Abstract
The dysfunction of multiple neurotransmitter systems is a striking pathophysiological feature of many mental disorders, schizophrenia in particular, but delineating the underlying mechanisms has been challenging. Here we show that manipulation of a single schizophrenia susceptibility gene, dysbindin, is capable of regulating both glutamatergic and dopaminergic functions through two independent mechanisms, consequently leading to two categories of clinically relevant behavioral phenotypes. Dysbindin has been reported to affect glutamatergic and dopaminergic functions as well as a range of clinically relevant behaviors in vertebrates and invertebrates but has been thought to have a mainly neuronal origin. We find that reduced expression of Drosophila dysbindin (Ddysb) in presynaptic neurons significantly suppresses glutamatergic synaptic transmission and that this glutamatergic defect is responsible for impaired memory. However, only the reduced expression of Ddysb in glial cells is the cause of hyperdopaminergic activities that lead to abnormal locomotion and altered mating orientation. This effect is attributable to the altered expression of a dopamine metabolic enzyme, Ebony, in glial cells. Thus, Ddysb regulates glutamatergic transmission through its neuronal function and regulates dopamine metabolism by regulating Ebony expression in glial cells.
Keywords: dystrobrevin binding protein 1, glutamate, glia
Schizophrenia is a debilitating mental disorder with intricate etiology and multidimensional pathophysiological and clinical features. Pathophysiologically, multiple neurotransmitter systems, including glutamate, dopamine, GABA, and serotonin, are disturbed in this morbid condition (1). Accordingly, schizophrenia has multiple clinical characteristics, including positive symptoms, negative symptoms, and cognitive impairments (2). However, how an imbalance of multiple neurotransmitter systems evolves and how the pathophysiological abnormalities give rise to the clinical features remain to be elucidated. Despite the complicated pathophysiology and associated symptoms, evidence is mounting that genetic factors contribute substantially to the development and expression of schizophrenia (3). Human genetic studies have identified a plethora of candidate genes linked to susceptibility for this disease (4). Notably, many susceptibility genes act by regulating the function or homeostasis of multiple neurotransmitter systems (5). Therefore, genetic manipulation of these susceptibility genes will help disclose the mechanisms underlying the genetic regulation of neurotransmitter systems as well as related behaviors and in turn will increase our understanding of the pathogenesis of the disease per se.
For this purpose, the current study began with an analysis of a Drosophila mutant of dysbindin (Ddysb), an ortholog of the human schizophrenia susceptibility gene, dystrobrevin binding protein 1 (DTNBP1, also known as “dysbindin”). The DTNBP1-encoded dysbindin-1 was identified initially as a member of the dystrophin-associated protein complex (6) and later as a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) (7). Despite some controversial results, the association between multiple variants in DTNBP1 and schizophrenia has been replicated by several independent studies (8, 9). Furthermore, reduced dysbindin expression has been reported in the prefrontal cortex and hippocampus of schizophrenia patients (10, 11). Although it is a null mutant, the spontaneously occurring deletion in the mouse homolog of DTNBP1 (7) leads to a range of schizophrenia-related pathophysiological and behavioral phenotypes, including reduced glutamatergic transmission, abnormalities in dopaminergic activities, locomotor hyperactivity, social withdrawal, and memory compromise (12, 13).
This study of the Drosophila mutant demonstrates that a 30–40% reduction in Ddysb expression is capable of recapitulating major features of schizophrenia-related pathophysiological changes observed in the null mutant of the so-called “sandy” mouse. Genetic manipulation allowed us to reveal distinct functions of Ddysb in neurons and in glial cells. Disruption of Ddysb function in neurons is responsible for hypoglutamatergic transmission and subsequent memory defects, whereas disruption of Ddysb function in glial cells causes hyperdopaminergic activity and locomotor hyperactivity via the reduction of the protein Ebony.
Results
Characterization of the Ddysb Mutant.
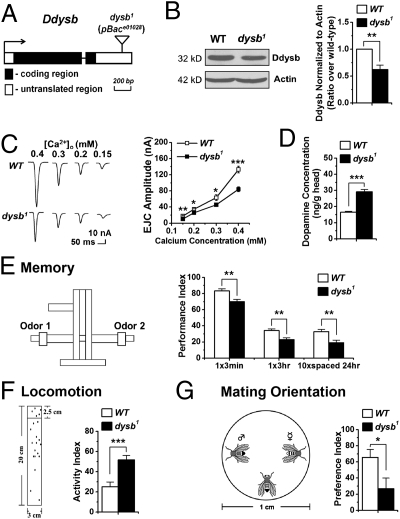
The Ddysb mutant studied here is a pBace01028 mutant carrying a piggyBac transposon in the 3′ UTR of Ddysb (Fig. 1A). Western blotting revealed that Ddysb expression in the adult head was reduced by ∼40% in this mutant (Fig. 1B). Immunohistochemistry also showed a consistent reduction of Ddysb signals in the mutant brain (Fig. S1A). In accordance with a recent report (14), these results suggest that pBace01028 is a hypomorphic allele of Ddysb and thus is referred to hereafter as “dysb1.”
Fig. 1.
Characterization of the Ddysb mutant. (A) Transposon insertion site. (B) Representative Western blots and group data showing reduced expression of Ddysb in the head of the dysb1 mutant (t test; P = 0.007; n = 5). (C) The dysb1 mutant shows decreased EJC amplitude (t test; P = 0.002, 0.02, 0.03, and 2.1E-6 for calcium concentrations of 0.15, 0.2, 0.3, and 0.4 mM, respectively; n = 7–14). (D) Dopamine concentration in whole-head extracts of adult flies is elevated significantly in the dysb1 mutant (t test; P = 1.9E-6; n = 8–10). (E) The dysb1 mutant shows memory defects in the Pavlovian olfactory aversive conditioning (t test; P = 0.004, 0.002, and 0.009; n = 7–8). The T-maze for memory test is illustrated. (F) dysb1 mutants show dramatically increased locomotor activity (t test; P = 2E-4; n = 17). The experimental paradigm for the locomotion test is illustrated on the left. (G) The dysb1 mutant shows mating disorientations (t test; P = 0.02; n = 28–32). The mating preference assay is illustrated. Data are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001.
