Abstract
The search for factors either promoting islets proliferation or survival during adult life is a major issue for both type 1 and 2 diabetes mellitus. Among factors with mitogenic activity on pancreatic β-cells, human placental lactogen (hPL) showed stronger activity when compared to the other lactogen hormones: growth hormone (GH) and prolactin (PRL). The aim of the present work is to elucidate the biological and molecular events of hPL isoform A (hPL-A) activity on human cultured islets. We used pure human pancreatic islets and insulinoma cell lines (βTC-1 and RIN, murine and rat respectively) stimulated with hPL-A recombinant protein and we compared hPL-A activity with that of hGH. We showed that hPL-A inhibits apoptosis, both in insulinoma and human islets, by the phosphorylation of AKT protein. Indeed, the antiapoptotic role of hPL-A was mediated by PI3K, p38 and it was independent by PKA, Erk1/2. Compared with hGH, hPL-A modulated at different intervals and/or intensity by the phosphorylation of JAKs/STATs and MAPKinases. Moreover, hPL-A induced PDX-1 intracellular expression, improving beta cell activity and ameliorating insulin secretion in response to high glucose stimulation. Our data support the idea that hPL-A is involved in the regulation of beta cells activity. Importantly, we found that hPL-A can preserve and improve the ability of purified human pancreatic islets cultured to secrete insulin in vitro.
Keywords: placental lactogen hormone, growth hormone, β-cell, apoptosis, islets survival, PDX-1, islets insulin secretion
Introduction
The complete or relative insufficiency of insulin secretion and/or insulin action present in diabetes mellitus induces derangement in carbohydrate, protein and fat metabolism. The management in achieving better metabolic control is sustained by insulin therapy, insulin enhancer action agents (insulin sensitizers; thiazolidinediones), and new-generation of insulin secretagogue: glimepiride, acarbose and designer insulin (lispro and aspart), ghrelin, GLP-1 and its analogous exenetide-4.1 Alternatively, patients, not responding to drug combinations, insulin infusion and affected by kidney failure, are treated with pancreas/kidney or islets transplantation. These procedures, although effective, are not suitable for the general diabetes population due to the inadequate supply of organs. Another problem is that transplantation procedures affect health and efficiency of islets.2
Evidence from molecular and epidemiological studies indicates that pancreatic β-cell dysfunction is crucial in both type 1 and type 2 diabetes mellitus.3,4 In both cases β-cell death is thought to occur by apoptosis. A number of pathological stimuli involved in the pathogenesis of type 1 and type 2 diabetes have been shown to elicit β-cell programmed death.5
Several laboratories have demonstrated that β-cells can proliferate in response to physiological and pathophysiological stimuli (hyperglycemia, pregnancy and pancreatectomy).6,7 New β-cells may proliferate both during fetal and adult life, leading to the formation of new islets or “islet neogenesis”, as a consequence of an increased physiological demand.8–10 In adult animals, a mechanism of balance between β-cell loss/proliferation and neogenesis seems to exist: in rodents about 3% of β-cells are renewed every day. In rodents and humans the hyperplasia of β-cells that occurs during pregnancy is depleted postpartum by apoptosis.11 In this context, a great number of molecules play a determinant role: i.e., signaling and cell cycle regulators, pancreatic islets specific transcription factors (PDX-1) and growth factors: parathyroid hormone-related protein (PTHrP), hepatic growth factor (HGF), placental lactogen (PL), fibroblast growth factor (FGF), glucagon-like peptide 1 (GLP-1), betacellulin (BTC), insulin-like growth factors (IGFs), islet neogenesis associated protein (INGAP), epidermal growth factor (EGF).12–14
Between the growth factors placental lactogen hormone (PL), produced only during pregnancy, is the factor mainly responsible for the mass increase of pancreatic islets and for improvement of their function.15 PL isoforms are members of the growth hormone (GH) and prolactin (PRL) family.16 It has been observed that targeted expression of PL in the β-cells of transgenic mouse induced an increment of β-cells proliferation, islets hyperplasia and hyperinsulinemia with resulting hypoglycemia.17 In humans, prolactin, PL and placental GH play a central role in the maternal food intake and insulin production, while placental GH is the major determinant of maternal insulin resistance during the second half of gestation.18 The molecular pathway involved in lactogens transduction signal is known (JAK/STAT, IRS-1 and 2, PI3-kinase, MAPKs),19–26 as well as the signal transduction mechanisms activated by PL in β-cells.27
Between the transcriptional molecules the pancreas/duodenum homeobox-1 (PDX-1) plays a critical role in the differentiation of the hormone-producing phenotype β-cell and modulates the expression of functional pancreatic genes including insulin.28 Indeed, PDX-1 is a homeodomain-containing transcription factor and binds several elements in the insulin promoter.29 Targeted disruption of PDX-1 in mice results in pancreatic agenesis, which precludes further analysis, but mice, with β-cell-restricted PDX-1 gene ablation, develop diabetes with age and exhibit impaired glucose tolerance. Furthermore, these mice showed abnormal islets architecture, reduction of insulin and glucose transporter 2 (GLUT-2) expressions.30 There are several evidences supporting the fact that PDX-1 plays a fundamental role in pancreas development and in adult life maintaining β-cell functionality.31–33
In this context of different therapeutic approaches in treating diabetes mellitus, in particular type 1, we show that hPL-A activates disparate signaling pathways in human pancreatic islets, yielding a pro-survival effect and ameliorating insulin response in cultured human islets.
Results
Cytofluorimetric analysis of apoptosis in insulinoma cell lines and human pancreatic islets treated with hPL-A.
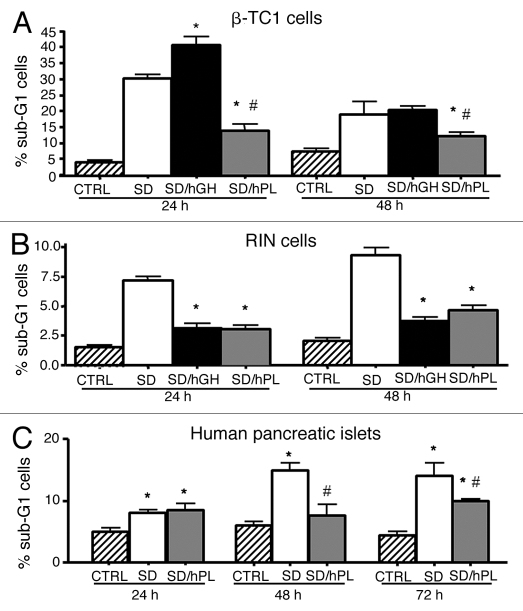
In order to evaluate the anti-apoptotic activity of hPL-A, we induced apoptosis by serum deprivation in mouse β-TC-1 and rat RIN insulinoma cells, and in isolated human pancreatic islets. We found that hPL-A was able to decrease about 50% the amount of apoptotic β-TC-1 cells in contrast to hGH (Fig. 1A). Similar results were obtained with rat insulinoma RIN cells that were protected in a similar fashion by hPL-A and hGH (Fig. 1B). We observed that hPL-A was able to protect human pancreatic islets from serum deprived-induced apoptosis (Fig. 1C). Due to the shortage of human islets we did not perform experiments with hGH.
