Abstract
A switch from oxidative phosphorylation to glycolysis is frequently observed in cancer cells and is linked to tumor growth and invasion, but the underpinning molecular mechanisms controlling the switch are poorly understood. In this report we show that Notch signaling is a key regulator of cellular metabolism. Both hyper- and hypoactivated Notch induce a glycolytic phenotype in breast tumor cells, although by distinct mechanisms: hyperactivated Notch signaling leads to increased glycolysis through activation of the phosphatidylinositol 3-kinase/AKT serine/threonine kinase pathway, whereas hypoactivated Notch signaling attenuates mitochondrial activity and induces glycolysis in a p53-dependent manner. Despite the fact that cells with both hyper- and hypoactivated Notch signaling showed enhanced glycolysis, only cells with hyperactivated Notch promoted aggressive tumor growth in a xenograft mouse model. This phenomenon may be explained by that only Notch-hyperactivated, but not -hypoactivated, cells retained the capacity to switch back to oxidative phosphorylation. In conclusion, our data reveal a role for Notch in cellular energy homeostasis, and show that Notch signaling is required for metabolic flexibility.
Cancer cells frequently rely on glycolysis rather than oxidative phosphorylation (OXPHOS) for energy generation. This phenomenon was first observed by Otto Warburg, who more than 80 y ago found that cancer cells, despite ample access to oxygen, prefer to metabolize glucose by aerobic glycolysis (1). The reason to opt for aerobic glycolysis is not fully understood, but it has been proposed that intermediates of the glycolytic pathway are important for biosynthesis required for rapid growth or to prime cancer cells for survival in hypoxic areas. Down-regulation of OXPHOS may also induce resistance to apoptosis by compromising intrinsic apoptotic programs. Recent data indicate that metabolic reprogramming promotes unrestricted growth and constitutes an essential component of the invasive phenotype (2–5).
The molecular mechanisms underlying the metabolic reprogramming are complex and only partially understood. Activation of oncogenic signals and the loss of tumor suppressors are critical modulators of tumor cell metabolism. Notably, activation of the phosphatidylinositol 3-kinase (PI3K)/AKT serine/threonine kinase pathway (6), Ras (7, 8), Myc (9), loss of the tumor suppressor p53 (10, 11), and activation of the cellular hypoxic response (12, 13) are linked to enhanced glycolysis. There is an emerging view that the glycolytic phenotype is not caused by permanent mitochondrial damage but that mitochondrial activity in many instances is retained (14), and that metabolic flexibility rather than a permanent switch to glycolysis is important for tumor progression. Cancer cells appear to have a substantial reserve capacity for OXPHOS (15). Recent data in fact suggest an important role for functional mitochondria in oncogenic transformation and tumor growth (16, 17).
In this report we have explored the role of Notch in metabolic control of tumor cells. The Notch pathway is important for differentiation in most cell types (18) and frequently deregulated in cancer (19, 20). Activating mutations in the Notch1 receptor are found in the majority of patients with acute lymphoblastic T-cell leukemia (T-ALL) (21), and deregulated Notch signaling is observed in solid tumors such as breast cancer (22–26). Notch signaling also cross-talks with the cellular hypoxic response, which is an important glycolysis driver (27, 28). We show that both activation and inhibition of Notch enhance glycolysis, although by different mechanisms. Activation of Notch resulted in activation of PI3K/AKT signaling, whereas inhibition of Notch reduced the activity of the mitochondrial respiratory chain and decreased p53 protein levels, accompanied by enhanced glycolysis. Notch inhibition rendered cells dependent on glucose and blocked growth under restricted conditions, whereas hyperactivated Notch signaling showed uncontrolled invasive tumor growth. The data indicate that Notch is important for maintenance of metabolic flexibility and that the glycolytic phenotype does not automatically enhance the tumorigenic potential.
Results
Hyperactive, but Not Hypoactive, Notch Signaling Promotes Tumor Growth and Invasiveness in Vivo.
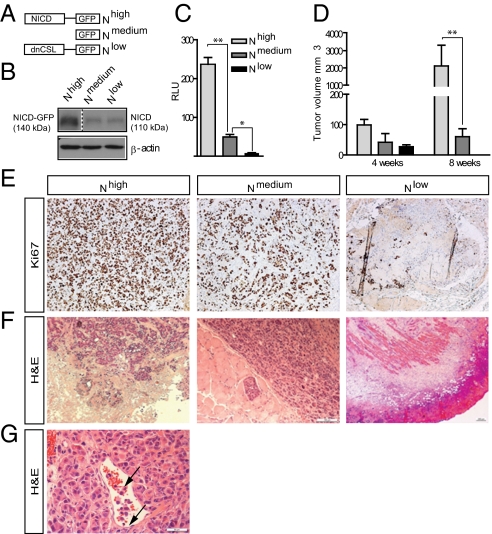
Notch signaling is activated by ligands on juxtaposed cells, liberating the Notch intracellular domain (NICD), which translocates to the nucleus where it interacts with the DNA-binding protein CSL (for CBF-1/Suppressor of Hairless/Lag-1) to regulate expression of downstream genes (18, 29). To explore the role of Notch signaling in breast tumor growth and cellular metabolism, we engineered MCF7 luminal-type breast cancer cells to express high, normal, and reduced Notch activity by stable expression of constructs NICD1-GFP, GFP, and dominant-negative CSL–GFP, respectively (Fig. 1 A–C). The cells are hereafter referred to as Nhigh, Nmedium, and Nlow cells, respectively.
