Abstract
Microbial fatty acid derivatives are emerging as promising alternatives to fossil fuel derived transportation fuels. Among bacterial fatty acid synthases (FAS), the Escherichia coli FAS is perhaps the most well studied, but little is known about its steady-state kinetic behavior. Here we describe the reconstitution of E. coli FAS using purified protein components and report detailed kinetic analysis of this reconstituted system. When all ketosynthases are present at 1 μM, the maximum rate of free fatty acid synthesis of the FAS exceeded 100 μM/ min. The steady-state turnover frequency was not significantly inhibited at high concentrations of any substrate or cofactor. FAS activity was saturated with respect to most individual protein components when their concentrations exceeded 1 μM. The exceptions were FabI and FabZ, which increased FAS activity up to concentrations of 10 μM; FabH and FabF, which decreased FAS activity at concentrations higher than 1 μM; and holo-ACP and TesA, which gave maximum FAS activity at 30 μM concentrations. Analysis of the S36T mutant of the ACP revealed that the unusual dependence of FAS activity on holo-ACP concentration was due, at least in part, to the acyl-phosphopantetheine moiety. MALDI-TOF mass spectrometry analysis of the reaction mixture further revealed medium and long chain fatty acyl-ACP intermediates as predominant ACP species. We speculate that one or more of such intermediates are key allosteric regulators of FAS turnover. Our findings provide a new basis for assessing the scope and limitations of using E. coli as a biocatalyst for the production of diesel-like fuels.
Due to their high energy density and low water solubility, fatty acids are arguably the most appropriate biofuel precursors. Therefore, fatty acid synthases (FASs) have emerged as attractive engineering targets in society’s recent quest for transportation fuels from renewable sources. Among different FASs, the Escherichia coli synthase is perhaps most well understood (1). However, notwithstanding extensive analysis of fatty acid biosynthesis and its regulation in E. coli (2–5), little is known about its steady-state kinetic properties. We therefore sought to undertake systematic kinetic analysis of the fully reconstituted E. coli FAS. It was anticipated that such analysis would provide a fundamentally new basis for assessing the scope and limitations of producing fatty acids using E. coli as a biocatalyst.
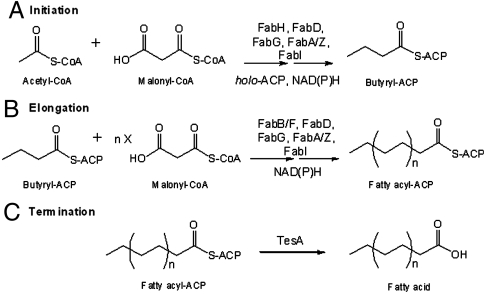
Fatty acid biosynthesis in E. coli is catalyzed by an enzyme system consisting of nine distinct proteins—FabA, FabB, FabD, FabF, FabG, FabH, FabI, FabZ, and ACP (Fig. 1). Together, they convert one equivalent of acetyl-CoA and 6–8 equivalents of malonyl-CoA into C14-C18 acyl-ACP species. One or two reducing equivalents of NAD(P)H are utilized in each round of chain elongation. Whereas most of the resulting fatty acyl chains are directly harnessed for phospholipid biosynthesis, the cytoplasmic mutant of the periplasmic thioesterase, TesA, is capable of releasing free fatty acids via hydrolysis of acyl-ACP species. Our enzymological analysis of the E. coli FAS therefore entails reconstitution of this 10-component system from individually purified proteins.
Fig. 1.
Catalytic cycle of the E. coli fatty acid synthase. (A) Initiation: In the presence of holo-ACP, NADPH and NADH, acetyl-CoA and malonyl-CoA undergo condensation and subsequent reduction to form butyryl-ACP. These reactions are catalyzed by the malonyl-CoA:ACP transacylase FabD, the ketosynthase FabH, the NADPH-dependent ketoreductase FabG, either the dual-function dehydratase/isomerase FabA or the monofunctional dehydratase FabZ, and the NADH-dependent enoyl reductase FabI. (B) Elongation: Butyryl-ACP is extended via 5–7 rounds of analogous reactions to produce a C14 to C18-ACP that is either fully saturated or monounsaturated. These extension cycles are catalyzed by either the ketosynthase FabB or FabF in collaboration with FabD, FabG, FabA or FabZ, and FabI. (C) Termination: The full-length fatty acid is released from the corresponding fatty acyl-ACP via hydrolysis by the thioesterase (TesA).
Results
Expression and Purification of the Components of the E. coli Fatty Acid Synthase.
All components of the E. coli FAS were individually overexpressed and purified from recombinant strains of E. coli. FabA, FabB, FabD, FabF, FabG, FabH, FabI, FabZ, and TesA were produced in E. coli BL21(DE3). Because overexpression of the ACP led to substantial accumulation of apo-ACP in this host, E. coli BAP1, a BL21(DE3) derivative with the sfp phosphopantetheinyl transferase gene from Bacillus subtilis integrated into its chromosome (6) was used to ensure complete conversion of the apo-protein into its holo form. To ensure high purity (Fig. S1), each protein was purified on two columns; in addition, residual apo-ACP was removed from purified holo-ACP preparations by an extra HPLC purification step.