Figure 1.
Cytofluorimetric Analysis of insulinoma cell lines and of human pancreatic islets stimulate with hormones. The insulinoma β-TC1 cells (A) were serum deprived for 18 h without BSA and stimulated with 500 ng/ml of hPL-A or hGH at different times (24 and 48 h). After treatments, attached and detached cells were harvested, stained by Propidium Iodide/Triton-X100 and analyzed by a BD FACSCalibur; *p < 0.050 vs. SD and #p < 0.050 vs. hGH (n = 4). Rat RIN cells (B) were serum deprived for 18 h without BSA and then stimulated with hPL-A or hGH at 24 and 48 h. After treatments, attached and detached cells were harvested, stained by Propidium Iodide/Triton-X100 and analyzed by a BD FACSCalibur; *p < 0.050 vs. SD (n = 4). Human pancreatic islets, at 90% of clinical purity, (C) were serum deprived for 18 h without BSA and stimulated with 500 ng/ml of hPL-A at different times (24, 48 and 72 h). After treatments, attached and detached cells were harvested, stained by Propidium Iodide/Triton-X100 and analyzed by a BD FACSCalibur; *p < 0.05 vs. CTRL and #p < 0.05 vs. SD (n = 4). Data represent four different experiment with islets from four different donors.
Western blot analysis of Akt, p38 and ERKs pathway involved in hPL-A and hGH signaling in β-TC-1 cells.
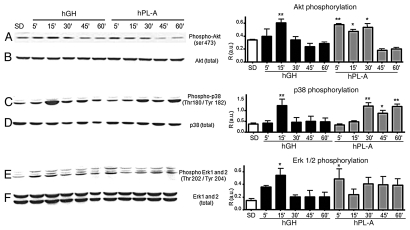
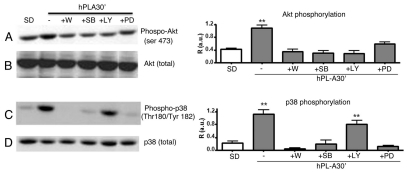
We analyzed the activation of relevant signaling nodes involved in cell metabolism, growth and survival drawing a time course protocol to identify differences of hPL signaling with respect to hGH. Whereas, in β-TC-1 cells model, hPL-A and GH activate in a similar fashion IRS-1, IRS-2 and 52 kDa Shc isoform (data not shown), Figure 2 shows that the down stream molecular pathway activation of Akt, p38, Erk1 and 2 respond differently to the two hormones stimulation. hPL-A induced the maximum of Akt phosphorylation up to an half of hour of hormone exposure, while hGH generated the maximum Akt activity at 15 min followed by a decrease in phosphorylation signal (Fig. 2A). Protein p38 was phosphorylated with a peak at 15 min by hGH and then the signal dropped; whereas hPL-A induced p38 activation at 30 min up to 1 hour (Fig. 2C). hGH leaded maximum phosphorylation of Erk proteins in the first quarter of time course; in contrast, hPL-A induced an earlier strong Erks activation and then the signal was maintained up to one hour (Fig. 2E). In order to elucidate the molecular pathways activated by hPL-A, we treated β-TC-1 cells with hPL-A in the presence of the following inhibitors: Wortmannin (W; a PI3Ks inhibitor), SB203580 (SB; a MAPKs inhibitor), LY294002 (LY; a strong PI3Ks inhibitor) and PD98059 (PD; a MEKs inhibitor). We found that W, SB, LY and PD inhibited Akt phosphorylation, while W, SB and PD strongly inhibited p38 phosphorylation (Fig. 3).
Figure 2.
Western blot analysis of AKT and Map-kinases proteins involved in the signal transduction pathway induced by hPL-A and by hGH in αTC-1 cells. αTC-1 cells were serum deprived (SD) and stimulated with 500 ng/ml of hPL-A and/or hGH at different times (5, 15, 30, 45 and 60 min). After cell lysis, 50 µg of protein lysates were separated by 10% SDS-PAGE and then transferred onto a nitrocellulose membrane. The phosphorylated proteins were analyzed using the anti-phospho-AKT, the anti-phospho-p38 and the anti-phospho-ERK1/2 antibodies (lane A, C and E, respectively). The protein amount was normalized utilizing the total antibody (lane B, D and F, respectively). Beside each couple of blots (A/B, C/D and E/F) we showed the relative densitometry analysis (R, Phosphorylated protein content/Total protein content; a.u., arbitrary units); *p < 0.050 and **p < 0.01 vs. SD (n = 3). One representative blot of three different biological experiments is visualized.
Figure 3.
Effect of protein inhibitors of the PI3K and MAPK molecular pathways on Akt and p38 phosphorylation induced by hPL-A. βTC-1 cells were serum deprived (SD) and treated for 30 min with 200 nM Wortmannin (W) or 50 µM SB203580 (SB), or 50 µM LY294002 (LY) or 20 µM PD98059 (PD) and then incubated for additional 30 min with 500 ng/ml of hPL-A. 50 µg of protein lysates were separated by 10% SDS-PAGE and analyzed by western-blot using the anti-phospho-AKT and the anti-phospho-p38 (lane A and C, respectively). The protein amount was normalized utilizing the total antibody (lane B and D, respectively). Beside each couple of blots (A/B and C/D) we showed the relative densitometry analysis (R, Phosphorylated protein content/Total protein content; a.u., arbitrary units); **p < 0.01 vs. SD (n = 3). One representative blot of three different biological experiments is visualized.
Western blot analysis of molecular pathway involved in hPL-A signaling in human pancreatic islets.