We first looked at pathophysiology-related changes, i.e., glutamatergic and dopaminergic activities. The two-electrode voltage-clamp method was used to assay excitatory junctional currents (EJCs) of glutamatergic synaptic transmission at the well-characterized larval neuromuscular junction (NMJ) (15). The dysb1 mutant showed significantly attenuated EJCs at various extracellular calcium concentrations (Fig. 1C) without significant morphological changes; this result is consistent with a recent independent study (14). To assay how dopamine activity might be affected, we measured the dopamine level in the heads of adult flies using ELISA (16). The dopamine concentration in dysb1 mutant heads was around twofold of that in WT flies (Fig. 1D). Therefore, reduced Ddysb expression leads to both hypoglutamatergic and hyperdopaminergic activities.
For pathophysiology-relevant behavioral phenotypes, we focused mainly on learning task and locomotor activity, because both are often assayed in rodent models of schizophrenia (17). For the learning task, adult flies were trained with Pavlovian olfactory aversive conditioning in which flies learn to avoid an odor previously associated with electric shock (Fig. 1E, Left) (18, 19). Memory performance immediately after one-session training was lower in dysb1 mutants than in WT flies. Memory retention at later time points, either 3 h after single-session training or 24 h after repetitive training, was also compromised in the dysb1 mutant (Fig. 1E, Right), but the sensorimotor responses necessary for performing the learning task were not altered (Fig. S1 B and C). For locomotor activity, we used a climbing assay to record how high flies climbed in response to light taps on the container (Fig. 1F, Left) (20). The dysb1 mutant exhibited remarkably elevated activity compared with WT flies of the same age (Fig. 1F, Right).
During the climbing assay, we observed that dysb1 males showed abnormal male–male courtship behavior. Mutant males courted females, as WT flies do (Fig. S1D), but also oriented the typical courtship ritual toward another male. To quantify this abnormal mating orientation, we adopted a mating preference assay, in which the testing male was allowed to choose between a decapitated WT male and female as a mating partner (Fig. 1G, Left) (21). WT males display a strong bias toward female flies, but this courtship tendency was reduced significantly in the dysb1 mutants (Fig. 1G, Right).
Thus dysb1 mutants showed hypoglutamatergic and hyperdopaminergic activities as well as memory defects, locomotor hyperactivity, and mating disorientation. The genetic specificity of the observed phenotypes was confirmed through rescuing and RNAi silencing experiments presented later.
Tissue-Specific Regulation of Changes in Neurotransmitter Systems.
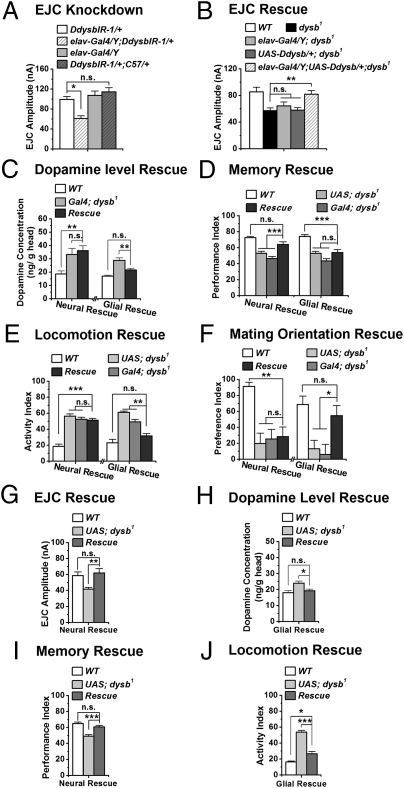
While determining the genetic specificity of the observed phenotypes, we discovered tissue-specific functions of Ddysb using the Gal4/Upstream Activation System (Gal4/UAS) binary system (22). Ddysb RNAi (DdysbIR) and UAS-Ddysb flies were used for Ddysb knockdown and overexpression (Fig. S2). At the NMJ, we first determined whether glutamatergic transmission was supported by a presynaptic or postsynaptic function of Ddysb. Transgenic RNAi was targeted to either presynaptic neurons or postsynaptic muscle cells. We found that silencing Ddysb functions pan-neuronally [embryonic lethal abnormal vision (elav)-Gal4/Y;DdysbIR-1/+], instead of in the muscle cells (DdysbIR-1/+;muscle Gal4 (C57)/+), caused a similarly reduced EJC amplitude in the dysb1 mutant (Fig. 2A). To confirm further the presynaptic role of Ddysb, we performed a rescuing experiment, showing that pan-neuronal expression of the UAS-Ddysb transgene in the dysb1 mutant background (elav-Gal4/Y;UAS-Ddysb/+;dysb1) successfully restored the EJC to the WT level (Fig. 2B). Moreover, pan-neuronal overexpression of Ddysb by two copies in the WT background (elav-Gal4;UAS-Ddysb) led to increased EJC amplitude (Fig. S3A). Thus, Ddysb regulates the glutamatergic synaptic transmission presynaptically in a dosage-dependent manner.
Fig. 2.