Messenger RNA and Protein Concentrations of Fatty Acid Synthase Subunits in E. coli.
To estimate the relative protein ratios of the reconstituted FAS, we first sought to measure the mRNA level of each subunit and protein levels of selected representative subunits in E. coli BL21(DE3) and its fatty acid overproducing derivative, TL101/pMSD8/pTL58. [TL101 is a fadE knockout strain, pMSD8 leads to overexpression of the E. coli acetyl-CoA carboxylase, and pTL58 is responsible for overexpression of the E. coli thioesterase as well as a medium chain thioesterase from the camphor plant (7).] To do so, we used quantitative PCR (qPCR) for mRNA measurements and Western blotting for protein concentration analysis. Results from qPCR experiments suggested that the genes encoding FAS components of E. coli BL21(DE3) could be divided into three groups: (i) acpP and tesA; (ii) fabB and fabF; and (iii) all the other fab genes (Fig. 2A). In each group, the mRNA levels are similar. The mRNA levels of acpP and tesA are approximately seven times higher than those of fabB and fabF, which in turn are slightly higher than all other fab genes. In contrast, in E. coli TL101/pMSD8/pTL58, the concentration of tesA mRNA is increased by more than 70-fold due to the presence of the plasmid pTL58; all other FAS genes are slightly upregulated.
Fig. 2.
(A) Relative mRNA levels of fab genes, acpP and tesA in E. coli BL21(DE3) (a representative native system) and TL101/pMSD8/pTL58 (a fatty-acid-overproducing derivative), determined by qPCR. (B) Concentrations of selected proteins in the two strains, based on Western blot analysis. Cell samples were collected at OD600 = 1.2 in the case of BL21(DE3) or 3 hr after induction in the case of TL101/pMSD8/pTL58. Results shown are the mean of three replicates with error bars representing standard deviations.
The proteins ACP, FabB, FabF, and FabI were selected as representative FAS subunits to investigate the relative protein levels in vivo by Western blot analysis (Fig. 2B). The intracellular concentration of FabI is the lowest, whereas FabB and FabF levels are slightly higher. The ACP level is the highest, being 4–5-fold higher than FabB and FabF and 20–30-fold higher than FabI. These results are consistent with qPCR analysis of E. coli BL21(DE3). Interestingly, when the acetyl-CoA carboxylase (ACC) and thioesterase (TE) genes were overexpressed, the expression levels of FabB, FabF, and FabI were not significantly changed, but the ACP level was increased by more than 60-fold.
Taken together, the results of qPCR and Western blot analysis suggest that in a native E. coli host, FabA, FabB, FabD, FabF, FabG, FabH, FabI, and FabZ are expressed at comparable levels, whereas ACP and TesA are expressed at considerably higher levels. Therefore, we initiated kinetic analysis of the reconstituted system by defining a reference condition as one that contained 1 μM each of FabA, FabB, FabD, FabF, FabG, FabH, FabI, and FabZ, and 10 μM each of holo-ACP and TesA.
Substrate and Cofactor Dependence of the Fatty Acid Synthase Activity.
Under steady-state conditions, the E. coli FAS synthesizes a variety of protein-bound C14-C18 acyl-ACP species from malonyl-CoA and acetyl-CoA; the corresponding free fatty acids can then be released by the thioesterase TesA (8, 9). Two cofactors are utilized in the process. FabG uses NADPH (10, 11), whereas FabI preferentially utilizes NADH (12, 13). To explore the dependence of the reconstituted FAS on the two acyl-CoA substrates and the two cofactors, we titrated these reactants individually into the system. No change was observed in the initial rate of free fatty acid synthesis when the concentration of malonyl-CoA was increased from 0.3 to 1.5 mM (Fig. 3A). We therefore selected an initial malonyl-CoA concentration of 500 μM for further investigations, to ensure a high initial reaction rate as well as an adequate substrate supply for at least the first 10 turnovers, which for a C16 fatty acid would be expected to consume 70 μM malonyl-CoA and 10 μM acetyl-CoA.
Fig. 3.
Titration of substrates and cofactors: (A) malonyl-CoA, (B) acetyl-CoA, (C) NADPH, and (D) NADH. The reaction conditions were: 1 μM each Fab, 10 μM holo-ACP, 10 μM TesA, and (A) 1 mM acetyl-CoA, 1 mM NADH, 1 mM NADPH and varying concentrations of malonyl-CoA; (B) 500 μM malonyl-CoA, 1 mM NADH, 1 mM NADPH and varying concentrations of acetyl-CoA; (C) 500 μM malonyl-CoA, 200 μM acetyl-CoA, 1 mM NADH, and varying concentrations of NADPH; and (D) 500 μM malonyl-CoA, 200 μM acetyl-CoA, 1 mM NADPH and varying concentrations of NADH.