Fig. 1.
Notch induces enhanced tumor growth and invasiveness. (A) Schematic depiction of the Nhigh, Nmedium, and Nlow cells. (B) NICD levels in Nhigh, Nmedium, and Nlow cells. NICD in Nhigh cells is expressed as a GFP fusion form. (C) Notch signaling activity from the 12xCSL-luc reporter in Nhigh, Nmedium, and Nlow cells. (D) Tumor volume measured at 4 and 8 wk after xenografting. (E) Expression of the proliferation marker Ki67 in Nhigh, Nmedium, and Nlow tumors at 8 wk. Nlow shows mainly connective tissue. (F) Histological analyses of Nhigh, Nmedium, and Nlow tumors showing invasive growth pattern of Nhigh tumors and the remaining scar tissue of Nlow tumors. (G) Infiltration of Nhigh tumor cells (arrows) into surrounding vessels. (Scale bar: E–G, 100 μm.) Values are significant at **P < 0.01 and *P < 0.05 as indicated in C and D. Values indicate the average of at least three independent experiments.
Upon orthotopical xenotransplantation, tumors developed from all three cell lines, and the Nhigh cell-derived tumors showed dramatically enhanced tumor size (Fig. 1D) coupled with increased proliferation compared with Nmedium cells (Fig. 1E). Furthermore, the Nhigh tumors exhibited an aggressive basal-like phenotype with a spindle-shaped cellular morphology, invasive growth pattern (including local invasion into blood vessels), and a blurred tumor-stroma boundary (Fig. 1 F and G).
In contrast, despite initial growth, Nlow tumors later regressed, and at 8 wk only scar tissue was visible (Fig. 1 E and F). In sum, these data show that elevated Notch signaling leads to enhanced tumor growth and a more aggressive tumor phenotype in vivo, whereas cancer progression was retarded in hypoactive Notch tumors.
Both Hyper- and Hypoactive Notch Signaling Induce a Glycolytic Switch in Vivo and in Vitro.
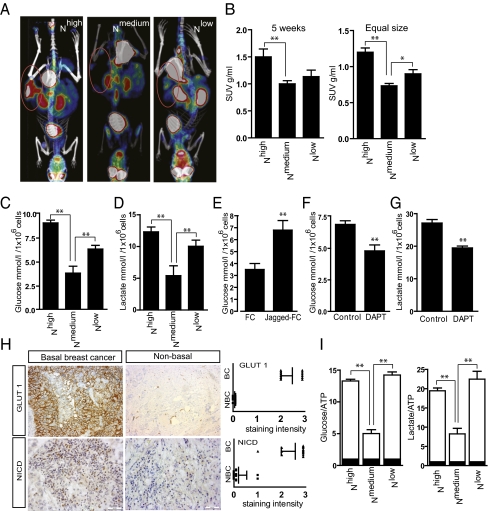
Analysis by 18F-fluorodeoxyglucose (18F-FDG) PET revealed that tumors from Nhigh cells showed increased glucose uptake, compared with Nmedium tumors (Fig. 2 A and B). Unexpectedly, we also observed higher uptake in the Nlow tumors (Fig. 2 A and B).
Fig. 2.
Tumors with hyper- and hypoactive Notch signaling show enhanced glucose uptake and lactate production. (A) Representative 18F-FDG PET images of mice 5 wk after grafting (tumor areas circled). (B) Standardized glucose uptake value (SUV) in Nhigh, Nmedium, and Nlow tumors 5 wk after transplantation (Left) or in tumors of equal size (Right). The graph summarizes three different experiments with four animals in each group. (C and D) Glucose consumption (C) and lactate production (D) in Nhigh, Nmedium, and Nlow cells. (E) Glucose consumption in naïve MFC7 cells with endogenous levels of Notch signaling (FC) and after Jagged ligand-induced activation (Jagged-FC). C–E represent the average of a minimum of three experiments with three technical replications within each experiment. (F and G) Glucose uptake (F) and lactate production (G) in glycolytically active MDA-MB-231 breast cancer cells treated with DAPT. (H Left) Representative images of GLUT1 and NICD expression in breast cancer tissue from patients with basal and nonbasal breast cancer. (Right) Scoring of staining intensities (n = 14). BC, basal cancer; NBC, nonbasal cancer. (I) Relative uptake of glucose (Left) or lactate (Right) production per unit of ATP content. Values are significant at **P < 0.01 and *P < 0.05 as indicated in B–I. Values indicate the average of at least three independent experiments.
We next analyzed the metabolic state in the different cell lines in vitro. In the Nhigh cell line, glucose consumption and lactate production were increased (Fig. 2 C and D), indicating that the glycolytic phenotype occurred independently of the in vivo context. Transient activation of Notch in naïve MCF7 by immobilized Notch ligands Jagged1 (Jagged-FC) yielded a similar increase in glucose consumption (Fig. 2E). Further, blocking Notch in breast cancer MDA-MB-231 cells with high endogenous Notch activity (Fig. S1) affected metabolism. MDA-MB-231 cells showed high glucose uptake and lactate production (Fig. S1), which was reduced by inhibition of Notch signaling (Fig. 2 F and G). In keeping with these data, tumor samples from patients with aggressive basal carcinoma showed increased expression of Notch1 ICD and glucose transporter 1 (GLUT1), a marker of glycolytic cancers, compared with a less aggressive luminal type of cancer. This finding further links Notch to glycolytic aggressive cancers and GLUT1 expression (Fig. 2H), and extends a recent report on Notch and glycolysis (30).