Tissue-specific regulation of changes in different neurotransmitter systems and behaviors. (A) EJC amplitude was reduced significantly by expression of the Ddysb RNAi presynaptically (elav-Gal4/Y; DdysbIR-1/+) but not postsynaptically (DdysbIR-1/+;C57/+) (ANOVA; n = 9–16). The calcium concentration for recording is 0.4 mM. (B) Neuronal expression of UAS-Ddysb (elav-Gal4/Y;UAS-Ddysb/+;dysb1) rescued the EJC deficit in the dysb1 mutant (ANOVA; n = 16–22). (C) Glial expression of Ddysb (UAS-Ddysb/+;Repo-Gal4 dysb1/dysb1), but not neuronal expression of Ddysb (UAS-Ddysb/+;elav-Gal4(III) dysb1/dysb1), restored the brain dopamine concentration to normal levels (ANOVA; n = 5–10). (D) Pan-neural expression of Ddysb (UAS-Ddysb/+;elav-Gal4(III) dysb1/dysb1) is sufficient to rescue the 3-min associative memory deficit in the dysb1 mutant. However, glia-specific expression of Ddysb (UAS-Ddysb/+;Repo-Gal4 dysb1/dysb1) failed to do so (ANOVA; n = 4–14). (E and F) Locomotor hyperactivity and mating disorientation were suppressed in dysb1 mutants expressing UAS-Ddysb in glia (UAS-Ddysb/+;Repo-Gal4 dysb1/dysb1) but not in neurons (UAS-Ddysb/+;elav-Gal4(III) dysb1/dysb1). (ANOVA; n = 8–10 for locomotor test; n = 22–51 for mating test.) Data are means ± SEM. (G and I) Pan-neuronal expression of Hdysb rescued the EJC amplitude (elav-Gal4/Y;UAS-Hdysb/+;dysb1) (ANOVA; n = 9–10) and the 3-min associative memory deficit (UAS-Hdysb/+;elav-Gal4(III) dysb1/dysb1) (ANOVA; n = 16) in the dysb1 mutant. Calcium concentration for recording is 0.4 mM. (H and J) Glial expression of Hdysb (UAS-Hdysb/+;Repo-Gal4 dysb1/dysb1) restored the brain dopamine level (ANOVA; n = 4–11) and partially suppressed the hyperactivity in the dysb1 mutant (ANOVA; n = 10). Data are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant (P > 0.05).
Unexpectedly, the neuronal expression of Ddysb in dysb1 mutant (UAS-Ddysb/+;elav-Gal4(III) dysb1/dysb1) did not rescue the dopamine level in the adult head (Fig. 2C, Left). This result prompted us to test the possibility that Ddysb might have nonneuronal functions. We found that expression of Ddysb with a glia-specific driver, Reversed polarity (Repo)-Gal4 (UAS-Ddysb/+;Repo-Gal4 dysb1/dysb1) suppressed the elevated dopamine level in the dysb1 mutant (Fig. 2C, Right). Therefore, Ddysb appears to regulate glutamatergic transmission and dopamine level through different mechanisms. This result led us to explore whether and how such distinct roles affect relevant behavioral phenotypes.
Tissue-Specific Regulation of Different Behaviors.
Pan-neuronal expression of Ddysb in the mutant background (UAS-Ddysb/+;elav-Gal4(III) dysb1/dysb1) rescued the short-term memory defect in the dysb1 mutant (Fig. 2D, Left), whereas glial expression (UAS-Ddysb/+;Repo-Gal4 dysb1/dysb1) failed to do so (Fig. 2D, Right).
Furthermore, pan-neuroanl overexpression of Ddysb (elav-Gal4/+;UAS-Ddysb/+) led to a similar defect in memory (Fig. S3B). Thus, all the data consistently supported the notion that intact Ddysb function in neurons is required for memory formation.
In contrast to the memory deficit, locomotor hyperactivity and the mating disorientation in the dysb1 mutant were suppressed by expressing Ddysb in glia (UAS-Ddysb/+;Repo-Gal4 dysb1/dysb1) but not in neurons (UAS-Ddysb/+;elav-Gal4(III) dysb1/dysb1) (Fig. 2 E and F). The specificity of this glial rescue was confirmed further via targeted RNAi silencing. Knocking down Ddysb pan-neuronally (elav-Gal4/Y;DdysbIR-1/+) had no effect on the mating orientation of males. However, expressing DdysbIR either ubiquitously (Actin-Gal4/DdysbIR-1) or specifically in glia (DdysbIR-1/+;Repo-Gal4/+) resulted in dramatically increased male–male courtship behavior, as shown in the dysb1 mutant (Fig. S3C).
Human Dysbindin Rescues the Phenotypes in dysb1 Mutant.
Ddysb shares 28% amino acid identity with human dysbindin-1 (Hdysb) (9), but it remains unknown whether the functions of dysbindin are conserved and whether the Hdysb also has the tissue-specific effects revealed above. Thus, we generated UAS-Hdysb transgenic flies (Fig. S4). Pan-neuronal expression of Hdysb successfully restored the glutamatergic transmission (elav-Gal4/Y;UAS-Hdysb/+;dysb1) and associative memory (UAS-Hdysb/+;elav-Gal4(III) dysb1/dysb1) in the dysb1 mutant (Fig. 2 G and I), whereas glial expression of Hdysb (UAS-Hdysb/+;Repo-Gal4 dysb1/dysb1) suppressed the elevated brain dopamine level and hyperactivity (Fig. 2 H and J). Thus, the evidence suggests considerable conservation between human and Drosophila dysbindin in function.
Endogenous Expression of Ddysb in Glial Cells.
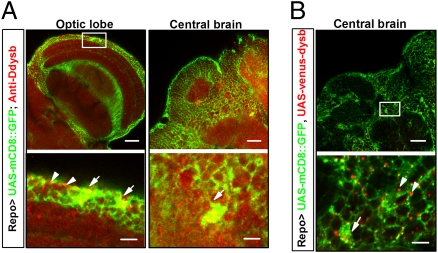
Earlier studies on dysbindin focused largely on its neuronal function (8). However, the experiments described above revealed an underestimated role of Ddysb in glia that could affect dopamine signaling, locomotion, and sex orientation. This finding led us to map the distribution of Ddysb in glial cells. We first attempted to visualize Ddysb in glial cells through immunohistochemical staining of Ddysb (in red) over GFP-labeled glia (UAS-mCD8::GFP/+;Repo-Gal4/+). The presence of endogenous Ddysb in glia was observed in the optic lobes and the central brain (merged yellow for Ddysb in glia; white arrows in Fig. 3A). To validate the detected signals, we expressed a Venus fluorescence protein (VFP)-tagged Ddysb (14) in GFP-labeled glia (UAS-mCD8::GFP/+;UAS-venus-dysb/Repo-Gal4). A similar pattern of Ddysb expression was observed (merged yellow at the cell body region; white arrows in Fig. 3B). However, we noted that a large fraction of VFP-Ddysb (red dots and white arrowheads in the lower panels of Fig. 3B and Fig. S5) was distributed over glial processes that were not merged with the GFP-tagged membrane protein, suggesting that the endogenous level of glial Ddysb was underestimated (Fig. 3A).
Fig. 3.