Another notable conclusion from the data shown in Fig. 3A is that product formation ceases after ca. 10 min, or ca. 40 turnovers of the catalytically active FAS subunits present in the reaction mixture. Given that the initial concentration of malonyl-CoA was varied, the observed cessation of the in vitro reaction cannot be explained by malonyl-CoA depletion. To test whether nonspecific deactivation of one or more active sites could explain this phenomenon, we preincubated the assay mixture for 10 min without any substrates or cofactors. No change in the initial reaction rate was observed, suggesting that nonspecific enzyme inactivation was not a significant factor. Together, these findings suggest that the buildup of one or more biosynthetic intermediates or end products inhibits the catalytic activity of the FAS in our reconstituted cell-free system.
To estimate the optimal concentration of acetyl-CoA in the reconstituted system, malonyl-CoA was held at 500 μM while the acetyl-CoA concentration was varied. The initial reaction rate peaked at 100 μM acetyl-CoA (Fig. 3B). We therefore used 200 μM acetyl-CoA in all further experiments. Similarly, the dependence of reaction rate on NADPH and NADH was assessed by fixing malonyl-CoA and acetyl-CoA concentrations at 500 μM and 200 μM, respectively, and varying the initial concentration of each cofactor individually. As predicted, fatty acid biosynthesis in the reconstituted system required the presence of NADPH. Maximum velocity was attained at NADPH concentrations above 0.5 mM (Fig. 3C); therefore the concentration of this cofactor was fixed at 1 mM in subsequent experiments. In contrast, although NADH is known to be the preferred cofactor of FabI, an appreciable rate of fatty acid production was achieved even in the absence of this cofactor (Fig. 3D). Again, 1 mM NADH was judged to be a suitable concentration in subsequent experiments in light of the beneficial effect of this cofactor on the reaction rate.
The ability of the cofactors NAD+ and NADP+ to inhibit fatty acid biosynthesis was also tested by titrating each oxidized cofactor into the reconstituted FAS assay. Neither metabolite had a significant effect on the reaction rate up to 5 mM concentration (Fig. S2).
Effects of Individual Protein Concentrations on the Steady-State Activity of the Fatty Acid Synthase.
To analyze the sensitivity of the turnover frequency of the E. coli fatty acid synthase toward the concentration of each subunit, FabA, FabB, FabD, FabF, FabG, FabH, FabI, FabZ, ACP, and TesA were individually titrated into the reference system described above. Although the FAS is known to yield a relatively heterogeneous mixture of saturated and unsaturated C14 to C18 fatty acids (7), for simplicity, the rate of fatty acid formation in vitro was quantified in units of palmitic acid equivalents synthesized per unit time. Titration results reveal that the FAS subunits fall into three categories. FabA, FabB, FabD, and FabG did not appreciably influence the rate of fatty acid synthesis, when their individual concentrations were varied relative to the reference concentration of 1 μM (Fig. 4 A and B and Fig. S3). In contrast, FabF and FabH inhibited fatty acid production at concentrations significantly higher 1 μM (Fig. 4 C and D). Perhaps most interestingly, FabI and FabZ enhanced the rate of fatty acid synthesis in a dose-dependent manner (Fig. 4 E and F). Whereas the effect of increasing FabI concentration above the reference concentration of 1 μM was relatively modest (twofold), FabZ had a more marked effect (sixfold) on the steady-state kinetics of the reconstituted system. The highest activity achievable was 100 μM palmitic acid equivalents/min, when the concentration of FabZ was more than 10-fold higher than those of other Fab enzymes.
Fig. 4.
Titration of individual FAS subunits: (A) FabA, (B) FabB, (C) FabF, (D) FabH, (E) FabI, (F) FabZ, (G) holo-ACP and its mutant S36T, and (H) TesA and its mutant S10A. Titration results of FabD and FabG are shown in Fig. S3. In the cases of A–F, each assay mixture included 10 μM holo-ACP, 10 μM TesA, and 1 μM of all other protein components except the one being titrated. In case G, for holo-ACP, the reaction condition was: 1 μM each Fab, 10 μM TesA, and varying concentrations of holo-ACP; for the mutant S36T, the condition was: 1 μM each Fab, 10 μM holo-ACP, 10 μM TesA, and varying concentrations of the mutant. For ACP S36T, the average and standard deviation of triplicate experiments are shown. In G, for TesA, the reaction condition was: 1 μM each Fab, 10 μM holo-ACP, and varying concentrations of TesA; for the mutant S10A, the condition was: 1 μM each Fab, 10 μM holo-ACP, 10 μM TesA, and varying concentrations of the mutant. In all the reactions, substrate and cofactor concentrations were as follows: 500 μM malonyl-CoA, 200 μM acetyl-CoA, 1 mM NADH, and 1 mM NADPH.
The two remaining components, holo-ACP and TesA, were also titrated into the reconstituted FAS. Supplementation of holo-ACP increased the steady-state rate of fatty acid synthesis up to a concentration of 32 μM, beyond which inhibition of FAS activity was observed (Fig. 4G). To determine whether inhibition is due to the apo-protein or the (free or acylated) phosphopantetheine arm, a similar titration experiment was performed using the S36T mutant of the ACP, which is incapable of undergoing phosphopantetheinylation (14). As observed in Fig. 4G, the S36T mutant is a weaker inhibitor of the FAS than holo-ACP, suggesting that the prosthetic group of the ACP contributes directly to its inhibitory characteristics at high concentrations. Alternatively, the lack of the prosthetic group could also change the conformation of the ACP, thereby influencing protein-protein interactions.