The Nlow cells also showed elevated glucose consumption and increased lactate production (Fig. 2 C and D), indicating that sustained Notch inhibition forces the cells into a glycolytic phenotype. Both Nhigh and Nlow cells were shown to use more glucose and produce more lactate to equivalent ATP content compared with Nmedium cells (Fig. 2I). Together, these data show that both hyper- and hypoactive Notch signaling boost glycolysis in a cell-intrinsic manner.
Hyperactive Notch Signaling Induces the Glycolytic Phenotype Through PI3K/AKT Signaling.
We next assessed whether Notch affected cellular metabolism via regulation of two signaling mechanisms known to induce glycolysis: the cellular hypoxic response and PI3K/AKT signaling. Notch signaling has been shown to cross-talk with the cellular hypoxic response in both stem cell differentiation and epithelial-to-mesenchymal transition (27, 28, 31), but up-regulation of the cellular hypoxic response in Nhigh cells was observed only during hypoxia, and was not significantly higher than in Nmedium cells (Fig. S2).
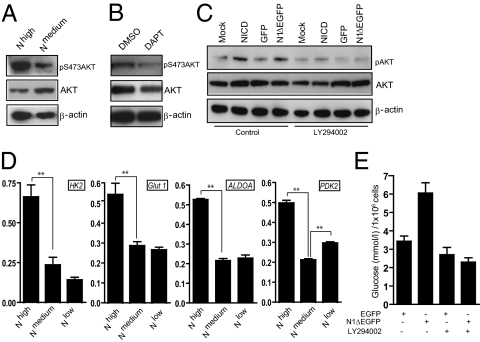
In contrast, Nhigh cells showed increased phosphorylation of AKT on serine 473 (Fig. 3A), accompanied by increased levels of PI3K class III and p110β (Fig. S3). Conversely, blocking Notch by N-[N-(3,5-difluorophenacetyl)-l-alanyl]-S-phenylglycine t-butyl ester (DAPT) reduced the amount of phosphorylated AKT in naïve MCF7 cells (Fig. 3B). An increase in S473-phosphorylated AKT levels was observed also after transient transfection of naïve MCF7 cells with Notch1 ICD or Notch1ΔE, a membrane-tethered form of Notch that generates Notch1 ICD after γ-secretase cleavage (Fig. 3C). PI3K/AKT signaling affects expression of several glycolytic genes, as well as the activity and localization of several rate-limiting glycolytic enzymes. Hexokinase 2 (HK2) was up-regulated on both protein and mRNA levels (Fig. 3D and Fig. S4), whereas GLUT1, aldolase A (ALDOA), and pyruvate dehydrogenase kinase 2 (PDK2) were up-regulated at the mRNA level (Fig. 3D). Furthermore, GLUT1 showed predominant membrane localization in Nhigh and Nlow cells, compared with Nmedium cells (Fig. S4). In contrast, in the Nlow cells, only PDK2 was up-regulated (Fig. 3D).
Fig. 3.
Notch induces glycolysis via PI3K/AKT signaling. (A) Immunoblotting of Nhigh and Nmedium cells with antibodies for activated (pS473), total AKT, and β-actin. (B) Immunoblotting of MCF7 cells with pS473, total AKT, and β-actin antibodies in the presence or absence of DAPT. (C) Immunoblotting of MCF7 cells transfected with Notch1 ICD or N1ΔEGFP with pS473 and total AKT antibodies in the presence or absence of the PI3K inhibitor LY294002. (D) Analysis of mRNA expression levels of HK2, GLUT1, ALDOA, and PDK2. Average of three different experiments. Values are significant at **P < 0.01 and *P < 0.05. Values indicate the average of at least three independent experiments. (E) Glucose consumption in MCF7 cells expressing EGFP or N1ΔEGFP in the presence or absence of LY294002. Average of two separate experiments, including three technical replications within each experiment.
We next tested whether PI3K/AKT signaling was required for the Notch-induced increase in AKT phosphorylation and glucose consumption. Phosphorylation of AKT by Notch required PI3K activity, as the PI3K inhibitor LY294002 significantly reduced the Notch-mediated increase in S473-phosphorylated AKT (Fig. 3C). Although the expression of GLUT1, HK2, and ALDOA was insensitive to PI3K/AKT inhibition, blocking of PI3K/AKT by LY294002 abrogated the Notch-induced increase in glucose consumption (Fig. 3E). Furthermore, AKT inhibition reduced proliferation and 3D growth, but did not affect the invasive capacity of Nhigh cells (Fig. S3). In sum, these data suggest that hyperactivated Notch signaling operates via the PI3K/AKT signaling pathway to induce glycolysis and growth.
Hypoactive Notch Signaling Induces Glycolysis by Altering Mitochondrial Function.