Endogenous expression of Ddysb in glial cells. Lower row shows enlarged views of boxed areas in upper row. (A) Ddysb is expressed extensively in both neurons and glia. Immunosignals of anti-Ddysb antibody (red), representing endogenous Ddysb, partially overlap (yellow) with Repo-Gal4–driven mCD8::GFP (green) at the glial cell bodies (white arrows).The dotted red signals also aggregate at the boundaries of neurons and glial processes (white arrowheads). (B) Coexpression of UAS-mCD8::GFP (green) and UAS-venus-dysb (red) in glia (UAS-mCD8::GFP/+;UAS-venus-dysb/Repo-Gal4). The immunosignals of Repo-Gal4–driven-Ddysb (red) localize at the glial cell body region (white arrow) and the boundaries of neurons and glial processes (white arrowheads), similar to the distribution of endogenous Ddysb in glia. (Scale bars: 20 μm Upper; 5 μm Lower.)
Although its expression in glia is scattered, Ddysb plays a critical role in development in addition to the physiological and behavioral roles described above. Silencing Ddysb specifically in glia with two copies of DdysbIR (DdysbIR-1/DdysbIR-2;Repo-Gal4/+) resulted in pupal lethality significantly higher than in the parental controls (Fig. S6). Driving even a single copy of RNAi by an astro-like glial Gal4 (NP3233) resulted in embryonic lethality similar to that seen with universal knockdown of Ddysb (Table S1).
Acute Induction of Ddysb Rescues the Phenotypes in dysb1 Mutants.
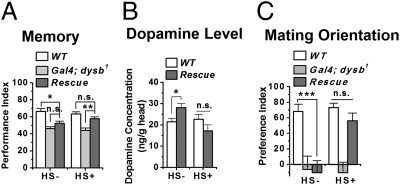
Because Ddysb might play a role in the development of the nervous system (as discussed above, and also see ref. 23 and 24), we wanted to determine the developmental contribution to the phenotypes observed. To this end, we tested the effects of acutely manipulated expression of Ddysb. Acute induction of Ddysb through a heat-shock Gal4 (hs-Gal4) driver (UAS-Ddysb/+;hs-Gal4 dysb1/dysb1) (Fig. S7) rescued the short-term memory defect (Fig. 4A) and suppressed the increased brain dopamine concentration (Fig. 4B) and the mating disorientation (Fig. 4C) in the dysb1 mutant. These results indicate that, apart from developmental functions, Ddysb also plays an acute physiological role in regulating neurotransmitters and behaviors in adult animals.
Fig. 4.
Acute induction of Ddysb rescues the phenotypes in the dysb1 mutant. Acute induction of Ddysb expression ubiquitously by a heat-shock promoter–Gal4 driver in the mutant background (UAS-Ddysb/+;hs-Gal4 dysb1/dysb1) rescued the 3-min associative memory deficit (A) (ANOVA; n = 6–12) and restored the brain dopamine level (B) (t test; P = 0.15; n = 7–14) and normal mating orientation (C) (ANOVA; n = 19–24). Data are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant (P > 0.05).
Hypoglutamate Is Responsible for Memory Defect.
Because both glutamatergic transmission at the NMJ and the memory capacity in the adult fly were restored by expressing WT Ddysb pan-neuronally in the dysb1 mutant background, we were interested in whether the function of Ddysb in glutamatergic transmission is related to its function in memory ability. First, we found that feeding flies with glycine, a NMDA receptor agonist and also a drug used to treat negative syndromes in schizophrenia (25), restored memory performance to the WT level in the adult fly, suggesting that the hypoglutamatergia in the dysb1 mutant is responsible for its memory deficit (Fig. 5A). To confirm the specificity of the pharmacological rescuing effect, we then expressed Ddysb with a glutamatergic neuron-specific Gal4 driver (vesicular glutamate transporter Gal4, VGluT-Gal4) in the mutant background (VGluT-Gal4/+;UAS-Ddysb/+;dysb1), which sufficiently restored the short-term memory to the normal level (Fig. 5D). Furthermore, overexpression of Ddysb in glutamatergic neurons (VGluT-Gal4/+;UAS-Ddysb/+) led to a defect in memory (Fig. S8) similar to that seen with pan-neuronal overexpression. Thus, all the data consistently show that the intact function of Ddysb in glutamatergic transmission is required for memory formation.
Fig. 5.
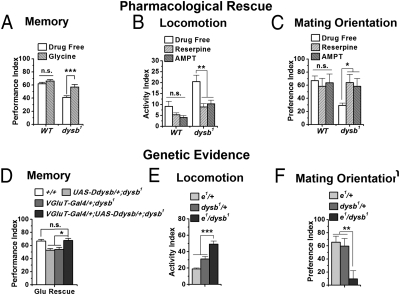
Hypoglutamate and hyperdopamine levels are responsible for different behavioral phenotypes. (A) Feeding flies with glycine, an NMDA receptor agonist, restored the 3-min associative memory in the dysb1 mutant (t test; P = 0.0007; n = 9–12) without significantly affecting WT flies (t test; P = 0.17; n = 12). (B and C) Feeding flies with reserpine, a vesicular monoamine transporter inhibitor, and AMPT, a TH inhibitor, suppressed the increased locomotion and disorientated mating behavior in the dysb1 mutant (ANOVA; n = 9–12 for locomotor test; n = 20–57 for mating test) but did not significantly influence WT flies. (D) Expression of Ddysb specifically in the glutamatergic neurons (VGluT-Gal4/+;UAS-Ddysb/+;dysb1) is sufficient to rescue the 3-min associative memory deficit in the dysb1 mutant (ANOVA; n = 14). (E and F) Genetic complementation test between dysb1 and ebony1 (e1) Compared with parental controls (e1/+ and dysb1/+), the double heterozygotes (e1/dysb1) showed significant locomotor hyperactivity (ANOVA; n = 15–16) and mating disorientation (ANOVA; n = 34–36). Data are means ± SEM. *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant (P > 0.05).
Elevated Dopamine Is Responsible for Hyperactivity and Mating Disorientation.