A similar trend was revealed when TesA was titrated into the reconstituted FAS system (Fig. 4H). The maximum reaction rate was achieved at a TesA concentration of 32 μM, and moderate inhibition was observed at higher TesA concentrations. We therefore investigated the effect of titrating the S10A active site mutant of TesA (7). This protein is catalytically inactive due to the mutation at the active site serine residue. Surprisingly, addition of the mutant enzyme to the reaction mixture resulted in up to 2.5-fold increase in FAS activity without any evidence of inhibition. Thus, the TesA protein appears to be able to enhance FAS activity in a manner that is independent of its catalytic activity.
Based on the results of these titration studies, we deduced that an optimal molar ratio of FabA∶FabB∶FabD∶FabF∶FabG∶FabH∶FabI∶FabZ∶holo-ACP∶TesA would be approximately 1∶1∶1∶1∶1∶1∶10∶10∶30∶30. To test this inference, two time courses were compared (Fig. 5). In each case, malonyl-CoA, acetyl-CoA, NADH and NADPH concentrations were increased by fivefold in order to extend the duration of fatty acid production. Under the optimized condition, 46 μM palmitic acid equivalents were synthesized per min at room temperature. Thus, the maximum turnover frequency of the E. coli FAS likely exceeds 1 s-1.
Fig. 5.
Comparison of fatty acid production at a FabA∶FabB∶FabD∶FabF∶FabG∶FabH∶FabI∶FabZ∶holo-ACP∶TesA molar ratio of 1∶1∶1∶1∶1∶1∶1∶1∶10∶10 (the reference condition, open circles) versus 1∶1∶1∶1∶1∶1∶10∶10∶30∶30 (the optimized condition, solid circles). Reactions contained 1 μM FabA and corresponding concentrations of other proteins. Substrate and cofactor concentrations were 2.5 mM malonyl-CoA, 1 mM acetyl-CoA, 5 mM NADH, and 5 mM NADPH.
To test whether the above results from a reconstituted FAS system could be extrapolated to a representative fatty acid overproducing strain, we supplemented cell-free extracts of E. coli XL100/pMSD8/pTL57, which overproduces fatty acids by ca. 30-fold relative to a native host (7), with increasing concentrations of FabA, FabB, FabD, FabF, FabG, FabH, FabI, and FabZ. When titrated individually, none of these proteins was able to substantially increase fatty acid productivity (Fig. S4). Importantly, FabH supplementation resulted in decreased fatty acid productivity. Thus it appears that fatty acid overproducing cell lines such as the aforementioned strain have molar protein ratios that are close to optimal and that further productivity improvements will likely require a coordinated increase in the levels of multiple proteins.
In Vivo Analysis of Fatty Acid Productivity.
Two sets of experiments were performed to demonstrate the relevance of the above biochemical analysis to fatty acid production in vivo. First, the intracellular concentration of FabF was increased in E. coli TL101/pTL58, a fadE knockout of BL21(DE3) that produces relatively high levels of fatty acids as a result of overexpression of both the native TesA thioesterase from E. coli as well as a medium chain-specific thioesterase from the camphor plant (7). Insertion of the fabF gene on pTL58 led to expression of this enzyme under control of the PBAD promoter, which, as predicted, markedly decreased fatty acid productivity (Fig. S5). Second, guided by the observation that FabI (enoyl reductase) and especially FabZ (dehydratase) increase FAS turnover in vitro (Fig. 4E and F), we expressed the full complement of reductive enzymes (FabG, FabZ, and FabI; Fig. 1) in the TL101/pTL58 background. The resulting strain produces ca. 50% more fatty acid, whereas a control strain expressing fabZ but not fabI or fabG does not (Fig. S5). Parenthetically, we also note that the observed relationship between TesA concentration and FAS activity also has precedence in our previous studies in vivo. Specifically, moderate overexpression of TesA in E. coli led to elevated fatty acid productivity, but high TesA levels suppressed product formation (7).
Molecular Analysis of acyl-ACP Intermediates.
The above biochemical analyses also suggested that one or more acyl-ACP intermediates could limit the turnover frequency and/or number of the fatty acid synthase. We therefore sought to analyze the identities and relative abundance of various acyl chains that accumulate on the ACP. Two methods were evaluated—conformation-dependent PAGE (14, 15) and mass spectrometry.
Cultures from E. coli BL21(DE3), TL101/pMSD8 (a fadE knockout of BL21(DE3) that overexpresses the acetyl-CoA carboxylase), and TL101/pMSD8/pTL58 (that overexpresses the same carboxylase as well as TesA and a second medium chain-specific thioesterase from the camphor plant ) (7, 16) were lysed and centrifuged. The supernatants were subjected to native PAGE, followed by Western blot analysis with an anti-ACP antibody. Multiple ACP species were observed in each sample (Fig. S6). Relative to E. coli BL21(DE3), TL101/pMSD8 contained a new acyl-ACP species, which was suppressed in TL101/pMSD8/pTL58. This species could be malonyl-ACP, which presumably accumulates as a result of acetyl-CoA carboxylase overexpression, or one or more longer chain acyl-ACP species that arose due to an increased carbon flux through the FAS in E. coli TL101/pMSD8. At the same time a notable increase was also observed in the abundance of the uppermost ACP species on this PAGE gel; this species could also be malonyl-ACP or a fatty acyl-ACP intermediate. Notwithstanding the inherent inability of native PAGE to provide molecular insights, these results highlight the substantial differences between fatty acid biosynthesis in wild-type and engineered strains of E. coli.