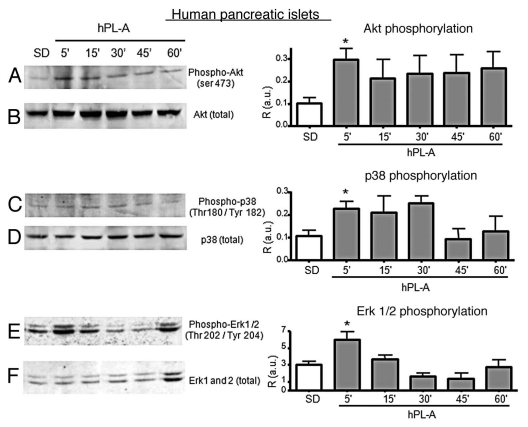
Due to the paucity of human material, we do not have analyzed hGH stimulus and human pancreatic islets were treated with hPL-A to study the intracellular signal only through Akt, Erks and p38 molecules. The cultured human islet at clinical purity were synchronized by serum deprivation and the osmolarity was stabilized by BSA addition. hPL-A hormone was added to islets cultures at different intervals. Figure 4 shows that hPL-A induced the Akt protein phosphorylation, with a maximum at 5 min, then the signal was maintained up to 60 min (Fig. 4A). Whereas protein p38 was phosphorylated at 5 min, from the hormonal stimulus, until 30 min (Fig. 4C) and hPL-A induced Erk proteins activation at 5 min of treatment, then the signal switched off after 15 min (Fig. 4E).
Figure 4.
Western blot analysis of proteins involved in the signal transduction pathway induced by hPL-A in human pancreatic islets. 90% clinical purity-grade human islets were serum deprived (SD) and incubated at different times (5, 15, 30, 45 and 60 min) with 500 ng/ml of hPL-A. Islets were then lysed and 50 µg of protein lysates were separated by 10% SDS-PAGE. The blotted proteins were incubated with the specific antibody anti-phospho-Akt, anti-phospho-p38 and anti-phospho-Erk1/2 (lane A, C and E, respectively). The protein amount was normalized utilizing the total antibody (lane B, D and F, respectively). Beside each couple of blots (A/B, C/D and E/F) we showed the relative densitometric analysis (R, Phosphorylated protein content/Total protein content; a.u., arbitrary units); *p < 0.05 vs. SD (n = 3). One representative blot of three different experiments performed with islets from three different donors is visualized.
Cytofluorimetric analysis of the anti-apoptotic molecular mechanism induced by hPL-A in insulinoma cell lines.
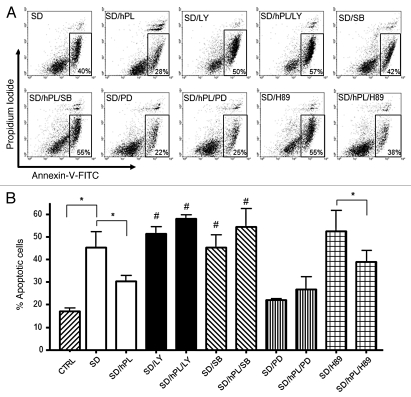
In order to elucidate the molecular mechanisms of the anti-apoptotic activity of hPL-A, we induced apoptosis in mouse β-TC-1 cells by serum deprivation for 48 h, and we then treated these cells with hPL-A and the following inhibitors: LY294002 (LY), SB203580 (SB), PD98059 (PD), H89 (a PKA inhibitor) and Wortmannin (W). We found that both LY and SB strongly inhibit the anti-apoptotic activity of hPL-A. The PD inhibitor was not able to reduce the anti-apoptotic activity of hPL, although we found that PD alone reduced the number of apoptotic cells induced by serum deprivation. Consistently we found that W, which was a less specific PI3K inhibitor, did not significantly influence the percentage of apoptotic cells (not shown). In contrast, H89, a specific inhibitor of PKA molecule, significantly did not inhibit hPL-A-mediated protection from apoptosis induced by serum deprivation in β-TC-1 cells (Fig. 5).
Figure 5.
Apoptosis protection induced by hPL-A was blocked by the inhibition of PI3K, p38 and MAPKs pathways, in insulinoma βTC1 cell line. The insulinoma β-TC1 cells were serum deprived (SD) for 48 h without BSA and stimulated with 500 ng/ml hPL-A and 50 nM LY294002 (LY) or 500 nM SB203580 (SB), or 200 nM PD98059 (PD) or 500 nM H89. After 48 h, both attached and detached cells were harvested and analyzed by a FACSCalibur, using the Annexin-V/Propidium Iodide apoptosis kit (Sigma-Aldrich), following the manufacturer's instruction. In (A) typical dot-plots are shown: early apoptotic cells were positive for Annexin-V-FITC only (box). For CTRL sample (not shown) we found that cells stained by Annexin-V-FITC were about 15%. The percentages of apoptotic cells are showed in (B); *p < 0.05; #p < 0.05 vs. SD/hPL (n = 4). One representative dot plot of four different experiments is visualized.
hPL-A increases PDX-1 expression and improves insulin secretion in cultured human islets.
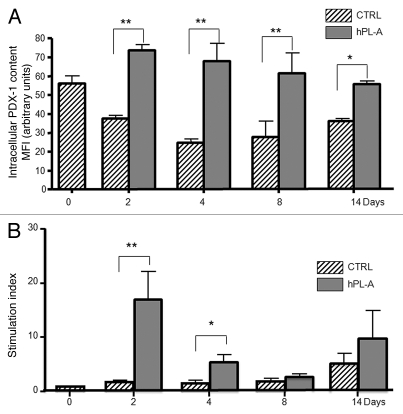
In order to evaluate the ability of hPL-A in recovering and improving human islets functionality, we treated cultured islets with 500 ng/ml hPL given every two days, and we evaluated PDX-1, insulin expression and Glucose-induced Insulin Secretion (GIIS) at basal conditions (0 day) and after 2, 4, 8 and 14 days. Cytofluorimetric analysis showed that hPL-A treatment induces a dramatic increase of PDX-1 expression with a slight decrease after 14 days of culture (Fig. 6A). Consistently, we found that hPL-A strongly improved glucose-induced insulin secretion (GIIS) up to four days of culture (Fig. 6B and Sup. 1). Then the concentration of secreted insulin in hPL-A treated samples decreased to that of control islets. After eight days we observed an increment in insulin secretion both in control and hPL-A stimulated islets. Even though, the lactogen hormone treated islets still secreted slightly more insulin than the untreated islets.
Figure 6.
hPL-A increases PDX-1 expression and improves Glucose induced insulin secretion (GIIS) in cultured human islets. Human islets were cultured in DMEM low glucose (5 mM) and 500 ng/ml of hPL-A was added every two days. After 2, 4, 8 and 14 days of treatment, cells and culture media were harvested and analyzed. We found (A) a dramatic increment in the intracellular expression of PDX-1, assayed by FACS; *p < 0.050 and **p < 0.010 (n = 4). Consistently (B) we found, by an RIA kit, that hPL-A improves insulin secretion after glucose (25 mM) challenge; *p < 0.050 and **p < 0.010 (n = 4). Data represent four different experiment with islets from four different donors.
