectins as modulators of tumour progression. Nat Rev Cancer. 2005;5:29–41. doi: 10.1038/nrc1527. [DOI] [PubMed] [Google Scholar]
- 4.Yang RY, Rabinovich GA, Liu FT. Galectins: Structure, function and therapeutic potential. Expert Rev Mol Med. 2008;10:e17. doi: 10.1017/S1462399408000719. [DOI] [PubMed] [Google Scholar]
- 5.Nakahara S, Oka N, Raz A. On the role of galectin-3 in cancer apoptosis. Apoptosis. 2005;10:267–275. doi: 10.1007/s10495-005-0801-y. [DOI] [PubMed] [Google Scholar]
- 6.Thijssen VL, Poirier F, Baum LG, Griffioen AW. Galectins in the tumor endothelium: Opportunities for combined cancer therapy. Blood. 2007;110:2819–2827. doi: 10.1182/blood-2007-03-077792. [DOI] [PubMed] [Google Scholar]
- 7.Rabinovich GA, Toscano MA. Turning ‘sweet’ on immunity: Galectin-glycan interactions in immune tolerance and inflammation. Nat Rev Immunol. 2009;9:338–352. doi: 10.1038/nri2536. [DOI] [PubMed] [Google Scholar]
- 8.Yang RY, Hsu DK, Yu L, Ni J, Liu FT. Cell cycle regulation by galectin-12, a new member of the galectin superfamily. J Biol Chem. 2001;276:20252–20260. doi: 10.1074/jbc.M010914200. [DOI] [PubMed] [Google Scholar]
- 9.Hotta K, et al. Galectin-12, an adipose-expressed galectin-like molecule possessing apoptosis-inducing activity. J Biol Chem. 2001;276:34089–34097. doi: 10.1074/jbc.M105097200. [DOI] [PubMed] [Google Scholar]
- 10.Kouadjo KE, Nishida Y, Cadrin-Girard JF, Yoshioka M, St-Amand J. Housekeeping and tissue-specific genes in mouse tissues. BMC Genomics. 2007;8:127. doi: 10.1186/1471-2164-8-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martin S, Parton RG. Lipid droplets: A unified view of a dynamic organelle. Nat Rev Mol Cell Biol. 2006;7:373–378. doi: 10.1038/nrm1912. [DOI] [PubMed] [Google Scholar]
- 12.Duncan RE, Ahmadian M, Jaworski K, Sarkadi-Nagy E, Sul HS. Regulation of lipolysis in adipocytes. Annu Rev Nutr. 2007;27:79–101. doi: 10.1146/annurev.nutr.27.061406.093734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9:367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fasshauer M, Klein J, Lossner U, Paschke R. Negative regulation of adipose-expressed galectin-12 by isoproterenol, tumor necrosis factor alpha, insulin and dexamethasone. Eur J Endocrinol. 2002;147:553–559. doi: 10.1530/eje.0.1470553. [DOI] [PubMed] [Google Scholar]
- 15.Yang RY, Hsu DK, Yu L, Chen HY, Liu FT. Galectin-12 is required for adipogenic signaling and adipocyte differentiation. J Biol Chem. 2004;279:29761–29766. doi: 10.1074/jbc.M401303200. [DOI] [PubMed] [Google Scholar]
- 16.Havel PJ. Update on adipocyte hormones: Regulation of energy balance and carbohydrate/lipid metabolism. Diabetes. 2004;53(Suppl 1):S143–S151. doi: 10.2337/diabetes.53.2007.s143. [DOI] [PubMed] [Google Scholar]
- 17.Piper RC, Hess LJ, James DE. Differential sorting of two glucose transporters expressed in insulin-sensitive cells. Am J Physiol. 1991;260:C570–C580. doi: 10.1152/ajpcell.1991.260.3.C570. [DOI] [PubMed] [Google Scholar]
- 18.Brasaemle DL, Subramanian V, Garcia A, Marcinkiewicz A, Rothenberg A. Perilipin A and the control of triacylglycerol metabolism. Mol Cell Biochem. 2009;326:15–21. doi: 10.1007/s11010-008-9998-8. [DOI] [PubMed] [Google Scholar]
- 19.Moore HP, Silver RB, Mottillo EP, Bernlohr DA, Granneman JG. Perilipin targets a novel pool of lipid droplets for lipolytic attack by hormone-sensitive lipase. J Biol Chem. 2005;280:43109–43120. doi: 10.1074/jbc.M506336200. [DOI] [PubMed] [Google Scholar]
- 20.Toh SY, et al. Up-regulation of mitochondrial activity and acquirement of brown adipose tissue-like property in the white adipose tissue of fsp27 deficient mice. PLoS One. 2008;3:e2890. doi: 10.1371/journal.pone.0002890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nishino N, et al. FSP27 contributes to efficient energy storage in murine white adipocytes by promoting the formation of unilocular lipid droplets. J Clin Invest. 2008;118:2808–2821. doi: 10.1172/JCI34090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh R, et al. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest. 2009;119:3329–3339. doi: 10.1172/JCI39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krintel C, et al. Ser649 and Ser650 are the major determinants of protein kinase A-mediated activation of human hormone-sensitive lipase against lipid substrates. PLoS One. 2008;3:e3756. doi: 10.1371/journal.pone.0003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Granneman JG, et al. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem. 2007;282:5726–5735. doi: 10.1074/jbc.M610580200. [DOI] [PubMed] [Google Scholar]