Among the different mass spectrometric methods evaluated, matrix-assisted laser desorption ionization–time of fly (MALDI-TOF) mass spectrometry proved to be most effective at identifying different acyl-ACP species present in a FAS reaction mixture. The reaction was allowed to occur under the reference conditions cited above. After 10 min, the assay mixture was rapidly subjected to MALDI-TOF mass spectrometric analysis. As a reference, a similar quantity of holo-ACP was analyzed using the same technique. Compared to the control sample, the intensities of the holo-ACP peaks were greatly reduced (Fig. 6). Instead, the most abundant ACP species appeared to be tethered to C6, C10, C16, and C18 chains. The resolution of the method was inadequate to differentiate between acyl chains with different degrees of saturation.
Fig. 6.
MALDI-TOF mass spectrometry of (A) 10 μM holo-ACP and (B) a FAS reaction mixture containing 10 μM holo-ACP. (A) The lower molecular weight (MW) species corresponds to the N+C terminally His-tagged holo-ACP, whereas the higher MW corresponds to the same ACP with a glucuronidated N terminus (17). (B) MALDI-TOF analysis of the same quantity of ACP from a FAS reaction mixture. The mass spectrum is shown on the same scale as A. From left to right, the peaks represent: holo-ACP, C6-, C10-, C16-, and C18-ACP species, and glucuronidated ACP.
Discussion
Among all known metabolic pathways in living systems, fatty acid biosynthesis yields the most energy dense products (18). Therefore, given the imperative to obtain transportation fuels from renewable sources, considerable effort has focused on the production of biofuels via the fatty acid biosynthetic pathway. A key prerequisite is to maximize the specific activity of the fatty acid synthase (FAS) in the biotransforming microbe. Indeed, recent efforts have shown that genetic manipulation of the E. coli FAS can improve its productivity of free fatty acids and their derivatives (7, 16, 19, 20). Notwithstanding these early successes, considerably greater improvements in atom economy and volumetric productivity are warranted, if fatty acid derivatives are to emerge as prominent fuels in the marketplace.
Like most other eubacterial and plant FASs, the E. coli FAS is comprised of several monofunctional proteins (MW < 50 kDa) that are used iteratively during sequential growth of the fatty acyl chain (Fig. 1). Whereas this multienzyme system has been extensively investigated at a genetic and enzymological level over the past five decades (1–5), to our knowledge, the fully reconstituted system has never been subjected to steady-state kinetic analysis. In preliminary work, we therefore sought to measure the key properties of the E. coli FAS in crude cell lysates derived from various engineered strains (7). Here we have assembled the complete FAS from individually purified components. The reconstituted system has enabled us to quantify its steady-state kinetic parameters and the extent to which they are influenced by substrate, cofactor, subunit, and product concentrations.
To obtain an initial frame of reference, we sought to mimic the intracellular concentrations of individual FAS components estimated from mRNA and protein measurements in wild-type and fatty acid overproducing strains. Under conditions where 1 μM each of FabA, FabB, FabD, FabF, FabG, FabH, FabI, and FabZ, and 10 μM each of holo-ACP and TesA were present, initial rates of fatty acid formation (on a palmitic acid basis) were in the range of 10 μM/ min. The highest velocities achieved in this study exceeded 100 μM/ min (Fig. 4). As a point of comparison, the fatty acid productivity of the most advanced strain of E. coli characterized by us thus far (XL100/pMSD8/pTL58) has an average specific productivity of 0.04 g h-1 g-1 dry cell mass (7), or a turnover rate of 0.02 s-1, assuming that dry cell mass contains 55% protein (21) and that the rate-limiting FAS component is expressed at 0.1% of total cellular protein. Thus, with an apparent kcat exceeding 1 s-1, it is evident that our reconstituted enzyme system accurately reflects intracellular FAS activity in E. coli.
The activity of the reconstituted E. coli FAS showed saturable dependence on each of its four substrates—acetyl-CoA, malonyl-CoA, NADPH, and NADH. FAS activity was not susceptible to modulation by the redox ratio (i.e., the ratio of NAD(P)H to NAD(P)+). These findings reinforce our earlier conclusion that, among these metabolites, only the intracellular concentration of malonyl-CoA is likely to be an attractive target for further engineering, if the goal is to improve fatty acid yield and/or productivity (22).