Discussion
Our data showed that the phosphorylation of Akt and MAPKs induced by hPL-A promotes cell survival and maintains the number of living cells at the control value, by inhibiting the apoptotic process induced by serum deprivation. hGH did not show a similar strong antiapoptotic effect in our experimental condition. This is in agreement with the evidences that the activation of the prolactin receptor protects from apoptosis. Therefore, the activation of the prolactin receptor that is induced by hPL-A could also plays an anti-apoptotic role on human islets.
According with the literature, the Akt phosphorylation signal pathway precedes the biological effects, such as apoptosis protection.34 The evidence that the phosphorylation of Akt and MAPKs is present within one hour after hPL-A stimulation is in agreement with apoptosis protection at 24–72 h. Indeed, first, hPL-A treatment was renewed every 24 h; second, the phosphorylation of Akt and MAPKs can activate a number of cell survival mechanisms that induce a prolonged cell protection; and, third, in some conditions, apoptosis protection is evident only in the presence of a significant number of dying cells (after 24 and 72 h). Moreover, we observed that human islets showed a slightly later response to the hormonal treatment than the insulinoma cell lines. Once again, this could be explained by the fact that apoptosis was appreciably present after 48 h of serum deprivation. Equal important is the finding that human islets behaved differently to the cell line model. In fact, hPL-A sustained the Akt phosphorylation up to 1 h of treatment, while, in mouse insulinoma cell line, we observed a decreased phopshorylation signal after half an hour of hormonal stimulus. These data may support the putative role of hPL-A in reducing the apoptotic events in cultured islets before transplantation. Moreover, in human islets, our data showed that the simultaneous decrease of p38 activity and of Erk1/2 phosphorylation, assured cell proliferation without undergoing uncontrolled replication and inflammation. In mouse insulinoma cells, the decrease of Akt activity inhibits a proliferation stimulus. We observed that MAPK phosphorylation showed a plateau for at least 1 h of hPL-A treatment. The balance between cell proliferation and apoptosis was supported by hPL-A, which maintained a low number of apoptotic events in insulinoma cells for at least two days. The different behavior between the cell line models and human islets could be explained by the complexity of interaction and dialog of different types of cells present in an islet in comparison to a single β-cell type model.
Downstream of the intracellular molecular pathway, we show that hPL-A and hGH induced the phosphorylation of AKT and MAPKs leading to the activation of metabolism, growth and proliferation. These molecular events can explain the physiological effects, mediated by lactogen hormones that have been reported in β-cells. Another intriguing aspect of this pathway is the role of p38 protein. We observed a strong p38 phosphorylation induced by hGH and/or hPL-A, then partial de-phosphorylation of the molecule with the activation of MAPKs. The switching on/off of p38 activity may control the biological role of this protein. This would exert an anti-caspase-3 role and promote cell-growth.35 Otherwise, it has been shown, that in pancreatic islets in vivo the prolonged activation of p38 may lead to a pro-inflammatory process induced by resident macrophages that release cytokines.36 Moreover, the preferential activation of p38 MAPK isoform may be regulated by different stimuli and/or signal strength.37
Also of interest here is our observation that in presence of protein inhibitors of the PI3K and MAPK molecular pathways both Akt and p38 activity were inhibited. This may suggest a connection between p38 and Akt not yet extensively described in reference 38, and requiring further investigation.
The phosphorylation of Akt by hPL-A in insulinoma cell lines and in human pancreatic islets may induce the inhibition of apoptotic events. The involvement of PI3K, and p38 pathways in the mechanism(s) of hPL-A-mediated apoptosis-protection was confirmed in the βTC1 cell model, by the observation that LY, and SB treatments did affect hPL-A antiapoptotic activity. Interestingly, we observed that the treatment with the MAPK inhibitor PD alone reduced serum deprivation-induced apoptosis: this phenomenon could be explained by the fact that the growth arrest induced by serum deprivation could be facilitated by MAPK inhibition, leading to a better survival or could be due to an additional effect of the inhibitor.
The role of lactogenic hormones (PRL, GH, PL) in the regulation of glucose homeostasis has been studied for many years.18,39 GH, PRL and PL are powerful inducers of pancreatic β-cell replication without compromising the ability of β-cells to secrete insulin.40,41 It has been shown that the replication signal induced by GH/PRL to pancreatic β-cells activated the protein complex Jak/Stat. In particular the intracellular signal activated by hGH involves different molecular pathways based on the specific cellular type (i.e., hepatocytes and adipocytes).20,42 It has been established that PRL and PL recognize a common PRL-Receptor and activate several downstream signaling pathways including JAK2/STAT5, PI3K/AKT, ERK1/2, Adenylyl cyclase/cAMP and Protein Kinase/Intracellular Calcium.27 Moreover, lactogens have been implicated in the pro-survival effect in cell types other than pancreatic β-cells.43 In particular, it has been demonstrated in a mouse pancreatic β-cell model, that lactogens drive their protective effects through the JAK2/STAT5 pathway.44 In our insulinoma cell line model, hPL-A treatment strongly resulted in a more prolonged and significant phosphorylation of the protein JAK2, whereas, in the same experimental condition, Jak1 and 3 were not phosphorylated by both the hormones (data not shown). We observed that hGH and hPL-A induced significant different response in the downstream JAK effectors STAT1 and STAT3. In fact, hPL-A induced a higher and more prolonged phosphorylation of STAT1 than hGH and STAT3 activation was significantly induced by hPL-A treatment only (data not shown). Moreover, we found that STAT5b, induced by hPL-A, reached higher and earlier activation than hGH (not shown). Based on the evidences reported about the molecular and functional role of PRL on human islets and islets insulin secretion,45–47 we observed that hPL-A and hGH in mouse insulinoma cell line model activated the same intracellular molecular pathway of insulin substrates cascade proteins, with a higher preferentially activated IRS1 pathway (data not shown). This is evident from the strong and prolonged MAPK phosphorylation induced by hPL-A. IRS-1 activation induced by hPL-A matches the fact that the transgenic mouse with targeted expression of PL in the β-cells, showed β-cell proliferation, hyperplasia, hyperinsulinemia and hypoglycemia.17 Protein Stat-1 was activated by hPL-A, whereas hGH induced Stat-1 phosphorylation only for a few minutes of hormonal treatment. Protein Stat-3 was significantly activated by hPL-A and not by hGH. It has been reported that Stat-1 supports the inflammation state of pancreatic β-cell in diabetes.48 However, our cell line model showed very healthy conditions. Therefore, it is possible that Stat-1 was counteracted by the prolonged activation of Stat-3 and 5, and MAPKs. Stat-3 has been shown to exert a relevant role in sustaining glucose levels, regulating the glucose sensor in pancreatic islets. The level of leptin in the central nervous system thus shows the effect of this molecule in supporting, under hPL-A stimulus, the sensitivity of β-cells to glucose variation.49
It has been reported that PRL is able to induce the upregulation of PDX-1.50 Here we showed that hPL-A treatment on human islets also induced a striking increment in the expression of PDX-1. This evidence is supported by published data which demonstrated a critical role for PI3K/AKT in PDX-1 expression in β-cell development.14 PDX-1 can activate insulin transcription, and it is required for maintaining the hormone-producing phenotype of β-cell. Furthermore, a number of new PDX-1 target genes have been recently identified, many of which contribute to energy sensing and insulin release in pancreatic β-cells.51 Therefore, the increment of PDX-1 strongly suggests that hPL-A can improve islets functionality. As reported, PDX-1 can induce and/or improve insulin synthesis.52 In agreement with these findings, hPL-A enhanced and sustained insulin secretion in human islets in culture, one of the main β-cell activities, for several days. The fact that at 14 days untreated islets also showed an increased stimulation index can be explained by the additional effects of culture stabilization. We consider that the improvement in insulin secretion may represent the physiological role of hPL-A.