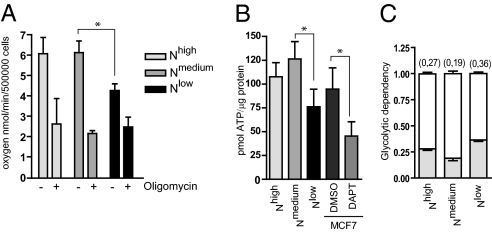
In Nlow cells, the PI3K/AKT signaling pathway was unaffected. In contrast, we observed that oxygen consumption was reduced in Nlow cells (Fig. 4A), which prompted us to assess mitochondrial function, as deregulated mitochondrial activity may lead to a glycolytic switch (14). OXPHOS is carried out by the respiratory complexes I–V in the inner mitochondrial membrane, where complexes I, III, and IV generate the proton gradient across the inner membrane, which is used to drive the ATP synthase activity of complex V for the generation of ATP (32, 33). Treatment of the cells with oligomycin, which inhibits the ATP synthase, resulted in a substantial decrease in oxygen consumption for Nhigh and Nmedium cells, but a considerably smaller decrease in Nlow cells (Fig. 4A). ATP levels were considerably reduced in Nlow cells and in naïve MCF7 cells treated with DAPT for 3 d (Fig. 4B). To corroborate the data, we analyzed an MCF7 cell line stably expressing dominant-negative Mastermind-like (dnMAML) and demonstrated that oxygen consumption and ATP production were reduced in these cells (Fig. S5). Interestingly, there was no significant reduction in oxygen consumption in Nhigh cells, indicating that OXPHOS was operative in these cells. When we measured glycolytic dependency, i.e., the relative contribution from glycolysis to the mitochondrial ATP budget, the Nlow cells showed the highest dependency (Fig. 4C). In sum, these data indicate that Nhigh cells are not dependent on glycolysis, and have the capacity to revert back to OXPHOS when needed, whereas Nlow cells have an impaired mitochondrial function and to a larger extent depend on glycolysis for energy production.
Fig. 4.
Oxygen consumption and ATP production are reduced in cells with blocked Notch signaling. (A) Oxygen consumption in untreated or oligomycin-treated Nhigh, Nmedium, and Nlow cells (B) ATP levels in Nhigh, Nmedium, and Nlow cells and in naïve MCF7 cells in the absence or presence of the Notch inhibitor DAPT. Values are significant at **P < 0.01 and *P < 0.05. Values indicate the average of at least three independent experiments. (C) Glycolytic dependency, i.e., the relative contribution of glycolysis to mitochondrial ATP production as calculated: lactate accumulation rate/lactate accumulation rate + 4.5× oxygen consumption rate.
Hypoactive Notch Signaling Attenuates the Activity of Respiratory Complexes I, IV, and V.
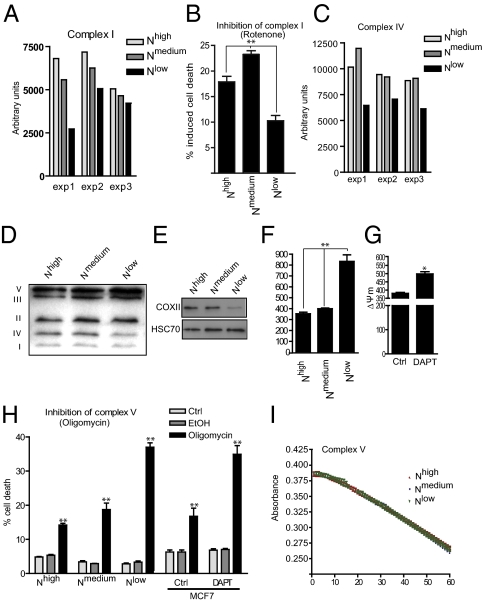
We next analyzed the activity of isolated mitochondrial complexes. The activity of complex I was reduced in Nlow cells (Fig. 5A), and the cells showed decreased sensitivity (i.e., reduced cell death) in response to the complex 1 inhibitor rotenone (Fig. 5B). The activity (Fig. 5C) and amount (Fig. 5D) of complex IV was also reduced in Nlow cells. Furthermore, we observed a decrease in the protein level of COXII (Fig. 5E), a subunit of complex IV previously shown to be linked to diminished activity of electron transfer chain complex IV in p53-deficient cancer cells (34).
Fig. 5.
Notch signaling is important for mitochondrial integrity and function. (A and B) The activity of the respiratory complex I (A) and the sensitivity to inhibition of complex I activity by rotenone (B) was determined on mitochondria isolated from Nhigh, Nmedium, and Nlow cells. (C) The activity of complex IV in isolated mitochondria. (D) Immunoblotting of mitochondrial preparations using antibodies against mitochondrial proteins for detection of different complexes. (E) Immunoblotting of whole-cell lysates of Nhigh, Nmedium, and Nlow cells with an antibody against subunit COXII. (F and G) The mitochondrial membrane potential in Nhigh, Nmedium, and Nlow cells (F) and in control or DAPT-treated naïve MCF7 cells (G). The cells were analyzed after 3 d of DAPT treatment. (H) Oligomycin-induced cell death (oligomycin 50 μM for 48 h) in Nhigh, Nmedium, and Nlow cells and in naïve MCF7 cells where Notch is inhibited with DAPT. (I) The hydrolyzing activity of complex V did not differ among Nhigh, Nmedium, and Nlow cells. Values are significant at **P < 0.01 and *P < 0.05. Values indicate the average of at least three independent experiments as indicated in C, F, G, and I.
To assess complex V function, we analyzed whether Notch signaling status affected the mitochondrial membrane potential (Δψm), which is built up if complex V functions suboptimally. The Δψm was significantly increased in Nlow cells (Fig. 5F), and a similar increase in Δψm was observed after DAPT treatment (Fig. 5G). Treatment with oligomycin resulted in increased cell death in Nlow cells and in DAPT-treated naïve MCF7 cells (Fig. 5H), further supporting a link between Notch and the activity of complex V. In contrast, we observed that the ATPase hydrolyzing activity in isolated mitochondria was not affected (Fig. 5I). Taken together, these data indicate that mitochondrial function is deregulated at several steps in the respiratory chain when Notch signaling is blocked.
