Abstract
Neural stem/progenitor cell proliferation and differentiation are required to replace damaged neurons and regain brain function after hypoxic-ischemic events. DNA base lesions accumulating during hypoxic-ischemic stress are removed by DNA glycosylases in the base-excision repair pathway to prevent cytotoxicity and mutagenesis. Expression of the DNA glycosylase endonuclease VIII-like 3 (Neil3) is confined to regenerative subregions in the embryonic and perinatal brains. Here we show profound neuropathology in Neil3-knockout mice characterized by a reduced number of microglia and loss of proliferating neuronal progenitors in the striatum after hypoxia-ischemia. In vitro expansion of Neil3-deficient neural stem/progenitor cells revealed an inability to augment neurogenesis and a reduced capacity to repair for oxidative base lesions in single-stranded DNA. We propose that Neil3 exercises a highly specialized function through accurate molecular repair of DNA in rapidly proliferating cells.
Keywords: DNA damage, formamidopyrimidine-DNA glycosylase/endonuclease VIII, hydantoins, neural stem cells, neuronal progenitor cells
The base-excision repair pathway (BER) maintains genomic integrity by removing base lesions caused by oxidation, alkylation, and deamination. DNA base lesions frequently are cytotoxic or mutagenic if not removed. BER is initiated by DNA glycosylases that recognize modified bases and catalyze cleavage of the N-glycosidic bond, creating an apurinic or apyrimidinic (AP) site. The exposed DNA backbone is cleaved by the AP lyase activity of bifunctional DNA glycosylases or by an AP endonuclease. Repair synthesis is completed by gap filling and ligation (1, 2).
Endonuclease VIII-like 3 (NEIL3) and endonuclease VIII-like 1 (NEIL1) are mammalian oxidized base-specific DNA glycosylases (3, 4). The function of NEIL3 has remained enigmatic, but recently the mouse ortholog was shown to remove a broad spectrum of oxidative base lesions on single-stranded DNA substrates with preference for spiroiminodihydantoin (Sp) and guanidinohydantoin (Gh), which are further oxidation products of one of the most common base lesions, 8-oxo-7,8-dihydroguanine (8ohG) (5). These findings suggest that NEIL3 serves as a DNA glycosylase to prevent accumulation of cytotoxic and mutagenic DNA lesions in mammalian cells, although the activity of NEIL1 far exceeds that of NEIL3 on most substrates.
In the late postnatal and adult brain, newborn neurons arise from neural stem/progenitor cells (NSPCs) in both the subgranular zone (SGZ) of the hippocampal dentate gyrus and in the subventricular zone (SVZ) (6). We previously reported a discrete expression pattern of Neil3 in the rodent SGZ and SVZ, confined to the embryonic and perinatal stages (7, 8). These observations indicate a role for Neil3 in proliferating cells in the brain. However, naïve Neil3-knockout mice generated by us and others (4) appear phenotypically normal. After perinatal hypoxic-ischemic (HI) and adult ischemic stroke, proliferation of SVZ NSPCs is enhanced, and differentiating progeny repopulate the striatum to restore the lost structures (9–12). Recently, ex vivo experiments demonstrated how brain injury activates microglia, the resident macrophages of the brain that induce NSPC proliferation (13) and striatal migration of neuronal progenitors from the SVZ (14). Thus, to elucidate a possible role of Neil3 after brain damage, we challenged perinatal mice with HI to analyze cellular damage, the induction of neurogenesis, and the biochemical properties of DNA repair capacity.
Results
Neil3 Is Involved In Stress-Induced Neurogenesis.
To penetrate the role of Neil3 during neurogenesis in vivo, we generated Neil3-knockout mice (Fig. S1). In agreement with a previous report (4), our Neil3−/− mice were viable, fertile, and healthy into adulthood.
To examine the role of Neil3 during stress-induced neuronal injury and subsequent neurogenesis, we applied the widely used Levine method (15), modified for use in perinatal mice (16). A combination of hypoxia and cerebral ischemia produces injury confined to the brain hemisphere ipsilateral to the occluded common carotid artery (CCA). In our hands, the model provided detectable histological injury in the cortex, hippocampus, striatum, and thalamus, whereas the contralateral hemisphere was indistinguishable from a sham-treated brain, constituting a morphologically accurate internal control (Fig. S2 A and B). Sham-treated animals were anesthetized and a skin incision was made, but the CCA was not occluded and they did not undergo hypoxia. After 3 d, Neil3 expression had increased 2.2-fold in the ipsilateral striatum and 1.8-fold in the hippocampus, whereas the SVZ displayed levels comparable with those in the sham-treated brain (Fig. S2C). This pattern suggested a proliferative response in the striatum.
