Abstract
Voltage-gated Na+ channels initiate action potentials during electrical signaling in excitable cells. Opening and closing of the pore of voltage-gated ion channels are mechanically linked to voltage-driven outward movement of the positively charged S4 transmembrane segment in their voltage sensors. Disulfide locking of cysteine residues substituted for the outermost T0 and R1 gating-charge positions and a conserved negative charge (E43) at the extracellular end of the S1 segment of the bacterial Na+ channel NaChBac detects molecular interactions that stabilize the resting state of the voltage sensor and define its conformation. Upon depolarization, the more inward gating charges R2 and R3 engage in these molecular interactions as the S4 segment moves outward to its intermediate and activated states. The R4 gating charge does not disulfide-lock with E43, suggesting an outer limit to its transmembrane movement. These molecular interactions reveal how the S4 gating charges are stabilized in the resting state and how their outward movement is catalyzed by interaction with negatively charged residues to effect pore opening and initiate electrical signaling.
Keywords: voltage-dependent gating, sliding helix
Electrical signaling in biology depends on the rapid flux of ions across phospholipid membranes conducted by voltage-gated ion channels (1). In eukaryotes, voltage-gated Na+ channels conduct fast inward currents that generate action potentials to initiate neurotransmission and muscle contraction (1). In prokaryotes, voltage-gated Na+ channels are involved in pH homeostasis, chemotaxis, and motility (2). Voltage-gated ion channels are tetrameric structures of four homologous transmembrane subunits or domains, which form an ion-permeable pore in their center (3–5). Each domain or subunit contains six α-helical transmembrane segments (S1–S6). The central pore is formed by the S5 and S6 segments and the membrane-reentrant P loop between them, whereas S1–S4 segments form the voltage-sensor module. Within the fourth transmembrane segment (S4) of voltage-gated Na+ channels, a conserved motif of a positively charged amino acid residue followed by two hydrophobic residues is linked in four to eight repeats extending across the membrane. Gating charges in all four S4 segments of sodium channels are important for activation (6). These gating charges move outward upon depolarization, as originally proposed for the “gating particles” of Na+ channels (7), and their outward movement initiates opening of the pore. Transmembrane movement of the gating charges has been detected as gating current (8–11), and approximately three to four positive charges per S4 segment move outward during activation of voltage-gated Na+ or K+ channels (12–15).
A major thermodynamic obstacle to activation of voltage sensors is stabilization of their gating charges in the transmembrane environment and catalysis of S4 movement across the membrane. Initially proposed in 1986 (16, 17) and later placed in structural context (18, 19), the sliding helix or helical screw models propose that the gating charges are neutralized in the transmembrane environment by formation of ion pairs with negatively charged amino acid residues in neighboring transmembrane segments and that S4 moves outward and rotates as its gating charges make sequential electrostatic interactions with negatively charged residues of the S1, S2, and S3 segments. This model provides a plausible mechanism for stabilization of the S4 segment in the membrane and for catalysis of its outward movement.
Previous studies using genetic complementation methods have shown that ion pair formation is required for biosynthesis and membrane insertion of the Shaker K+ channel (20, 21) and have identified interacting amino acid partners in the KAT channel in yeast (22). Sequential formation of ion pairs between gating charges and negatively charged amino acid residues in the S2 segment during activation of the homotetrameric bacterial Na+ channel NaChBac (23, 24) has been detected using the disulfide-locking method (25, 26). This method has also been used to detect a large outward movement of the S4 segment relative to the S3 segment during activation of the voltage sensor of the Shaker K+ channel (27). A critical feature of the sliding helix model of gating is formation of ion pairs with gating charges in the resting state, which stabilize the resting conformation of the voltage sensor, followed by exchange of those ion pair partners upon depolarization, as the S4 segment moves outward during activation (28). However, no ion pair interactions that stabilize the gating charges in the resting state have been documented previously, and exchange of ion pair partners during the transition from resting to activated states has not been demonstrated. Here we apply the disulfide-locking strategy to capture ion pair formation in resting and activated states of a voltage sensor in real time and chart the movement of the S4 segment in relation to the conserved negatively charged residue E43 at the extracellular end of the S1 segment of the cysteine-free Na+ channel NaChBac. Our results show how the gating charges are stabilized in the resting state and how they make specific and sequential molecular interactions with E43 in the resting, intermediate, and activated states of the voltage sensor.
Results
Disulfide Locking T0 and R1 in the Resting State.
Disulfide bond formation between substituted cysteine residues requires sulfur atoms to approach within 2 Å, providing a high-resolution method for analysis of intraprotein interactions (25, 29–31). NaChBac has T110 (T0) and four conserved arginine residues (R1–R4) positioned at three-residue intervals in its S4 segment (Fig. S1). We studied their interaction with a highly conserved negative charge (E43) near the extracellular end of the S1 helix. According to the Rosetta Membrane sliding helix model, the S4 gating charges and this negative charge form ion pairs sequentially during activation (18), but this prediction has not been tested.
