Abstract
The size composition of human islet preparations has been attributed to functional potency, islet survival and transplantation outcomes. In the early post-transplantation phase islets are supplied with oxygen by diffusion only and are at risk of critical hypoxia. The high rate of early islet graft dysfunction is in part attributed to this condition. It has been presumed that islets with smaller diameter, and therefore smaller diffusion distance, are superior to large islets regarding early survival rate and graft function. In this study we aimed to evaluate Complex Object Parametric Analysis and Sorting (COPAS) as a device for automated sorting of human islets. The use of COPAS was validated for accuracy and sensitivity using polystyrene beads of known diameters. Based on time of flight relative to particle isolated islets were then automatically sorted and analyzed for viability and function using handpicked islets as control. Our results suggest that COPAS enables the automated and accurate sorting of islets with no negative impact on their integrity and viability. Thus, COPAS is an adequate tool for size-specific analysis of pancreatic islets and may be considered as part of a platform for automated high-throughput screening of pancreatic islets.
Key words: islet size, automated sorting, COPAS, glucose response, insulin secretion
Introduction
Pancreatic islet transplantation has evolved into a relevant treatment option for patients with type 1 diabetes. During the isolation procedure islets are deprived of their naïve endothelial glomerular-like network1,2 and microenvironment and then transplanted “naked” into the portal system of the liver. Engraftment and revascularization of the islets is a slow process and a relevant blood-flow is re-established not earlier than 10–14 days after transplantation.3,4 Until then, islets are supplied with oxygen by diffusion only, and therefore, are highly susceptible to hypoxic damage. Moreover the intraportal environment shows naturally a rather low oxygen tension in the range of 5 mmHg, which is only a fraction of the oxygen tension within the native pancreas.1,2,4 Recent studies demonstrated that islets with smaller diameter are superior to larger islets during the early engraftment period, presumably due to the better gas exchange (O2, CO2) as a consequence of the shorter diffusion distance. Accordingly, it has been reported that smaller islets display greater O2 consumption and insulin secretion rates than larger islets.4–7
The COPAS (Complex Object Parametric Analysis and Sorting) technology is based on traditional single cell flowcytometry, but it is designed for the analysis and sorting of objects with diameter from 20–700 µm. Current applications include beads, small seeds, Drosophila embryos, other like sized model organisms and large cells/cellular clusters. The continuous flow system can analyze objects using five parameters: size, optical density and spectrums of fluorescence. Objects are aligned in the centre of a sheath fluid, which produces a stable laminar flow and carries the object to the flow cell. Here, they pass axially and individually through the focus of an Argon 488 nm laser beam. The resulting signals are then measured and recorded by forward scatter and fluorescence detectors. The relative size of each object is measured by an axial light-loss detector, which calculates the time the light blockage signal remains above a pre-set threshold level: this parameter corresponds to the time of flight (TOF). TOF is related to an object axial length and was the essential parameter used for this study. Sorting and dispensing decisions are based on user-selected ranges of TOF. In the case an object meets the pre-defined criteria, the airflow is briefly paused and the object is sorted to the collection dish.
In the present study we aimed to validate the potential of the COPAS instrument for automated size-based sorting of islets, a crucial pre-requisite for accurate study of their size-dependent characteristics.
Results
Calibration of the COPAS.
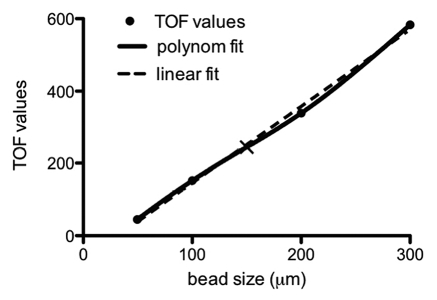
Initial validation of sensitivity and accuracy of the COPAS was performed using polystyrene particles whose diameter range mimicked that of a typical mixture of isolated islets. Particles were run on the COPAS and TOF values were collected. Correlation of the data obtained with given particle sizes allowed for polynomial function analysis and calculation of a standard curve8 (Fig. 1). Based on this standard curve, it was determined that the cut off TOF value for sorting islets smaller and larger than 150 µm was 244. The sorting gates were set accordingly for islet sorting (Fig. 2A and B).