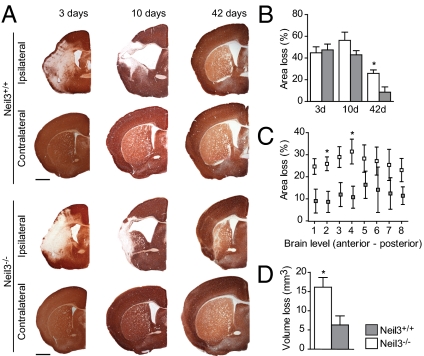
Neuronal injury caused by ischemic brain damage results in a nearly total loss of MAP2 immunoreactivity (17, 18). We analyzed brain sections from perinatal mice subjected to HI at three time points after injury (Fig. 1 A and B). In the subacute phase, 3 and 10 d after injury, a pronounced loss of MAP2 was detected in the ipsilateral hemispheres of both Neil3−/− and Neil3+/+ mice, but no difference in neuronal injury was observed between the two genotypes. After 10 d the anterior part of the hippocampus had disappeared, cystic lesions appeared in the cortex, and the lateral ventricle was dilated. Beyond this time point, neuronal tissue consisted, to some degree, of neurons generated post HI (11, 19). Forty-two days after injury the damaged brain structures were partially restored in both genotypes, but with reduced volume and extensive scarring in the ipsilateral hemisphere. The neuronal tissue deficit was significantly greater in Neil3−/− brains than in Neil3+/+ brains (74.1% and 91.3% viable neuronal tissue in the ipsilateral hemispheres, respectively). Similar results were obtained from the posterior parts of the forebrain, but the hippocampus sustained severe and incessant damage (Fig. S3). We conducted serial cross-sectioning of the entire forebrain and found the most profound differences between the two genotypes in the anterior sections (Fig. 1C). Moreover, the total infarct volume was 2.6-fold larger in Neil3−/− forebrains than in Neil3+/+ forebrains (Fig. 1D).
Fig. 1.
Neil3 deficiency reduces neuronal regeneration after perinatal HI. (A and B) Infarction size calculated as reduction in the MAP2 immunoreactive area (brown) in the ipsilateral hemispheres as a percentage of the contralateral hemisphere at bregma 0.4. (Scale bars, 1 mm.) *P = 0.024. (C) Area loss in coronal sections extending from brain level 1–8. *P < 0.05. (D) Loss of volume calculated from 16 evenly spaced sections throughout the forebrain. *P = 0.029. (B–D) n = 5–6 mice per time point. Data represent mean ± SEM.
To elucidate further the degree of cellular damage and death, we quantified double-strand breaks (DSBs) 3 d after HI by immunoreactivity to γH2AX antibody. Overall, sparse amounts of DSBs were present, but the ipsilateral hemisphere of both genotypes displayed an increase as compared with the contralateral hemisphere (6.4- and 5.0-fold increases in Neil3−/− and Neil3+/+, respectively) (Fig. S4A). There were no significant differences in the frequency of DSBs in any region of the Neil3−/− brain as compared with the Neil3+/+ brain. Very few γH2AX+ cells were present in the SVZ. Residing within the SVZ are cells that are immunopositive to doublecortin (DCX), a microtubule-associated protein transiently expressed in proliferating and migrating neuronal progenitors during development (20, 21) and injury-induced neurogenesis (9, 11, 12). DCX+ cells typically appear in clusters in the SVZ (Fig. S4A). We could not detect any γH2AX+/DCX+ cells. In contrast, HI provided widespread apoptotic cell death as measured by TUNEL, indicating free 3′-OH termini in DNA (Fig. S4B). In Neil3-deficient brains apoptotic cell death was reduced by 24% in subregions of the hippocampus and somewhat less and not significantly in striatal and thalamic nuclei. No TUNEL+ cells were detected in the SVZ.
In summary, we did not detect increased cellular damage or death in vivo in Neil3-deficient mice at an early stage after HI, but we found a significant deficit in reconstituted neuronal tissue after 42 d. These observations warranted investigation of the regenerative response.
Neil3 Deficiency Decreases the Number of Neuronal Progenitors After HI.
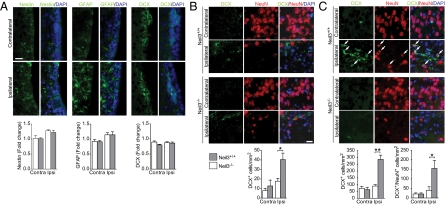
NSPCs reside in the SVZ and SGZ throughout life and are responsible for constitutive neurogenesis in the juvenile and adult forebrain (22). After perinatal HI (11, 12) and adult stroke (23), symmetric cell division contributes to expansion of the NSPC pool. Evidence suggests that neuronal progenitors in the SVZ are the predominant source of the new striatal neurons (19). We hypothesized that reduced neurogenesis in Neil3−/− animals was caused by a depletion of NSPCs in the SVZ. In agreement with previous reports (11, 12), we detected increased nestin expression within the ipsilateral SVZ after HI. Additionally, immunoreactivity to GFAP was augmented. GFAP is a marker for differentiated and reactivated astrocytes but also is expressed in NSPCs in the adult SVZ and SGZ (22, 24–26). To prove our hypothesis wrong, no differences could be detected between Neil3+/+ and Neil3−/− brains after HI (Fig. 2A). A more prominent increase in GFAP expression was detected in the SGZ of the hippocampal dentate gyrus in both genotypes (Fig. S5); however, the hippocampus sustained severe and permanent damage (Fig. S3).
Fig. 2.
Neuronal progenitor cell numbers are reduced in the Neil3-deficient striatum after HI. (A) Cellularity in the SVZ after HI. The images represent Neil3+/+. Bar graphs display densiometric quantification of fluorescent intensity measured in two nonadjacent coronal sections from each animal. Fold change from sham-treated animals was calculated. (B and C) Neuronal progenitors (DCX+) in striatal tissue 3 d (B) and 10 d (C) after injury. NeuN+/DCX+ neuronal progenitors are indicated by arrows. (B) *P = 0.027. (C) *P = 0.049; **P = 0.011. Cells were counted in two nonadjacent sections from each animal. (A–C) n = 4–5 mice. Data represent mean ± SEM. (Scale bars, 20 μm.)
