Abstract
The definitive measure of β-cell quality in an islet is the measurement of β-cell function, i.e., the ability of the islets to release insulin in a controlled manner in response to minute changes in ambient glucose levels. Continuous flow or dynamic perifusion of the solution containing glucose and secretagogues through the islets is the most accurate assessment of regulated insulin release in vitro. Here, we describe in detail a low cost, mini-perifusion system that can be adapted to any laboratory to assess islet function by examining dynamic insulin release in response to elevated glucose concentrations and addition of secretagogues. Human islets with purity >80% and viability >90% were perifused with low glucose (1 mM) and subsequently challenged with high glucose (16.8 mM ± KCl, 25 mM). A prototypical biphasic response to elevated glucose concentrations was observed with an average 8-fold (above basal) increase in insulin concentration at peak values. Similarly, perifusion with carbachol or exendin-4 (Byetta) with glucose (6 mM) resulted in 1.32- and 1.35-fold increase in insulin secretion above basal. Islets could be maintained in the perifusion apparatus and continued to respond to glucose for up to 3 h. At minimal financial cost and technical expertise, this apparatus can be set-up in any biological laboratory to evaluate regulated hormone release from many cell types in less than 6 h. This will allow other laboratories to measure insulin responses to their drug or modifier of interest in vitro, in a manner that better approximates islet function in vivo.
Key words: human islets of Langerhans, insulin release, glucose, exendin-4
Introduction
Clinical islet transplantation has offered new hope for patients with severe forms of type 1 diabetes, particularly after the successful reversal of diabetes using the Edmonton immunosuppressive protocol.1 Three important factors contributed to this success. First, an improvement in the islet isolation procedure, by use of highly purified collagenase in conjunction with thermolysin or neutral protease followed by purification with continuous density gradients, which yielded an improvement in islet quantity and quality2–4 and in turn, enabled a positive clinical outcome in some patients with the use of 1–3 cadaveric pancreata. Second, was the application of low-dose immunosuppressive regiments to prevent allograft rejection.1 Third, and most important, was increased attention to proper post-transplant management.5,6 Since then, several centers globally have successfully repeated the Edmonton protocol with and without modifications.2,7,8 However, long-term insulin-independence due to loss of graft function still remains a limiting factor. This can, in part, be attributed to transplantation of islets with sub-optimal insulin secretory responses to elevations in ambient glucose concentrations. The ability of the β-cell in the islet to sense minute changes in blood glucose concentrations and to secrete insulin proportionally is central to tight blood glucose control. There is, therefore, a need for a reliable, quick and simple method of evaluating islet function prior to islet transplantation.
Perifusion systems to challenge islets with glucose and measure dynamic insulin release have been utilized for more than 35 y and remain a valid procedure used by many research laboratories to test islet function in a more physiologically relevant way.9–11 Also, this method is useful for determining regulation of hormone release in response to various drugs and agents.12–17 However, every laboratory has its unique perifusion system.18 Currently there is no standardized, inexpensive apparatus and protocol that can be replicated easily in any laboratory. Here, we describe a step-by-step procedure for optimal assessment of islet functionality and response to glucose and secretagogues in an in vitro flow through system. We have used this protocol to test various drugs and methods to characterize islet function in different conditions.19,20
Results
High glucose and potassium chloride stimulation.
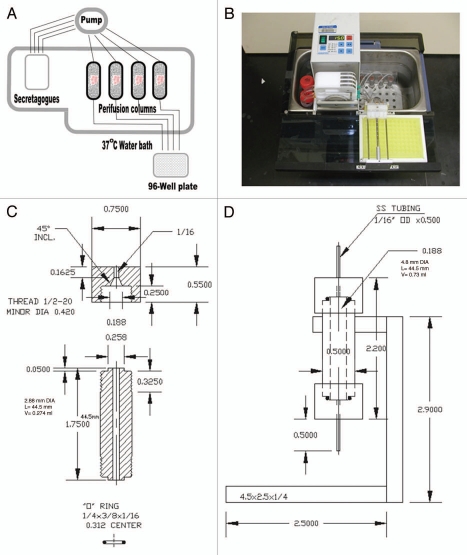
Figure 1 shows a simplified schema (Fig. 1A) and photograph (Fig. 1B) of the entire perifusion apparatus and technical specifications for the perifusion columns (Fig. 1C) described in the material and methods section below. Each of the four columns was loaded with 200 human islet equivalents (IEQ) and all of the four columns shown were run simultaneously in all protocols.