Hypoactive Notch Signaling Induces Glucose Addiction in Tumor Growth.
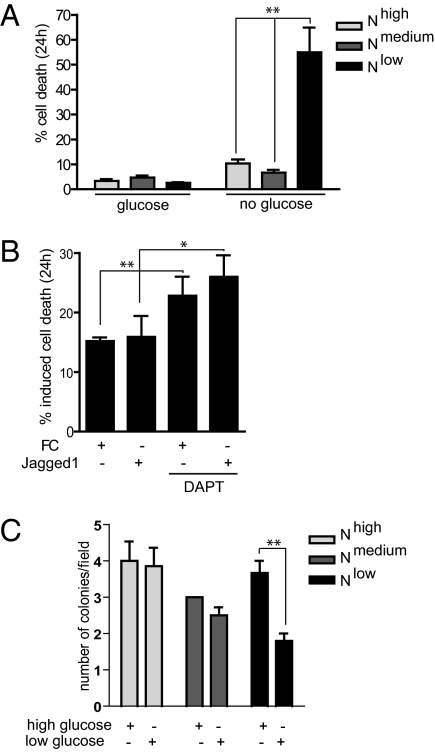
We next subjected the cells to glucose deprivation, to test whether the switch to glycolysis was irreversible or if OXPHOS could be resumed. Nmedium cells were not sensitive to glucose deprivation, in keeping with the nonglycolytic phenotype of these cells. In contrast, the Nlow cells demonstrated significantly increased cell death upon glucose deprivation (Fig. 6A). Similarly, blocking of Notch signaling by DAPT increased sensitivity to glucose withdrawal in ligand-induced naïve MCF7 cells and in control cells (Fig. 6B). Cell death in Nhigh cells or naïve MCF7 cells cultured on Jagged1 ligand was not significantly elevated (Fig. 6 A and B). When cultured in a 3D growth assay, the growth and survival of both Nmedium and Nlow cells was not significantly hampered by low glucose levels (Fig. 6C), but the Nlow cells developed considerably fewer colonies. Together, these data indicate that the mitochondrial defects in Nlow cells are irreversible, whereas glycolytic Nhigh cells have a respiratory backup function under conditions when glucose is scarce but oxygen is available.
Fig. 6.
Block of Notch signaling induces glucose addiction. (A and B) Cell death upon glucose deprivation in Nhigh, Nmedium, and Nlow cells (A), or in naïve control (FC) or ligand (FC-Jagged)-activated MCF7cells grown in the absence or presence of the Notch inhibitor DAPT (B). Average of three different experiments is shown. (C) Tumor growth of Nhigh, Nmedium, and Nlow cells in high- or low-glucose conditions as assessed by a 3D growth assay. Values are significant at **P < 0.01 and *P < 0.05. Values indicate the average of at least three independent experiments.
Hypoactive Notch Signaling Reduces p53 Levels.
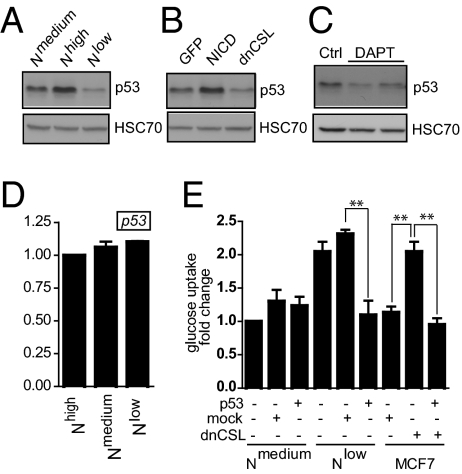
Loss of p53 has been associated with increased glycolysis, mitochondrial dysfunction, and deregulation of COXII (35, 36), which was also observed in Nlow cells (Fig. 5E). We therefore analyzed whether reduced Notch signaling affected p53. Nlow cells exhibited reduced p53 levels, whereas Nhigh cells showed elevated p53 protein levels, compared with Nmedium cells (Fig. 7A). Transient transfection of Notch1 ICD into naïve MCF7 cells increased the p53 protein level (Fig. 7B), whereas introduction of dominant-negative CSL (dnCSL) or treatment with DAPT for 5 d led to a reduction in the amount of p53 protein (Fig. 7 B and C). The p53 mRNA levels were not significantly altered by altering the level of Notch signaling (Fig. 7D). Reintroducing p53 into Nlow cells reversed the increase in glucose consumption (Fig. 7E). Conversely, knockdown of p53 by siRNA in Nmedium cells increased the mitochondrial membrane potential (Fig. S6). Furthermore, transient transfection of dnCSL into naïve MCF7 cells led to an increase in glucose uptake, which could be abrogated by simultaneously introducing p53 (Fig. 7E). In sum, the data show that reduced Notch signaling lowers p53 protein levels, and that the elevated glucose consumption resulting from low Notch signaling can be abrogated by restoring high p53 levels in cells.
Fig. 7.
Notch regulates p53 protein levels. (A and B) Immunoblotting of Nhigh, Nmedium, and Nlow cells (A) or naïve MCF7 cells transiently transfected with GFP, NICD, or dnCSL (B) using an antibody against p53. (C) Western blot of naïve MCF7 cells cultured in the presence or absence of DAPT. HCS70 was used as loading control. (D) Quantitative PCR of p53 mRNA levels in response to activation of blocking of Notch. (E) Fold change in glucose uptake in Nmedium and Nlow cells and in naïve MCF7 cells transfected with dnCSL in the presence and absence of reintroduced p53 as related to glucose uptake in nontransfected Nmedium cells (which was set at 1). Values are significant at **P < 0.01 and *P < 0.05. Values indicate the average of at least three independent experiments.
