Abstract
Cystic fibrosis (CF) is caused by mutations in the CF transmembrane conductance regulator (CFTR) gene that impair the function of CFTR, an epithelial chloride channel required for proper function of the lung, pancreas, and other organs. Most patients with CF carry the F508del CFTR mutation, which causes defective CFTR protein folding and processing in the endoplasmic reticulum, resulting in minimal amounts of CFTR at the cell surface. One strategy to treat these patients is to correct the processing of F508del-CFTR with small molecules. Here we describe the in vitro pharmacology of VX-809, a CFTR corrector that was advanced into clinical development for the treatment of CF. In cultured human bronchial epithelial cells isolated from patients with CF homozygous for F508del, VX-809 improved F508del-CFTR processing in the endoplasmic reticulum and enhanced chloride secretion to approximately 14% of non-CF human bronchial epithelial cells (EC50, 81 ± 19 nM), a level associated with mild CF in patients with less disruptive CFTR mutations. F508del-CFTR corrected by VX-809 exhibited biochemical and functional characteristics similar to normal CFTR, including biochemical susceptibility to proteolysis, residence time in the plasma membrane, and single-channel open probability. VX-809 was more efficacious and selective for CFTR than previously reported CFTR correctors. VX-809 represents a class of CFTR corrector that specifically addresses the underlying processing defect in F508del-CFTR.
Keywords: cystic fibrosis transmembrane modulator, VX-770
Cystic fibrosis (CF) is a fatal autosomal-recessive genetic disorder caused by loss-of-function mutations in the CFTR gene, which encodes for the CFTR protein (CF transmembrane conductance regulator) (1, 2). CFTR is a chloride ion channel that regulates epithelial salt and fluid transport in numerous tissues, including the lung, pancreas, intestine, reproductive tract, and sweat gland (3). CFTR mutations that reduce CFTR protein function cause accumulation of thick, sticky mucus in the bronchi of the lungs, loss of exocrine pancreatic function, impaired intestinal secretion, and an increase in the concentration of chloride in the sweat (4, 5). Patients with CF require numerous therapies to manage these symptoms (3), including mucolytic and antibiotic agents and chest physiotherapy to treat the airway disease and digestive enzymes to replace the loss of exocrine pancreatic function. These and other interventions have increased life expectancy dramatically, but improvement is needed to reduce the high treatment burden and increase survival (6, 7).
Since the discovery that a loss of CFTR function causes CF (1, 2), there have been efforts to restore CFTR function with gene therapy or drugs to ameliorate the disease (8). In support of this approach, the CFTR potentiator VX-770 (9) improved in vivo measures of chloride transport and lung function in patients with CF with the G551D CFTR channel gating mutation (10). Although these results support increasing CFTR function as a strategy to treat CF, the G551D CFTR mutation is present in fewer than 5% of patients with CF (11).
To restore or improve CFTR function in the majority of the population of patients with CF, it will likely be necessary to target the underlying molecular defect in CFTR caused by the F508del CFTR mutation, which is present in 90% of patients with CF (1, 11). The F508del CFTR mutation impairs CFTR processing in the endoplasmic reticulum (ER) by preventing the protein from folding properly (12–14). Misfolded F508del-CFTR is retained by the ER and degraded, reducing F508del-CFTR delivery to the cell surface (15). In addition, the small amount of F508del-CFTR that is delivered to the cell surface exhibits defective channel gating and increased turnover (16, 17). To enhance chloride transport via F508del-CFTR, drugs that increase the delivery of functional F508del-CFTR to the cell surface may be required. Such agents are called CFTR correctors (18, 19). Our goal was to discover CFTR correctors that could advance to clinical studies in patients with CF with the F508del CFTR mutation.
Results
Discovery of VX-809.
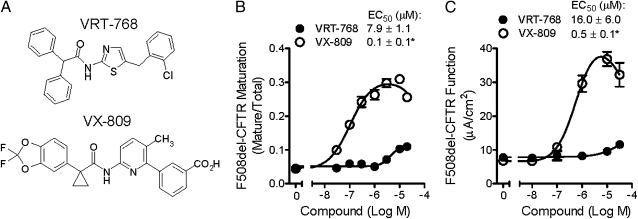
To discover CFTR correctors, we screened 164,000 small molecules for compounds that increased F508del-CFTR–mediated chloride transport in a recombinant cell-based assay (18). Active compounds were prioritized based on evidence of improved F508del-CFTR processing in the ER and increased functional F508del-CFTR at the cell surface. Immunoblot techniques were used to measure F508del-CFTR exit from the ER and passage through the Golgi, which is characterized by an increase in the molecular weight of CFTR (from a 135–140-kDa band to a 170–180-kDa band) as a result of glycosylation (20). After CFTR is processed by the Golgi, the mature, complex-glycosylated CFTR form is delivered to the cell surface. To allow sufficient time for de novo synthesis, ER processing, and cellular trafficking of F508del-CFTR to reach steady state, cells were incubated with compounds for 48 h before measurement. One active compound, VRT-768 (Fig. 1A), increased F508del-CFTR maturation by 2.5 ± 0.1 fold (EC50, 16 ± 6 μΜ; n = 4) and enhanced chloride transport (EC50, 7.9 ± 1.1 μM; n = 4) compared with vehicle-treated controls in Fischer rat thyroid (FRT) cells expressing F508del-CFTR (Fig. 1B and Fig. S1A).
Fig. 1.