Because the memory defect was associated specifically with Ddysb functions in glutamatergic neurons, we then tested whether glia-dependent hyperdopaminergic activity, locomotor hyperactivity, and mating disorientation are related. In fact, there are reports that hyperdopaminergia in the fruit fly can lead to male–male courtship (26) and hyperactivity (27). We fed flies with two inhibitors of dopamine signaling: the vesicular monoamine transporter inhibitor reserpine (28) and the tyrosine hydroxylase (TH) inhibitor α-methyl-p-tyrosine (AMPT) (29). Compared with the drug-free group, both reserpine and AMPT significantly suppressed locomotor hyperactivity and mating disorientation in the dysb1 mutant but at the tested concentration had no significant effects on WT flies (Fig. 5 B and C).
The specificity of the pharmacological assay was confirmed by a genetic interaction experiment between Ddysb and ebony, which encodes a glia-specific β-alanyl biogenic amine synthetase that metabolically inactivates biogenic amines in the nervous system. The dopamine sequestered by glia is inactivated by Ebony to form N-β-alanyldopamine (NBAD) (30). Defective Ebony increases brain dopamine level and perturbs multiple behaviors including courtship (26, 31). As expected, we revealed a genetic interaction between Ddysb and ebony. The locomotor hyperactivity and mating disorientation were observed in double-heterozygous mutants (ebony1/dysb1), although single heterozygotes (ebony1/+ or dysb1/+) showed no phenotypes (Fig. 5 E and F). Therefore, evidence from pharmacological rescue and genetic interaction corroborates the contribution of elevated dopamine signaling to locomotor hyperactivity and mating disorientation.
Ddysb Regulates Dopamine Level via Ebony in Glia.
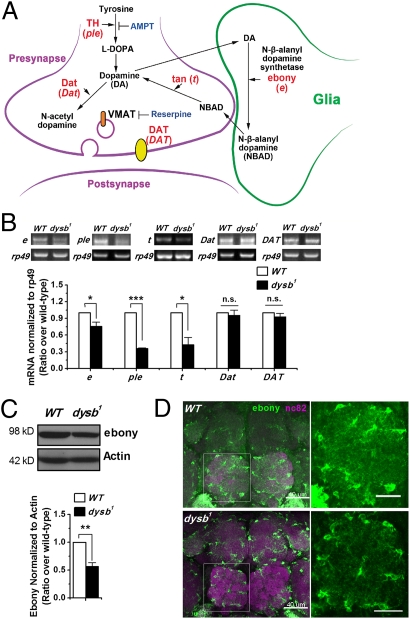
To gain insights into how Ddysb regulates dopaminergic activity in glial cells, we examined the transcription profiles of major genes involved in the synthetic and metabolic pathway of dopamine in Drosophila (Fig. 6A and refs. 32 and 33). RT-PCR results showed that, among the genes examined, the transcription levels of pale [the genetic locus for TH, the rate-limiting enzyme in dopamine biosynthesis (Fig. 6A and ref. 32)], and tan [encoding Tan, the enzyme for hydrolysis of NBAD to dopamine (Fig. 6A and ref. 34)] were decreased dramatically in dysb1 mutants (Fig. 6B). However, the changes in expression of these two genes could not be responsible for hyperdopaminergic activity, because (i) both TH and Tan are expressed specifically in neurons; and (ii) their reduction is a change in the opposite direction of producing hyperdopaminergic activity. In addition to these two genes, expression of ebony is also reduced significantly at both mRNA and protein levels in the dysb1 mutant (Fig. 6 B and C). As mentioned earlier, a reduction in Ebony function leads to hyperdopaminergic activity (26). The genetic interaction between Ddysb and ebony (Fig. 5 E and F) corroborates nicely with biochemical data, indicating that a reduction in Ddysb activity leads to reduced Ebony activity, which in turn leads to hyperdopaminergic activity.
Fig. 6.
Ddysb regulates dopamine level via Ebony in glial cells. (A) Dopamine (DA) synthetic and metabolic pathway. The actions of proteins, their encoding genes, and inhibitors are shown. DAT (dopamine transporter) is a dopamine transmembrane transporter. Dat (dopamine N acetyltransferase) is an N-acetyltransferase to metabolize dopamine to N-acetyl-dopamine. VMAT (vesicular monoamine transporter) is a synaptic vesicle amine transmembrane transporter. (B) Representative RT-PCR and group data show decreased mRNA levels of ebony (e), pale (ple), and tan (t) in the dysb1 mutant (t test; P = 0.02, 1.7E-5, and 0.02; n = 4), whereas the transcripts of DAT and Dat did not have significant changes compared with WT. (C) Representative Western blot and group data showing decreased Ebony protein level in the dysb1 mutant (t test; P = 0.002; n = 7). (D) Ebony is expressed in glial cells. Neuropils were stained with mAb nc82 (magenta). Ebony immunosignals (green) were weaker in the dysb1 mutant than in the WT. (Scale bars: 40 μm Left; 20 μm Right.) *P < 0.05; **P < 0.01; ***P < 0.001; n.s., not significant (P > 0.05).
Further immunohistochemical analysis of the adult brain confirmed the changes in Ebony. The Ebony signals were decreased in both the cell body and in the processes region of the Ebony-expressing glial cells (Fig. 6D), with more visible reduction in the processes.
Discussion
The current study investigated functions of Ddysb to explore how the altered expression of a single schizophrenia susceptibility gene relates to the pathophysiology and clinically relevant phenotypes. The function of this gene is highly conserved from Drosophila to vertebrates and even to humans. The observed pattern of Ddysb expression in the Drosophila brain (Fig. 3A and Fig. S1A) is very similar to that reported in the vertebrate brain: widespread and enriched in neurons (6). Loss-of-function mutations and RNAi knockdown of Ddysb in Drosophila produced phenotypes similar to those observed in the sandy mouse, including attenuated glutamatergic transmission, hyperdopaminergic activity, memory defects, and locomotor hyperactivity. Moreover, the human DTNBP1 gene was capable of rescuing dysb1 mutant phenotypes in Drosophila (Fig. 2 G–J). With the help of genetic tools exclusively available in Drosophila, however, we gained surprising insights, as outlined below.