NSPCs respond to injury with a multilineage cytogenic response. Progeny of nestin+ cells from the SVZ predominantly differentiate into GFAP+ astrocytes to repopulate the striatum, but DCX+ cells, NeuN+ terminally differentiated neurons, and O4+ oligodendrocytes also are offspring of the SVZ nestin+ cells (27). Some DCX+ cells retain their multipotentiality, but only cells restricted to the neuronal lineage express high levels of DCX and can be visualized in immunohistochemically processed tissue (28). Henceforward, we therefore define DCX+ cells as neuronal progenitors.
The number of neuronal progenitor cells in the SVZ did not increase after HI (Fig. 2A). However, when examining the adjacent striatal parenchyma, DCX+ cells with migratory profiles (29) were detected in increased numbers (Fig. 2 B and C). Quantification in predetermined striatal regions 3 and 10 d after HI revealed 56.0% (Fig. 2B) and 69.1% (Fig. 2C) reductions in Neil3−/− brains, respectively. After 10 d, DCX+/NeuN+ cells were present, demonstrating that a limited number of neuronal progenitors differentiated into postmitotic neurons to repopulate the injured site (Fig. 2C). The total number of DCX+/NeuN+ cells was significantly lower in Neil3-deficient striata, but the DCX+/NeuN+ over DCX+/NeuN-ratio was not (Neil3+/+ 54.3% vs. Neil3−/− 45.1%).
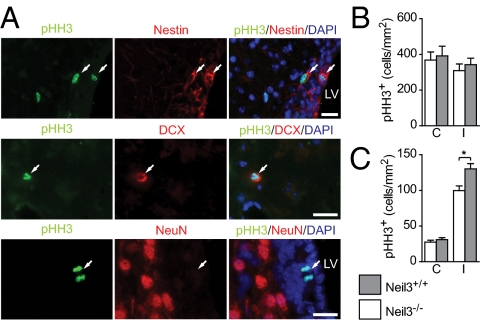
To address the distribution of proliferating cells after HI, we used the mitosis-specific antibody to phosphorylated histone H3 (pHH3) (Fig. 3 A–C). BrdU and Ki67 labeling was avoided because of evidence that BrdU+/NeuN+/TUNEL+ neurons may persist up to 7–14 d after HI and also express Ki67 before disappearing (30). We feared that labeling dying neurons could cause an overestimation of proliferation in Neil3+/+ mice from the data presented in Fig. S4B. Additionally, BrdU incorporation resulting from DNA repair, although in general negligible in an adult rodent brain (31), cannot be ruled out as a confounder in a DNA repair-deficient model. Using the pHH3 antibody, we detected a 23.1% reduction in the total number of dividing cells in the striatum of Neil3−/− animals 3 d after HI, as compared with Neil3+/+ animals (Fig. 3C). No differences in pHH3+ cell numbers were observed between Neil3+/+ and Neil3−/− animals in the SVZ (Fig. 3B). Furthermore, no NeuN+/pHH3+ cells were observed, but we detected nestin+/pHH3+ in the SVZ and DCX+/pHH3+ cells in the striatum, confirming the presence of proliferating NSPCs.
Fig. 3.
Mitotic cell numbers are reduced in the Neil3-deficient striatum. (A) Mitotic (white arrows) nestin+ cells in the SVZ, a mitotic DCX+ cell in the striatum, and NeuN+/pHH3− neurons adjacent to a mitotic cell in the SVZ 3 d after HI. LV, lateral ventricle. (Scale bars, 20 μm.) (B and C) The number of pHH3+ cells was quantified in the SVZ (B) and striatum (C) in two nonadjacent sections from each animal. C, contralateral hemisphere; I, ipsilateral hemisphere. *P = 0.019. n = 4–5 mice. Data represent mean± SEM.
In summary, increased nestin and GFAP immunoreactivity in the SVZ indicated that both Neil3+/+ and Neil3−/− mice responded to HI by expanding the NSPC pool. A population of DCX+ cells was depleted, and the number of dividing cells was reduced in the striatum of Neil3-deficient brains.
Neil3-Deficient Neurospheres Exhibit Poor Growth and Skewed Differentiation.
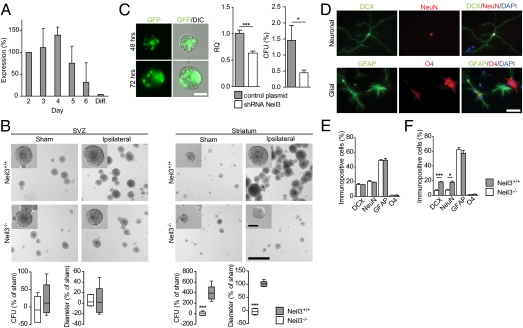
We used the neurosphere assay to examine further the proliferation defect following loss of Neil3. The neurosphere assay identifies NSPCs in vitro according to their self-renewal capacity and multipotency (32–34). Naïve wild-type spheres showed increasing Neil3 expression during propagation (Fig. 4A). The maximum expression was reached at day 4 after passaging, followed by a rapid decrease. Expression was diminished in differentiated cells, suggesting a primary role of Neil3 in proliferating cells.
Fig. 4.