VRT-768 and VX-809 increased F508del-CFTR maturation and chloride transport in FRT cells. (A) Chemical structures of VRT-768 and VX-809. (B) F508del-CFTR maturation, expressed as a ratio of mature CFTR to total CFTR (mature and immature), in FRT cells treated for 48 h with the indicated VRT-768 (•) or VX-809 (○) concentrations (n = 4). (C) F508del-CFTR function in FRT cells preincubated for 48 h with the indicated VRT-768 (•) or VX-809 (○) concentrations (n = 3). Forskolin was added to activate CFTR through the cAMP/PKA pathway, and CFTR function was measured as transepithelial currents in the presence of basolateral to apical chloride gradient. Asterisks indicate significant difference vs. VRT-768 (P < 0.05; t test).
Extensive medicinal chemistry and structure activity analyses were used to improve in vitro potency, efficacy, and other drug-like properties of the chemical scaffold represented by VRT-768. By using the central amide bond to synthesize analogues, the role of the amine and the acid moieties were probed independently, resulting in the identification of several distinct chemical scaffolds, one of which led to VX-809 (Fig. 1A). Compared with VRT-768, VX-809 had greater potency and improved in vitro efficacy as determined by enhanced F508del-CFTR maturation and chloride transport (Fig. 1B). In FRT cells, VX-809 improved F508del-CFTR maturation by 7.1 ± 0.3 fold (n = 3) compared with vehicle-treated cells (EC50, 0.1 ± 0.1 μM; n = 3: Fig. 1B and Fig. S1A) and enhanced F508del-CFTR–mediated chloride transport by approximately fivefold (EC50, 0.5 ± 0.1 μM; n = 3: Fig. 1C). At VX-809 concentrations greater than 10 μM, the response was reduced, resulting in a bell-shaped dose–response relationship with an IC50 of approximately 100 μM. VX-809 was orally bioavailable in rats and achieved in vivo plasma levels significantly above concentrations required for in vitro efficacy (SI Materials and Methods).
VX-809 Corrected Folding and Processing Defect of F508del-CFTR.
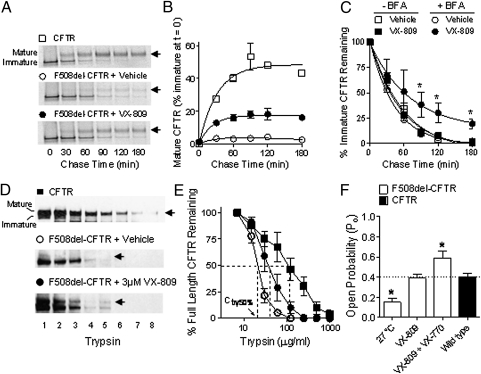
The efficiency of F508del-CFTR correction in the presence of VX-809 was determined by measuring the fractional conversion of the ER-associated, core-glycosylated CFTR form to the mature, complex-glycosylated CFTR form by using metabolic pulse-chase techniques (20). In HEK-293 cells expressing F508del-CFTR, 24-h treatment with 3 μM VX-809 increased F508del-CFTR exit from the ER by sixfold compared with vehicle-treated cells, reaching levels comparable to 34 ± 4% (n = 3) of CFTR (Fig. 2 A and B). F508del-CFTR that did not exit the ER in the presence of VX-809 appeared to be degraded at a similar rate compared with vehicle-treated cells (Fig. 2C and Fig. S1B). These data suggest that VX-809 allowed proper processing of a fraction of the F508del-CFTR in the ER (12, 21). Consistent with this hypothesis, blocking ER export to the Golgi with brefeldin A (BFA) (22) revealed that 28% ± 10% (n = 3) of the F508del-CFTR trapped in the ER was resistant to degradation in the presence of VX-809, as the rate of decay was slower compared with vehicle-treated cells (Fig. 2C and Fig. 1B). The effect of VX-809 did not appear to be caused by inhibition of the proteasome degradation pathway, as VX-809 did not inhibit the degradation of a proteosome reporter substrate (Fig. S1C). Taken together, these results suggested that VX-809 increased the conformational stability of a fraction of F508del-CFTR in the ER, allowing it to exit the ER and traffic to the cell surface.
Fig. 2.
Biochemical and functional data suggest that VX-809 acted at the level of the ER to allow a fraction of the F508del-CFTR pool to adopt a properly folded form. (A) Representative gels from a pulse-chase experiment shows the conversion of immature to mature CFTR in HEK-293 cells expressing CFTR (□) or F508del-CFTR following 24-h incubation with vehicle (○) or 3 μM VX-809 (•). Arrows indicate mature CFTR. (B) Quantification of mature F508del-CFTR shown in A (n = 4). (C) Quantification of immature CFTR during the 180-min chase in cells pretreated with vehicle (open symbols) or 3 μM VX-809 (filled symbols) in the presence (circles) and absence of BFA (squares). (D) Immunoblot of trypsin-digested microsomes from cells expressing CFTR or F508del-CFTR pretreated with vehicle or 3 μM VX-809 for 24 h. Trypsin concentrations were 0, 15, 30, 60, 120, 240, 480, and 960 μg/mL (labeled 1–8, respectively). Arrows indicate mature CFTR. (E) Quantification of the data in D. The trypsin concentration value required to eliminate 50% of the full-length (mature and immature) CFTR protein (Ctry50%) is indicated by the dotted lines. (F) Quantification of the Po of CFTR channels in excised membranes from NIH 3T3 cells expressing CFTR or F508del-CFTR. Cells expressing F508del-CFTR were pretreated for 48 h with vehicle or 3 μM VX-809 at 37 °C or vehicle at 27 °C. To activate CFTR, 1 mM ATP and 100 U/mL PKA were added to the bath. Acute VX-770 addition further increased the Po of F508del-CFTR following VX-809 treatment. Asterisks indicate significant difference vs. CFTR (dotted line).