Figure 1.
Perifusion system. (A) A simplified diagram of the perifusion system showing the basic elements of the four column apparatus. (B) Photograph of the perifusion system, consisting of a water bath, ismatec digital pump, cooling tray for 96-well plate, tubing and columns. (C) Blue print of the 0.73 cm3 volume islet perifusion column with technical specifications. (D) Blue print of the islet perifusion column shown with rack that is fabricated from polycarbonate plastic.
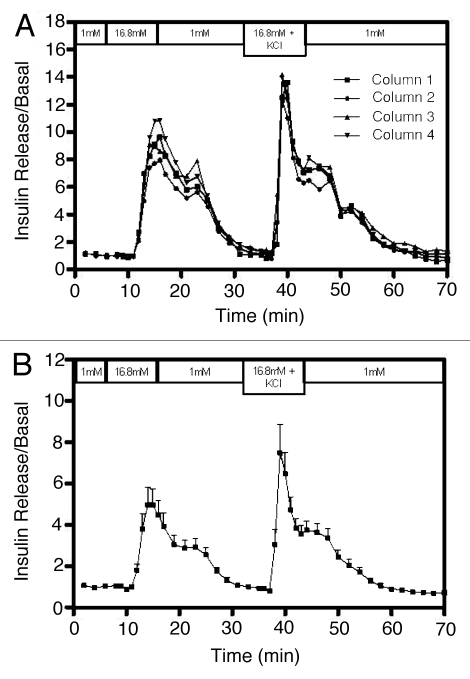
To assess intra-assay variation in this mini-perifusion apparatus, 200 human islet equivalents (IEQ) isolated from the same cadaveric donor were placed in each of the four perifusion chambers and were perifused in the same manner (Fig. 2). Figure 2A shows how tightly matched the values for insulin concentration in each of the replicates was during the entire protocol. The SI value, when challenged with high glucose (16.8 mM), was 7.8 ± 0.45. Further, when islets were exposed to high glucose in the presence of KCl (25 mM), insulin release was enhanced to give an SI of 9.1 ± 0.34 fold. These results were consistent with previously published studies in references 14, 18, 21 and 22 and are typical of the known physiological function of islets. More importantly, the values for the four columns correlated highly with each other and were statistically significant (R = 0.987, p < 0.0001). In addition, the reproducibility of the data from this mini-perifusion system was further confirmed when we retrospectively analyzed insulin release values from 12 different donor islets (Fig. 2B) (R = 0.901, p < 0.0001). The mean SI value was 3.88 ± 1.12 and the variability of SI depends on the donor and islet quality perifused. Table 1 shows the donors and pancreas characteristics that were used for islet isolation and performed in this study.
Figure 2.
Validation of perifusion assay to assess human islet functional quality. 200 IEQ/column each from the same cadaveric donor were perifused for 1 h with 1 mM glucose prior to fraction collection. They were then stimulated with 16.8 mM glucose for a period of 10 min. This was followed by a 17 min perifusion with 1 mM glucose. Subsequently, the islets were then stimulated with 16.8 mM glucose + 25 mM KCl for an additional 10 min. Collected fractions were measured for insulin by ELISA. Insulin measurements were divided by basal release to obtain normalized values (stimulation index, SI). (A) Values for insulin measured from the fractions collected from each of the four columns run in parallel using the same islet preparation. Normalized values of insulin release are consistent across replicate columns. (B) Retrospective analysis of perifusion data from 12 different donor islet preparations showing the mean ± SEM of each point.
Table 1.
Donor and islet characteristics used in this study
| Parameter | Mean ± SEM |
| Age (years) | 42 ± 3.3 |
| Weight (lb) | 221 ± 11 |
| BMI (kg/m2) | 32.2 ± 1.7 |
| Length of hospitalization (days) | 3.4 ± 0.8 |
| Cold ischemia time (hours) | 8.7 ± 1.1 |
| Pancreas weight (g) | 101.0 ± 7.3 |
| Digested pancreas weight (g) | 62.6 ± 6.3 |
| IEQ/Digested pancreas weight | 3407 ± 759 |
| Viability (%) | 95.0 ± 1.1 |
| Purity (%) | 78.7 ± 1.9 |
The mean and standard error of the mean (SEM) for donor and islet characteristics were calculated from 12 human donor pancreata.