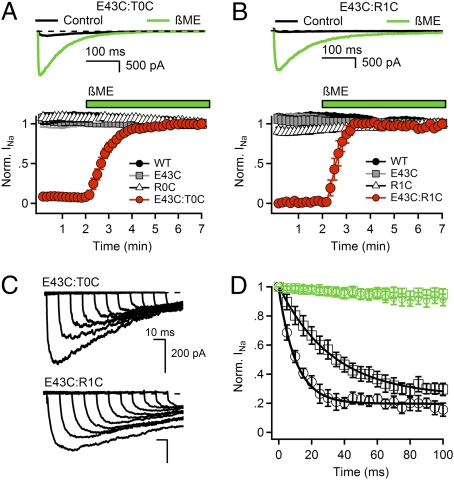
We expressed single- and double-cysteine mutants of T0C, R1C, R2C, R3C, R4C, and E43C and investigated functional effects of disulfide bond formation. For double-cysteine mutants that form disulfide bonds that lock them in the resting state, we expected that no Na+ current (INa) would be observed under control conditions, but INa would appear after reduction of disulfide bonds. Repetitive depolarization of WT or the single cysteine mutants from −140 to 0 mV for 500 ms resulted in inward INa of constant size (Fig. 1 A and B), but no current was observed for double mutant E43:T0C (Fig. 1A) or double mutant E43C:R1C (Fig. 1B). Treatment with the sulfhydryl-reducing reagents β-mercaptoethanol (βME) or DTT or the phosphine-reducing agent Tris(2-carboxyethyl)phosphine (TCEP) restored INa to steady-state amplitude within 3 min (Fig. 1 A and B and Fig. S2), and deactivation of INa was rapid upon repolarization (Fig. S3 A–D). Reducing agents did not affect INa of WT or single mutants (Fig. 1 A and B). We hypothesize that the lack of INa for the E43C:T0C and E43C:R1C double mutants is caused by disulfide locking of the voltage sensors in a deactivated conformation.
Fig. 1.
Disulfide locking of the voltage sensors in the resting state of E43C:T0C and E43C:R1C channels. (A and B) Upper: INa from the first pulse in control conditions (black) and last pulse in the presence of reducing agent (green) elicited by a 0.1-Hz train of 500-ms depolarizations to 0 mV from a holding potential of −140 mV. Lower: Effects of 10 mM βME on the mean normalized peak currents (±SEM) recorded during trains (n = 6). (C) Rate of relocking in the resting state. E43C:T0C and E43C:R1C channels were unlocked by a 5-s pulse to −180 mV in the presence of 2 mM βME. Cells were then returned to βME-free solution for 5 min, depolarized to −120 mV for 0, 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 ms, and INa was measured by a 500-ms test pulse to 0 mV. (D) Rates of relocking of the voltage sensors in the resting state at −120 mV. βME (2 mM) was removed (black) or retained (green) during this phase of the experiment. Mean normalized peak currents are plotted vs. time at −120 mV (±SEM, n = 4). E43C:T0C (black circles), τ = 10.3 ms; E43C:R1C (black squares), τ = 32.5 ms; E43C:T0C + βME (green circles); E43C:R1C + βME (green squares).
To examine state-dependent disulfide locking more directly, we hyperpolarized cells expressing E43C:T0C or E43C:R1C to −180 mV in the presence of βME to electrically “unlock” the voltage sensors (SI Results and Discussion, refs. 25 and 26), removed βME, waited for 5 min, and then returned to −120 mV, where all NaChBac channels are in the resting state. Substantial INa appeared at −180 mV, and return to −120 mV caused progressive relocking as reflected by the reduction in INa (Fig. 1C). Relocking at −120 mV was more rapid for E43C:T0C than for E43C:R1C (Fig. 1D), as expected if outward movement of S4 after depolarization from −180 mV to −120 mV leads first to interaction of T0 with E43 and later allows interaction of R1 with E43. No relocking was observed at −120 mV in the continued presence of βME (Fig. 1D).
Disulfide Locking R2 and R3 but Not R4 in the Activated State.
Disulfide locking D60 in S2 with R3 and R4 leads to channel opening and then permanent inactivation (25, 26). Therefore, in contrast to our results for T0 and R1, we expected that double cysteine mutants of R2 and R3 with E43 would conduct INa normally under control conditions but would be disulfide-locked after activation, open once, and then permanently inactivate (Fig. S3 E–G; Table S1) (25, 26). Consistent with these expectations, a single depolarization of E43C:R2C activated a large INa, but there was nearly total loss of INa in subsequent test pulses and no recovery after 5 min at −140 mV (Fig. 2A). In contrast, repetitive depolarization had no effect on WT or single cysteine mutants (Fig. 2A). Even after treatment with 2 mM hydrogen peroxide (H2O2) to enhance disulfide bond formation, INa remained stable if the voltage sensor was not activated but was lost immediately when E43C:R2C channels were activated (Fig. 2B). Consistent with disulfide bond formation, perfusion with βME, DTT, or TCEP reversed disulfide locking and restored INa (Fig. 2C and Fig. S2).
Fig. 2.