Figure 1.
Mathematical correlation between polystyrene particle size and time of flight (TOF). The cut off point of 244 for a particle with diameter of 150 µm is indicated by the slanted black line.
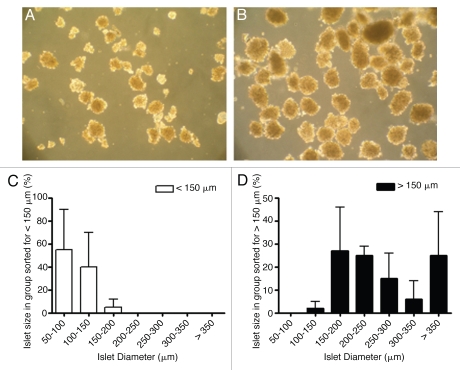
Figure 2.
(A and B) Representative bright field pictures (20× magnification) of COPAS sorted islets with diameter <150 µm (A) and >150 µm (B). (C and D) Size distribution of sorted islets with diameters <150 µm (C) and >150 µm (D) as assessed by visual inspection. (n = 3).
Accuracy of islet sorting.
Since COPAS cannot discriminate between aggregates of exocrine cells and islets, the purity of the islet preparation prior to sorting is a critical factor. Accordingly, COPAS sorting was carried out on three independent islet isolation whose purity was >85% pure, as estimated by microscopy analysis after dithizone staining. The outcome of the automated islet sorting was also validated by visual inspection of each islet group using a microscope equipped with an eyepiece grid. Ninety five percent of the small islets sorted with COPAS had a diameter smaller than 150 µm, whereas in the group of larger islets 98% had a diameter bigger than 150 µm (Fig. 2C and D). The corresponding 5 and 2% erroneously sorted islets were found in the next neighboring size category.
Islet viability.
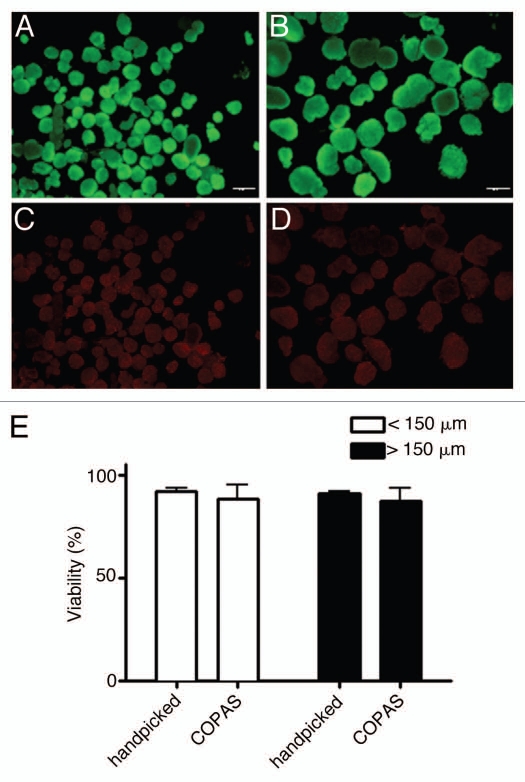
The membrane integrity of handpicked and COPAS sorted islets was determined by FDA/PI staining and fluorescent microscopy (Fig. 3A–D). The viability of manually and automated sorted islets did not significantly differ (<150 µm, handpicked: 92 ± 1.5%, COPAS: 88 ± 7.2%; >150 µm, handpicked: 91 ± 1.3%, COPAS: 87 ± 6.3%) (Fig. 3E), suggesting that the COPAS procedure was not harmful. This viability assay, however, has limitations as cells at early stages of apoptosis, i.e., prior to damage of their plasma membrane, may escape detection.20–22
Figure 3.
(A–D) Representative fluorescence microscopy pictures (40× magnification) of COPAS sorted islets <150 µm (A and C) or >150 µm (B and D) stained with FDA (A and B) and PI (C and D). (E) Viability of handpicked or COPAS sorted islets. (n = 3).