Effect of prolonged perifusion times with low glucose.
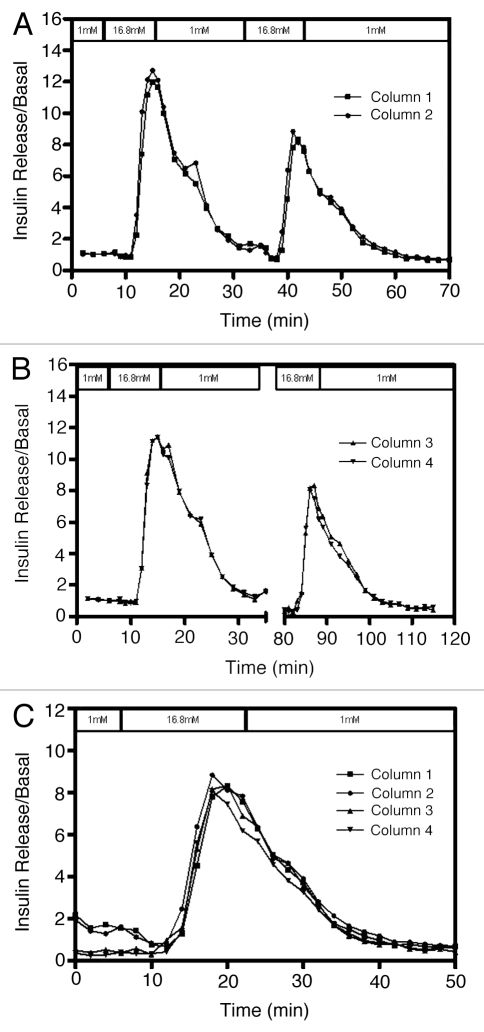
In order to test the effect of prolonged exposure time to low glucose, islets were stimulated with high glucose twice, with different time intervals between the first and second high glucose stimulation (Fig. 3). The first two columns had a 17 min low glucose exposure time (Fig. 3A), while the other two columns were perifused with low glucose for 60 min between the high glucose stimulations (Fig. 3B). SI values were lower in all cases for the second stimulation (column 1 SI = 4.36, column 2 SI = 4.94, column 3 SI = 11.82 and column 4 SI = 16.45) and there was no difference in the responses to the second stimulation with either interval times (Fig. 3C).
Figure 3.
Effect of second glucose stimulation at different time points on the same islets. Human islets (200 IEQ/column) were perifused for 1 h with 1 mM glucose prior to collection of samples for insulin measurement. Islets were then stimulated twice with 16.8 mM glucose for 10 min each. The time between the two stimulations was (A) 17 min (first two columns) and (B) 60 min (last two columns). (C) The second stimulation with data from all four columns superimposed on another. Although lower than the first, the second stimulations clearly overlap with each other indicating that islets from the same donor have similar physiological response when challenged twice with glucose at different time points. Insulin measurements were divided by basal release to obtain normalized values of insulin release.
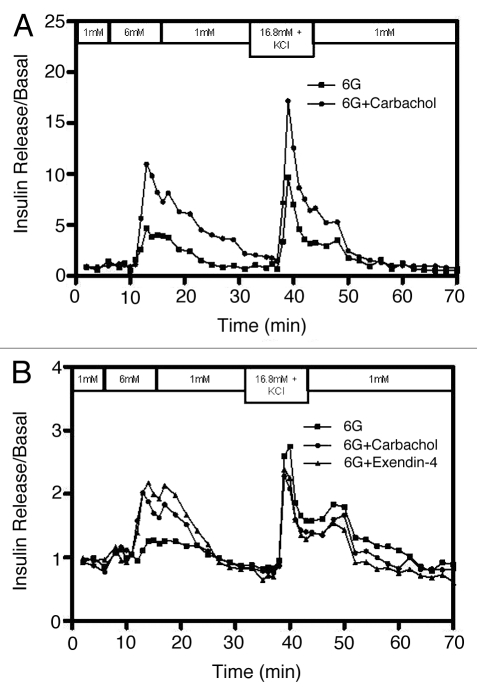
Stimulation with compounds that enhance glucose-induced insulin secretion.