Discussion
In this report, we show that deregulation of Notch signaling resets cellular metabolism, and that both hyper- and hypoactivation of Notch induces a glycolytic phenotype, although by distinct mechanisms. Hyperactive Notch enhanced glycolysis in a PI3K/AKT-dependent manner. These data extend previous observations in normal and leukemic T cells, where AKT was activated by Notch, accompanied by increased glucose uptake (37) and a prosurvival function of Notch1-PI3K/AKT under metabolic challenge in malignant mesothelioma (38), suggesting that Notch and PI3K/AKT signaling synergize in different tumor cell types. High Notch activity boosted glycolysis without significantly lowering mitochondrial activity. This combination of glycolysis and retained mitochondrial activity may ensure a balance between the need for building blocks and the need for energy production through ATP synthesis to promote a highly proliferative state.
Notch signaling can occur in a canonical form that requires CSL, and in a noncanonical form that bypasses CSL or is independent of the ligand (18). A recent report indicates that canonical Notch signaling is dispensable for mammary tumorigenesis in mice (39). Notch1 ICD lacking the CSL binding domain did not revert the metabolic phenotype of Nlow cells (Fig. S6), suggesting that canonical Notch signaling is required for metabolic regulation in cancer. Whether this difference reflects species difference between human and mouse mammary tumors, or activation of Notch in endogenous mammary cells vs. the use of established cell lines, remains to be analyzed.
Canonical Notch signaling was required for mitochondrial integrity, and mitochondrial function was compromised at several steps in the respiratory chain in cells expressing dnCSL. The cells were dependent on glycolysis for energy production and more sensitive to glucose deprivation, because they could not revert to OXPHOS when glucose was scarce. Our study links Notch activity to mitochondrial respiration, and may shed light on the observation that patients with cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy harboring Notch3 mutations exhibit mitochondrial dysfunction (40, 41).
Our data implicate p53 in the metabolic effects in Nlow cells and show that blocking canonical Notch signaling diminishes p53 levels, adding a new link to the multifaceted cross-talk between Notch and p53 (42). In keeping with this finding, Notch was recently shown to restore p53 in glioblastoma (43) and to enhance p53 protein levels in hepatocellular carcinoma (44). Activated Notch1 has been reported to directly bind p53 and inhibit its phosphorylation and transactivation (45), and a direct interaction between NICD4, p53, and murine double minute 2 (Mdm2) was recently shown to be important for Mdm2-mediated degradation of Notch (46). Conversely, modulation of p53 levels directly altered Notch levels in hepatocellular carcinoma, and p53 was required for Notch-induced growth and invasiveness (47). Notch may also be part of p53's antiapoptotic activity (48). Collectively, our findings suggest the existence of an intricate cross-talk between Notch and p53, and, although the precise details remain to be worked out, this may be of interest for possible future combination therapies (48). There is also an emerging view that p53 regulates mitochondrial functions. In p53-deficient mice, mitochondrial biogenesis is impaired, accompanied by reduced oxygen consumption and increased glycolysis (11, 35). Moreover, parkin, a novel p53 target gene, mediates the effect of p53 on metabolism, and parkin deficiency leads to mitochondrial dysfunction and reduced mitochondrial respiration (49). p53 has also been shown to regulate the level of COXII (34), which is in keeping with the observed reduction of COXII after blocking Notch signaling.
Though Nhigh and Nlow cells both enhanced glycolysis, Nhigh cells generated rapidly growing, metastasizing tumors, whereas Nlow cells grew only transiently, and then regressed. This finding shows that a glycolytic phenotype alone is not sufficient for promotion of tumor growth. Growth of Nlow in vitro was hampered under glucose-restricted conditions, and the cells showed increased sensitivity to glucose deprivation, which suggests that metabolic flexibility is more important than glycolysis for tumor progression. Though it was initially thought that mitochondrial dysfunction accompanied aerobic glycolysis in tumor cells (1), there is accumulating evidence that tumor cells have intact mitochondria (14, 15, 50, 51) and can revert to OXPHOS in oxygenated regions to more efficiently use the limited amount of glucose available (17, 52).
In summary, our data show that Notch signaling plays an important role in cellular energy homeostasis, which is crucial for better understanding the physiological effects of deregulated Notch signaling in tumors and inspiring new strategies for cancer therapy.
Materials and Methods
Details for plasmid constructs; cell culture; metabolic and mitochondrial assays; quantitative RT-PCR; immunohistochemistry and cytochemistry; Western blot analysis; and tumor xenotransplantation are given in SI Materials and Methods.
Supplementary Material
Acknowledgments
We are grateful to the Turku PET Centre; Helena Saarento, Natalie Råtts, and Susanne Bergstedt for technical assistance; and the University of Turku, Department of Pathology, for histological analyses. Support for this work was provided by the Academy of Finland (C.M.S.), Sigrid Juselius Foundation (C.M.S. and V.M.), the Swedish Cancer Society, the Swedish Research Council (Developmental Biology for Regenerative Medicine Project Grant), the European Consortium projects EuroSyStem and NotchIT, and the Karolinska Institutet's Breast Cancer Theme Center, Theme Center in Regenerative Medicine, and Distinguished Professor Award (to U.L.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1104943108/-/DCSupplemental.