Limited proteolysis assays were used to test whether the increased ER export of F508del-CFTR following VX-809 treatment was associated with changes in F508del-CFTR protein conformation. This technique is based on the premise that folded proteins are more compact and therefore typically more resistant to proteolytic digestion than unfolded or partially folded proteins and has been used to probe differences in protein folding between CFTR and F508del-CFTR (12, 23–25). As previously shown (3, 12), full-length F508del-CFTR and the second nucleotide binding domain (NBD2) fragment (∼25-kDa band) of F508del-CFTR were more susceptible to proteolytic digestion than those of CFTR (Fig. 2 D and E and Fig. S2). The trypsin concentrations required to eliminate 50% (Ctry50%) of both full-length and the NBD2 fragment of F508del-CFTR were significantly higher in VX-809–treated cells compared with vehicle-treated cells (Fig. 2 D and E and Fig. S2). These data indicate that VX-809 allowed a fraction of the F508del-CFTR in the ER to form a more compact protease-resistant conformation, consistent with improved folding of F508del-CFTR.
Defects in F508del-CFTR folding have been linked to its impaired channel gating (26–28). To determine whether correction of F508del-CFTR by VX-809 resulted in CFTR protein with normal channel gating, the channel open probability (Po) of F508del-CFTR was assessed by using single-channel patch-clamp techniques (Fig. 2F and Fig. S2D). The F508del-CFTR delivered to the cell surface following treatment (24–48 h) with 3 μM VX-809 had a Po of 0.39 ± 0.04 (n = 9), which was indistinguishable from that of CFTR (Po, 0.40 ± 0.04; n = 6) and higher than that reported for uncorrected F508del-CFTR [Po, 0.1 (27)]. The Po of F508del-CFTR in VX-809–treated cells was also higher than the Po of F508del-CFTR in cells incubated at 27 °C for 24 to 48 h (Po, 0.15 ± 0.04; n = 9), a treatment previously shown to improve F508del-CFTR processing but not channel gating (16). Acute addition of VX-809 had no effect on F508del-CFTR function (Fig. S3), suggesting that VX-809 is not a CFTR potentiator. Acute addition of the CFTR potentiator VX-770 further increased the Po of F508del-CFTR following VX-809 treatment (Po, 0.59 ± 0.07; n = 9), indicating that VX-770 potentiated the channel gating activity of VX-809-corrected F508del-CFTR.
VX-809 Enhanced Chloride and Fluid Transport in Cultures of CF Airway Epithelial Cells.
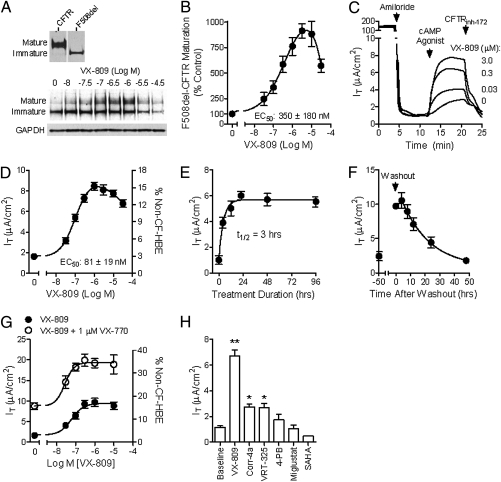
The pharmacology of VX-809 was assessed in cultured human bronchial epithelial (HBE) cells isolated from the lungs of seven patients with CF homozygous for the F508del-CFTR mutation (F508del-HBE). Incubation of F508del-HBE with VX-809 for 48 h increased CFTR maturation by approximately eightfold, with an EC50 of 350 ± 180 nM, and enhanced chloride transport by approximately fourfold, from 1.9 ± 0.4 μA/cm2 to 7.8 ± 1.3 μA/cm2, with an EC50 of 81 ± 19 nM (Fig. 3 A–D and Table S1). This corresponded to an increase in chloride transport from 3.4 ± 0.7% to 13.9 ± 2.3% of that measured in HBE isolated from four non-CF donor lungs (56 ± 6 μA/cm2; Fig. 3D and Table S1). The increased chloride transport in VX-809–corrected F508del-HBE exhibited pharmacological hallmarks of normal CFTR, such as dependence on stimulation by the cAMP/protein kinase A (PKA) signaling pathway and inhibition by a specific CFTR blocker (Fig. 3C). The maximum effect of VX-809 occurred following 24 h of treatment (t1/2, ∼3 h; Fig. 3E), consistent with the time required for newly synthesized F508del-CFTR to reach a steady state at the cell surface (17). Following VX-809 washout, chloride transport returned to uncorrected levels within 48 h (t1/2, ∼13 h; Fig. 3F). The t1/2 of VX-809–corrected F508del-CFTR was similar to that of CFTR [t1/2, 16–24 h (17)] and considerably longer than vehicle-treated (t1/2, <4 h) or 27 °C-corrected F508del-CFTR (Fig. S4). The long residence time in the plasma membrane suggests that VX-809–corrected F508del-CFTR is not recognized as unfolded by the peripheral protein quality control mechanism (29), supporting the biochemical and functional data that VX-809 improved F508del-CFTR protein folding.
Fig. 3.