In conclusion, hPL-A showed an apoptosis protective effect through PI3K pathway and a secretagogue effect on cultured human islets improving β-cell insulin secretion. Taken together these data highlight hPL-A as a promising molecule in the field of pancreatic islet survival.
Materials and Methods
Materials.
The plasmid phPL4828 (human placental lactogen), coding for the recombinant protein hPL (isoform A) was kindly provided by Genentech and purified in our laboratory as previously described in reference 53. The human growth hormone (hGH) and all the powder and chemical compounds were purchased from Sigma-Aldrich. The cell culture and molecular biology products were purchased from Gibco-Invitrogen.
Anti-Akt, anti-Erk1/2 and anti-p38 and antibodies against phosphorylated forms of AKT (Ser473), p38, (Thr180/Tyr182), Erk1/2 (Thr202/Tyr204) were purchased from Cell Signaling Technology. The anti-phosphotyrosine RC-20 antibody was furnished by Transduction Laboratories. The primary antibodies IRS-1, IRS-2, Jak-1, -2 and -3 were purchased from Upstate Biotechnology. The primary antibody STAT-5b was purchased from Santa Cruz Biotechnology. The secondary antibody anti-rabbit-horseradish-peroxidase conjugate, the FPLC instrument and the chromatographic columns were purchased from General Electric HealthCare (GE Healthcare). The molecular inhibitors LY294002 (LY), SB203580 (SB), PD98059 (PD), H89, Wortmannin (W) were purchased from Calbiochem.
Cell lines and human islets.
The murine βTC-1 and rat RINr1046-38 insulinoma cell lines were kindly provided by Dr A.L. Notkins (NIH, USA) and S. Efrat (Tel Aviv University, Israel). Human islets were isolated using the automated method as previously described in reference 54.
Cytofluorimetric analysis.
Apoptosis was analyzed by fluorescence-activated cell sorting (FACS) analysis of DNA content and by Annexin-V staining. The βTC-1 or RINr1046-38 cells (0.5 × 106) were plated in T25 flasks (Falcon), incubated in serum-free medium DMEM (low glucose) (GIBCO, Invitrogen Corporation) without BSA for 18 h in 5% CO2/93% air at 37°C and stimulated with or without human growth factor (hGH) (Sigma) and hPL-A (500 ng/ml) at 24 and 48 h. Human pancreatic islets, at 90% of clinical purity, were serum deprived with no BSA, for 18 h and stimulated with hPL-A 500 ng/ml at 24, 48 and 72 h. After treatments, insulinoma cells and human islets were trypsinized (trypsin-EDTA 1x, GIBCO), centrifuged at 1,200 rpm for 15 min at 4°C and stained with propidium iodide (100 µg/ml in PBS) for 20 min at 37°C in the dark. Analysis of DNA contents in propidium iodide-stained cells was performed by FACSCalibur (Laser Argon 488 nm, BD). Cells with a lower DNA content (sub-G1 region) were considered apoptotic. The presence of necrosis phenomena were excluded by trypan blue staining (data not shown).
We studied the mechanism of hPL-A protection from apoptosis by FACSCalibur, using the Annexin-V-FITC/Propidium Iodide method (Sigma-Aldrich). Cells were serum depleted over night and the day after treated with 500 ng/ml of hPL-A and/or the following inhibitors: 50 nM LY294002; 500 nM SB203580; 200 nM PD98059; 2 nM Wortmannin; 500 nM H89. Inhibitors were added 30 min before hPL-A. Then treated cells were maintained in serum-free medium for 48 h before analysis.
The intracellular expression of PDX-1 was assayed by cytofluorimetric analysis. Briefly, at the end of each treatment, cells were harvested, fixed by paraformaldheyde (2%) and permeabilyzed by Tween-20 (0.1%). After treatment with blocking solution, cells were incubated with rabbit-anti-human PDX-1 (Chemicon). Isotype identical antibodies served as control. Then cells were washed, incubated with the appropriate fluorescent secondary antibody and analyzed by FACS.
Immunoprecipitation and immunoblotting.
To determine the molecules involved in the intracellular β-cell signalling cascade mediated by hPL-A and/or hGH, the βTC-1 cells were serum starved for 18 h in the presence of 0.1% BSA and stimulated with 500 ng/ml of hPL-A or hGH for different times (5, 15, 30, 45 and 60 min). Then the cells were washed with ice-cold wash buffer (137 mM NaCl, 20 mM Tris pH 7.6, 1 mM MgCl2) containing proteinase inhibitor (100 µM Na3VO4), they were then lysed with 300 µl of Lysis-Buffer (wash buffer, 1.5% NP 40, 10% glycerol, 2 mM EDTA), containing proteinase inhibitors (2 mM PMSF, 2 mM Na3VO4, 10 mM NaPP, 10 mM NaF, 8 µg/ml leupeptin) and incubated on ice for 20 min. The protein concentrations were then measured using the Bradford method.55 Soluble proteins (1.0 or 2.0 mg) were incubated over-night at 4°C on a rotating device with the antibody JAK-2, IRS-1 and -2, SHC, STAT-1, -3 and -5b and then added with 100 µl Protein A (GE Healthcare) for 2 h at 4°C. The proteins immunoprecipitated (IP) were analyzed by 7% SDS-PAGE. The blots were first probed with the horseradish peroxidase-conjugated anti-phosphotyrosine antibody RC20H (Transduction Laboratories), stripped with stripping solution (PIERCE), and then reprobed with specifics antibodies. To determine activation of AKT and MAPKs (Erk1/2 and p38), the proteins were separated by 10% SDS-PAGE and probed with anti-AKT, Erk1/2 and p38. The proteins were visualized by ECL (GE Healthcare). To analyze signal induced in βTC-1 cells through PI3-Kinase and MAPKs we used 50 µg of βTC-1 protein lysates, obtained from cells serum deprived (SD), treated for 30 min with 200 nM Wortmannin or 50 µM SB203580, or 50 µM LY294002, or 20 µM PD98059 and then incubated for 30 min with 500 ng/mL of hPL-A.