References
- 1.Warburg O. Ueber den stoffwechsel der tumoren. Biochemische Zeitschrift. 1924;152:319–344. [Google Scholar]
- 2.Gatenby RA, Gillies RJ. Why do cancers have high aerobic glycolysis? Nat Rev Cancer. 2004;4:891–899. doi: 10.1038/nrc1478. [DOI] [PubMed] [Google Scholar]
- 3.Kroemer G, Pouyssegur J. Tumor cell metabolism: Cancer's Achilles’ heel. Cancer Cell. 2008;13:472–482. doi: 10.1016/j.ccr.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Vander Heiden MG, Cantley LC, Thompson CB. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 6.Manning BD, Cantley LC. AKT/PKB signaling: Navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dang CV, Semenza GL. Oncogenic alterations of metabolism. Trends Biochem Sci. 1999;24:68–72. doi: 10.1016/s0968-0004(98)01344-9. [DOI] [PubMed] [Google Scholar]
- 8.Ramanathan A, Wang C, Schreiber SL. Perturbational profiling of a cell-line model of tumorigenesis by using metabolic measurements. Proc Natl Acad Sci USA. 2005;102:5992–5997. doi: 10.1073/pnas.0502267102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gordan JD, Thompson CB, Simon MC. HIF and c-Myc: Sibling rivals for control of cancer cell metabolism and proliferation. Cancer Cell. 2007;12:108–113. doi: 10.1016/j.ccr.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bensaad K, et al. TIGAR, a p53-inducible regulator of glycolysis and apoptosis. Cell. 2006;126:107–120. doi: 10.1016/j.cell.2006.05.036. [DOI] [PubMed] [Google Scholar]
- 11.Matoba S, et al. p53 regulates mitochondrial respiration. Science. 2006;312:1650–1653. doi: 10.1126/science.1126863. [DOI] [PubMed] [Google Scholar]
- 12.Gordan JD, Simon MC. Hypoxia-inducible factors: Central regulators of the tumor phenotype. Curr Opin Genet Dev. 2007;17:71–77. doi: 10.1016/j.gde.2006.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Semenza GL. Hypoxia-inducible factor 1 (HIF-1) pathway. Sci STKE. 2007 doi: 10.1126/stke.4072007cm8. 10.1126/stke.4072007cm8. [DOI] [PubMed] [Google Scholar]
- 14.Frezza C, Gottlieb E. Mitochondria in cancer: Not just innocent bystanders. Semin Cancer Biol. 2009;19:4–11. doi: 10.1016/j.semcancer.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 15.Fantin VR, St-Pierre J, Leder P. Attenuation of LDH-A expression uncovers a link between glycolysis, mitochondrial physiology, and tumor maintenance. Cancer Cell. 2006;9:425–434. doi: 10.1016/j.ccr.2006.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Gough DJ, et al. Mitochondrial STAT3 supports Ras-dependent oncogenic transformation. Science. 2009;324:1713–1716. doi: 10.1126/science.1171721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sonveaux P, et al. Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Invest. 2008;118:3930–3942. doi: 10.1172/JCI36843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kopan R, Ilagan MX. The canonical Notch signaling pathway: Unfolding the activation mechanism. Cell. 2009;137:216–233. doi: 10.1016/j.cell.2009.03.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Radtke F, Raj K. The role of Notch in tumorigenesis: Oncogene or tumour suppressor? Nat Rev Cancer. 2003;3:756–767. doi: 10.1038/nrc1186. [DOI] [PubMed] [Google Scholar]
- 20.Rizzo P, et al. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27:5124–5131. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 21.Weng AP, et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science. 2004;306:269–271. doi: 10.1126/science.1102160. [DOI] [PubMed] [Google Scholar]
- 22.Callahan R, Egan SE. Notch signaling in mammary development and oncogenesis. J Mammary Gland Biol Neoplasia. 2004;9:145–163. doi: 10.1023/B:JOMG.0000037159.63644.81. [DOI] [PubMed] [Google Scholar]
- 23.Callahan R, Raafat A. Notch signaling in mammary gland tumorigenesis. J Mammary Gland Biol Neoplasia. 2001;6:23–36. doi: 10.1023/a:1009512414430. [DOI] [PubMed] [Google Scholar]
- 24.Lee CW, et al. A functional Notch–survivin gene signature in basal breast cancer. Breast Cancer Res. 2008 doi: 10.1186/bcr2200. 10.1186/bcr2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Politi K, Feirt N, Kitajewski J. Notch in mammary gland development and breast cancer. Semin Cancer Biol. 2004;14:341–347. doi: 10.1016/j.semcancer.2004.04.013. [DOI] [PubMed] [Google Scholar]
- 26.Stylianou S, Clarke RB, Brennan K. Aberrant activation of notch signaling in human breast cancer. Cancer Res. 2006;66:1517–1525. doi: 10.1158/0008-5472.CAN-05-3054. [DOI] [PubMed] [Google Scholar]
- 27.Gustafsson MV, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev Cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Lendahl U, Lee KL, Yang H, Poellinger L. Generating specificity and diversity in the transcriptional response to hypoxia. Nat Rev Genet. 2009;10:821–832. doi: 10.1038/nrg2665. [DOI] [PubMed] [Google Scholar]