Neil3-deficient NSPCs are indifferent to HI and show reduced differentiation in the neuronal lineage. (A) Neil3 expression in naïve wild-type neurospheres by qRT-PCR. Each graph represents the mean ± SEM of two independent experiments. Diff., differentiated cells. (B) Neurosphere cultures 4 d after first passage. (Scale bars, 500 μm; Inset, 100 μm.) CFU estimates from the ipsilateral hemisphere after HI are shown as change from sham-treated cultures of the same genotype. Whiskers indicate minimum and maximum of five counted areas. Each graph is representative of two independent experiments. ***P < 0.001. (C) Knockdown of Neil3 in neurospheres. *P = 0.047; ***P < 0.001. (Scale bar, 50 μm.) (D) Cells differentiated from Neil3+/+ SVZ neurospheres. (Scale bar, 50 μm.) (E and F) Quantification of cells differentiated from neurospheres from contralateral (E) and ipsilateral (F) SVZ. Values represent mean ± SEM of three technical replicates. Each graph is representative of two independent experiments. *P = 0.017; ***P < 0.001.
In line with current knowledge (11, 12), expansion of NSPCs from the ipsilateral hemispheres of Neil3+/+ brains was augmented after HI (Fig. 4B). No stroke-induced proliferation could be detected in Neil3−/− cultures. We experienced some variation in naïve colony formation, probably related to the cellular composition of primary cultures, and therefore used shRNA to knock down Neil3 (Fig. 4C). Attempts to transfect primary cell cultures resulted in impaired proliferation resulting from the transfection procedure; however, transfection was successful in stable high-passage SVZ neurospheres. A 37% reduction in Neil3 expression was achieved in naïve neurospheres, and we observed a 69.2% reduction of colony formation 72 h after transfection. A GFP control plasmid revealed heterogeneous expression, indicating that the transfection efficiency did not reach 100%. However, we observed a profound effect on proliferation in knockdown spheres, suggesting a selective incorporation of the plasmid in a subpopulation of cells important for proliferation. This result supports the notion that Neil3 is required for efficient proliferation of NSPCs.
We next analyzed the effect of Neil3 loss on differentiation of NSPCs in vitro. Single-cell suspensions derived from first- and second-passage neurospheres were induced to differentiate. Cells in the glial and neuronal lineages were identified by immunoreactivity to GFAP and O4 or to DCX and NeuN, respectively (Fig. 4D). In the contralateral hemisphere a total of 24.0% of both Neil3−/− and Neil3+/+ differentiated cells were committed to a neuronal lineage and were DCX+, NeuN+, or DCX+/NeuN+ (Fig. 4E). In the ipsilateral hemisphere a mere 9.9% of the Neil3−/− differentiated cells were neuronal, in contrast to 25.3% of the Neil3+/+ cells (Fig. 4F). In total, Neil3-deficient NSPCs displayed a 61% reduction of cells differentiating in the neuronal lineage after HI compared with Neil3+/+.
In summary, the in vitro data recapitulate the phenotype observed in Neil3−/− mice after HI, implying a defect in proliferation of neural progenitors and a subsequent skewed differentiation pattern. In the following section we provide evidence that these observations may be attributed to DNA repair deficiency.
Neil3 Is the Primary Contributor to Repair of Single-Stranded Hydantoin Lesions in Neural Stem/Progenitor Cells.
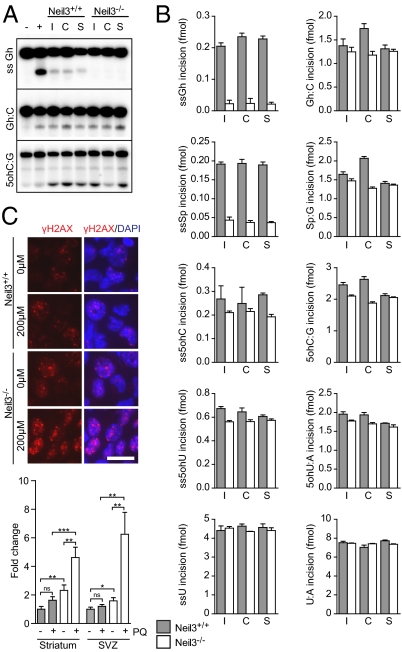
Stroke exacerbates oxidative stress and induces oxidative base lesions in DNA (35). Although the bulk of these lesions are repaired efficiently by the BER pathway, increased mutation rates indicate that not all oxidized bases are removed after ischemia (35). We therefore examined the repair capacity of total protein extracts from NSPCs at day 4 after first passaging and from brain tissue isolated 3 d post HI. Neil3−/− NSPCs from the SVZ showed normal capacity for repair of all double-stranded substrates [Sp, Gh, 5-hydroxycytosin (5ohC), and 5-hydroxyuracil (5ohU)] and a slightly reduced repair of 5ohC and 5ohU in ssDNA (about 90% of wild-type activity) (Fig. 5 A and B). In contrast, repair of Sp and Gh lesions in ssDNA was strongly reduced in Neil3−/− NSPCs (about 10% of wild-type activity). Similar results were obtained with NSPC extracts derived from striatal tissue. Brain tissue extracts from the SVZ and striatum had repair capacity similar to that of neurospheres on double-stranded substrates and single-stranded 5ohC and 5ohU. However, repair of Sp and Gh in ssDNA was below detection level. These results suggest that Neil3-expressing cells are enriched in neurosphere cultures but represent only a small fraction of the total cell mass in the brain, as shown previously by in situ hybridization experiments (7, 8).
Fig. 5.