- 25.Haemmerle G, et al. Defective lipolysis and altered energy metabolism in mice lacking adipose triglyceride lipase. Science. 2006;312:734–737. doi: 10.1126/science.1123965. [DOI] [PubMed] [Google Scholar]
- 26.Zimmermann R, et al. Fat mobilization in adipose tissue is promoted by adipose triglyceride lipase. Science. 2004;306:1383–1386. doi: 10.1126/science.1100747. [DOI] [PubMed] [Google Scholar]
- 27.Haemmerle G, et al. Hormone-sensitive lipase deficiency in mice causes diglyceride accumulation in adipose tissue, muscle, and testis. J Biol Chem. 2002;277:4806–4815. doi: 10.1074/jbc.M110355200. [DOI] [PubMed] [Google Scholar]
- 28.Kitamura T, et al. Insulin-induced phosphorylation and activation of cyclic nucleotide phosphodiesterase 3B by the serine-threonine kinase Akt. Mol Cell Biol. 1999;19:6286–6296. doi: 10.1128/mcb.19.9.6286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi YH, et al. Alterations in regulation of energy homeostasis in cyclic nucleotide phosphodiesterase 3B-null mice. J Clin Invest. 2006;116:3240–3251. doi: 10.1172/JCI24867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lazar MA. How obesity causes diabetes: Not a tall tale. Science. 2005;307:373–375. doi: 10.1126/science.1104342. [DOI] [PubMed] [Google Scholar]
- 31.Banerjee RR, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- 32.Tansey JT, et al. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA. 2001;98:6494–6499. doi: 10.1073/pnas.101042998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wagner A. Distributed robustness versus redundancy as causes of mutational robustness. Bioessays. 2005;27:176–188. doi: 10.1002/bies.20170. [DOI] [PubMed] [Google Scholar]
- 34.Jarnaess E, Taskén K. Spatiotemporal control of cAMP signalling processes by anchored signalling complexes. Biochem Soc Trans. 2007;35:931–937. doi: 10.1042/BST0350931. [DOI] [PubMed] [Google Scholar]
- 35.Zaccolo M, Pozzan T. Discrete microdomains with high concentration of cAMP in stimulated rat neonatal cardiac myocytes. Science. 2002;295:1711–1715. doi: 10.1126/science.1069982. [DOI] [PubMed] [Google Scholar]
- 36.Lehnart SE, et al. Phosphodiesterase 4D deficiency in the ryanodine-receptor complex promotes heart failure and arrhythmias. Cell. 2005;123:25–35. doi: 10.1016/j.cell.2005.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brasaemle DL. Thematic review series: Adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 38.Puri V, Czech MP. Lipid droplets: FSP27 knockout enhances their sizzle. J Clin Invest. 2008;118:2693–2696. doi: 10.1172/JCI36554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–1783. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Viswanadha S, Londos C. Optimized conditions for measuring lipolysis in murine primary adipocytes. J Lipid Res. 2006;47:1859–1864. doi: 10.1194/jlr.D600005-JLR200. [DOI] [PubMed] [Google Scholar]
- 41.Fine JB, DiGirolamo M. A simple method to predict cellular density in adipocyte metabolic incubations. Int J Obes Relat Metab Disord. 1997;21:764–768. doi: 10.1038/sj.ijo.0800469. [DOI] [PubMed] [Google Scholar]
- 42.Brasaemle DL, Wolins NE. Isolation of lipid droplets from cells by density gradient centrifugation. Curr Protoc Cell Biol. 2006;3:1–12. doi: 10.1002/0471143030.cb0315s29. [DOI] [PubMed] [Google Scholar]
- 43.Rosen ED, et al. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes Dev. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ohsaki Y, Maeda T, Fujimoto T. Fixation and permeabilization protocol is critical for the immunolabeling of lipid droplet proteins. Histochem Cell Biol. 2005;124:445–452. doi: 10.1007/s00418-005-0061-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.