Insulin secretion analysis.
Before treatment, human islets were analyzed for their viability (propidium iodide), purity (dithizone staining) and quality (insulin and PDX-1 expression). About 120 islets were plated in each well (9.6 cm2) and cultured in DMEM low glucose (5 mM) plus 10% FBS and 1% Penn/Strep, in a humidified incubator at 30°C with 5% CO2. After 24 h, 500 ng/mL hPL-A was added, and this treatment was renewed every 2 days. After 2, 4, 8 and 14 days of treatment, both control and hPL-treated islets were starved for 3 h in glucose-free HEPES-Krebs buffer and then challenged with HEPES-Krebs buffer 25 mM glucose for 1 h. Secreted insulin was estimated by an RIA kit (Institute of Isotopes Co., Ltd.), and normalized with protein concentration. In order to assess the sensitivity of islet β-cells to glucose stimulation, we calculated the stimulation index using the ratio of the insulin secretion stimulated by 25 mM glucose for 1 h, to zero glucose.
Statistical analysis.
Data are expressed as means ± standard deviation. Statistical analysis was performed by one way ANOVA followed by the Newman-Keuls's post hoc test. Values of p < 0.050 were considered significant.
Acknowledgments
We thank for suggestions and discussion: G. Sconocchia (CNR, Italy); G. Sesti and M.L. Hirbal (University of Catanzaro, Italy); G. Melino, M. Ranalli and F. Ferrelli (University of Rome Tor Vergata); G. Barbato, M. Mattu, F. Talamo and L. Orsatti (IRBM, Rome, Italy). We also thank Graziano Bonelli (University of Rome Tor Vergata) for artwork. This work was supported by grants from CNR (5% Biotechnology Project 95/95), from Italian Ministry of Health and Research (Project N.210/2003, N.200267482_006 and N. 2004067425_003), from Fondazione Roma, and from ASI (Agenzia Spaziale Italiana). Human islets were provided from the Islet Processing Facility Milan Program through the JDRF award 6-2005-1178 (ECIT; Islet for Research program).
Abbreviations
- BTC
betacellulin
- FGFs
fibroblast growth factors
- EGF
epidermal growth factor
- GLP-1
glucagon-like peptide 1
- GLUT-2
glucose transporter 2
- GH
growth hormone
- HGF
hepatic growth factor
- IGFs
insulin-like growth factors
- INGAP
islet neogenesis associated protein
- PDX-1
pancreas/duodenum homeobox-1
- PTHrP
parathyroid hormone-related protein
- PL
placental lactogen
- PRL
prolactin
Supplementary Material
References
- 1.Tripathi BK, Srivastava AK. Diabetes mellitus: complications and therapeutics. Med Sci Monit. 2006;12:130–147. [PubMed] [Google Scholar]
- 2.Hogan A, Pileggi A, Ricordi C. Transplantation: current developments and future directions; the future of clinical islet transplantation as a cure for diabetes. Front Biosci. 2008;13:1192–1205. doi: 10.2741/2755. [DOI] [PubMed] [Google Scholar]
- 3.Lee MS, Chang I, Kim S. Death effectors of beta-cell apoptosis in type 1 diabetes. Mol Genet Metab. 2004;83:82–92. doi: 10.1016/j.ymgme.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Rhodes CJ. Type 2 diabetes-a matter of beta-cell life and death? Science. 2005;307:380–384. doi: 10.1126/science.1104345. [DOI] [PubMed] [Google Scholar]
- 5.Meier JJ, Ritzel RA, Maedler K, Gurlo T, Buttler PC. Increased vulnerability of newly forming beta cells to cytokine induced cell death. Diabetologia. 2006;49:83–89. doi: 10.1007/s00125-005-0069-3. [DOI] [PubMed] [Google Scholar]
- 6.Fontaine MJ, Fan W. Islet cell transplantation as a cure for insulin dependent diabetes: current improvements in preserving islet cell mass and function. Hepatobiliary Pancreat Dis Int. 2003;2:170–179. [PubMed] [Google Scholar]
- 7.Yamada S, Kojima I. Regenerative medicine of the pancreatic beta cells. J Hepatobiliary Pancreat Surg. 2005;12:218–226. doi: 10.1007/s00534-005-0983-2. [DOI] [PubMed] [Google Scholar]
- 8.Bonner-Weir S, Weir GC. New sources of pancreatic beta-cells. Nat Biotech. 2005;23:857–861. doi: 10.1038/nbt1115. [DOI] [PubMed] [Google Scholar]
- 9.Lee CS, De León DD, Kaestner KH, Stoffers DA. Regeneration of pancreatic islets after partial pancreatectomy in mice does not involve the reactivation of neurogenin-3. Diabetes. 2006;55:269–272. [PubMed] [Google Scholar]
- 10.Trucco M. Regeneration of the pancreatic beta cell. J Clin Invest. 2005;15:5–12. doi: 10.1172/JCI23935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowens L, Rooman I. Regulation of pancreatic beta-cell mass. Physiol Rev. 2005;85:1255–1270. doi: 10.1152/physrev.00025.2004. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Cao X, Li LX, Brubaker PL, Edlund H, Drucker DJ. Beta-cell Pdx1 expression is essential for the glucoregulatory, proliferative and cytoprotective actions of glucagon-like peptide-1. Diabetes. 2005;54:482–491. doi: 10.2337/diabetes.54.2.482. [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Ocana A, Vasavada RC, Cebrian A, Lopez-Talavera JC, Stewart AF. Using beta-cell growth factors to enhance human pancreatic Islet transplantation. J Clin Endocrinol Metab. 2001;86:984–988. doi: 10.1210/jcem.86.3.7315. [DOI] [PubMed] [Google Scholar]
- 14.Watanabe H, Saito H, Ueda J, Evers M. Regulation of pancreatic duct cell differentiation by phosphatidylinositol-3-kinase. Biochem Biophys Res Commun. 2008;370:33–37. doi: 10.1016/j.bbrc.2008.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brelje TC, Scharp DW, Lacy PE, Ogren L, Talamantes F, Robertson M, et al. Effect of homologous placental lactogens, prolactins and growth hormones on islet beta-cell division and insulin secretion in rat, mouse and human islets: implication for placental lactogen regulation of islet function during pregnancy. Endocrinology. 1993;132:879–887. doi: 10.1210/endo.132.2.8425500. [DOI] [PubMed] [Google Scholar]