As is often the case in multistep biosynthetic pathways (23, 24), the steady-state flux of carbon precursors and reducing equivalents into fatty acids is sensitive to the activity of only a few enzymes. In particular, productivity increases with increasing concentrations of FabI and FabZ in the 1–10 μM range. FabI is the enoyl reductase, whereas FabZ is one of two dehydratases in the E. coli FAS. It is unlikely that these reactions are rate limiting in every chain elongation cycle of the FAS. A plausible explanation for their influence on its turnover frequency is that FabI and FabZ have poor specificity toward an intermediate with a defined chain length and/or degree of unsaturation. Although earlier studies have highlighted a role for both enzymes in the regulation of fatty acid biosynthesis in E. coli (25, 26), direct biochemical evidence for the effects of these enzymes on FAS kinetics has thus far been lacking. Our findings suggest that it may be possible to further improve the intracellular carbon flux through the fatty acid biosynthetic pathway by identifying and alleviating the mechanistic underpinnings of these kinetic bottlenecks.
In contrast to FabI and FabZ, which influence FAS activity in a typical hyperbolic fashion, increasing quantities of FabF, FabH, holo-ACP, or TesA enhance FAS activity at low concentrations but inhibit activity at higher concentrations. FabH is the ketosynthase responsible for chain initiation, whereas FabF catalyzes chain elongation (Fig. 1). We hypothesize that, at high concentrations, the affinity of these ketosynthases for the holo-ACP leads to sequestration of the carrier protein. Such a mechanism could also explain the observed inhibition of the FAS in the presence of a high concentration of the holo-ACP. If so, then the inability of the S36T mutant to inhibit the FAS would also suggest that the acyl-phosphopantetheine moiety contributes substantially to this mode of inhibition. Direct structural evidence for KS-ACP interactions has been reported (27, 28, 29), as have other in vitro assays revealing acyl-ACP inhibition of FabH (15, 30). Similarly, changing concentrations of TesA could also alter the relative distribution of certain acyl-ACP species, which in turn could modulate the turnover frequency of the FAS. This is supported by the observation that the inactive S10A mutant of TesA is noninhibitory. The modest rate increase in response to titration of the S10A mutant may be due to its ability to alter the steady-state distribution of acyl-ACP species by preferentially binding to, but not hydrolyzing, a subset of these biosynthetic intermediates.
Given the potential role of acyl-ACP intermediates in regulating FAS activity, we sought to develop a method for monitoring the distribution of these adducts under typical reactive conditions. Both electrospray ionization mass spectrometry and MALDI-TOF analysis were evaluated; the latter was found to be more informative. As seen in Fig. 6, free holo-ACP is a minor constituent of the reaction mixture; acyl-ACPs in the C6 to C18 range are more abundant. The structures and properties of these intermediates warrant detailed characterization, as one or more acyl-ACPs could prove to be important positive or negative regulators of FAS activity in E. coli.
The metabolic relevance of at least some of our observations in vitro has been tested through targeted manipulations in vivo of FabF, FabG, FabI, FabZ (Fig. S5), and TesA (7). In each case, we were able to recapitulate the essential features of our cell-free data in genetically engineered strains of E. coli. In addition to underscoring the predictive power of the reconstituted FAS, our findings suggest specific combinatorial manipulations that are warranted in order optimize the specific productivity of the fatty acid biosynthetic power of E. coli. For example, multivariate experiments that focus on coordinately manipulating FabI, FabZ, holo-ACP, and TesA levels are likely to be a promising avenue. Analogous experiments have yielded dramatic improvements in terpene biosynthesis in E. coli (31).
In conclusion, we have successfully reconstituted the E. coli fatty acid synthase and highlighted the utility of our cell-free system for interrogating its properties under steady-state reactive conditions. Future studies along these lines with E. coli or other bacterial FASs promise to yield valuable insights into those aspects of the system that merit further engineering with the goal of enhancing bioenergy production from renewable sources.
Methods and Materials
For details, see SI Text.
Plasmid Construction.
For details, see SI Text.
Protein Purification.
For details see SI Text.
Preparation of Cell-Free Lysates for Fatty Acid Synthesis Assays.
Cell-free lysates from E. coli XL100/pMSD8/pTL57 were prepared as previously reported (7).
Kinetic Analysis of Fatty Acid Synthase Activity.
Radioactive assays of the cell-free system were performed as previously described (9). In brief, the protein components, substrates and cofactors (and cell lysate when applicable) were added to a reaction buffer containing 100 mM sodium phosphate buffer (pH 7.5) and 1 mM TCEP at the indicated concentrations. The reaction was initiated by addition of [2-14C] malonyl-CoA (or [1-14C] acetyl-CoA when titrating into a lysate-based system) (American Radiolabeled Chemicals). At various time intervals, 20 μL reaction mixture was withdrawn and quenched with 250 μL 4∶1 (v∶v) isopropanol∶acetic acid and 230 μL water. To extract free fatty acids, 500 μL hexanes was added, and mixed, whereafter which 400 μL of the organic layer was removed and dried. Dried samples were resuspended in 20 μL hexanes, spotted on a silica gel TLC plate, and chromatographed in a 70∶30∶2 (v/v) mixture of hexanes∶ether∶acetic acid. Radioactivity was quantified on a Packard Phosphorimager, using [1-14C] myristic acid, [1-14C] acetyl-CoA, and [2-14C] malonyl-CoA as calibration standards. Origin v6.0 software was used for data fitting.
Quantifying the Expression of fab Genes in Wild-Type and Engineered E. coli Strains.