Glucose-stimulated insulin secretion.
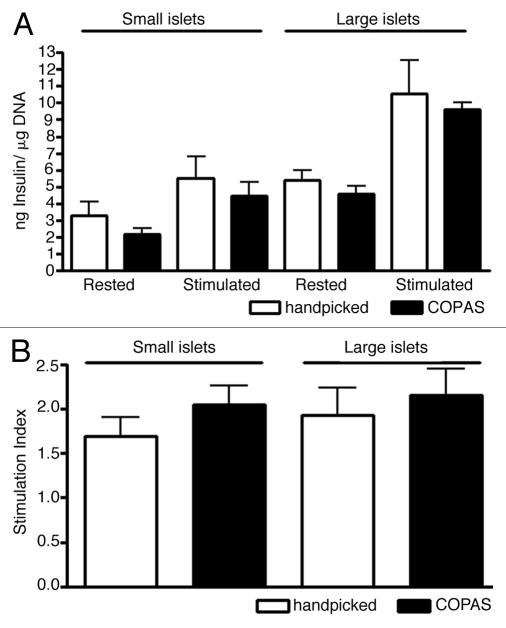
The functional integrity of handpicked and COPAS sorted islets was further assessed by comparing their insulin secretion. Manually and automatically sorted small islets exposed to 3.3 mM glucose for 1 h secreted 3.31 ± 0.82 ng insulin/µg DNA and 2.19 ± 0.35 ng insulin/µg DNA, respectively. Upon stimulation with 16.7 mM glucose for 1 h insulin secretion increased to 5.52 ± 1.26 ng insulin/µg DNA for handpicked and 4.47 ± 0.83 ng insulin/µg DNA. Handpicked and COPAS sorted large human islets secreted at rest 5.41 ± 0.55 ng insulin/µg DNA and 4.57 ± 0.47 ng insulin/µg DNA, respectively. After stimulation with 16.7 mM glucose their insulin secretion increased to 10.52 ± 2 ng insulin/µg DNA and 9.6 ± 0.4 ng insulin/µg DNA (Fig. 4A). Stimulation indices showed no significant difference between islet groups based either on their size or method of sorting (Fig. 4B). Hence, the sorting method did not appear to alter insulin secretion.
Figure 4.
(A and B) Insulin secretion (A) and stimulation index (B) of islets <150 µm or >150 µm after manual or automated sorting.
Discussion
Islet size has an influence on islet transplantation outcome and survival. Recent publications show that mouse smaller islets perform better than larger islets when exposed to hypoxia followed syngeneic transplantation under the kidney capsule.4,5,12 The smaller islet diameter implies a shorter diffusion distance and thereby a more effective supply of oxygen and nutrients. Evidence that the COPAS is not detrimental for islet function and viability opens the opportunity of applying this technology for the sorting of thousands of islets of homogeneous size within a few hours—a task that is unfeasible by manual sorting. COPAS-mediated islet sorting could therefore pave the way for high throughput screening of islets from large mammals, such as pigs, and thereby advance the field of islet research and drug development for diabetes therapy.
Materials and Methods
Islet isolation.
Human islets (n = 3) were isolated from donor organs with consent obtained for research by next of kin and authorization through Eurotransplant. Islets were isolated following the modified Ricordi method using collagenase NB1 and neutral protease (Serva). Purification was performed with continuous-density Biocoll gradient (Biochrom AB) centrifugation using a COBE 2991 cell processor. Islets were cultured for 24 h in M1A media (Mediatech) supplemented with 32.5 mM L-glutathione (Sigma) prior to experimentation. The purity of the preparations was assessed by microscopy after dithizone staining of the islets.
Islet sorting based upon size by “handpicking” or large particle flowcytometry (COPAS).
For handpicking experiments the islet size was visually determined using an eyepiece grid integrated into an inverted microscope. The islets were manually separated into two groups with diameter smaller and larger than 150 µm. Small and large islets were separate according to sorting gates pre-determined with polystyrene particles of diameters of 49.8, 100, 200 and 300 µm (Union Biometrica). For automated sorting the islets were loaded on the COPAS at a dilution that would result in the detection of 10–30 events/second. In our experience this minimizes the likelihood of two particles being separated simultaneously, thus allowing for the reliable size sorting of 35,000–100,000 islets within 1 h. Following the sorting procedure, the islets were cultured over night prior to further experimentation.