Neil3−/− NSPCs are DNA-repair deficient. (A) Results of DNA glycosylase activity assay. +, purified core catalytic domain of Neil3; C, contralateral; I, ipsilateral; S, sham-treated. (B) DNA glycosylase activities of NSPCs derived from the SVZ of Neil3+/+ and Neil3−/− mice. Values represent mean ± SEM of three technical replicates. Each graph is representative of two independent experiments. 5ohC, 5-hydroxycytosin; 5ohU, 5-hydroxyuracil; Gh, guanidinohydantoin; Sp, spiroiminodihydantoin; U, uracil. (C) DNA strand breaks (γH2AX+) in dissociated naïve neurospheres after exposure to paraquat. Micrographs show SVZ-derived cells. (Scale bar, 20 μm.) Bar graph displays densiometric measurements of fluorophore-labeled γH2AX. ns, not significant; PQ, paraquat. Values represent mean ± SEM of 30 cell nuclei as fold change from nonexposed Neil3-proficient neurosphere cultures. *P < 0.05; **P < 0.01; ***P < 0.001.
If DNA repair is the mode of action for Neil3 in proliferating cells, Neil3-deficient neurospheres should exhibit abnormal DNA-damage response. We exposed proliferating neurospheres to the DNA-oxidizing agent paraquat and investigated the accumulation of DNA fragmentation (Fig. 5C and Fig. S6). Neil3-deficient cells exhibited increased accumulation of strand breaks detected as γH2AX immunoreactivity both in micrographs and on Western blots compared with Neil3-proficient cells, suggesting that Neil3 functions in repair of oxidative base lesions in NSPCs.
Our results show that Neil3 is the primary DNA glycosylase for removal of single-stranded Gh and Sp in NSPCs. Our data are in agreement with Liu, et al. (5), demonstrating that hydantoins are preferred substrates for Neil3. The next obvious step would be to monitor accumulation of hydantoins in brain tissue and neurospheres after HI. However, to our knowledge there is no available protocol with the sensitivity to detect hydantoins in mammalian tissue.
Microglial Response Is Decreased in Neil3-Deficient Brains.
Microglia may act on stroke-induced neurogenesis in a tightly regulated temporal and spatial manner (13, 36). They possess a dual role, being either neurotoxic or neuroprotective (37). The cells represent a mixture of intrinsically activated microglia and infiltrating blood cells breaching through the damaged blood–brain barrier (38). Because activated microglia also proliferate, we examined the microglia response after HI in Neil3-deficient mice.
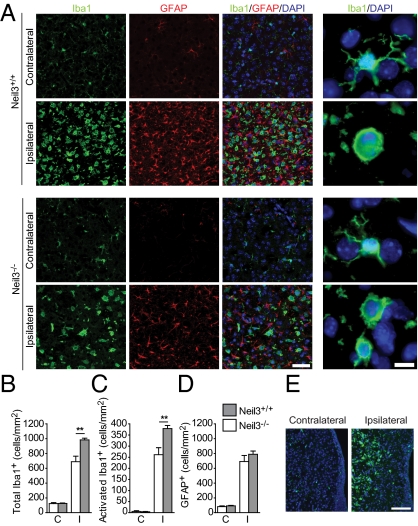
Microglial activation occurs early in the striatal peri-infarct area, whereas the presence of these cells peaks first after 6 wk within the SVZ (36). In agreement with this observation, we detected a profound increase in activated microglia in the ipsilateral striatum but not in the SVZ 3 d after injury (Fig. 6 A and E). In the striatum of Neil3−/− animals the total number of microglia was reduced by 30.0% compared with Neil3+/+ (Fig. 6B). We quantified the numbers of ramified surveying and round activated microglia based on morphological appearance (37). Comparable to the total count, there was a 31.1% deficit in the number of activated microglia (Fig. 6C). Thus, there were no differences in the composition of activation states between genotypes. The presence of GFAP+ astrocytes was not affected significantly by loss of Neil3 (Fig. 6D).
Fig. 6.
The microglial response to HI is reduced in Neil3-deficient animals. (A) Detection of microglia [ionized calcium-binding adaptor molecule 1-positive (Iba1+)] and astrocytes (GFAP+) in striatal tissue 3 d after HI. (Scale bar, 50 μm.) Enlargements (Right column) demonstrate a ramified, surveying morphology in the contralateral hemisphere and round, activated microglia in the ipsilateral hemisphere. (Scale bar, 10 μm.) (B–D) Quantification of the total number of Iba1+ cells (**P = 0.004) (B), activated Iba1+ cells (C), and GFAP+ cells (D) per square millimeter in striatum. (P = 0.009). C, contralateral; I, ipsilateral. n = 4–5 mice. Values represent mean ± SEM. (E) Identification of microglia (green) with activated morphology in the ipsilateral striatum but not in the SVZ of a Neil3+/+ mouse 3 d after HI. (Scale bar, 100 μm.)
Discussion
In the present study we used Neil3−/− mice to study the cytogenic response after perinatal HI. Neil3 belongs to the formamidopyrimidine-DNA glycosylase/endonuclease VIII superfamily of DNA glycosylases which, during the course of evolution, seems to have diverisfied alongside the increasing complexity of the central nervous system (4, 7, 8, 39). As opposed to Neil1 expression, which is widely distributed in the perinatal brain, only a fraction of cells in regenerative subregions express Neil3, and we and others have not previously detected a profound phenotype in naïve Neil3−/− mice (4, 7). Here we show that under stress Neil3-deficient mice failed to produce neuronal progenitors and replace damaged tissue to the same degree as Neil3-proficient mice. Neuronal damage sustained from HI was not increased by Neil3 deficiency. This observation implies that Neil3, in contrast to uracil-DNA glycosylase (40) and 8-oxoguanine DNA glycosylase (41), has no significant function in postmitotic cells but rather plays a role in regeneration. We found that, even in the presence of Neil1, Neil3 is the main DNA glycosylase for removal of the single-stranded hydantoin products Gh and Sp in proliferating NSPCs amplified from the perinatal mouse brain. Additionally, the NSPCs display an abnormal DNA-damage response, supporting the proposal that Neil3 functions in repair of oxidative DNA base lesions.