For details, see SI Text.
Analysis of Total Fatty Acids from a Fermentation-Derived Sample.
For details, see SI Text.
ACP Analysis via Nondenaturing PAGE.
An earlier protocol was used to separate individual acyl-ACP species in the reaction mixture (15). In brief, a 12% nondenaturing PAGE (containing 2.5 M urea) was prepared, as outlined in Table S4. Protein samples were mixed with equal volumes of Laemmli sample buffer (Bio-Rad) without β-mercaptoenthanol, and separated at 60 V. The Western blot procedure, described above, was used to visualize each ACP species.
Mass Spectrometric Analysis of ACP Species.
The reaction was set up and initiated in the same way as the radioactive assays described above, except that nonradioactive material was used. An aliquot (3 μL) of the reaction mixture was withdrawn and loaded on to the matrix for MALDI-TOF mass spectrometric analysis, 10 min after the initiation of the reaction. For pure ACP, the solution was incubated for 10 min before matrix loading. The analyses were conducted in Stanford Protein and Nucleic Acid Facility. The instrument used was an ABI Voyager DE-RP, and the matrix was sinapinic acid. The analyses were performed in delayed, linear, and positive mode; the parameters used were: acceleration voltage = 25 kV, grid voltage = 92%, guide wire = 0.15%, delay time = 350 ns.
Supplementary Material
Acknowledgments.
We thank Shripa Patel for help with MALDI-TOF MS. This research was supported by a grant from LS9, Inc to C.K. Additional funding was provided by the 973 Project from the Ministry of Science and Technology of China (2011CBA00806) and by the National Science Foundation of China (31040086) to T. Liu.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1110852108/-/DCSupplemental.
References
- 1.Cronan JE, Jr, Rock CO. In: Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. Neidhardt FC, editor. Washington: ASM Press; 1996. pp. 612–636. [Google Scholar]
- 2.Rock CO, Jackowski S. Forty years of bacterial fatty acid synthesis. Biochem Biophys Res Commun. 2002;292:1155–1166. doi: 10.1006/bbrc.2001.2022. [DOI] [PubMed] [Google Scholar]
- 3.White SW, Zheng J, Zhang YM, Rock CO. The structural biology of type II fatty acid biosynthesis. Annu Rev Biochem. 2005;74:791–831. doi: 10.1146/annurev.biochem.74.082803.133524. [DOI] [PubMed] [Google Scholar]
- 4.Lu YJ, Zhang YM, Rock CO. Product diversity and regulation of type II fatty acid synthases. Biochem Cell Biol. 2004;82:145–155. doi: 10.1139/o03-076. [DOI] [PubMed] [Google Scholar]
- 5.Zhu K, Zhang YM, Rock CO. Transcriptional regulation of membrane lipid homeostasis in Escherichia coli. J Biol Chem. 2009;284:34880–34888. doi: 10.1074/jbc.M109.068239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pfeifer BA, Admiraal SJ, Gramajo H, Cane DE, Khosla C. Biosynthesis of complex polyketides in a metabolically engineered strain of E. coli. Science. 2001;291:1790–1792. doi: 10.1126/science.1058092. [DOI] [PubMed] [Google Scholar]
- 7.Liu T, Vora H, Khosla C. Quantitative analysis and engineering of fatty acid biosynthesis in E. coli. Metab Eng. 2010;12:378–386. doi: 10.1016/j.ymben.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 8.Jiang P, Cronan JE., Jr Inhibition of fatty acid synthesis in Escherichia coli in the absence of phospholipid synthesis and release of inhibition by thioesterase action. J Bacteriol. 1994;176:2814–2821. doi: 10.1128/jb.176.10.2814-2821.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho H, Cronan JE., Jr Defective export of a periplasmic enzyme disrupts regulation of fatty acid synthesis. J Biol Chem. 1995;270:4216–4219. doi: 10.1074/jbc.270.9.4216. [DOI] [PubMed] [Google Scholar]
- 10.Price AC, Zhang YM, Rock CO, White SW. Structure of beta-ketoacyl-[acyl carrier protein] reductase from Escherichia coli: Negative cooperativity and its structural basis. Biochemistry. 2001;40:12772–12781. doi: 10.1021/bi010737g. [DOI] [PubMed] [Google Scholar]
- 11.Price AC, Zhang YM, Rock CO, White SW. Cofactor-induced conformational rearrangements establish a catalytically competent active site and a proton relay conduit in FabG. Structure. 2004;12:417–428. doi: 10.1016/j.str.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 12.Baldock C, et al. A mechanism of drug action revealed by structural studies of enoyl reductase. Science. 1996;274:2107–2110. doi: 10.1126/science.274.5295.2107. [DOI] [PubMed] [Google Scholar]