Disulfide locking of E43:R2C and E43C:R3C requires activation. Mean normalized peak currents during trains of 500-ms depolarizations to V = V1/2 + 40 mV from a holding potential of −140 mV. (A–C) E43C:R2C and single mutants as indicated. (A) Disulfide locking of E43C:R2C. After 1 min, pulsing was stopped for 5 min to test for recovery of INa. (B) Effect of H2O2 on disulfide locking of E43C:R2C. Disulfide locking in the presence of 2 mM H2O2 for 2 min at −140 mV without pulsing followed by 2 min with a train of 500-ms depolarizations. (C) Effect of reduction with βME on E43C:R2C. After 2 min of stimulation, cells were exposed to 10 mM βME, and stimulation was continued (n = 5). Current Insets are the first pulse in the presence of H2O2 (black), the last pulse in the presence of H2O2 (gray), and the last pulse in the presence of reducing agent (green). (D–F) E43C:R2C and single mutants as indicated. (D) Effect of 2 mM H2O2 on disulfide locking of E43C:R2C. (E) Requirement for depolarization for disulfide locking of E43C:R2C. Cells were stimulated with 500-ms depolarizations for 1 min, exposed to H2O2 for 1 min at −140 mV without pulsing, and pulsing was resumed. (F) Reversal of disulfide locking with 10 mM βME. Cells were stimulated with 500-ms depolarizations for 2 min, H2O2 was added for 3 min with continued stimulation, H2O2 was washed out for 3 min, and 10 mM βME was added for 5 min (n = 4). Mean ± SEM. Current Insets are the first pulse in the presence of H2O2 (black), the last pulse in the presence of H2O2 (blue), and the last pulse in the presence of reducing agent (green).
If a fraction of the voltage sensors are disulfide-locked at the resting membrane potential, that fraction would be unavailable for activation upon depolarization but would become available after disulfide bond reduction. As expected for partial disulfide locking, only 34% of maximal INa of E43C:R2C was observed with the initial depolarization (Fig. 2C). This INa was lost during repetitive stimulation, but treatment with βME restored INa to its maximum level (Fig. 2C). The overshoot above the starting level of INa reflects reduction of disulfide bonds formed at the resting potential (66% ± 4%). Thus, activation increases disulfide locking of E43C:R2C, but significant disulfide locking of R2 and E43 occurs at the resting membrane potential.
Continuing along the activation pathway, S4 would move further outward where R3 and then R4 might encounter E43. INa conducted by E43C:R3C channels was stable during repetitive stimulation under control conditions (Fig. 2D). However, upon application of the extracellular oxidizing agents H2O2 or copper phenanthroline, INa from E43C:R3C diminished rapidly with repetitive pulsing (Fig. 2D and Fig. S2D). INa of WT and the single mutants E43C and R3C was not affected (Fig. 2D). Disulfide locking of E43C:R3C required activation of the voltage sensor, because perfusion of H2O2 for 2 min at −140 mV had no effect until the channel was activated (Fig. 2E). The loss of INa caused by disulfide locking was reversed by treatment with βME or TCEP (Fig. 2F and Fig. S2D). These results demonstrate that activation of E43C:R3C immediately induces disulfide bond formation, which locks the channel in a nonconducting state. Reduction returns INa to the control level, indicating that no disulfide locking occurs at the resting membrane potential before activation (Fig. 2F and Fig. S2D).
Unlike E43C:R2C and E43C:R3C, INa from the E43C:R4C channels was stable during repetitive depolarizations in the presence of oxidizing or reducing agents (Fig. S4). Thus, the cysteine substituted for E43 will not form disulfide bonds with R4C, the last gating charge in the S4 segment.
Overall, these results indicate that interactions of T0 and R1 with E43 take place in the resting state, whereas interactions of R2 and R3 with E43 are enhanced by activation. We propose that R1 forms an ion pair with E43 in a resting state of NaChBac. R2 interacts with E43 to form an ion pair, and formation of this ion pair is increased by activation, suggesting that this interaction takes place in an intermediate activated state. R3 interacts with E43 only after activation of the voltage sensor, suggesting that this interaction only occurs in the activated state. R4 is unable to interact with E43 during activation, even though it forms ion pair interactions with E70 and D60 in the S2 segment during activation (26). These results suggest that R4 moves outward but not far enough for a substituted cysteine to reach E43.
Kinetics of Disulfide Locking of E43C with R2C and R3C.