The pathogenesis of HI brain injury is complex and multifactorial. The degree of brain maturity and the timing and severity of the asphyxia are determinants of the initial outcome. Inflammatory responses, reactivation of glial cells, and angiogenesis and neurogenesis during the recovery phase are factors determining the final outcome. Increased oxidative stress is an early feature of HI, and apoptotic cell death following oxidative stress-regulated release of proapoptotic factors is more prominent in the immature brain (42). Inflammatory-activated microglia are proposed to promote apoptotic cell death via production of peroxynitrite and neurotoxic factors (43). Thus, the reduced number of apoptotic cells observed in Neil3-deficient brains may be explained by a decrease in the microglial response.
The oxidative burst from inflammatory macrophages after HI may produce excessive amounts of the oxidative lesion 8ohG. Notably, peroxynitrite is almost 1,000 times more reactive toward 8ohG than guanine, resulting in secondary oxidation products such as Sp and Gh (44, 45). These lesions are potent sources of replication blocks in vivo (46). The presence of hydantoin lesions remains to be determined in mammalian DNA, but endonuclease VIII-deficient Escherichia coli accumulates 20-fold more Sp lesions than WT when exposed to an oxidizing agent (47). Neil3-deficient NSPCs showed impaired ability to repair Sp and Gh in ssDNA and failed to augment proliferation in response to HI. We propose that accumulation of hydantoins in these cells inhibit replication and consequently impair proliferation and alter differentiation. The build-up of strand breaks in Neil3-deficient neurospheres exposed to an oxidizing agent support this proposal; however, we cannot exclude the possibility that impaired neurogenesis in Neil3−/− mice is partly epiphenomenal to alterations in the inflammatory response. Reactive astrocytes and activated microglia proliferate in response to ischemic injury and produce factors that mediate proliferation and migration of NSPCs (14, 48, 49). Thus, the decrease of neuronal progenitors in the striatum also could be attributed to a reduced microglial response.
Injury to the immature brain by HI, as seen after birth asphyxia and in preterm neonates, is a significant cause of severe and longstanding neurological disabilities. The SVZ is a promising therapeutic target, and insight into mechanisms for preservation of DNA integrity in NSPCs seems imperative for control of proliferation and differentiation. Grafting with human neural stem cells in perinatal rats enhances endogenous repair through improved neurotrophic support, gliogenesis, and neurogenesis after HI, possibly as a result of altered microglial response (50). DCX expression increases massively, and transgenic ablation of DCX-expressing cells worsens outcome (50, 51). Herein we provide evidence that ablation of Neil3 depletes DCX-expressing cells and microglia. We argue that Neil3 has a role in regeneration, involving repair of hydantoins in proliferating cells (NSPCs, neuronal progenitors, reactive astrocytes, and activated microglia) during the compound response to HI. This role provides motivation for examining NEIL3 DNA glycosylase in human neural stem cell grafts.
Materials and Methods
All methods are described in detail in SI Materials and Methods.
Generation of Neil3-Knockout Mice.
Neil3-deficient mice were generated by germline deletion of exons 3–5.
Perinatal HI.
Cerebral HI was produced in Neil3+/+ and Neil3−/− mice at postnatal day 9 (15, 16), by permanent occlusion of the CCA followed by hypoxia.
Culturing of Neurospheres, Immunocytochemistry, and Activity Assays.
Neurosphere cultures were established and propagated as described, and differentiation, immunocytochemistry, and activity assays were performed according to established protocols (3, 52, 53).
Supplementary Material
Acknowledgments
This work was supported by grants from the Research Council of Norway (NevroNor Research Program, Stem Cells Research Program), the Norwegian Cancer Society (Ragnvarda F. Soervik and Haakon Starheim's Foundation), The Laerdal Foundation for Acute Medicine, and the National Institutes of Health Grant CA090689 (to C.J.B.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. S.S.W. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1106880108/-/DCSupplemental.