- 13.Baldock C, Rafferty JB, Stuitje AR, Slabas AR, Rice DW. The X-ray structure of Escherichia coli enoyl reductase with bound NAD+ at 2.1 A resolution. J Mol Biol. 1998;284(5):1529–1546. doi: 10.1006/jmbi.1998.2271. [DOI] [PubMed] [Google Scholar]
- 14.Keating DH, Carey MR, Cronan JE., Jr The unmodified (apo) form of Escherichia coli acyl carrier protein is a potent inhibitor of cell growth. J Biol Chem. 1995;270:22229–22235. doi: 10.1074/jbc.270.38.22229. [DOI] [PubMed] [Google Scholar]
- 15.Heath RJ, Rock CO. Inhibition of beta-ketoacyl-acyl carrier protein synthase III (FabH) by acyl-acyl carrier protein in Escherichia coli. J Biol Chem. 1996;271:10996–11000. doi: 10.1074/jbc.271.18.10996. [DOI] [PubMed] [Google Scholar]
- 16.Lu X, Vora H, Khosla C. Overproduction of free fatty acids in E. coli: Implications for biodiesel production. Metab Eng. 2008;10:333–339. doi: 10.1016/j.ymben.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 17.Geoghegan KF, et al. Spontaneous alpha-N-6-phosphogluconoylation of a “His tag” in Escherichia coli: The cause of extra mass of 258 or 178 Da in fusion proteins. Anal Biochem. 1999;267:169–184. doi: 10.1006/abio.1998.2990. [DOI] [PubMed] [Google Scholar]
- 18.Liu T, Khosla C. Genetic engineering of Escherichia coli for biofuel production. Annu Rev Genet. 2010;44:53–69. doi: 10.1146/annurev-genet-102209-163440. [DOI] [PubMed] [Google Scholar]
- 19.Lennen RM, Braden DJ, West RA, Dumesic JA, Pfleger BF. A process for microbial hydrocarbon synthesis: overproduction of fatty acids in Escherichia coli and catalytic conversion to alkanes. Biotechnol Bioeng. 2010;106:193–202. doi: 10.1002/bit.22660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Steen EJ, et al. Microbial production of fatty-acid-derived fuels and chemicals from plant biomass. Nature. 2010;463:559–562. doi: 10.1038/nature08721. [DOI] [PubMed] [Google Scholar]
- 21.Neidhardt FC, Umbarger HE. In: Escherichia coli and Salmonella typhimurium: Cellular and Molecular Biology. Neidhardt FC, editor. Washington: ASM Press; 1996. Chapter 3, table 1. [Google Scholar]
- 22.Davis MS, Solbiati J, Cronan JE., Jr Overproduction of acetyl-CoA carboxylase activity increases the rate of fatty acid biosynthesis in Escherichia coli. J Biol Chem. 2000;275:28593–28598. doi: 10.1074/jbc.M004756200. [DOI] [PubMed] [Google Scholar]
- 23.Kacser H, Burns JA. The control of flux. Biochem Soc Trans. 1995;23:341–366. doi: 10.1042/bst0230341. [DOI] [PubMed] [Google Scholar]
- 24.Heinrich R, Rapoport TA. A linear steady-state treatment of enzymatic chains. General properties, control and effector strength. Eur J Biochem. 1974;42:89–95. doi: 10.1111/j.1432-1033.1974.tb03318.x. [DOI] [PubMed] [Google Scholar]
- 25.Heath RJ, Rock CO. Enoyl-acyl carrier protein reductase (fabI) plays a determinant role in completing cycles of fatty acid elongation in Escherichia coli. J Biol Chem. 1995;270:26538–26542. doi: 10.1074/jbc.270.44.26538. [DOI] [PubMed] [Google Scholar]
- 26.Heath RJ, Rock CO. Roles of the FabA and FabZ beta-hydroxyacyl-acyl carrier protein dehydratases in Escherichia coli fatty acid biosynthesis. J Biol Chem. 1996;271:27795–27801. doi: 10.1074/jbc.271.44.27795. [DOI] [PubMed] [Google Scholar]
- 27.Gajiwala KS, et al. Crystal structures of bacterial FabH suggest a molecular basis for the substrate specificity of the enzyme. FEBS Lett. 2009;583:2939–2946. doi: 10.1016/j.febslet.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 28.Musayev F, Sachdeva S, Scarsdale JN, Reynolds KA, Wright HT. Crystal structure of a substrate complex of Mycobacterium tuberculosis beta-ketoacyl-acyl carrier protein synthase III (FabH) with lauroyl-coenzyme A. J Mol Biol. 2005;346:1313–1321. doi: 10.1016/j.jmb.2004.12.044. [DOI] [PubMed] [Google Scholar]
- 29.Davies C, Heath RJ, White SW, Rock CO. The 1.8 A crystal structure and active-site architecture of beta-ketoacyl-acyl carrier protein synthase III (FabH) from Escherichia coli. Structure. 2000;8:185–195. doi: 10.1016/s0969-2126(00)00094-0. [DOI] [PubMed] [Google Scholar]
- 30.Heath RJ, Rock CO. Regulation of fatty acid elongation and initiation by acyl-acyl carrier protein in Escherichia coli. J Biol Chem. 1996;271:1833–1836. doi: 10.1074/jbc.271.4.1833. [DOI] [PubMed] [Google Scholar]
- 31.Ajikumar PK, et al. Isoprenoid pathway optimization for Taxol precursor overproduction in Escherichia coli. Science. 2010;330:70–74. doi: 10.1126/science.1191652. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.