In addition to measuring β-cell functionality, this mini-perifusion system has a further advantage of testing various drugs and agents as shown when carbachol or exendin-4 (a potent glucagon-like peptide-1 receptor agonist) were used to challenge human islets in the presence of 6 mM glucose. Figure 4 shows the response of the islets in individual columns to 6 mM glucose alone, or a combination of 6 mM glucose and carbachol (50 µM) or exendin-4 (50 nM). Islets exposed to carbachol had a stimulation index of 7.19 compared with 3.15 for the control (Fig. 4A). Islets from a different preparation had an SI of 1.63 when exposed to carbachol (Fig. 4B). This same preparation had an SI of 1.23 and 1.90 for the control and exendin-4 respectively. Insulin release from the control islets that were perifused with 6 mM glucose alone was minimal; however, insulin release was optimal upon subsequent stimulation of the same islets with high glucose and KCl.
Figure 4.
Effect of carbachol and exendin-4 on islet insulin release.
Discussion
A reliable, highly reproducible and simple method to assess islet function is highly advantageous. The majority of the methods currently being used to assess islets are expensive or cumbersome and the data are difficult to reproduce.23–28 The current standard method to assess islet quality is to transplant islets into diabetic immunodeficient rodents.29,30 However, the results are typically not available until long after islets are transplanted into a patient, given the time required to reverse diabetes in animal models. Having an alternative standardized procedure to assess islet quality and predict function in transplanted patients would not only be more convenient, but may also become a necessity for the approval of transplantable islets under a biological release license from the Food and Drug Administration.31 The main objective of this study was to establish a step-by-step procedure and to set a simple and highly reproducible mini-perifusion system from in vitro assessment of whole human islet function. The system is confined with the columns submerged in a 37°C water bath, which provide a consistent temperature environment that resemble in vivo situation. And, this eliminates the variable of temperature fluctuation when islets were being assayed across different laboratories. Furthermore, the collected fractions were cooled immediately post-stimulation to 4°C and this step is important to protect the integrity of eluted insulin. Also, it is suggested that the addition of protease inhibitor during islet perifusion may protect islet function and prevent insulin degradation. In fact, recent study had shown that the addition of protease inhibitor, α-1 antitrypsin, prevented insulin degradation and improved islet function and survival.32 Taken together, features provided by our perifusion system are clearly not achievable if a static incubation were to be used.
In addition, our result demonstrated that functional islets can survive in the columns for at least 2–3 h after loading and islets remained functional in releasing insulin upon glucose stimulation (Fig. 3). Also, we noted that the second peaks, although very similar to one another, were lower than the first peaks. This decrease in insulin production may be attributed to a refractory period during which insulin content within the islet is recouped. It is also conceivable that the absence of appropriate amounts of nutrients, vitamins, amino acids, O2 and CO2 during prolonged perifusion may have additionally contributed to this effect.
Furthermore, our perifusion data generated from 12 different donor islets showed remarkable consistency, with a correlation coefficiency of R = 0.901, p < 0.0001. In addition to the reduction of the variable in temperature fluctuation, another important contributing factor to this high correlation coefficiency is the random sampling method that we employed; islet equivalents were counted by random sampling of the whole cell suspension in our study. In several islet transplant centers, it is a common practice to use handpicked islets for quality assessment and specifically select on the basis of size and appearance; the result would not be an optimum performance of islet preparation. Elimination of human bias for larger islets, to overestimate islet function results in an inconclusive assessment of in vitro and in vivo functional assays for clinical transplantation application. The variability in response to stimulation by enhancers of insulin secretion is particularly evident in the 4-fold difference in the response to carbachol between two separate preparations. Thus, this suggests that agents that augment glucose-stimulated insulin secretion should also be incorporated into the standardized assessment of islet function. Also, the islets in the perifusion system behaved as expected in terms of their physiological responses to glucose (Figs. 2 and 3) and known potentiators and secretagogues (Fig. 4). Once loaded, the islets remained viable and functional in the columns for at least 2–3 h.