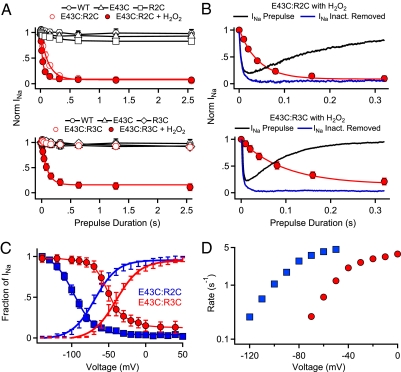
As in our previous experiments (25, 26), disulfide locking of R2C and R3C to E43C prevents rapid deactivation of the voltage sensor and leads to permanent inactivation (Fig. S3 E–G). Inactivation and disulfide locking can be reversed by strong hyperpolarization, which catalyzes reduction of the disulfide bond and returns the channel to its resting state (SI Results and Discussion and Fig. S5) (25, 26). Such voltage-dependent unlocking allows analysis of the kinetics of disulfide locking (Fig. 3). After a 5-s pulse to −170 mV to unlock a fraction of the channels, we measured the rate of disulfide locking of E43C:R2C at V ≈ V1/2 + 40 mV, using irreversible loss of INa after repolarization to −140 mV as a criterion (Fig. 3A, Upper). For E43C:R2C channels, depolarization caused disulfide locking and loss of INa, but depolarization had no effect on WT or single cysteine mutants (Fig. 3A, Upper). The rapid loss of INa for E43C:R2C caused by disulfide locking occurred on the millisecond time scale like activation of INa as measured by the time constant for pore opening (H2O2: τO = 13 ± 2 ms; τS-S = 55 ± 2 ms; Fig. 3B, Upper). As described above, no disulfide locking occurs without an oxidizing agent for the E43C:R3C channel, and no change in INa was observed under control conditions (Fig. 3A, Lower). However, depolarization of E43C:R3C channels to V ≈ V1/2 + 40 mV in the presence of 2 mM H2O2 caused disulfide locking and 93% loss of INa, with a 1.4-fold slower rate (H2O2: τO = 7 ms ± 2; τS-S = 77 ms ± 2; Fig. 3B, Lower). Thus, at potentials where voltage sensors are fully activated, the E43C:R2C interaction occurred 1.4-fold more rapidly than the E43C:R3C interaction when both were measured in the presence of H2O2. Because the intrinsic rate of activation of INa for the mutant E43C:R3C is 1.9-fold more rapid than the mutant E43:R2C (τO = 7 ms vs. 13 ms), the actual ratio of interaction rates for R2 with E43 vs. R3 with E43 may be as high as 2.6-fold.
Fig. 3.
Time course and voltage dependence of disulfide locking of E43C:R2C and E43C:R3C channels. (A) E43C:R2C (Upper) and E43C:R3C channels (Lower) were unlocked by a 5-s prepulse to −170 mV. Cells were then depolarized by prepulses to V1/2 + 40 mV of the indicated durations. After 5 s at −140 mV, disulfide-locked channels were assayed with a 100-ms test pulse to V1/2 + 40 mV (WT, 0 mV; E43C, 60 mV; R2C, −20 mV; E43C:R2C, −30 mV; E43C:R3C, 0 mV). Peak test pulse currents were normalized to the test pulse current in the absence of a prepulse. Mean values (±SEM) were plotted vs. prepulse duration (n = 6). (B) Comparison of the rate of disulfide locking and channel activation (blue line, after subtraction of the exponential effect of inactivation) for E43C:R2C and E43C:R3C in 2 mM H2O2. (C) The voltage dependence of channel activation (lines with error bars) and of disulfide locking (symbols) measured in the presence of 2 mM H2O2. To measure the voltage dependence of disulfide locking of E43C:R2C (n = 9), channels were unlocked by a 5-s pulse to −170 mV, then depolarized with 500-ms prepulses to the indicated potentials. After 5 s at −140 mV, the number of channels locked during the prepulse was assessed with a 100-ms test pulse to 0 mV. Because H2O2-induced disulfide locking of E43C:R3C channels cannot be reversed by hyperpolarization, disulfide locking could only be tested at one potential for each cell (n = 5–6 for each potential). (D) Rate of disulfide locking as a function of membrane potential estimated from the data in C at potentials where the fraction of channels locked was between 0.1 and 0.9 as ln[(Fraction of INa)]/(−0.5 s).
Voltage Dependence of Disulfide Locking of E43C with R2C and R3C.
We induced disulfide locking of E43C:R2C and E43C:R3C in the presence of H2O2 during 500-ms prepulses to a range of voltages and recorded the extent of disulfide locking after repolarization to −140 mV for 5 s (Fig. 3C). The voltage dependence of disulfide locking (V1/2(S-S) = −97 ± 2 mV) for the E43C:R2C channel was 27 mV more negative than the voltage dependence of pore opening (V1/2(O) = −71 ± 3 mV). By comparison, the voltage for half-maximal disulfide locking of E43C:R3C was −50 mV, only 11 mV more negative than the voltage dependence of pore opening (V1/2(O) = −39 ± 2 mV). These data suggest that the interaction of E43 with R2 is more likely to occur at an earlier step in the S4 activation pathway than interaction of E43 with R3. This voltage-dependent process was also analyzed by plotting the rate constant for disulfide locking vs. voltage over the range of 10–90% reaction completion (Fig. 3D). This analysis shows that the rate of disulfide locking of E43C:R2C is more rapid at each voltage than E43C:R3C, as expected if the R3 gating charge must move farther outward to engage E43.
Mutant Cycle Analysis of Gating Charge Interactions.