References
- 1.Hegde ML, Hazra TK, Mitra S. Early steps in the DNA base excision/single-strand interruption repair pathway in mammalian cells. Cell Res. 2008;18:27–47. doi: 10.1038/cr.2008.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Robertson AB, Klungland A, Rognes T, Leiros I. DNA repair in mammalian cells: Base excision repair: The long and short of it. Cell Mol Life Sci. 2009;66:981–993. doi: 10.1007/s00018-009-8736-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morland I, et al. Human DNA glycosylases of the bacterial Fpg/MutM superfamily: An alternative pathway for the repair of 8-oxoguanine and other oxidation products in DNA. Nucleic Acids Res. 2002;30:4926–4936. doi: 10.1093/nar/gkf618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Torisu K, Tsuchimoto D, Ohnishi Y, Nakabeppu Y. Hematopoietic tissue-specific expression of mouse Neil3 for endonuclease VIII-like protein. J Biochem. 2005;138:763–772. doi: 10.1093/jb/mvi168. [DOI] [PubMed] [Google Scholar]
- 5.Liu M, et al. The mouse ortholog of NEIL3 is a functional DNA glycosylase in vitro and in vivo. Proc Natl Acad Sci USA. 2010;107:4925–4930. doi: 10.1073/pnas.0908307107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Landgren H, Curtis MA. Locating and labeling neural stem cells in the brain. J Cell Physiol. 2011;226:1–7. doi: 10.1002/jcp.22319. [DOI] [PubMed] [Google Scholar]
- 7.Rolseth V, et al. Widespread distribution of DNA glycosylases removing oxidative DNA lesions in human and rodent brains. DNA Repair (Amst) 2008;7:1578–1588. doi: 10.1016/j.dnarep.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hildrestrand GA, et al. Expression patterns of Neil3 during embryonic brain development and neoplasia. BMC Neurosci. 2009;10:45. doi: 10.1186/1471-2202-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- 10.Yamashita T, et al. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang Z, Levison SW. Hypoxia/ischemia expands the regenerative capacity of progenitors in the perinatal subventricular zone. Neuroscience. 2006;139:555–564. doi: 10.1016/j.neuroscience.2005.12.059. [DOI] [PubMed] [Google Scholar]
- 12.Felling RJ, et al. Neural stem/progenitor cells participate in the regenerative response to perinatal hypoxia/ischemia. J Neurosci. 2006;26:4359–4369. doi: 10.1523/JNEUROSCI.1898-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deierborg T, Roybon L, Inacio AR, Pesic J, Brundin P. Brain injury activates microglia that induce neural stem cell proliferation ex vivo and promote differentiation of neurosphere-derived cells into neurons and oligodendrocytes. Neuroscience. 2010;171:1386–1396. doi: 10.1016/j.neuroscience.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 14.Yan YP, Lang BT, Vemuganti R, Dempsey RJ. Osteopontin is a mediator of the lateral migration of neuroblasts from the subventricular zone after focal cerebral ischemia. Neurochem Int. 2009;55:826–832. doi: 10.1016/j.neuint.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Levine S. Anoxic-ischemic encephalopathy in rats. Am J Pathol. 1960;36:1–17. [PMC free article] [PubMed] [Google Scholar]
- 16.Sheldon RA, Sedik C, Ferriero DM. Strain-related brain injury in neonatal mice subjected to hypoxia-ischemia. Brain Res. 1998;810:114–122. doi: 10.1016/s0006-8993(98)00892-0. [DOI] [PubMed] [Google Scholar]
- 17.Kitagawa K, et al. Microtubule-associated protein 2 as a sensitive marker for cerebral ischemic damage—immunohistochemical investigation of dendritic damage. Neuroscience. 1989;31:401–411. doi: 10.1016/0306-4522(89)90383-7. [DOI] [PubMed] [Google Scholar]
- 18.Ballough GP, et al. Microtubule-associated protein 2 (MAP-2): A sensitive marker of seizure-related brain damage. J Neurosci Methods. 1995;61:23–32. doi: 10.1016/0165-0270(95)00019-q. [DOI] [PubMed] [Google Scholar]
- 19.Yang Z, Levison SW. Perinatal hypoxic/ischemic brain injury induces persistent production of striatal neurons from subventricular zone progenitors. Dev Neurosci. 2007;29:331–340. doi: 10.1159/000105474. [DOI] [PubMed] [Google Scholar]
- 20.Francis F, et al. Doublecortin is a developmentally regulated, microtubule-associated protein expressed in migrating and differentiating neurons. Neuron. 1999;23:247–256. doi: 10.1016/s0896-6273(00)80777-1. [DOI] [PubMed] [Google Scholar]
- 21.Gleeson JG, Lin PT, Flanagan LA, Walsh CA. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron. 1999;23:257–271. doi: 10.1016/s0896-6273(00)80778-3. [DOI] [PubMed] [Google Scholar]
- 22.Garcia AD, Doan NB, Imura T, Bush TG, Sofroniew MV. GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain. Nat Neurosci. 2004;7:1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- 23.Zhang R, et al. Activated neural stem cells contribute to stroke-induced neurogenesis and neuroblast migration toward the infarct boundary in adult rats. J Cereb Blood Flow Metab. 2004;24:441–448. doi: 10.1097/00004647-200404000-00009. [DOI] [PubMed] [Google Scholar]
- 24.Doetsch F, Caillé I, Lim DA, García-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 25.Merkle FT, Tramontin AD, García-Verdugo JM, Alvarez-Buylla A. Radial glia give rise to adult neural stem cells in the subventricular zone. Proc Natl Acad Sci USA. 2004;101:17528–17532. doi: 10.1073/pnas.0407893101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buffo A, et al. Origin and progeny of reactive gliosis: A source of multipotent cells in the injured brain. Proc Natl Acad Sci USA. 2008;105:3581–3586. doi: 10.1073/pnas.0709002105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li L, et al. Focal cerebral ischemia induces a multilineage cytogenic response from adult subventricular zone that is predominantly gliogenic. Glia. 2010;58:1610–1619. doi: 10.1002/glia.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Walker TL, Yasuda T, Adams DJ, Bartlett PF. The doublecortin-expressing population in the developing and adult brain contains multipotential precursors in addition to neuronal-lineage cells. J Neurosci. 