Previous studies have reported that the sensitivity of β-cells to glucose was species specific.33 Islet function of mice and rat was increased upon exposure to high glucose (28 mM) indicating that rodent islets can tolerate high glucose upon exposure.33 The deleterious effects of human islets were markedly observed by diminishing insulin secretion and content when challenged with 28 mM glucose. In contrast, rat islets tolerated extremely high glucose (56 mM) which had similar effect when human islets were exposed to 11 mM glucose. These data further substantiate that human islets are indeed different from rodent islets at both physiological level and, as shown recently, at cytoarchitectural level.34
Additionally, Henquin et al. have shown that human islets perifused with 1 mM glucose responded better than islets perifused with higher glucose.14 We observed insulin leakage when human islets were perifused with 3 mM glucose (unpublished data). Therefore, in this study, using 1 mM for perifusion of human islets with low glucose was shown to be optimum. We expect that this system will not only be useful for clinical islet assessment, but also for basic research addressing a direct effect of novel agents on human islets in vitro.
In conclusion, we described a simple, fast, flexible, well-controlled and easily reproducible perifusion system for measuring islet response to various secretagogues. The system is a robust tool that can used to evaluate human islet functional quality prior to transplantation. The mini-column could be scaled down to a smaller volume facilitating perifusion without the need for the polyacrylamide gel and the total insulin content can be extracted and measured. Furthermore, the system can be adapted for stimulating other islet endocrine cells such as glucagon secreting α-cells.35
Materials and Methods
Human islet isolation.
Human cadaveric pancreata from brain-dead donors were obtained from organ procurement organizations and preserved in UW solution or by Two-Layer Method (TLM).36 Only organs from donors with approved consent for research from the next of kin were used to perform this study. Also, an approval for the use of human tissue was obtained from the Institutional Review Board at City of Hope National Medical Center and Beckman Research Institute before commencing the project. Pancreata were processed using standard Liberase HI collagenase digestion to free the islets. The islets were then purified from mass acinar tissue using a cold Cobe 2991 machine with Biocoll continuous density gradients. Islet count and purity were determined using DTZ staining with count expressed as the number of islet equivalents to 150 µm (IEQ).37,38 Viability was determined microscopically using Fluorescein Diaacetete and Ethidium Bromide as previously described in reference 39. Islets with purity >70% and viability >80% were cultured in serum free medium at 37°C, 5% CO2 for 24–72 h were used to perform this study.
Mini-perifusion system.
Our mini-perifusion system, as shown in Figure 1A and B, basically consisted of a four channel ismatec digital pump from Cole Parmer (Vernon Hills, IL) connected to four individual perifusion columns. The exact technical specifications for an individual column are shown in Figure 1C. The columns have an internal diameter and length of 0.48 and 4.45 cm respectively allowing for a total internal volume of 630 µl. Similar chamber sizes can now be purchased from or made by Biorep Technologies, Miami, FL (http://www.biorep.com). This is the equivalent to a packed volume of 200 human islet equivalents (IEQ) and 600 µl of polyacrylimide P4 BioGel fine (Biorad, Hercules, CA). The reservoir for perifusion medium is a simple 50 ml conical tube with a hole punched in the lid to accommodate the tubing. Both the reservoir tubes and the perifusion columns were submerged in a water bath at 37°C as shown (Fig. 1B). Effluent from the columns were collected in a 96-well plate, polypropylyne (Nunc, Denmark) with a specially designed stabilizing rig (Fig. 1B) to allow for collection of fractions from the individual columns such that outflow from the four tubes could be collected. This allowed for ease of movement of the 96-well plate once every minute to permit collection of fractions with a total volume of 300 µl. Tygon R-3603 autoanalyzer tubing (Saint Gobain Performance Plastics, Garden Grove, CA) was used throughout the apparatus to connect the pump to the reservoir and perifusion columns, and on the outflow from the columns to the 96-well plate. The total lag time for perifusion from the reservoir through the column to the collection plate was approximately 4 min. The 96-well plate is kept in a cold plate constructed of polypropylene to protect integrity of the insulin in the collected fractions and to ensure against heat-induced degradation. Individual fractions were stored at −20°C until insulin was measured by ELISA assay.
All experiments described herein commenced with a 60 min equilibration period during which the islets were continuously perifused with Modified Krebs-Ringer Buffer (MKRB) supplemented with 1 mM glucose at 37°C. Time zero, as shown in Figures 2–4, were defined as the time after 1 h of perifusion with 1 mM glucose in which fraction collection commenced. Insulin levels were measured in fractions collected every two minutes. Basal levels of insulin were defined as the amount of insulin released in response to low glucose (1 mM). Also, the basal level was determined by taking the mean of the values in the initial 10 min incubation period of the experiment. Changes in the perifusion medium in each protocol are as outlined in each of the Figures 2–4. The stimulation index (SI) was calculated by dividing the average values of insulin concentrations in response to any given stimulus by the basal value in that same run, which is usually from 14–23 min.