- 16.Goffin V, Shiverik KT, Kelly PA, Martial JA. Sequence-function relationships within the expanding family of prolactin, growth hormone, placental lactogen and related proteins in mammals. Endocrine Rev. 1996;17:385–410. doi: 10.1210/edrv-17-4-385. [DOI] [PubMed] [Google Scholar]
- 17.Vasavada RC, Garcia-Ocaña A, Zawalich WS, Sorenson RL, Dann P, Syed M, et al. Targeted expression of placental lactogen in the beta cells of transgenic mice results in beta cell proliferation, islet mass augmentation and hypoglycemia. J Biol Chem. 2000;275:15399–15406. doi: 10.1074/jbc.275.20.15399. [DOI] [PubMed] [Google Scholar]
- 18.Freemark M. Regulation of maternal metabolism by pituitary and placental hormones: roles in fetal development and metabolic programming. Horm Res. 2006;65:41–49. doi: 10.1159/000091505. [DOI] [PubMed] [Google Scholar]
- 19.Chow JC, Ling PR, Qu Z, Laviola L, Ciccarone AM, Bristian BR, et al. Growth hormone stimulates tyrosine phpsphorylations of JAK2 and STAT5, but not Insulin Receptor Substrate-1 or SHC proteins in liver and Skeletal muscle of normal rats in vivo. Endocrinology. 1996;137:2280–2286. doi: 10.1210/endo.137.7.8770909. [DOI] [PubMed] [Google Scholar]
- 20.Ridderstrale M, Tornquist H. Effects of tyrosine kinase inhibitors on tyrosine phpsphorilations and the insulin-like effects in response to human growth hormone in isolated rat adipocytes. Endocrinology. 1996;137:4650–4656. doi: 10.1210/endo.137.11.8895329. [DOI] [PubMed] [Google Scholar]
- 21.Friedrichsen BN, Galsgaard ED, Nielsen JH, Moldrup A. Growth hormone and prolactin-induced proliferation of insulinoma cells, INS-1, depends on activation of STAT-5 (signal transducer and activator of transcription 5) Endocrinology. 2001;15:136–148. doi: 10.1210/mend.15.1.0576. [DOI] [PubMed] [Google Scholar]
- 22.Amaral MEC, Cunha DA, Anhκ GF, Ueno M, Carneiro EM, Velloso LA, et al. Participation of prolactin receptors and phosphatidylinositol-3-kinase and MAP kinase pathways in the increase in pancreatic islet mass and sensitivity to glucose during pregnancy. J Endocrinol. 2004;183:469–476. doi: 10.1677/joe.1.05547. [DOI] [PubMed] [Google Scholar]
- 23.Dickson LM, Rhodes CJ. Pancreatic cell growth and survival in the onset of type 2 diabetes: a role for protein kinase B in the Akt? Am J Physiol Endocrinol Metab. 2004;287:192–198. doi: 10.1152/ajpendo.00031.2004. [DOI] [PubMed] [Google Scholar]
- 24.Brelje TC, Stout LE, Bhagroo NV, Sorenson RL. Distinctive roles for prolactin and growth hormone in the activation of signal transducer and activator of transcription 5 in pancreatic islets of Langerhans. Endocrinology. 2004;145:4162–4175. doi: 10.1210/en.2004-0201. [DOI] [PubMed] [Google Scholar]
- 25.Gorogowa SI, Fujitani Y, Kaneto H, Hazama Y, Watada H, Miyamoto Y, et al. Insulin secretory defects and impaired islet architecture in pancreatic beta-cell-specific STAT 3 knockout mice. Biochem Biophys Res Commun. 2004;319:1159–1217. doi: 10.1016/j.bbrc.2004.05.095. [DOI] [PubMed] [Google Scholar]
- 26.Chilton BS, Hewetson A. Prolactin and growth hormone signaling. Curr Top Dev Biol. 2005;68:1–23. doi: 10.1016/S0070-2153(05)68001-5. [DOI] [PubMed] [Google Scholar]
- 27.Vasavada RC, Gonzalez-Pertusa JA, Fujinaka Y, Taesch-Fiaschi N, Cozar-Castellano I, Garcia-Ocana A. Growth factors and beta-cell replication. Int J Biochem Cell Biol. 2006;38:931–950. doi: 10.1016/j.biocel.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Hui H, Perfetti R. Pancreas duodenum homeobox-1 regulates pancreas development during embryogenesis and islet cell function in adulthood. Eur J Endocrinol. 2002;146:129–141. doi: 10.1530/eje.0.1460129. [DOI] [PubMed] [Google Scholar]
- 29.Melloul D, Marshak S, Cerasi E. Regulation of insulin gene transcription. Diabetologia. 2002;45:309–326. doi: 10.1007/s00125-001-0728-y. [DOI] [PubMed] [Google Scholar]
- 30.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Beta-cell-specific inactivation of the mouse Ipf1/Pdx1 gene results in loss of the beta-cell phenotype and maturity onset diabetes. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Edlund H. Transcribing pancreas. Diabetes. 1998;47:1817–1823. doi: 10.2337/diabetes.47.12.1817. [DOI] [PubMed] [Google Scholar]
- 32.Sander M, German MS. The beta cell transcription factors and development of the pancreas. J Mol Med. 1997;75:327–340. doi: 10.1007/s001090050118. [DOI] [PubMed] [Google Scholar]
- 33.Habener JF, Stoffers DA. A newly discovered role of transcription factors involved in pancreas development and the pathogenesis of diabetes mellitus. Proc Assoc Am Physicians. 1998;110:12–21. [PubMed] [Google Scholar]
- 34.Wang X, Liu Y, Yang Z, Zhang Z, Zhou W, Ye Z, et al. Glucose metabolism-related protein 1 (GMRP1) regulates pancreatic beta cell proliferation and apoptosis via activation of Akt signalling pathway in rats and mice. Diabetologia. 2011;54:852–863. doi: 10.1007/s00125-011-2048-1. [DOI] [PubMed] [Google Scholar]