Viability staining.
Islet aliquots were transferred to phosphate-buffered saline (PBS) and fluorescein diacetate (FDA) and propidium iodide (PI) were added to a final concentration of 0.5 and 75 µM, respectively. Cell viability was estimated using fluorescence microscopy by calculating the percentage of viable (FDA-positive, green) versus non-viable (PI-positive, red) cells within each islet. A minimum of 80 islets was analyzed for each sample.
Glucose stimulated insulin release.
Islet samples were transferred to Krebs Ringer Buffer (KRB, 137 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO2·7H2O, 2.5 mM CaCl2·2H2O, 25 mM NaHCO3) and equilibrated in 3.3 mM glucose for a minimum of 30 min. The islets were then transferred into fresh medium containing either 3.3 mM (resting condition) or 16.7 mM (stimulation condition) glucose for 1 h in a gently shaking water bath at 37°C. Insulin secreted in the supernatant was measured by radioimmunoassay (RIA, Millipore) and normalized to DNA content, as determined after purification with the DNeasy Kit (Qiagen). The stimulation index was calculated as quotient of insulin secreted upon stimulation versus insulin secreted in resting conditions.
Statistical analysis.
Results and figures are expressed as mean ± standard error of the mean (SEM).
Acknowledgments
We are grateful to Klaus Knoch for discussion and Katja Pfriem for administrative help. This project was supported in part with funds from the BMBF-Network of Competence on Diabetes Mellitus to M.S. and from a Med-Drive Grant from the Carl Gustav Carus Medical Faculty at TUD to B.L.
References
- 1.Carlsson PO, Palm F, Andersson A, Liss P. Markedly decreased oxygen tension in transplanted rat pancreatic islets irrespective of the implantation site. Diabetes. 2001;50:489–495. doi: 10.2337/diabetes.50.3.489. [DOI] [PubMed] [Google Scholar]
- 2.Carlsson PO, Liss P, Andersson A, Jansson L. Measurements of oxygen tension in native and transplanted rat pancreatic islets. Diabetes. 1998;47:1027–1032. doi: 10.2337/diabetes.47.7.1027. [DOI] [PubMed] [Google Scholar]
- 3.Jansson L, Carlsson PO. Graft vascular function after transplantation of pancreatic islets. Diabetologia. 2002;45:749–763. doi: 10.1007/s00125-002-0827-4. [DOI] [PubMed] [Google Scholar]
- 4.Lehmann R, Zuellig RA, Kugelmeier P, Baenninger PB, Moritz W, Perren A, et al. Superiority of small islets in human islet transplantation. Diabetes. 2007;56:594–603. doi: 10.2337/db06-0779. [DOI] [PubMed] [Google Scholar]
- 5.Nam KH, Yong W, Harvat T, Adewola A, Wang S, Oberholzer J, et al. Size-based separation and collection of mouse pancreatic islets for functional analysis. Biomed Microdevices. 2010;12:865–874. doi: 10.1007/s10544-010-9441-2. [DOI] [PubMed] [Google Scholar]
- 6.MacGregor RR, Williams SJ, Tong PY, Kover K, Moore WV, Stehno-Bittel L. Small rat islets are superior to large islets in in vitro function and in transplantation outcomes. Am J Physiol Endocrinol Metab. 2006;290:771–779. doi: 10.1152/ajpendo.00097.2005. [DOI] [PubMed] [Google Scholar]
- 7.Papas KK, Long RC, Jr, Constantinidis I, Sambanis A. Effects of oxygen on metabolic and secretory activities of beta TC3 cells. Biochim Biophys Acta. 1996;1291:163–166. doi: 10.1016/0304-4165(96)00062-1. [DOI] [PubMed] [Google Scholar]
- 8.Fernandez LA, Hatch EW, Armann B, Odorico JS, Hullett DA, Sollinger HW, et al. Validation of large particle flow cytometry for the analysis and sorting of intact pancreatic islets. Transplantation. 2005;80:729–737. doi: 10.1097/01.tp.0000179105.95770.cd. [DOI] [PubMed] [Google Scholar]