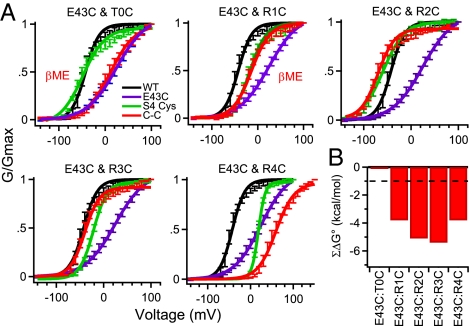
Our disulfide locking results suggest that T0 and R1 interact physically with E43 sequentially in the resting states, whereas R2 and R3 interact sequentially as channels activate. If these residues do indeed interact physically with each other, they may have a significant energy of interaction during the activation process. Mutant cycle analysis measures the energy of interaction of pairs of substituted amino acid residues in sets of single and double mutants (SI Methods) (32). Because the E43C:T0C and E43C:R1C channels are disulfide-locked in a resting state, the voltage dependence of activation could only be assessed after βME treatment. Double-cysteine mutant cycle analysis did not reveal a significant [>±1 kcal/mol (32)] energy of interaction for E43C:T0C in the presence of βME (Fig. 4; E43C:T0C, −0.1 kcal/mol). Thus, the side chains of these Glu and Thr residues do not have a significant energetic interaction in the aqueous environment of the outer vestibule of the gating pore, even though they are physically close to each other. On the other hand, E43C:R1C did have a significant energy of interaction in the presence of βME (E43C:R1C, −3.8 kcal/mol). Mutant cycle analysis of E43C:R2C and E43C:R3C channels in the absence of βME also revealed a substantial energy of interaction, −5.1 kcal/mol and −5.4 kcal/mol, respectively (Fig. 4 and Table S2 ). Evidently, interaction of these residues favors activation of these mutant channels. The E43C:R4C channel also showed significant energy coupling (−3.8 kcal/mol), even though Cys residues substituted for E43 and R4 do not move close enough to form a disulfide bond during channel activation (Fig. 4 and Table S2). These results suggest that the long, flexible side chains of Glu and Arg can stretch to make a significant ionic interaction, even though the shorter, less flexible side chains of the Cys residues substituted for them cannot form a disulfide bond. These mutant cycle analysis results give additional perspective on the chemistry of interaction of the gating charges in resting and activated states. Interaction of T0 with E43 does not have a major effect on the energy of activation, interaction of R1, R2, and R3 with E43 generates a favorable energy of activation, and interaction of R4 with E43 also favors activation, even though substituted Cys residues do not move close enough to E43 to form a disulfide bond.
Fig. 4.
Mutant cycle analysis of cysteine pairs. (A) Conductance–voltage relationships for WT, double-mutant channels, and the E43C and gating charge single-mutant channels. Conductance–voltage relationships for double mutants E43C:T0C and E43C:R1C were measured in the presence of 10 mM βME. Conductance–voltage relationships were calculated from peak INa elicited by 100-ms depolarizations to the indicated potentials (n ≥ 9; ±SEM) as described in SI Methods. (B) Coupling energy (ΣΔG°) for each residue pair as described in SI Methods. Mean ± SEM.
Specificity of Disulfide Locking and Energy Coupling.
The structures of the KV1.2–2.1 chimera (33) and NavAb (34) show the side chain of the residue corresponding to I42, which immediately precedes E43 in the amino acid sequence (Fig. S1A), to be facing away from the gating pore (Fig. 5 and Fig. S1 B and C). Accordingly, no evidence for state-dependent disulfide locking or energy coupling was found for I42C with any gating charge (Figs. S6 and S7). These results provide strong support for the specificity of interaction of E43 with T0, R1, R2, and R3.
Fig. 5.
Transmembrane view of disulfide-locked Rosetta Membrane structural models of the voltage-sensing domain of NaChBac. Segments S1 through S4 colored individually and labeled. Side chains of cysteine mutants of E43, T0, R1, R2, and R3 are shown in space-filling representation and colored yellow. Cβ atoms of gating-charge-carrying arginines (blue; labeled R1 through R4) in S4 and D60 and E70 (red) in S2 shown as spheres. Models were generated with the Rosetta Membrane modeling system (SI Methods). This figure was generated using Chimera (43).
Discussion
Gating Charge Interactions in the Resting State.
Our results provide initial experimental insights into the molecular interactions of gating charges in the resting states of a voltage sensor. In the KV1.2–2.1 chimera structure (33), the voltage sensor is hypothesized to be in an activated state, and the carboxyl side chain of E43 of S1 would face into the gating pore (Fig. S1). However, the side chains of residues equivalent to T0 and R1 are positioned above the gating pore in that structure (Fig. S1). For T0 and R1 to interact with E43 in the resting state, the S4 segment would need to slide down into the gating pore during deactivation. Our disulfide-locking results indicate that E43 does indeed interact with T0 and R1 in a resting state, because chemical reduction is required to release the voltage sensors and allow activation. Moreover, relocking of these substituted cysteine residues takes place in the resting state, and cysteine substituted for T0 relocks more rapidly than cysteine substituted for R1. These results require that the S4 segment move inward as a sliding helix through the gating pore during deactivation at hyperpolarized membrane potentials and return to its disulfide-locked position in the resting state at −120 mV. The molecular interactions of T0 and R1 with E43 in the resting state shown here are consistent with previous structure–function studies (27, 35, 36). Our mutant cycle analysis indicates that single substitutions for T0, R1, and E43 all inhibit the overall activation process, as indicated by positive ΔG° values for these mutations (Table S2). Substitution of a second cysteine complements the original mutation, resulting in significant coupling energy for R1 but not for T0. Therefore, both disulfide locking and mutant cycle analyses support interactions of R1 with E43 in the resting state, whereas close proximity of T0 with E43 in the resting state is indicated by their disulfide locking, but their interaction does not have a significant energetic effect.