- 35.Ehses JA, Casilla VR, Doty T, Pospisilik JA, Winter KD, Demuth AU, et al. Glucose-dependent insulinotropic polypeptide promotes beta-(INS-1) cell survival via cyclic adenosine monophosphate-mediated caspase-3 inhibition and regulation of p38 mitogen-activated protein kinase. Endocrinology. 2003;144:4433–4445. doi: 10.1210/en.2002-0068. [DOI] [PubMed] [Google Scholar]
- 36.Matsuda T, Omori K, Vuong T, Pascual M, Valiente L, Ferreri K, et al. Inhibition of p38 pathway suppresses human islet production of pro-inflammatory cytokines and improves islet graft function. Am J Transplant. 2005;5:484–493. doi: 10.1046/j.1600-6143.2004.00716.x. [DOI] [PubMed] [Google Scholar]
- 37.Piquer S, Barcelò-Battlori S, Julià M, Marzo N, Nadal B, Guinivart JJ, et al. Phosphorylation events implicating p38 and PI3K mediate tungstate-effects in MIN6 beta cells. Biochem Biophys Res Comm. 2007;358:385–391. doi: 10.1016/j.bbrc.2007.04.143. [DOI] [PubMed] [Google Scholar]
- 38.Alonso G, Ambrosino C, Jones M, Nebrada AR. Differential activation of p38 mitogen-activated protein kinase isoforms depending on signal strength. J Biol Chem. 2000;275:40641–40648. doi: 10.1074/jbc.M007835200. [DOI] [PubMed] [Google Scholar]
- 39.Soares M. The prolactin and growth hormone families: pregnancy-specific hormones-cytokines at the maternal-fetal interface. Reprod Biol Endocrinol. 2004;2:51. doi: 10.1186/1477-7827-2-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Billestrup N, Nielsen JH. The stimulatory effect of growth hormone, prolactin and placental lactogen on beta-cell proliferation is not mediated by insulin-like growth factor-I. Endocrinology. 1991;129:883–888. doi: 10.1210/endo-129-2-883. [DOI] [PubMed] [Google Scholar]
- 41.Fleenor D, Oden J, Kelly PA, Mohan S, Alliouachene S, Pende M, et al. Roles of the lactogens and somatogens in perinatal and postnatal metabolism and growth: studies of a novel mouse model combining lactogen resistance and growth hormone deficiency. Endocrinology. 2005;146:103–112. doi: 10.1210/en.2004-0744. [DOI] [PubMed] [Google Scholar]
- 42.Cao J, Gowry PM, Ganguly TC, Wood M, Hyde JF, Talamantes F, et al. PRL, placental lactogen and GH induce NA(+)/taurocholate-cotransporting polypeptide gene expression by activating signal transducer and activator of transcription-5 in liver cells. Endocrinology. 2001;142:4212–4222. doi: 10.1210/endo.142.10.8456. [DOI] [PubMed] [Google Scholar]
- 43.Heito JJ, Karmik K, Kim SK. Intrinsic regulators of pancreatic beta-cell proliferation. Annu Rev Cell Dev Biol. 2006;22:311–338. doi: 10.1146/annurev.cellbio.22.010305.104425. [DOI] [PubMed] [Google Scholar]
- 44.Fujiamaka Y, Takane K, Yamashito H, Vasavada RC. Lactogens promote beta cell survival through JAK2/STAT5 activation and Bcl-XL upregulation. J Biol Chem. 2007;282:30707–30717. doi: 10.1074/jbc.M702607200. [DOI] [PubMed] [Google Scholar]
- 45.Yamamoto T, Ricordi C, Mita A, Miki A, Sakuma Y, Molano RD, et al. Beta-cell specific cytoprotection by prolactin on human islets. Transplant Proc. 2008;40:382–383. doi: 10.1016/j.transproceed.2008.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Labriola L, Ferreira GB, Montor WR, Demasi MAA, Pimenta DC, Lojudice FH, et al. Prolactin-induced changes in protein expression in human pancreatic islets. Mol Cell Endocrinol. 2007;264:16–27. doi: 10.1016/j.mce.2006.10.004. [DOI] [PubMed] [Google Scholar]
- 47.Labriola L, Montor WR, Krogh K, Lojudice FH, Genzini T, Goldberg AC, et al. Benefical effects of prolactin and laminin on human pancreatic islets-cell cultures. Mol Cell Endocrinol. 2007;263:120–133. doi: 10.1016/j.mce.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 48.Callewaert HI, Gysemans CA, Ladriere L, D'Hertog W, Hagenbrock J, Overbergh L, et al. Deletion of STAT-1 pancreatic islets protects against streptozotocin-induced diabetes and early graft failure but not against late rejection. Diabetes. 2007;56:2169–2173. doi: 10.2337/db07-0052. [DOI] [PubMed] [Google Scholar]
- 49.Stephanou A, Latchman DS. Opposing actions of STAT-1 and STAT-3. Growth Factors. 2005;23:177–182. doi: 10.1080/08977190500178745. [DOI] [PubMed] [Google Scholar]
- 50.Nasir I, Kedees MH, Ehrlich ME, Teitelman G. The role of pregnancy hormones in the regulation of Pdx-1 expression. Mol Cell Endocrinol. 2005;233:1–13. doi: 10.1016/j.mce.2005.02.001. [DOI] [PubMed] [Google Scholar]
- 51.Keller DM, McWeeney S, Arsenlis A, Drouin J, Wright CVE, Wang H, et al. Characterization of pancreatic transcription factor Pdx-1 binding sites using promoter microarray and serial analysis of chromatin occupancy. J Biol Chem. 2007;282:32084–32092. doi: 10.1074/jbc.M700899200. [DOI] [PubMed] [Google Scholar]
- 52.Macfarlane WM, Shepherd RM, Cosgrove KE, James RFL, Dunne MJ, Docherty K. Glucose modulation of insulin mRNA levels is dependent on trascription factor PDX-1 and occurs indipendently of changhes in intracellular Ca2+ Diabetes. 2000;49:418–423. doi: 10.2337/diabetes.49.3.418. [DOI] [PubMed] [Google Scholar]
- 53.Lowman HB, Cunningham BC, Wells JA. Mutational analysis and protein engineering of receptor-binding determinants in human placental lactogen. J Biol Chem. 1991;266:10982–10988. [PubMed] [Google Scholar]
- 54.Bertuzzi F, Grohovaz F, Maffi P, Caumo A, Aldrighetti L, Nano R, et al. Successful transplantation of human islets in recipients bearing a kidney graft. Diabetologia. 2002;45:77–84. doi: 10.1007/s125-002-8247-2. [DOI] [PubMed] [Google Scholar]
- 55.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.