VX-809 increased CFTR maturation and chloride secretion in cultured F508del-HBE. (A) Glycosylation pattern of CFTR (Upper) and F508del-CFTR pretreated for 48 h with VX-809 at the indicated concentrations (Lower). (B) Quantification of the data in A (n = 3) expressed as a percentage of the mature/total CFTR in the absence of VX-809 (as percent of control). (C) Representative recording of the forskolin (10 μM)-stimulated IT in F508del-HBE pretreated for 48 h with VX-809 at the indicated concentrations. Before adding forskolin, amiloride was added to block the epithelial Na+ channel. A basolateral-to-apical chloride gradient was used for Ussing chamber experiments. (D) Quantification of the forskolin-stimulated IT in F508del-HBE isolated from seven patients with CF homozygous for the F508del-CFTR mutation (left y axis). Right y axis shows the IT normalized to the 10 μM forskolin-stimulated IT in non-CF HBE. (E) The onset of VX-809 action was determined by measuring the CFTR-mediated IT in F508del-HBE pretreated with 3 μM VX-809 for the indicated times (n = 6; data from single donor lung). (F) Cell surface turnover of F508del-CFTR was determined by first incubating F508del-HBE for 48 h with 3 μM VX-809 and then measuring the forskolin-stimulated IT at the indicated times after VX-809 washout (data from single donor lung; n = 6). (G) Concentration response curve for VX-809 in the absence (•) and presence of 1 μM VX-770 (○) in F508del-HBE from a single donor. (H) Mean (±SEM) forskolin-stimulated IT values in F508del-HBE pretreated for 48 h with VX-809 (3 μM), 4-phenylbutyrate (4-PB; 1,500 μM), Corr-4a (10 μM), and VRT-325 (6.7 μM) for 8 d with 1 μM SAHA or 2 to 4 h with 100 μM miglustat. The concentration and treatment duration for each compound were based on the maximally effective experimental conditions published for the previously described CFTR corrector (18, 30, 43, 44). Asterisks indicate significant (P < 0.05; paired t test) increase in IT vs. untreated levels.
To enhance chloride transport through F508del-CFTR corrected by VX-809, the CFTR potentiator VX-770 (ivacaftor) (9) was added to maximize the Po of the CFTR channel. Acute application of 1 μM VX-770 increased forskolin-stimulated chloride transport in cultured F508del-HBE pretreated with VX-809 for 48 h (Fig. 3G). At the maximally effective concentrations of both compounds, F508del-CFTR–mediated chloride transport reached levels equivalent to approximately 25% of that measured in non-CF HBE (Fig. 3G and Table S1). These results are consistent with the increase in Po of VX-809–corrected F508del-CFTR by VX-770 in recombinant cells (Fig. 2F).
The in vitro efficacy of VX-809 was compared with several previously described CFTR correctors and low-temperature correction in Ussing chamber studies by using cultured F508del-HBE. These included drugs approved for non-CF indications [e.g., 4-phenylbutyrate, miglustat, sildenafil, suberoylanilide hydroxamic acid (SAHA)] (19, 30,31–32) and compounds identified through high-throughput screening (e.g., corr-4a, VRT-325) (18, 19). The cultured F508del-HBE cells were preincubated with each compound at the maximally effective concentration and treatment duration (Fig. 3H). In cultured F508del-HBE, VX-809 (3 μM) was significantly (P < 0.05; ANOVA followed by Tukey multiple-comparison test) more efficacious than Corr-4a (10 μM) and VRT-325 (6.7 μM; Fig. 3H), as well as 27 °C-corrected F508del-CFTR (Fig. S4B). No significant correction of F508del-CFTR was observed in cultured F508del-HBE for the other known CFTR correctors (Fig. 3H), although SAHA did increase forskolin-stimulated chloride secretion in recombinant FRT cells expressing F508del-CFTR (Fig. S5). The ability of some CFTR correctors to act in recombinant cells expressing CFTR but not in native HBE cultures has been previously reported (33) and highlights the importance of confirming activity in native epithelial cells.
In the CF lung, the loss of CFTR-mediated chloride transport is believed to cause an imbalance between fluid secretion and absorption, resulting in airway surface dehydration (4). Confocal microscopy was used to test if VX-809 increased the airway surface liquid (ASL) height in cultured F508del-HBE. To activate CFTR, vasoactive intestinal peptide was added to the basolateral surface throughout the treatment period (9). Addition of VX-809 to the basolateral surface for 5 d increased the ASL height from 4.5± 0.2 μm to 6.7 ± 0.5 μm, indicating less fluid absorption, more secretion, or both. Addition of 3 μM VX-770 with VX-809 further increased the ASL height to 9.2 ± 0.2 μm (Fig. S6), which was consistent with the additive effects of VX-770 and VX-809 on chloride transport (Fig. 3G).
VX-809 Showed Selectivity for Correction of CFTR Processing.
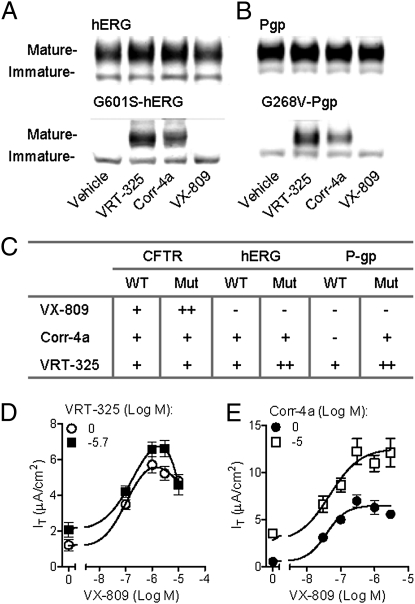
To assess the selectivity of VX-809 action, we compared the ability of VX-809, VRT-325, and Corr-4a to correct the normal and ER-arrested mutant forms of CFTR (CFTR and F508del-CFTR), the human ether-à-go-go–related K+ channel (hERG; G601S-hERG), and P-glycoprotein (P-gp; G268V-P-gp or Y490del-P-gp) in HEK-293 cells. VX-809 corrected CFTR and F508del-CFTR (Fig. 1 and Fig. S7), but did not improve the processing of the normal or mutant forms of hERG or P-gp (Fig. 4 A–C and Fig. S7). Furthermore, VX-809 did not correct other disease-causing mislocalized proteins, including α1-antitrypsin Z mutant (E342K-α1-AT) or N370S-β-glucosidase (Fig. S7). In contrast, VRT-325 and Corr-4a increased the processing of all proteins tested (Fig. 4 and Fig. S7). Although the amount of F508del-CFTR correction by VRT-325 and Corr-4a was similar (Fig. 4A), VRT-325 was more effective in increasing the processing of non-CFTR proteins. Also, all compounds tested showed greater effects on the processing of mutant vs. normal proteins.