Gating Charge Interactions in Activated State.
Our disulfide-locking studies show that gating charges R2 and R3 interact with E43 in the activated voltage sensor, as suggested previously for R2 from mutant cycle analysis (37). Measurements of rate and voltage-dependence of interaction indicate that R2 interacts with E43 rapidly, but the voltage sensor can transition to a further activated state in which R3 interacts with E43. This conclusion is also supported by the extent of disulfide bond formation at the resting membrane potential for these double mutants. More than half of E43C:R2C is disulfide-locked at the beginning of our experiments, whereas none of E43C:R3C is disulfide-locked. We have previously shown that R4 interacts sequentially with E70 and D60 in S2 (25, 26), but our present results suggest that R4 may not move past D60 during activation, and therefore substituted Cys residues are not able to disulfide lock with E43.
Sliding Helix Movement of the S4 Segment During Activation.
Because the S4 segment is a rigid helix, amino acid residues in each turn of S4 must interact with E43 in sequence. Our results are consistent with this outward movement of S4 past E43. First, T0 interacts with E43 faster than R1 in the resting state (Fig. 1 C and D). Second, T0 and R1 interact with E43 completely at −120 mV, whereas depolarization from −120 mV to more depolarized voltages stimulates disulfide locking of R2 and R3 (Fig. 3C). Third, R2 interacts with E43 significantly at the resting membrane potential (Fig. 2C), whereas R3 does not (Fig. 2D). Finally, R2 interacts with E43 more rapidly and at more negative membrane potentials than R3 (Fig. 3 A–D). These independent lines of evidence support a model in which T0, R1, R2, and R3 form sequential interactions with E43 as the S4 segment moves outward during activation. Assuming the S4 segment is a mobile but rigid helical structure, the E43 interactions with T0, R1, R2, and R3 require the S4 segment to move ≈10 Å through the gating pore relative to S1. However, the minimum distance of transmembrane movement by S4 required for ion pair interactions of the native Arg and Glu side chains could be 8 Å, given their length when fully extended. The lack of disulfide locking of E43C:R4C suggests that the sulfur atoms of cysteines at these positions do not approach within 2 Å of each other and therefore define the upper limit of S4 movement. The outward movement of the S4 segment and the sequential exchange of ion pair partners observed in this work fit closely with the predictions of the sliding helix model of voltage sensor function but are not compatible with the paddle model, as discussed in detail in a recent review (28).
Structural Basis for Voltage Sensor Function.
We developed structural models of resting and activated states of the voltage-sensing domain of NaChBac using the Rosetta Membrane algorithm (SI Methods). Our model of our most negative resting state (resting state 1) based on disulfide locking between E43C and T0C predicts that D60 (in S2) interacts with gating charges R1 and R2 and that E70 (in S2) interacts with R3 (Fig. 5, Resting 1). R1 interaction with D60 is in agreement with data for the Shaker K+ channel, suggesting close proximity of R1 to the homolog of D60 in the resting state (38). The model of the second most negative resting state (resting state 2) based on disulfide locking between E43C and R1C predicts that D60 (in S2) interacts with R2 and that E70 (in S2) interacts with R4 (Fig. 5, Resting 2). Activated state 1 was defined in our previous work on the basis of interaction of R4 with E70 (26). The activated state 2 model (Fig. 5) is based on disulfide locking between E43C and R2C and predicts that D60 (in S2) interacts with R3 in agreement with our previous data (25). The activated state 3 model is based on disulfide locking between E43C and R3C and predicts that D60 (in S2) interacts with R4 (Fig. 5, Activated 3), in agreement with our previous data (26). R1–R4 are all exposed to the extracellular aqueous environment in activated state 3 (Fig. 5), which would potentially allow movement of all four gating charges across the membrane electric field, in agreement with previous experimental estimates of movement of four gating charges per voltage sensor in NaChBac (15). Comparison of resting state 1 with activated state 3 suggests that transmembrane movement of S4 is ≈8–10 Å, in reasonable agreement with 6–8 Å of S4 vertical movement predicted in the voltage-sensing domain of Shaker potassium channels (39). These structural models are in agreement with our results that the E43C:R2C and E43C:R3C only interact to form disulfide bonds when the voltage sensors are activated. Interestingly, the S4 segment has moved one helical turn farther in the extracellular direction from activated state 2 to activated state 3, suggesting that the interactions of E43–R2 and E43–R3 would occur sequentially. This prediction is confirmed by our disulfide-locking results showing that the interaction of R2 with E43 occurs earlier in the activation pathway, in both the time and voltage domains, than the interaction of R3 with E43. Together, these structural models reveal probable conformational transitions of the voltage sensor in sequential activated states in the voltage-dependent activation pathway (Fig. 5). These results fit well with expectations of a sliding-helix model of voltage sensing, in which ion pairs are formed in the resting state to stabilize the gating charges in the transmembrane environment, and the S4 segment moves outward and exchanges ion pair partners upon depolarization, which catalyzes movement of the gating charges and initiates the conformational change that mediates pore opening.
Comparison with the Structure of the NavAb Channel.