First, although Ddysb is widely expressed in the brain, restoring Ddysb in glutamatergic neurons alone was sufficient to rescue hypoglutamatergic transmission and memory defects. Second, Ddysb's functions in glial cells are essential for normal dopaminergic activity and associated behaviors, including locomotion and mating orientation. Third, all observed pathophysiological and behavioral phenotypes were rescued with acute genetic or pharmacological treatments in adults.
We devoted special attention to validating the phenotypes observed, including maintaining an isogenic background for all genotypes (Materials and Methods), balancing the behavioral assays (19), and confirming the manifested phenotypes by different genetic manipulations (mutations, genetic rescuing, and RNAi knockdown). In the following sections, we address the significance and implications of our findings.
Glutamatergic Functions and Memory Defects.
An increasing number of studies suggest that genetic variation in DTNBP1 in normal human populations affects verbal and visual memories as well as working memory (35, 36). This association is supported by studies on the sandy mouse, which is defective in a range of memory tasks, including spatial memory, novel object recognition, and contextual fear conditioning (12, 13). However, the physiological causes of such memory defects are not clearly defined.
In this study, we show that altered Ddysb function in glutamatergic neurons alone is responsible for attenuated glutamatergic transmission and for the memory defect. It is interesting that this memory defect is not a developmental phenotype and could be rescued acutely both by feeding flies with the NMDA receptor agonist glycine (Fig. 5A) and by expressing Ddysb only in glutamatergic neurons (Fig. 5D). Such a result is consistent with reports showing that NMDA receptors in the Drosophila brain are involved in memory formation (37, 38).
Glial Functions and Regulation of Dopaminergic Activity.
Before the current study, the expression and function of dysbindin were considered to occur primarily, if not exclusively, in neurons (8). However, recent reports have demonstrated that in mouse and rat brains the expression level of dysbindin in glia is comparable with, if not higher than, its expression in neurons (23, 39), although its glial functions remained to be determined. Genetic tools available for Drosophila allowed us not only to define the function of dysbindin in glia but also to gain insight into the underlying mechanisms.
Anatomically, we showed that immunohistochemical signals of Ddysb (in red) were detected in glial cells labeled by GFP-tagged membrane proteins, with sparse Ddysb distribution in cell bodies and the majority of glial Ddysb signals in glial processes or in thin layers surrounding individual neuronal cell bodies (Fig. 3A). This observation was supported by the distribution pattern of VFP-tagged Ddysb in GFP-labeled glial cells (Fig. 3B and Fig. S5).
Evidence supporting a functional role of Ddysb in glia is very strong. We showed that the escalated dopamine level in the dysb1 mutant could be rescued by targeted expression of the Ddysb or human DTNBP1 transgene only in glial cells but not in neurons (Fig. 2 C and H). In addition, the hyperdopaminergia-elicited behaviors, including locomotor hyperactivity and mating disorientation, were rescued only through targeted glial expression of Ddysb or human DTNBP1 transgenes (Fig. 2 E, F, and J). More convincingly, knocking down Ddysb universally or in glia but not in neurons resulted in embryonic or pupal lethality, respectively (Fig. S6 and Table S1).
Our further investigation suggests that mutations of Ddysb cause hyperdopaminergic activity by down-regulating the expression of Ebony. Our biochemical data profiling mRNA and protein expression corroborated well with genetic observations, supporting the idea that Ebony plays critical role in mediating the effects of Ddysb in glial cells. It is likely that this Ddysb/Ebony-produced hyperdopaminergic activity somehow leads to reduced TH and Tan expression in neurons through a negative feedback mechanism for maintaining the homeostasis of dopaminergic activity.
How Ddysb regulates expression of Ebony remains to be determined. One possibility comes from reports that human dysbindin can function as a nucleocytoplasmic shuttling protein that regulates the transcription of several genes either directly or by binding with other transcription-related factors (40–42). Here, we analyzed the Ddysb protein sequence with the PSORT II Prediction WWW Server (http://psort.ims.u-tokyo.ac.jp/form2.html) and found that the probability that Ddysb localizes to the nucleus is 94.1%. Thus, it is plausible that Ddysb in glia plays a role in regulating gene transcription.
Alternatively, Ddysb might regulate the dopamine level in glial cells by affecting the stability of the Ebony protein. The dysbindin-containing BLOC-1 complex is a component of the endosomal protein sorting and compartmental machinery (43, 44). Abnormalities in Ebony protein sorting may lead to abnormalities in ubiquitylation, protein instability, or malfunction of the enzyme.
Implications for Disease.
Although the possibility of generating fly models of schizophrenia has been raised recently (45), the intent of this study is not to model schizophrenia in Drosophila. Instead, we are interested in whether and how a single mild genetic alteration, similar to those observed in cases of schizophrenia, gives rise to complex phenotypes at the neurotransmitter regulation and behavioral levels. Our study led to two interesting observations.
First, we were surprised to see that a rather mild 30–40% reduction in Ddysb expression led to significant alterations in both glutamatergic transmission and dopaminergic activity. Most schizophrenia susceptibility genes reported to date are identified not from mutations but from single-nucleotide polymorphisms or haplotypes, which are believed to produce only mild alterations at the gene expression level (5). It therefore is debatable how strong the contribution of an individual genetic variant is and whether multiple genetic components acting in concert are needed for the effects. Here we show that a mild reduction of at least one of the susceptibility genes is sufficient to cause complex changes in multiple neurotransmitter systems through very different mechanisms. Our findings suggest that these susceptibility genes might play such critical roles in neurotransmitter regulation that a mild change in expression is sufficient to cause detectable behavioral phenotypes.
Second, although a developmental role of dysbindin has been reported earlier (23, 24) and is supported, as mentioned above (Fig. S6 and Table S1), both neurotransmitter and behavioral phenotypes examined in this study could be rescued through acute treatments (Figs. 4 and 5 A–C). Schizophrenia is considered a neurodevelopmental disorder (46), a notion that is supported by animal model studies of development and by genetic mouse models of neurodevelopmental candidate genes and susceptibility genes (47). However, this study suggests that, to some extent, some of the genetically relevant phenotypes are reversible or could be treated in adults.