Fig. 4.
VX-809 did not improve the processing of non-CFTR misfolded proteins. The effects of 48 h treatment with 10 μM VX-809, 6.7 μM VRT-325 (18), and 10 μM Corr-4a (19) on the maturation of normal and mutant hERG (G601S-hERG) (A) and P-gp (G268V-P-gp) (B) transiently expressed in HEK-293-cells. (C) Summary of the effects of VX-809, Corr-4a, and VRT-325 on the cellular processing of normal (i.e., WT) and mutant (mut) CFTR, P-gp, and hERG (Fig. S7). Dash indicates no significant difference vs. vehicle-treated controls. A single plus sign indicates a significant difference compared with vehicle-treated controls, whereas a double plus sign indicates a significant difference between vehicle-treated controls and the other compound treatments (ANOVA followed by Tukey multiple-comparison test; n = 3–5). (D and E) Additive effects of VX-809 and VRT-325 (D) or Corr-4a (E) at the indicated concentrations on CFTR-mediated chloride transport in cultured F508del-HBE isolated from a single donor bronchi (n = 4).
The different in vitro selectivity profiles of VX-809, VRT-325, and Corr-4a suggest that they may act through distinct pathways and/or molecular targets to correct F508del-CFTR. Consistent with this hypothesis, VX-809 was additive with VRT-325 and Corr-4a in Ussing chamber studies of CFTR-mediated chloride transport in cultured F508del-HBE (Fig. 4 D and E).
Discussion
Several CFTR correctors have been previously reported to be active in vitro, including drugs approved for non-CF indications (e.g., 4-phenylbutyrate, miglustat, sildenafil, SAHA) (19, 30,31–32) and compounds identified through high-throughput screening (e.g., corr-4a, VRT-325) (18, 19). Therapies for CF have not advanced from these efforts, possibly due to insufficient restoration of CFTR function and/or poor selectivity for processing of CFTR compared with other proteins and other off-target actions. VX-809 represents a class of CFTR correctors, distinguished from previously described CFTR correctors by the magnitude of its increase in F508del-CFTR-mediated chloride transport in cultures of F508del-HBE and its selectivity for CFTR vs. other normal and misfolded proteins. Based on its favorable in vitro and in vivo profiles, VX-809 was advanced into clinical studies evaluating the safety and efficacy of VX-809 alone and in combination with VX-770 in patients with CF homozygous for the F508del-CFTR mutation.
Several lines of evidence suggest that VX-809 works by promoting the proper folding of a fraction of F508del-CFTR during its biogenesis and processing in the ER, allowing it to exit the ER, traffic to the cell surface, and function normally. First, VX-809 increased the efficiency of F508del-CFTR ER export, suggesting that a fraction of F508del-CFTR attained a more stable protein conformation that was not recognized as defective by the ER quality control pathways (12, 21, 34, 35). Second, VX-809 decreased the proteolytic sensitivity of full-length F508del-CFTR and the NBD2 fragment of F508del-CFTR, consistent with a more compact protein conformation (12, 23, 24). Third, the channel gating activity of VX-809–corrected F508del-CFTR was normal, indicating that VX-809 promoted the proper domain–domain interactions previously shown to be essential for normal channel gating (26, 28). Fourth, the cell surface stability of VX-809–corrected F508del-CFTR was similar to that of CFTR, suggesting that it was not recognized as defective by the peripheral quality control pathways and degraded (29).
Under the conditions tested, the maximal level of chloride transport in cultured F508del-HBE treated with VX-809 reached 14% of non-CF HBE. In recombinant HEK cells, we observed that approximately 34% of the F508del-CFTR pool showed improved processing in the ER. Thus, while VX-809 is affecting a fraction of the F508del-CFTR pool, it does not correct F508-del CFTR processing to normal levels. It is not clear if this reflects a fundamental limitation of the mechanism of VX-809 action or if additional optimization of the VX-809 chemical scaffold would lead to compounds that further improved processing. However, the additivity of VX-809 with Corr-4a or VRT-325 suggests that the available pool of F508del-CFTR in the ER is not limiting the efficacy of CFTR correction by small molecules.
A notable feature of VX-809 compared with previously described CFTR correctors was its improved ability to increase the processing of normal and F508del-CFTR selectively. Although only a small number of proteins were included in the present study, they represented proteins that use similar trafficking pathways as CFTR (hERG, G601S-hERG) or are from the same superfamily as CFTR (G268V-P-gp, Y490del-P-gp), as well as other ER-arrested misfolded proteins that likely use chaperone pathways distinct from CFTR (α1-ATZ and N370S-β-glucosidase) (36–39). The effect of VX-809 on F508del-CFTR and Corr-4a and VRT-325 on F508del-CFTR and other mutant proteins was greater than that observed for the normal protein forms. This is likely a result of the efficient processing of normally folded proteins compared with misfolded proteins in the ER. These results suggest that VX-809 acts directly with CFTR and/or CFTR-associated proteins, whereas VRT-325 and Corr-4a may act on multiple proteins or on a more general mechanism involved in protein processing. Additional studies are required to determine the mechanism of action of VX-809.