We have recently determined the structure of the NavAb channel, a homolog of NaChBac, in a preopen state with all four voltage sensors activated but the pore closed, at 2.7 Å resolution (34). The structure of the voltage sensor of NavAb agrees closely with the structure of activated state 1 and provides additional support for the interactions defined for that state by disulfide locking and mutant cycle analysis.
Methods
Mutants of NaChBac were constructed and analyzed by whole-cell voltage clamp. The structures of NaChBac in resting and activated states were determined using the Rosetta ab initio modeling algorithm (18, 40–42). These techniques are described in SI Methods.
Supplementary Material
Acknowledgments
We thank Dr. Fredrik Elinder (Linkoping University) for insightful comments on a draft of the manuscript. This work was supported by National Institutes of Health Research Grants R01 NS15751 (to W.A.C.) and T32 GM07270 (to P.G.D.).
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1116449108/-/DCSupplemental.
References
- 1.Hille B. Ion Channels of Excitable Membranes. 3rd Ed. Sunderland, MA: Sinauer; 2001. [Google Scholar]
- 2.Ito M, et al. The voltage-gated Na+ channel NaVBP has a role in motility, chemotaxis, and pH homeostasis of an alkaliphilic Bacillus. Proc Natl Acad Sci USA. 2004;101:10566–10571. doi: 10.1073/pnas.0402692101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu FH, Catterall WA. The VGL-chanome: A protein superfamily specialized for electrical signaling and ionic homeostasis. Sci STKE. 2004;2004:re15. doi: 10.1126/stke.2532004re15. [DOI] [PubMed] [Google Scholar]
- 4.Yi BA, Jan LY. Taking apart the gating of voltage-gated K+ channels. Neuron. 2000;27:423–425. doi: 10.1016/s0896-6273(00)00052-0. [DOI] [PubMed] [Google Scholar]
- 5.Yellen G. The voltage-gated potassium channels and their relatives. Nature. 2002;419:35–42. doi: 10.1038/nature00978. [DOI] [PubMed] [Google Scholar]
- 6.Kontis KJ, Rounaghi A, Goldin AL. Sodium channel activation gating is affected by substitutions of voltage sensor positive charges in all four domains. J Gen Physiol. 1997;110:391–401. doi: 10.1085/jgp.110.4.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952;117:500–544. doi: 10.1113/jphysiol.1952.sp004764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armstrong CM, Bezanilla F. Charge movement associated with the opening and closing of the activation gates of the Na channels. J Gen Physiol. 1974;63:533–552. doi: 10.1085/jgp.63.5.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bezanilla F. The voltage sensor in voltage-dependent ion channels. Physiol Rev. 2000;80:555–592. doi: 10.1152/physrev.2000.80.2.555. [DOI] [PubMed] [Google Scholar]
- 10.Bezanilla F, Armstrong CM. Gating currents of the sodium channels: Three ways to block them. Science. 1974;183:753–754. doi: 10.1126/science.183.4126.753. [DOI] [PubMed] [Google Scholar]
- 11.Keynes RD, Rojas E. Kinetics and steady-state properties of the charged system controlling sodium conductance in the squid giant axon. J Physiol. 1974;239:393–434. doi: 10.1113/jphysiol.1974.sp010575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoppa NE, McCormack K, Tanouye MA, Sigworth FJ. The size of gating charge in wild-type and mutant Shaker potassium channels. Science. 1992;255:1712–1715. doi: 10.1126/science.1553560. [DOI] [PubMed] [Google Scholar]
- 13.Sigworth FJ. Charge movement in the sodium channel. J Gen Physiol. 1995;106:1047–1051. doi: 10.1085/jgp.106.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aggarwal SK, MacKinnon R. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 1996;16:1169–1177. doi: 10.1016/s0896-6273(00)80143-9. [DOI] [PubMed] [Google Scholar]
- 15.Kuzmenkin A, Bezanilla F, Correa AM. Gating of the bacterial sodium channel, NaChBac: Voltage-dependent charge movement and gating currents. J Gen Physiol. 2004;124:349–356. doi: 10.1085/jgp.200409139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Catterall WA. Molecular properties of voltage-sensitive sodium channels. Annu Rev Biochem. 1986;55:953–985. doi: 10.1146/annurev.bi.55.070186.004513. [DOI] [PubMed] [Google Scholar]
- 17.Guy HR, Seetharamulu P. Molecular model of the action potential sodium channel. Proc Natl Acad Sci USA. 1986;83:508–512. doi: 10.1073/pnas.83.2.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yarov-Yarovoy V, Baker D, Catterall WA. Voltage sensor conformations in the open and closed states in ROSETTA structural models of K+ channels. Proc Natl Acad Sci USA. 2006;103:7292–7297. doi: 10.1073/pnas.0602350103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shafrir Y, Durell SR, Guy HR. Models of voltage-dependent conformational changes in NaChBac channels. Biophys J. 2008;95:3663–3676. doi: 10.1529/biophysj.108.135335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Papazian DM, et al. Electrostatic interactions of S4 voltage sensor in Shaker K+ channel. Neuron. 1995;14:1293–1301. doi: 10.1016/0896-6273(95)90276-7. [DOI] [PubMed] [Google Scholar]
