Abstract
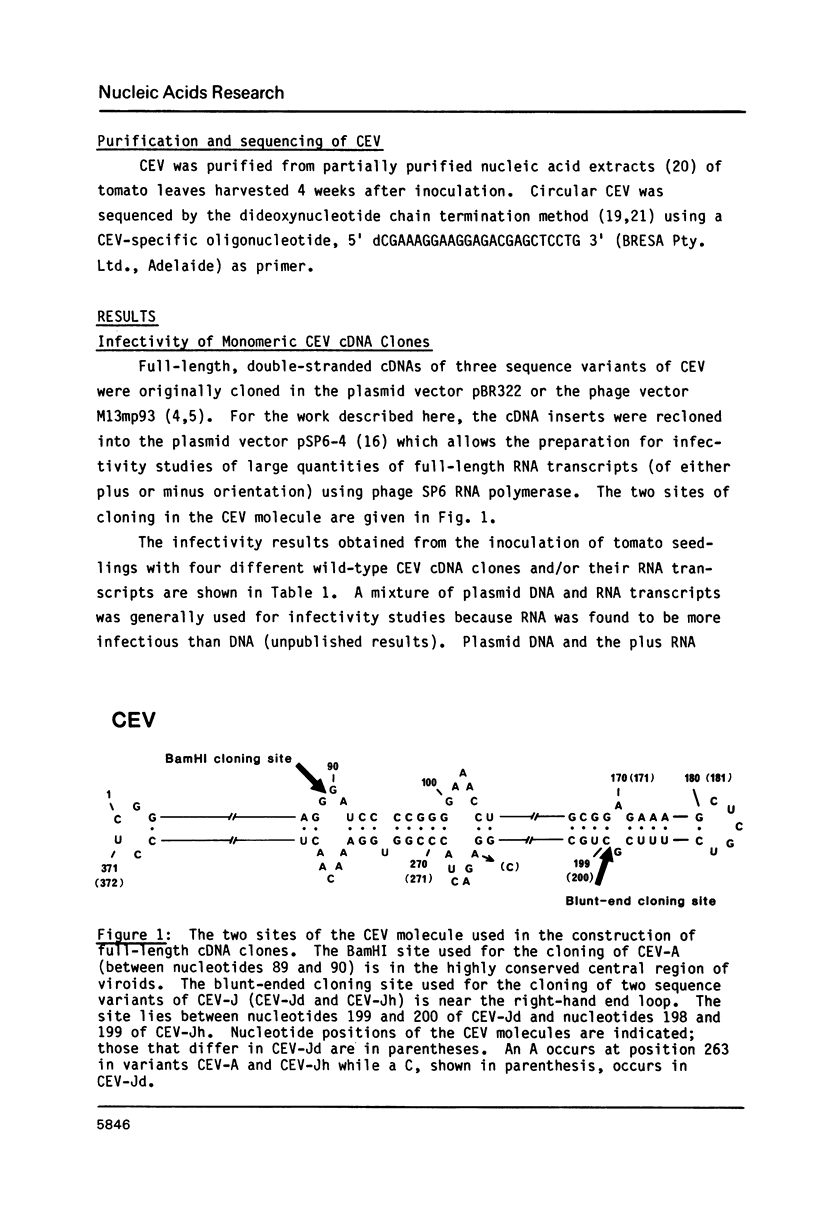
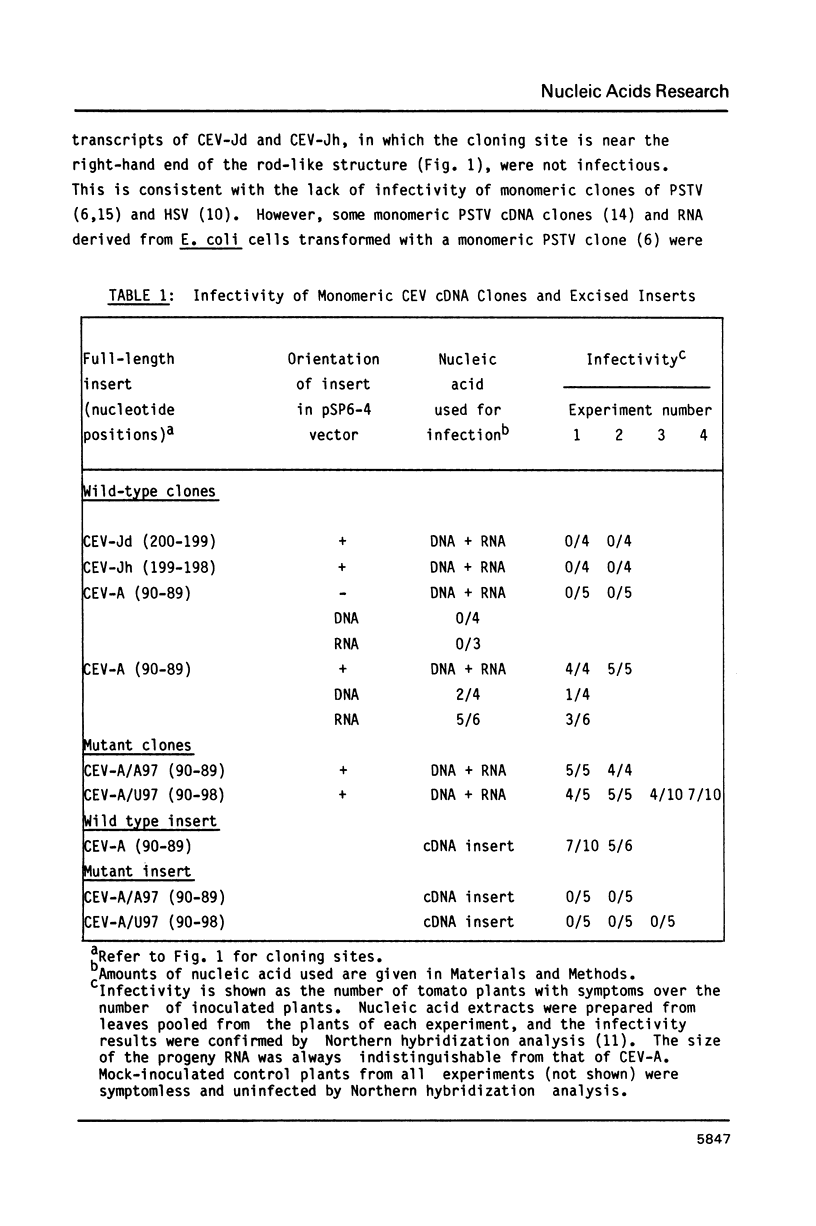
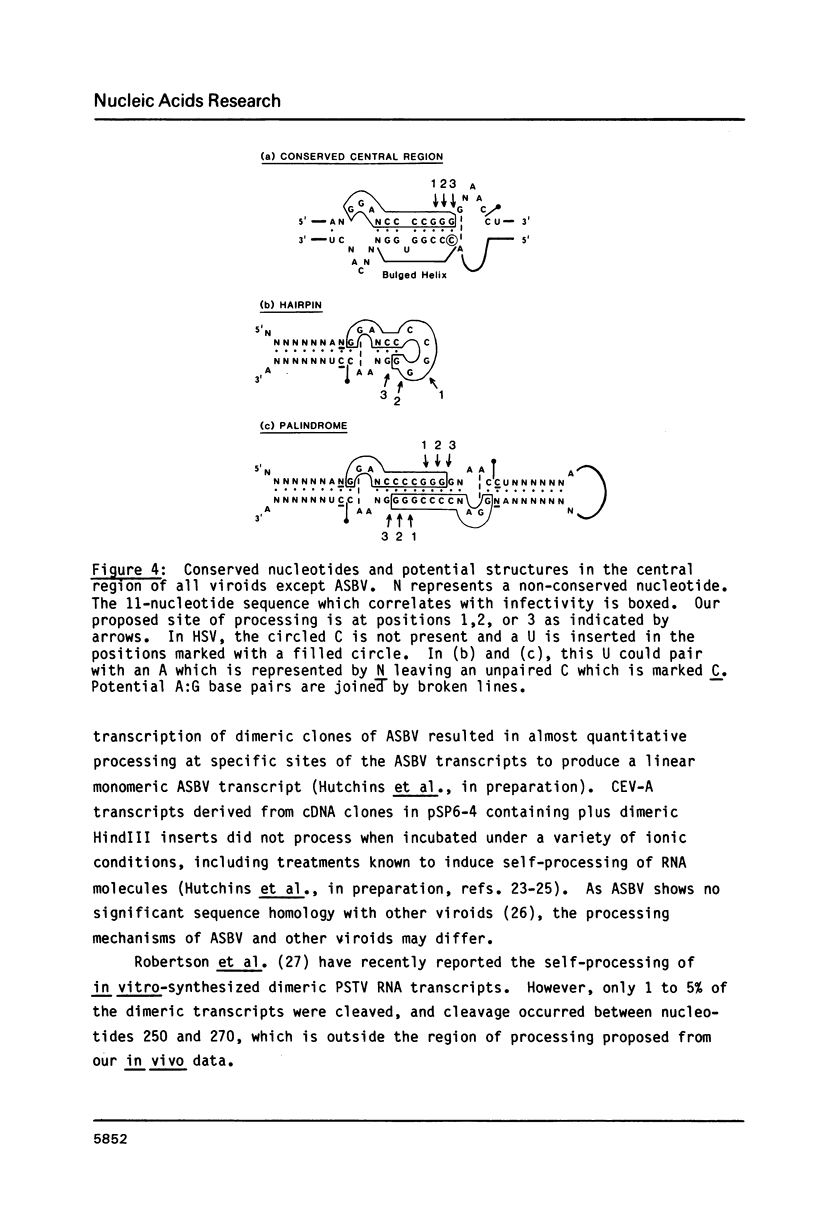
Monomeric cDNA clones of citrus exocortis viroid (CEV) were constructed in the plasmid vector pSP6-4 and the infectivity of the clones plus in vitro-synthesized RNA transcripts determined by inoculation onto tomato seedlings. Infectivity was dependent on the site of the viroid molecule used for cloning and the orientation of the cDNA insert. Only the plus BamHI cDNA clone was infectious and produced progeny viroid with wild-type sequence at the region corresponding to the BamHI cloning site. Infectivity correlated with the terminal repetition of 11 nucleotides of viroid sequence, 5'GGATCCCCGGG 3', in the vector adjacent to the insert. The 11-nucleotide sequence lies within the highly conserved central region of viroids. Site-directed mutagenesis of a single nucleotide in the repeat at the 5'-end of the CEV insert to 5' GGATCCCC(T,A)GG 3' gave two point mutants. The two mutant CEV inserts, when excised from the vector, were not infectious. However, plasmid DNA and RNA transcripts from non-excised mutant CEV inserts were infectious. The progeny of one of these clones was examined and contained wild-type sequence. It was concluded that in vivo processing of longer-than-unit-length CEV occurs at one of three adjacent sites in the 11 nucleotide sequence and that the G nucleotide at position 97 is important for viroid replication.