- 29.Fortini ME. Notch signaling: The core pathway and its posttranslational regulation. Dev Cell. 2009;16:633–647. doi: 10.1016/j.devcel.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Xing F, et al. Hypoxia-induced Jagged2 promotes breast cancer metastasis and self-renewal of cancer stem-like cells. Oncogene. 2011;30:4075–4086. doi: 10.1038/onc.2011.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bellance N, Lestienne P, Rossignol R. Mitochondria: From bioenergetics to the metabolic regulation of carcinogenesis. Front Biosci. 2009;14:4015–4034. doi: 10.2741/3509. [DOI] [PubMed] [Google Scholar]
- 33.Gogvadze V, Orrenius S, Zhivotovsky B. Mitochondria in cancer cells: What is so special about them? Trends Cell Biol. 2008;18:165–173. doi: 10.1016/j.tcb.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Zhou S, Kachhap S, Singh KK. Mitochondrial impairment in p53-deficient human cancer cells. Mutagenesis. 2003;18:287–292. doi: 10.1093/mutage/18.3.287. [DOI] [PubMed] [Google Scholar]
- 35.Cheung EC, Vousden KH. The role of p53 in glucose metabolism. Curr Opin Cell Biol. 2010;22:186–191. doi: 10.1016/j.ceb.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 36.Vousden KH, Ryan KM. p53 and metabolism. Nat Rev Cancer. 2009;9:691–700. doi: 10.1038/nrc2715. [DOI] [PubMed] [Google Scholar]
- 37.Ciofani M, Zúñiga-Pflücker JC. Notch promotes survival of pre-T cells at the beta-selection checkpoint by regulating cellular metabolism. Nat Immunol. 2005;6:881–888. doi: 10.1038/ni1234. [DOI] [PubMed] [Google Scholar]
- 38.Graziani I, et al. Opposite effects of Notch-1 and Notch-2 on mesothelioma cell survival under hypoxia are exerted through the Akt pathway. Cancer Res. 2008;68:9678–9685. doi: 10.1158/0008-5472.CAN-08-0969. [DOI] [PubMed] [Google Scholar]
- 39.Raafat A, et al. Rbpj conditional knockout reveals distinct functions of Notch4/Int3 in mammary gland development and tumorigenesis. Oncogene. 2009;28:219–230. doi: 10.1038/onc.2008.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de la Peña P, et al. Mitochondrial dysfunction associated with a mutation in the Notch3 gene in a CADASIL family. Neurology. 2001;57:1235–1238. doi: 10.1212/wnl.57.7.1235. [DOI] [PubMed] [Google Scholar]
- 41.Dotti MT, et al. A novel NOTCH3 frameshift deletion and mitochondrial abnormalities in a patient with CADASIL. Arch Neurol. 2004;61:942–945. doi: 10.1001/archneur.61.6.942. [DOI] [PubMed] [Google Scholar]
- 42.Dotto GP. Crosstalk of Notch with p53 and p63 in cancer growth control. Nat Rev Cancer. 2009;9:587–595. doi: 10.1038/nrc2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lin H, et al. Notch-1 activation-dependent p53 restoration contributes to resveratrol-induced apoptosis in glioblastoma cells. Oncol Rep. 2011;26:925–930. doi: 10.3892/or.2011.1380. [DOI] [PubMed] [Google Scholar]
- 44.Wang C, et al. Notch1 signaling sensitizes tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in human hepatocellular carcinoma cells by inhibiting Akt/Hdm2-mediated p53 degradation and up-regulating p53-dependent DR5 expression. J Biol Chem. 2009;284:16183–16190. doi: 10.1074/jbc.M109.002105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Kim SB, et al. Activated Notch1 interacts with p53 to inhibit its phosphorylation and transactivation. Cell Death Differ. 2007;14:982–991. doi: 10.1038/sj.cdd.4402083. [DOI] [PubMed] [Google Scholar]
- 46.Sun Y, et al. Trp53 regulates Notch 4 signaling through Mdm2. J Cell Sci. 2011;124:1067–1076. doi: 10.1242/jcs.068965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lim SO, et al. Notch1 differentially regulates oncogenesis by wildtype p53 overexpression and p53 mutation in grade III hepatocellular carcinoma. Hepatology. 2011;53:1352–1362. doi: 10.1002/hep.24208. [DOI] [PubMed] [Google Scholar]
- 48.Wickremasinghe RG, Prentice AG, Steele AJ. p53 and Notch signaling in chronic lymphocytic leukemia: Clues to identifying novel therapeutic strategies. Leukemia. 2011;25:1400–1407. doi: 10.1038/leu.2011.103. [DOI] [PubMed] [Google Scholar]
- 49.Zhang C, et al. Parkin, a p53 target gene, mediates the role of p53 in glucose metabolism and the Warburg effect. Proc Natl Acad Sci USA. 2011;108:16259–16264. doi: 10.1073/pnas.1113884108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bonnet S, et al. A mitochondria-K+ channel axis is suppressed in cancer and its normalization promotes apoptosis and inhibits cancer growth. Cancer Cell. 2007;11:37–51. doi: 10.1016/j.ccr.2006.10.020. [DOI] [PubMed] [Google Scholar]
- 51.Rossignol R, et al. Energy substrate modulates mitochondrial structure and oxidative capacity in cancer cells. Cancer Res. 2004;64:985–993. doi: 10.1158/0008-5472.can-03-1101. [DOI] [PubMed] [Google Scholar]
- 52.Semenza GL. Tumor metabolism: Cancer cells give and take lactate. J Clin Invest. 2008;118:3835–3837. doi: 10.1172/JCI37373. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.