- 21.Tiwari-Woodruff SK, Lin MA, Schulteis CT, Papazian DM. Voltage-dependent structural interactions in the Shaker K+ channel. J Gen Physiol. 2000;115:123–138. doi: 10.1085/jgp.115.2.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grabe M, Lai HC, Jain M, Jan YN, Jan LY. Structure prediction for the down state of a potassium channel voltage sensor. Nature. 2007;445:550–553. doi: 10.1038/nature05494. [DOI] [PubMed] [Google Scholar]
- 23.Ren D, et al. A prokaryotic voltage-gated sodium channel. Science. 2001;294:2372–2375. doi: 10.1126/science.1065635. [DOI] [PubMed] [Google Scholar]
- 24.Koishi R, et al. A superfamily of voltage-gated sodium channels in bacteria. J Biol Chem. 2004;279:9532–9538. doi: 10.1074/jbc.M313100200. [DOI] [PubMed] [Google Scholar]
- 25.DeCaen PG, Yarov-Yarovoy V, Zhao Y, Scheuer T, Catterall WA. Disulfide locking a sodium channel voltage sensor reveals ion pair formation during activation. Proc Natl Acad Sci USA. 2008;105:15142–15147. doi: 10.1073/pnas.0806486105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeCaen PG, Yarov-Yarovoy V, Sharp EM, Scheuer T, Catterall WA. Sequential formation of ion pairs during activation of a sodium channel voltage sensor. Proc Natl Acad Sci USA. 2009;106:22498–22503. doi: 10.1073/pnas.0912307106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broomand A, Elinder F. Large-scale movement within the voltage-sensor paddle of a potassium channel-support for a helical-screw motion. Neuron. 2008;59:770–777. doi: 10.1016/j.neuron.2008.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Catterall WA. Ion channel voltage sensors: structure, function, and pathophysiology. Neuron. 2010;67:915–928. doi: 10.1016/j.neuron.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Siedler F, Rudolph-Böhner S, Doi M, Musiol HJ, Moroder L. Redox potentials of active-site bis(cysteinyl) fragments of thiol-protein oxidoreductases. Biochemistry. 1993;32:7488–7495. doi: 10.1021/bi00080a021. [DOI] [PubMed] [Google Scholar]
- 30.Clarke J, Fersht AR. Engineered disulfide bonds as probes of the folding pathway of barnase: Increasing the stability of proteins against the rate of denaturation. Biochemistry. 1993;32:4322–4329. doi: 10.1021/bi00067a022. [DOI] [PubMed] [Google Scholar]
- 31.Wahlberg E, Härd T. Conformational stabilization of an engineered binding protein. J Am Chem Soc. 2006;128:7651–7660. doi: 10.1021/ja060933g. [DOI] [PubMed] [Google Scholar]
- 32.Yifrach O, MacKinnon R. Energetics of pore opening in a voltage-gated K+ channel. Cell. 2002;111:231–239. doi: 10.1016/s0092-8674(02)01013-9. [DOI] [PubMed] [Google Scholar]
- 33.Long SB, Tao X, Campbell EB, MacKinnon R. Atomic structure of a voltage-dependent K+ channel in a lipid membrane-like environment. Nature. 2007;450:376–382. doi: 10.1038/nature06265. [DOI] [PubMed] [Google Scholar]
- 34.Payandeh J, Scheuer T, Zheng N, Catterall WA. The crystal structure of a voltage-gated sodium channel. Nature. 2011;475:353–358. doi: 10.1038/nature10238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Campos FV, Chanda B, Roux B, Bezanilla F. Two atomic constraints unambiguously position the S4 segment relative to S1 and S2 segments in the closed state of Shaker K channel. Proc Natl Acad Sci USA. 2007;104:7904–7909. doi: 10.1073/pnas.0702638104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Khalili-Araghi F, et al. Calculation of the gating charge for the Kv1.2 voltage-activated potassium channel. Biophys J. 2010;98:2189–2198. doi: 10.1016/j.bpj.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paldi T, Gurevitz M. Coupling between residues on S4 and S1 defines the voltage-sensor resting conformation in NaChBac. Biophys J. 2010;99:456–463. doi: 10.1016/j.bpj.2010.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tombola F, Pathak MM, Isacoff EY. Voltage-sensing arginines in a potassium channel permeate and occlude cation-selective pores. Neuron. 2005;45:379–388. doi: 10.1016/j.neuron.2004.12.047. [DOI] [PubMed] [Google Scholar]
- 39.Pathak MM, et al. Closing in on the resting state of the Shaker K+ channel. Neuron. 2007;56:124–140. doi: 10.1016/j.neuron.2007.09.023. [DOI] [PubMed] [Google Scholar]
- 40.Barth P, Schonbrun J, Baker D. Toward high-resolution prediction and design of transmembrane helical protein structures. Proc Natl Acad Sci USA. 2007;104:15682–15687. doi: 10.1073/pnas.0702515104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cooper S, et al. Predicting protein structures with a multiplayer online game. Nature. 2010;466:756–760. doi: 10.1038/nature09304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yarov-Yarovoy V, Schonbrun J, Baker D. Multipass membrane protein structure prediction using Rosetta. Proteins. 2006;62:1010–1025. doi: 10.1002/prot.20817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pettersen EF, et al. UCSF Chimera—a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.