The different in vitro efficacy and selectivity profiles of VX-809, Corr-4a, and VRT-325 along with the functional additivity of the CFTR correctors in cultured F508del-HBE show that several distinct pathways and/or molecular targets are amenable to small-molecule correction of F508del-CFTR. Previous studies have shown that effects of Corr-4a and VRT-325 on the processing of F508del-CFTR are additive (40). These in vitro results support the rationale of combining corrector compounds as one strategy to achieve more correction of F508del-CFTR.
Genotype-to-phenotype studies correlating in vivo CFTR function, as determined by nasal potential difference techniques, with disease severity suggest that more than 10% of normal CFTR chloride transport is associated with milder CF as characterized by a lower incidence of pancreatic insufficiency, later age at diagnosis, a more moderate lung function decline, and lower sweat chloride levels (∼80 mmol/L) compared with those with minimal (<10% normal CFTR function) CFTR chloride transport (i.e., patients with F508del-homozygous CF) (41, 42). The in vitro results presented here show that VX-809 increased chloride transport in cultured F508del-HBE to approximately 14% of that measured in non-CF HBE, which was sufficient to increase the ASL height. These results suggest that VX-809 correction might reach levels that affect airway epithelial function and therefore may be clinically meaningful. Recently, results from a 28-d study of up to 200 mg/d of VX-809 in CF subjects homozygous for F508del-CFTR demonstrated evidence of increased transport as determined by a decrease in sweat chloride. However, no change in lung function was observed (45). Additional clinical studies are planned to determine if VX-809 can produce clinical benefit in patients with CF. If additional efficacy is required, the in vitro results presented here suggest that combinations with CFTR potentiators, such as VX-770, and/or different classes of CFTR correctors could further increase F508del-CFTR–mediated chloride transport. The discovery of an efficacious and selective CFTR corrector suitable for advancement into clinical studies represents a step forward in the development of potential new therapies to treat the basic defect in patients with CF with the F508del CFTR mutation.
Materials and Methods
Cell Culture and Media.
FRT cells or HEK-293 cells stably expressing human normal or F508del-CFTR were cultured as previously described (9, 18) (SI Materials and Methods). HBE cells were isolated from the bronchi of lungs obtained from non-CF or F508del-homozygous subjects with CF and cultured as previously described (9,18).
Electrophysiology.
Ussing chamber techniques with FRT and HBE cells were used to record the transepithelial current (IT) resulting from CFTR-mediated chloride transport (9). The single-channel activity of CFTR was measured by using excised inside-out membrane patch recordings (9) Further details are provided in SI Materials and Methods.
CFTR Immunoblot Analysis.
Immunoblot techniques using the monoclonal CFTR antibody 769 were used to measure CFTR maturation in FRT, HEK-293, or HBE cells expressing CFTR or F508del-CFTR (18). Pulse-chase studies using HEK-293 cells expressing CFTR or F508del-CFTR were performed as previously described (18). Limited proteolysis studies were performed by using HEK-293 cells expressing F508del-CFTR or CFTR and the primary CFTR antibodies, 660 or 596 (all CFTR antibodies provided by John R. Riordan, University of North Carolina, Chapel Hill, NC). Further details are provided in SI Materials and Methods.
Measurement of ASL Volume.
Confocal studies were used to monitor the ASL height by using 10,000 kDa dextran conjugated to Alexa Fluor 488 (SI Materials and Methods).
P-gp and hERG Immunoblot Analysis.
HEK-293 cells were transiently transfected with a pcDNA3.1-based vector containing cDNA for P-gp, G268V-P-gp, or Y490del-P-gp and processed for Western blotting by using monoclonal P-gp antibody C219 (Abcam). HEK-293 cells stably expressing hERG or G601S-hERG were processed for Western blot by using a hERG antibody 373500 (Merck). Further details are provided in SI Materials and Methods.
α1-Antitrypsin Metabolic Pulse-Chase Analysis.
HeLa cells were transiently transfected with a pcDNA3.1-based vector containing cDNA for the E342K form of the α1-antitrypsin (AAT) Z mutant (gift from W. E. Balch, Scripps Research Institute, La Jolla, CA) and processed for metabolic pulse-chase analysis (SI Materials and Methods).
β-Glucosidase Assay.
Primary skin fibroblasts isolated from a male type I Gaucher disease patient (GM00372) were obtained from Coriell Cell Repositories, and the enzymatic activity of β-glucosidase was measured as described in SI Materials and Methods.
Statistical Analyses.
Statistical comparisons were made by using ANOVA followed by Tukey multiple-comparison test or Student t test (Prism 5; GraphPad Software). All data are presented as mean ± SEM.
Supplementary Material
Acknowledgments
We thank Robert Beall, Preston Campbell, John Chabala, Eric Gordon, Diana Wetmore, and Christopher M. Penland for guidance; John Riordan for anti-CFTR antibodies; Bill Balch for the z-AAT construct; Mike Welsh for FRT cell lines; and Neil Bradbury for HEK-293 cell line expressing F508del-CFTR. This study was supported by Cystic Fibrosis Foundation Therapeutics.
Footnotes
Conflict of interest statement: F.V.G., S.H., P.D.J.G., B.B., J.H.S., K.S.S., C.J.D., M.M., J.M., E.R.O., and P.A.N. are employees of Vertex Pharmaceuticals, which is evaluating VX-809 as a potential treatment for cystic fibrosis.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1105787108/-/DCSupplemental.