Materials and Methods
Fly stocks and detailed procedures for the generation of constructs and antibody, germ-line transformation, Western blot, RT-PCR, electrophysiology, drug treatments, dopamine level determination, immunohistochemistry, heat-shock regimen, and behavior assays including Pavlovian olfactory conditioning, the locomotor activity test, mating orientation test, and statistical analysis are described in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. K. Talbot (University of Pennsylvania) for human dysbindin antibodies; Dr. S. Carroll (University of Wisconsin–Madison) for Ebony antibody; Dr. S. Matsuzaki (Osaka University) for the pEGFP-C1-dysbindin vector; Drs. T. Awasaki and G. Davis for sharing flies; and Dr. H. Luo and L. Huang (Tsinghua University) for technical support. This work was supported by Grants 2006CB500806 and 2009CB941301 from the National Basic Research Project (Program 973) of the Ministry of Science and Technology of China, by Grant Z07000200540705 from the Project of Beijing Municipal Science and Technology Plan, and by a grant from the Tsinghua-Yue-Yuen Medical Sciences Fund (to Y. Zhong).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1114569108/-/DCSupplemental.
References
- 1.Keshavan MS, Tandon R, Boutros NN, Nasrallah HA. Schizophrenia, “just the facts”: What we know in 2008 Part 3: Neurobiology. Schizophr Res. 2008;106:89–107. doi: 10.1016/j.schres.2008.07.020. [DOI] [PubMed] [Google Scholar]
- 2.Tandon R, Nasrallah HA, Keshavan MS. Schizophrenia, “just the facts” 4. Clinical features and conceptualization. Schizophr Res. 2009;110:1–23. doi: 10.1016/j.schres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 3.Tandon R, Keshavan MS, Nasrallah HA. Schizophrenia, “just the facts” what we know in 2008. 2. Epidemiology and etiology. Schizophr Res. 2008;102:1–18. doi: 10.1016/j.schres.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 4.Owen MJ, Craddock N, O'Donovan MC. Schizophrenia: Genes at last? Trends Genet. 2005;21:518–525. doi: 10.1016/j.tig.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Harrison PJ, Weinberger DR. Schizophrenia genes, gene expression, and neuropathology: On the matter of their convergence. Mol Psychiatry. 2005;10(1):40–68. doi: 10.1038/sj.mp.4001558. image 45. [DOI] [PubMed] [Google Scholar]
- 6.Benson MA, Newey SE, Martin-Rendon E, Hawkes R, Blake DJ. Dysbindin, a novel coiled-coil-containing protein that interacts with the dystrobrevins in muscle and brain. J Biol Chem. 2001;276:24232–24241. doi: 10.1074/jbc.M010418200. [DOI] [PubMed] [Google Scholar]
- 7.Li W, et al. Hermansky-Pudlak syndrome type 7 (HPS-7) results from mutant dysbindin, a member of the biogenesis of lysosome-related organelles complex 1 (BLOC-1) Nat Genet. 2003;35:84–89. doi: 10.1038/ng1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talbot K, et al. Dysbindin-1 and Its Protein Family. Handbook of Neurochemistry and Molecular Neurobiology. New York: Springer; 2009. pp. 107–241. [Google Scholar]
- 9.Guo AY, et al. The dystrobrevin-binding protein 1 gene: Features and networks. Mol Psychiatry. 2009;14:18–29. doi: 10.1038/mp.2008.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Talbot K, et al. Dysbindin-1 is reduced in intrinsic, glutamatergic terminals of the hippocampal formation in schizophrenia. J Clin Invest. 2004;113:1353–1363. doi: 10.1172/JCI20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang J, et al. Dysbindin-1 in dorsolateral prefrontal cortex of schizophrenia cases is reduced in an isoform-specific manner unrelated to dysbindin-1 mRNA expression. Hum Mol Genet. 2009;18:3851–3863. doi: 10.1093/hmg/ddp329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Talbot K. The sandy (sdy) mouse: A dysbindin-1 mutant relevant to schizophrenia research. Prog Brain Res. 2009;179:87–94. doi: 10.1016/S0079-6123(09)17910-4. [DOI] [PubMed] [Google Scholar]
- 13.Cox MM, et al. Neurobehavioral abnormalities in the dysbindin-1 mutant, sandy, on a C57BL/6J genetic background. Genes Brain Behav. 2009;8:390–397. doi: 10.1111/j.1601-183X.2009.00477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dickman DK, Davis GW. The schizophrenia susceptibility gene dysbindin controls synaptic homeostasis. Science. 2009;326:1127–1130. doi: 10.1126/science.1179685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhong Y, Wu CF. Altered synaptic plasticity in Drosophila memory mutants with a defective cyclic AMP cascade. Science. 1991;251:198–201. doi: 10.1126/science.1670967. [DOI] [PubMed] [Google Scholar]
- 16.Liu T, et al. Reduction of dopamine level enhances the attractiveness of male Drosophila to other males. PLoS ONE. 2009;4:e4574. doi: 10.1371/journal.pone.0004574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Arguello PA, Gogos JA. Modeling madness in mice: One piece at a time. Neuron. 2006;52:179–196. doi: 10.1016/j.neuron.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 18.Tully T, Preat T, Boynton SC, Del Vecchio M. Genetic dissection of consolidated memory in Drosophila. Cell. 1994;79:35–47. doi: 10.1016/0092-8674(94)90398-0. [DOI] [PubMed] [Google Scholar]
- 19.Tully T, Quinn WG. Classical conditioning and retention in normal and mutant Drosophila melanogaster. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 1985;157:263–277. doi: 10.1007/BF01350033. [DOI] [PubMed] [Google Scholar]