- 9.Fujita Y, Takita M, Shimoda M, Itoh T, Sugimoto K, Noguchi H, et al. Large human islets secrete less insulin per islet equivalent than smaller islets in vitro. Islets. 2011;3:1–5. doi: 10.4161/isl.3.1.14131. [DOI] [PubMed] [Google Scholar]
- 10.Huang HH, Novikova L, Williams SJ, Smirnova IV, Stehno-Bittel L. Low insulin content of large islet population is present in situ and in isolated islets. Islets. 2011;3:6–13. doi: 10.4161/isl.3.1.14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janette Williams S, Huang HH, Kover K, Moore W, Berkland C, Singh M, et al. Reduction of diffusion barriers in isolated rat islets improves survival, but not insulin secretion or transplantation outcome. Organogenesis. 2010;6:115–124. doi: 10.4161/org.6.2.10373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su Z, Xia J, Shao W, Cui Y, Tai S, Ekberg H, et al. Small islets are essential for successful intraportal transplantation in a diabetes mouse model. Scand J Immunol. 2010;72:504–510. doi: 10.1111/j.1365-3083.2010.02466.x. [DOI] [PubMed] [Google Scholar]
- 13.Kikugawa R, Katsuta H, Akashi T, Yatoh S, Weir GC, Sharma A, et al. Differentiation of COPAS-sorted non-endocrine pancreatic cells into insulin-positive cells in the mouse. Diabetologia. 2009;52:645–652. doi: 10.1007/s00125-009-1260-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Watson DA, Gaskill DF, Brown LO, Doorn SK, Nolan JP. Spectral measurements of large particles by flow cytometry. Cytometry A. 2009;75:460–464. doi: 10.1002/cyto.a.20706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Doitsidou M, Flames N, Lee AC, Boyanov A, Hobert O. Automated screening for mutants affecting dopaminergic-neuron specification in C. elegans. Nature. 5:869–872. doi: 10.1038/nmeth.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morton E, Lamitina T. A suite of MATLAB-based computational tools for automated analysis of COPAS Biosort data. Biotechniques. 2010;48:15–30. doi: 10.2144/000113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Korsgren O, Lundgren T, Felldin M, Foss A, Isaksson B, Permert J, et al. Optimising islet engraftment is critical for successful clinical islet transplantation. Diabetologia. 2008;51:227–232. doi: 10.1007/s00125-007-0868-9. [DOI] [PubMed] [Google Scholar]
- 18.Zhang N, Richter A, Suriawinata J, Harbaran S, Altomonte J, Cong L, et al. Elevated vascular endothelial growth factor production in islets improves islet graft vascularization. Diabetes. 2004;53:963–970. doi: 10.2337/diabetes.53.4.963. [DOI] [PubMed] [Google Scholar]
- 19.Mattsson G, Jansson L, Nordin A, Andersson A, Carlsson PO. Evidence of functional impairment of syngeneically transplanted mouse pancreatic islets retrieved from the liver. Diabetes. 2004;53:948–954. doi: 10.2337/diabetes.53.4.948. [DOI] [PubMed] [Google Scholar]
- 20.Hanson MS, Park EE, Sears ML, Greenwood KK, Danobeitia JS, Hullett DA, et al. A simplified approach to human islet quality assessment. Transplantation. 2010;89:1178–1188. doi: 10.1097/TP.0b013e3181d54bce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boyd V, Cholewa OM, Papas KK. Limitations in the use of fluorescein diacetate/propidium iodide (FDA/PI) and cell permeable nucleic acid stains for viability measurements of id islets of Langerhans. Curr Trends Biotechnol Pharm. 2008;2:66–84. [PMC free article] [PubMed] [Google Scholar]
- 22.Papas KK, Suszynski TM, Colton CK. Islet assessment for transplantation. Curr Opin Organ Transplant. 2009;14:674–682. doi: 10.1097/MOT.0b013e328332a489. [DOI] [PMC free article] [PubMed] [Google Scholar]