References
- 1.Castellani C, et al. Consensus on the use and interpretation of cystic fibrosis mutation analysis in clinical practice. J Cyst Fibros. 2008;7:179–196. doi: 10.1016/j.jcf.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Riordan JR, et al. Identification of the cystic fibrosis gene: Cloning and characterization of complementary DNA. Science. 1989;245:1066–1073. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- 3.Farrell PM, et al. Cystic Fibrosis Foundation Guidelines for diagnosis of cystic fibrosis in newborns through older adults: Cystic Fibrosis Foundation consensus report. J Pediatr. 2008;153:S4–S14. doi: 10.1016/j.jpeds.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boucher RC. Cystic fibrosis: A disease of vulnerability to airway surface dehydration. Trends Mol Med. 2007;13:231–240. doi: 10.1016/j.molmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Quinton PM. Cystic fibrosis: Lessons from the sweat gland. Physiology (Bethesda) 2007;22:212–225. doi: 10.1152/physiol.00041.2006. [DOI] [PubMed] [Google Scholar]
- 6.Cystic Fibrosis Foundation . US Cystic Fibrosis Patient Registry. Bethesda: Cystic Fibrosis Foundation; 2002. [Google Scholar]
- 7.Sawicki GS, Sellers DE, Robinson WM. High treatment burden in adults with cystic fibrosis: Challenges to disease self-management. J Cyst Fibros. 2009;8:91–96. doi: 10.1016/j.jcf.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ashlock MA, et al. A pipeline of therapies for cystic fibrosis. Semin Respir Crit Care Med. 2009;30:611–626. doi: 10.1055/s-0029-1238919. [DOI] [PubMed] [Google Scholar]
- 9.Van Goor F, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci USA. 2009;106:18825–18830. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Accurso FJ, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bobadilla JL, Macek M, Jr, Fine JP, Farrell PM. Cystic fibrosis: A worldwide analysis of CFTR mutations—correlation with incidence data and application to screening. Hum Mutat. 2002;19:575–606. doi: 10.1002/humu.10041. [DOI] [PubMed] [Google Scholar]
- 12.Du K, Sharma M, Lukacs GL. The DeltaF508 cystic fibrosis mutation impairs domain-domain interactions and arrests post-translational folding of CFTR. Nat Struct Mol Biol. 2005;12:17–25. doi: 10.1038/nsmb882. [DOI] [PubMed] [Google Scholar]
- 13.Thibodeau PH, Brautigam CA, Machius M, Thomas PJ. Side chain and backbone contributions of Phe508 to CFTR folding. Nat Struct Mol Biol. 2005;12:10–16. doi: 10.1038/nsmb881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas PJ, Ko YH, Pedersen PL. Altered protein folding may be the molecular basis of most cases of cystic fibrosis. FEBS Lett. 1992;312:7–9. doi: 10.1016/0014-5793(92)81399-7. [DOI] [PubMed] [Google Scholar]
- 15.Denning GM, Ostedgaard LS, Welsh MJ. Abnormal localization of cystic fibrosis transmembrane conductance regulator in primary cultures of cystic fibrosis airway epithelia. J Cell Biol. 1992;118:551–559. doi: 10.1083/jcb.118.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Denning GM, et al. Processing of mutant cystic fibrosis transmembrane conductance regulator is temperature-sensitive. Nature. 1992;358:761–764. doi: 10.1038/358761a0. [DOI] [PubMed] [Google Scholar]
- 17.Lukacs GL, et al. The delta F508 mutation decreases the stability of cystic fibrosis transmembrane conductance regulator in the plasma membrane. Determination of functional half-lives on transfected cells. J Biol Chem. 1993;268:21592–21598. [PubMed] [Google Scholar]
- 18.Van Goor F, et al. Rescue of DeltaF508-CFTR trafficking and gating in human cystic fibrosis airway primary cultures by small molecules. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1117–L1130. doi: 10.1152/ajplung.00169.2005. [DOI] [PubMed] [Google Scholar]
- 19.Verkman AS, Galietta LJ. Chloride channels as drug targets. Nat Rev Drug Discov. 2008;8:153–171. doi: 10.1038/nrd2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng SH, et al. Defective intracellular transport and processing of CFTR is the molecular basis of most cystic fibrosis. Cell. 1990;63:827–834. doi: 10.1016/0092-8674(90)90148-8. [DOI] [PubMed] [Google Scholar]
- 21.Kowalski JM, Parekh RN, Mao J, Wittrup KD. Protein folding stability can determine the efficiency of escape from endoplasmic reticulum quality control. J Biol Chem. 1998;273:19453–19458. doi: 10.1074/jbc.273.31.19453. [DOI] [PubMed] [Google Scholar]
- 22.Lukacs GL, et al. Conformational maturation of CFTR but not its mutant counterpart (delta F508) occurs in the endoplasmic reticulum and requires ATP. EMBO J. 1994;13:6076–6086. doi: 10.1002/j.1460-2075.1994.tb06954.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cui L, et al. Domain interdependence in the biosynthetic assembly of CFTR. J Mol Biol. 2007;365:981–994. doi: 10.1016/j.jmb.2006.10.086. [DOI] [PubMed] [Google Scholar]
- 24.Fontana A, et al. Probing protein structure by limited proteolysis. Acta Biochim Pol. 2004;51:299–321. [PubMed] [Google Scholar]