- 20.Iijima K, et al. Dissecting the pathological effects of human Abeta40 and Abeta42 in Drosophila: A potential model for Alzheimer's disease. Proc Natl Acad Sci USA. 2004;101:6623–6628. doi: 10.1073/pnas.0400895101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grosjean Y, Grillet M, Augustin H, Ferveur JF, Featherstone DE. A glial amino-acid transporter controls synapse strength and courtship in Drosophila. Nat Neurosci. 2008;11:54–61. doi: 10.1038/nn2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 23.Ghiani CA, et al. The dysbindin-containing complex (BLOC-1) in brain: Developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Mol Psychiatry. 2010;15(2):115. doi: 10.1038/mp.2009.58. 204–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito H, et al. Dysbindin-1, WAVE2 and Abi-1 form a complex that regulates dendritic spine formation. Mol Psychiatry. 2010;15:976–986. doi: 10.1038/mp.2010.69. [DOI] [PubMed] [Google Scholar]
- 25.Heresco-Levy U, et al. Efficacy of high-dose glycine in the treatment of enduring negative symptoms of schizophrenia. Arch Gen Psychiatry. 1999;56:29–36. doi: 10.1001/archpsyc.56.1.29. [DOI] [PubMed] [Google Scholar]
- 26.Liu T, et al. Increased dopamine level enhances male-male courtship in Drosophila. J Neurosci. 2008;28:5539–5546. doi: 10.1523/JNEUROSCI.5290-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kong EC, et al. A pair of dopamine neurons target the D1-like dopamine receptor DopR in the central complex to promote ethanol-stimulated locomotion in Drosophila. PLoS ONE. 2010;5:e9954. doi: 10.1371/journal.pone.0009954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kanner BI, Fishkes H, Maron R, Sharon I, Schuldiner S. Reserpine as a competitive and reversible inhibitor of the catecholamine transporter of bovine chromaffin granules. FEBS Lett. 1979;100:175–178. doi: 10.1016/0014-5793(79)81158-8. [DOI] [PubMed] [Google Scholar]
- 29.Sjoerdsma A, Engelman K, Spector S, Udenfriend S. Inhibition of catecholamine synthesis in man with alpha-methyl-tyrosine, an inhibitor of tyrosine hydroxylase. Lancet. 1965;2:1092–1094. doi: 10.1016/s0140-6736(65)90062-0. [DOI] [PubMed] [Google Scholar]
- 30.Richardt A, et al. Ebony, a novel nonribosomal peptide synthetase for beta-alanine conjugation with biogenic amines in Drosophila. J Biol Chem. 2003;278:41160–41166. doi: 10.1074/jbc.M304303200. [DOI] [PubMed] [Google Scholar]
- 31.Jacobs ME. Influence of beta-alanine on mating and territorialism in Drosophila melanogaster. Behav Genet. 1978;8:487–502. doi: 10.1007/BF01067478. [DOI] [PubMed] [Google Scholar]
- 32.Monastirioti M. Biogenic amine systems in the fruit fly Drosophila melanogaster. Microsc Res Tech. 1999;45:106–121. doi: 10.1002/(SICI)1097-0029(19990415)45:2<106::AID-JEMT5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 33.Jackson FR, Haydon PG. Glial cell regulation of neurotransmission and behavior in Drosophila. Neuron Glia Biol. 2008;4:11–17. doi: 10.1017/S1740925X09000027. [DOI] [PubMed] [Google Scholar]
- 34.Borycz J, Borycz JA, Loubani M, Meinertzhagen IA. tan and ebony genes regulate a novel pathway for transmitter metabolism at fly photoreceptor terminals. J Neurosci. 2002;22:10549–10557. doi: 10.1523/JNEUROSCI.22-24-10549.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashimoto R, et al. A genetic variation in the dysbindin gene (DTNBP1) is associated with memory performance in healthy controls. World J Biol Psychiatry. 2010;11:431–438. doi: 10.1080/15622970902736503. [DOI] [PubMed] [Google Scholar]
- 36.Wolf C, Jackson MC, Kissling C, Thome J, Linden DE. Dysbindin-1 genotype effects on emotional working memory. Mol Psychiatry. 2011;16:145–155. doi: 10.1038/mp.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia S, et al. NMDA receptors mediate olfactory learning and memory in Drosophila. Curr Biol. 2005;15:603–615. doi: 10.1016/j.cub.2005.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu CL, et al. Specific requirement of NMDA receptors for long-term memory consolidation in Drosophila ellipsoid body. Nat Neurosci. 2007;10:1578–1586. doi: 10.1038/nn2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Iijima S, et al. Immunohistochemical detection of dysbindin at the astroglial endfeet around the capillaries of mouse brain. J Mol Histol. 2009;40:117–121. doi: 10.1007/s10735-009-9221-6. [DOI] [PubMed] [Google Scholar]
- 40.Fei E, et al. Nucleocytoplasmic shuttling of dysbindin-1, a schizophrenia related protein, regulates synapsin I expression. J Biol Chem. 2010;285:38630–38640. doi: 10.1074/jbc.M110.107912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oyama S, et al. Dysbindin-1, a schizophrenia-related protein, functionally interacts with the DNA- dependent protein kinase complex in an isoform-dependent manner. PLoS ONE. 2009;4:e4199. doi: 10.1371/journal.pone.0004199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Okuda H, et al. Dysbindin regulates the transcriptional level of myristoylated alanine-rich protein kinase C substrate via the interaction with NF-YB in mice brain. PLoS ONE. 2010;5:e8773. doi: 10.1371/journal.pone.0008773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mullin AP, Gokhale A, Larimore J, Faundez V. Cell biology of the BLOC-1 complex subunit dysbindin, a schizophrenia susceptibility gene. Mol Neurobiol. 2011;44:53–64. doi: 10.1007/s12035-011-8183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lloyd V, Ramaswami M, Krämer H. Not just pretty eyes: Drosophila eye-colour mutations and lysosomal delivery. Trends Cell Biol. 1998;8:257–259. doi: 10.1016/s0962-8924(98)01270-7. [DOI] [PubMed] [Google Scholar]
- 45.Furukubo-Tokunaga K. Modeling schizophrenia in flies. Prog Brain Res. 2009;179:107–115. doi: 10.1016/S0079-6123(09)17912-8. [DOI] [PubMed] [Google Scholar]
- 46.Jarskog LF, Miyamoto S, Lieberman JA. Schizophrenia: New pathological insights and therapies. Annu Rev Med. 2007;58:49–61. doi: 10.1146/annurev.med.58.060904.084114. [DOI] [PubMed] [Google Scholar]
- 47.Chen J, Lipska BK, Weinberger DR. Genetic mouse models of schizophrenia: From hypothesis-based to susceptibility gene-based models. Biol Psychiatry. 2006;59:1180–1188. doi: 10.1016/j.biopsych.2006.02.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.