2007;27:3734–3742. doi: 10.1523/JNEUROSCI.5060-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang RL, et al. Neuroblast division during migration toward the ischemic striatum: A study of dynamic migratory and proliferative characteristics of neuroblasts from the subventricular zone. J Neurosci. 2007;27:3157–3162. doi: 10.1523/JNEUROSCI.4969-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuan CY, et al. Hypoxia-ischemia induces DNA synthesis without cell proliferation in dying neurons in adult rodent brain. J Neurosci. 2004;24:10763–10772. doi: 10.1523/JNEUROSCI.3883-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cooper-Kuhn CM, Kuhn HG. Is it all DNA repair? Methodological considerations for detecting neurogenesis in the adult brain. Brain Res Dev Brain Res. 2002;134:13–21. doi: 10.1016/s0165-3806(01)00243-7. [DOI] [PubMed] [Google Scholar]
- 32.Potten CS, Loeffler M. Stem cells: Attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 33.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 34.Reynolds BA, Tetzlaff W, Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci. 1992;12:4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu PK, et al. Damage, repair, and mutagenesis in nuclear genes after mouse forebrain ischemia-reperfusion. J Neurosci. 1996;16:6795–6806. doi: 10.1523/JNEUROSCI.16-21-06795.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thored P, et al. Long-term accumulation of microglia with proneurogenic phenotype concomitant with persistent neurogenesis in adult subventricular zone after stroke. Glia. 2009;57:835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- 37.Hanisch UK, Kettenmann H. Microglia: Active sensor and versatile effector cells in the normal and pathologic brain. Nat Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 38.Lyons SA, et al. Distinct physiologic properties of microglia and blood-borne cells in rat brain slices after permanent middle cerebral artery occlusion. J Cereb Blood Flow Metab. 2000;20:1537–1549. doi: 10.1097/00004647-200011000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Takao M, et al. Human Nei-like protein NEIL3 has AP lyase activity specific for single-stranded DNA and confers oxidative stress resistance in Escherichia coli mutant. Genes Cells. 2009;14:261–270. doi: 10.1111/j.1365-2443.2008.01271.x. [DOI] [PubMed] [Google Scholar]
- 40.Endres M, et al. Increased postischemic brain injury in mice deficient in uracil-DNA glycosylase. J Clin Invest. 2004;113:1711–1721. doi: 10.1172/JCI20926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liu D, et al. Evidence that OGG1 glycosylase protects neurons against oxidative DNA damage and cell death under ischemic conditions. J Cereb Blood Flow Metab. 2011;31:680–692. doi: 10.1038/jcbfm.2010.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blomgren K, Hagberg H. Free radicals, mitochondria, and hypoxia-ischemia in the developing brain. Free Radic Biol Med. 2006;40:388–397. doi: 10.1016/j.freeradbiomed.2005.08.040. [DOI] [PubMed] [Google Scholar]
- 43.Brown GC, Neher JJ. Inflammatory neurodegeneration and mechanisms of microglial killing of neurons. Mol Neurobiol. 2010;41:242–247. doi: 10.1007/s12035-010-8105-9. [DOI] [PubMed] [Google Scholar]
- 44.Uppu RM, Cueto R, Squadrito GL, Salgo MG, Pryor WA. Competitive reactions of peroxynitrite with 2′-deoxyguanosine and 7,8-dihydro-8-oxo-2′-deoxyguanosine (8-oxodG): Relevance to the formation of 8-oxodG in DNA exposed to peroxynitrite. Free Radic Biol Med. 1996;21:407–411. doi: 10.1016/0891-5849(96)00220-1. [DOI] [PubMed] [Google Scholar]
- 45.Niles JC, Wishnok JS, Tannenbaum SR. Spiroiminodihydantoin and guanidinohydantoin are the dominant products of 8-oxoguanosine oxidation at low fluxes of peroxynitrite: Mechanistic studies with 18O. Chem Res Toxicol. 2004;17:1510–1519. doi: 10.1021/tx0400048. [DOI] [PubMed] [Google Scholar]
- 46.Henderson PT, et al. The hydantoin lesions formed from oxidation of 7,8-dihydro-8-oxoguanine are potent sources of replication errors in vivo. Biochemistry. 2003;42:9257–9262. doi: 10.1021/bi0347252. [DOI] [PubMed] [Google Scholar]
- 47.Hailer MK, Slade PG, Martin BD, Sugden KD. Nei deficient Escherichia coli are sensitive to chromate and accumulate the oxidized guanine lesion spiroiminodihydantoin. Chem Res Toxicol. 2005;18:1378–1383. doi: 10.1021/tx0501379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Miller JT, et al. The neuroblast and angioblast chemotaxic factor SDF-1 (CXCL12) expression is briefly up regulated by reactive astrocytes in brain following neonatal hypoxic-ischemic injury. BMC Neurosci. 2005;6:63. doi: 10.1186/1471-2202-6-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan YP, et al. Monocyte chemoattractant protein-1 plays a critical role in neuroblast migration after focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:1213–1224. doi: 10.1038/sj.jcbfm.9600432. [DOI] [PubMed] [Google Scholar]
- 50.Daadi MM, et al. Human neural stem cell grafts modify microglial response and enhance axonal sprouting in neonatal hypoxic-ischemic brain injury. Stroke. 2010;41:516–523. doi: 10.1161/STROKEAHA.109.573691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin K, Wang X, Xie L, Mao XO, Greenberg DA. Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice. Proc Natl Acad Sci USA. 2010;107:7993–7998. doi: 10.1073/pnas.1000154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Machon O, et al. Forebrain-specific promoter/enhancer D6 derived from the mouse Dach1 gene controls expression in neural stem cells. Neuroscience. 2002;112:951–966. doi: 10.1016/s0306-4522(02)00053-2. [DOI] [PubMed] [Google Scholar]
- 53.Rappa G, et al. Efficient expansion and gene transduction of mouse neural stem/progenitor cells on recombinant fibronectin. Neuroscience. 2004;124:823–830. doi: 10.1016/j.neuroscience.2003.11.030. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.