- 25.Zhang F, Kartner N, Lukacs GL. Limited proteolysis as a probe for arrested conformational maturation of delta F508 CFTR. Nat Struct Biol. 1998;5:180–183. doi: 10.1038/nsb0398-180. [DOI] [PubMed] [Google Scholar]
- 26.Cui L, et al. The role of cystic fibrosis transmembrane conductance regulator phenylalanine 508 side chain in ion channel gating. J Physiol. 2006;572:347–358. doi: 10.1113/jphysiol.2005.099457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dalemans W, et al. Altered chloride ion channel kinetics associated with the delta F508 cystic fibrosis mutation. Nature. 1991;354:526–528. doi: 10.1038/354526a0. [DOI] [PubMed] [Google Scholar]
- 28.Serohijos AW, et al. Phenylalanine-508 mediates a cytoplasmic-membrane domain contact in the CFTR 3D structure crucial to assembly and channel function. Proc Natl Acad Sci USA. 2008;105:3256–3261. doi: 10.1073/pnas.0800254105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okiyoneda T, et al. Peripheral protein quality control removes unfolded CFTR from the plasma membrane. Science. 2010;329:805–810. doi: 10.1126/science.1191542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hutt DM, et al. Reduced histone deacetylase 7 activity restores function to misfolded CFTR in cystic fibrosis. Nat Chem Biol. 2010;6:25–33. doi: 10.1038/nchembio.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rubenstein RC, Egan ME, Zeitlin PL. In vitro pharmacologic restoration of CFTR-mediated chloride transport with sodium 4-phenylbutyrate in cystic fibrosis epithelial cells containing delta F508-CFTR. J Clin Invest. 1997;100:2457–2465. doi: 10.1172/JCI119788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rubenstein RC, Zeitlin PL. A pilot clinical trial of oral sodium 4-phenylbutyrate (Buphenyl) in deltaF508-homozygous cystic fibrosis patients: partial restoration of nasal epithelial CFTR function. Am J Respir Crit Care Med. 1998;157:484–490. doi: 10.1164/ajrccm.157.2.9706088. [DOI] [PubMed] [Google Scholar]
- 33.Pedemonte N, Tomati V, Sondo E, Galietta LJ. Influence of cell background on pharmacological rescue of mutant CFTR. Am J Physiol Cell Physiol. 2010;298:C866–C874. doi: 10.1152/ajpcell.00404.2009. [DOI] [PubMed] [Google Scholar]
- 34.Nagy JK, Sanders CR. Destabilizing mutations promote membrane protein misfolding. Biochemistry. 2004;43:19–25. doi: 10.1021/bi035918s. [DOI] [PubMed] [Google Scholar]
- 35.Wilson MH, Highfield HA, Limbird LE. The role of a conserved inter-transmembrane domain interface in regulating alpha(2a)-adrenergic receptor conformational stability and cell-surface turnover. Mol Pharmacol. 2001;59:929–938. doi: 10.1124/mol.59.4.929. [DOI] [PubMed] [Google Scholar]
- 36.Ficker E, Dennis A, Kuryshev Y, Wible BA, Brown AM. HERG channel trafficking. Novartis Found Symp. 2005;266:57–69. [PubMed] [Google Scholar]
- 37.Burrows JA, Willis LK, Perlmutter DH. Chemical chaperones mediate increased secretion of mutant alpha 1-antitrypsin (alpha 1-AT) Z: A potential pharmacological strategy for prevention of liver injury and emphysema in alpha 1-AT deficiency. Proc Natl Acad Sci USA. 2000;97:1796–1801. doi: 10.1073/pnas.97.4.1796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Loo TW, Bartlett MC, Clarke DM. Rescue of DeltaF508 and other misprocessed CFTR mutants by a novel quinazoline compound. Mol Pharm. 2005;2:407–413. doi: 10.1021/mp0500521. [DOI] [PubMed] [Google Scholar]
- 39.Yu L, et al. Alpha-1-C-octyl-1-deoxynojirimycin as a pharmacological chaperone for Gaucher disease. Bioorg Med Chem. 2006;14:7736–7744. doi: 10.1016/j.bmc.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 40.Wang Y, Loo TW, Bartlett MC, Clarke DM. Additive effect of multiple pharmacological chaperones on maturation of CFTR processing mutants. Biochem J. 2007;406:257–263. doi: 10.1042/BJ20070478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rowe SM, Accurso F, Clancy JP. Detection of cystic fibrosis transmembrane conductance regulator activity in early-phase clinical trials. Proc Am Thorac Soc. 2007;4:387–398. doi: 10.1513/pats.200703-043BR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Strausbaugh SD, Davis PB. Cystic fibrosis: A review of epidemiology and pathobiology. Clin Chest Med. 2007;28:279–288. doi: 10.1016/j.ccm.2007.02.011. [DOI] [PubMed] [Google Scholar]
- 43.Noël S, Wilke M, Bot AG, De Jonge HR, Becq F. Parallel improvement of sodium and chloride transport defects by miglustat (n-butyldeoxynojyrimicin) in cystic fibrosis epithelial cells. J Pharmacol Exp Ther. 2008;325:1016–1023. doi: 10.1124/jpet.107.135582. [DOI] [PubMed] [Google Scholar]
- 44.Pedemonte N, et al. Small-molecule correctors of defective DeltaF508-CFTR cellular processing identified by high-throughput screening. J Clin Invest. 2005;115:2564–2571. doi: 10.1172/JCI24898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Clancy JP, et al. Results of a Phase 2a Study of VX-809, an investigational CFTR corrector compound in subjects, homozygous for the F508del-CFTR mutation. Thorax. 2011 doi: 10.1136/thoraxjnl-2011-200393. 10.1136/thoraxjnl-2011-200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.