Full text
PDF









Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Branch A. D., Robertson H. D. A replication cycle for viroids and other small infectious RNA's. Science. 1984 Feb 3;223(4635):450–455. doi: 10.1126/science.6197756. [DOI] [PubMed] [Google Scholar]
- Branch A. D., Robertson H. D., Greer C., Gegenheimer P., Peebles C., Abelson J. Cell-free circularization of viroid progeny RNA by an RNA ligase from wheat germ. Science. 1982 Sep 17;217(4565):1147–1149. doi: 10.1126/science.217.4565.1147. [DOI] [PubMed] [Google Scholar]
- Cress D. E., Kiefer M. C., Owens R. A. Construction of infectious potato spindle tuber viroid cDNA clones. Nucleic Acids Res. 1983 Oct 11;11(19):6821–6835. doi: 10.1093/nar/11.19.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diener T. O. Viroids. Adv Virus Res. 1983;28:241–283. doi: 10.1016/s0065-3527(08)60725-3. [DOI] [PubMed] [Google Scholar]
- Ishikawa M., Meshi T., Ohno T., Okada Y., Sano T., Ueda I., Shikata E. A revised replication cycle for viroids: the role of longer than unit length RNA in viroid replication. Mol Gen Genet. 1984;196(3):421–428. doi: 10.1007/BF00436189. [DOI] [PubMed] [Google Scholar]
- Kan L. S., Chandrasegaran S., Pulford S. M., Miller P. S. Detection of a guanine X adenine base pair in a decadeoxyribonucleotide by proton magnetic resonance spectroscopy. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4263–4265. doi: 10.1073/pnas.80.14.4263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meshi T., Ishikawa M., Ohno T., Okada Y., Sano T., Ueda I., Shikata E. Double-stranded cDNAs of hop stunt viroid are infectious. J Biochem. 1984 May;95(5):1521–1524. doi: 10.1093/oxfordjournals.jbchem.a134761. [DOI] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezaian M. A., Williams R. H., Gordon K. H., Gould A. R., Symons R. H. Nucleotide sequence of cucumber-mosaic-virus RNA 2 reveals a translation product significantly homologous to corresponding proteins of other viruses. Eur J Biochem. 1984 Sep 3;143(2):277–284. doi: 10.1111/j.1432-1033.1984.tb08370.x. [DOI] [PubMed] [Google Scholar]
- Rich A., RajBhandary U. L. Transfer RNA: molecular structure, sequence, and properties. Annu Rev Biochem. 1976;45:805–860. doi: 10.1146/annurev.bi.45.070176.004105. [DOI] [PubMed] [Google Scholar]
- Riesner D., Henco K., Rokohl U., Klotz G., Kleinschmidt A. K., Domdey H., Jank P., Gross H. J., Sänger H. L. Structure and structure formation of viroids. J Mol Biol. 1979 Sep 5;133(1):85–115. doi: 10.1016/0022-2836(79)90252-3. [DOI] [PubMed] [Google Scholar]
- Sanger F., Coulson A. R., Barrell B. G., Smith A. J., Roe B. A. Cloning in single-stranded bacteriophage as an aid to rapid DNA sequencing. J Mol Biol. 1980 Oct 25;143(2):161–178. doi: 10.1016/0022-2836(80)90196-5. [DOI] [PubMed] [Google Scholar]
- Symons R. H. Avocado sunblotch viroid: primary sequence and proposed secondary structure. Nucleic Acids Res. 1981 Dec 11;9(23):6527–6537. doi: 10.1093/nar/9.23.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabler M., Sänger H. L. Cloned single- and double-stranded DNA copies of potato spindle tuber viroid (PSTV) RNA and co-inoculated subgenomic DNA fragments are infectious. EMBO J. 1984 Dec 20;3(13):3055–3062. doi: 10.1002/j.1460-2075.1984.tb02257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub W., Sussman J. L. Adenine-guanine base pairing ribosomal RNA. Nucleic Acids Res. 1982 Apr 24;10(8):2701–2708. doi: 10.1093/nar/10.8.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader J. E., Gould A. R., Bruening G. E., Symons R. H. Citrus exocortis viroid: nucleotide sequence and secondary structure of an Australian isolate. FEBS Lett. 1982 Jan 25;137(2):288–292. doi: 10.1016/0014-5793(82)80369-4. [DOI] [PubMed] [Google Scholar]
- Visvader J. E., Symons R. H. Eleven new sequence variants of citrus exocortis viroid and the correlation of sequence with pathogenicity. Nucleic Acids Res. 1985 Apr 25;13(8):2907–2920. doi: 10.1093/nar/13.8.2907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson N., Gurevitz M., Ford J., Apirion D. Self cleavage of a precursor RNA from bacteriophage T4. J Mol Biol. 1984 Jan 25;172(3):301–323. doi: 10.1016/s0022-2836(84)80028-5. [DOI] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis using M13-derived vectors: an efficient and general procedure for the production of point mutations in any fragment of DNA. Nucleic Acids Res. 1982 Oct 25;10(20):6487–6500. doi: 10.1093/nar/10.20.6487. [DOI] [PMC free article] [PubMed] [Google Scholar]