Perifusion of human islets.
The following is a detailed description of the procedure for the set-up of a single column after the initial set up of the apparatus. The perifusion top and bottom endcaps of column was detached from circuit (keep short piece of tubing attached) and were unscrewed. A hole punch was used to make a small circle of Whatman filter paper (∼7 mm diameter) which was placed in the bottom cap so as to evenly cover the hole. The column (with rubber gasket) was screwed back onto the bottom endcap and placed in stand with paper towels underneath to catch the outflow. The column was filled with low glucose (1 mM) using a 200 µl micropipetor. Using a transfer pipette, drops of P4 Bio-Gel were added until the layer was almost halfway up the column. The islets (200 IEQ) were washed with 1 ml of low glucose and placed in the column using a 1,000 µl micropipetor. Once the islets settled, the remainder of the column was filled with P4 Bio-Gel and the tubing attached to the bottom endcap was clamped using a mini binder clip. The top endcap was attached to tubing from the pump and 1 mM or 1 G was pumped through at a rate of approximately 130 µl/min. The endcap was inverted to fill with solution. Once filled the top endcap was attached to the top of the column in one rapid movement (to avoid introduction of air bubbles), and the binder clip on the bottom tubing was immediately removed. The collection tubing was then connected to the bottom endcap tubing, completing the circuit. The column was placed in its holder and the running system was monitored for 10 min to confirm there were no leakages. The data was expressed as fold change of insulin release as compared with basal levels. The basal level of insulin release is defined as the average of the insulin concentrations from the first seven fractions of buffer with low glucose. The stimulation index (SI) was calculated by dividing the average value of the peak by the basal value within the same run.
Measurement of insulin by ELISA assay.
Insulin was measured using the human insulin ELISA kit (Mercodia, Uppsala, Sweden) according to the manufacturer's instructions. We found that diluting the samples 1:2.5 in modified Krebs-Ringer Perifusion Buffer (MKRPB consisting of 125 mM NaCl, 5.9 mM KCl, 1.28 mM CaCl2, 1.2 mM MgCl2, 25 mM HEPES and 1 g/L BSA; pH 7.4) gave a final concentration of insulin in the unknown samples that was within the range of the linear region of the standard curve of the Mercodia kit. The mean of insulin values for the first seven time points were calculated to obtain the value for insulin release in the quiescent state. In a separate column, each value from the original insulin release column was divided by the baseline to obtain the normalized insulin release value. The normalized values were plotted against time as shown in our figures.
Sources of reagents.
Byetta or Exendin-4 (Amylin Pharmaceuticals, Inc., San Diego, CA) and carbachol (Sigma-Aldrich, St. Louis, MO) were used in this study.
Statistical analysis.
Results are represented as the mean ± standard error of the mean. The difference between the variables was calculated using Pearson correlation coefficients and p values ≤ 0.05 was considered significant.
Acknowledgments
The authors wish to thank the Islet Cell Resource Consortium (ICR) of City of Hope National Medical Center and the islet isolation team of the Southern California Islet Cell Resources Center (SCICRC) for provision of islets. Thanks to Bioinstrumentation Services, particularly Miro Rusnick at City of Hope for fabrication of the perifusion columns. We also wish to thank Dr. K.D. Shiang for statistical advice and Dr. Silvia da Costa for help in editing this protocol. M.D. is supported by the Sanford Career Award from the Juvenile Diabetes Research Foundation in collaboration with the Sanford Project, Sanford Health, Sioux Falls, SD. Byetta samples were obtained from Amylin Pharmaceuticals, Inc., San Diego, CA. This work was supported by a grant from the National Institute of Health (NIH), The Islet Cell Resources (ICR) 5U42RR016607 and City of Hope National Medical Center.
References
- 1.Shapiro AM, Lakey JR, Ryan EA, Korbutt GS, Toth E, Warnock GL, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Shapiro AM, Ricordi C, Hering BJ, Auchincloss H, Lindblad R, Robertson RP, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. doi: 10.1056/NEJMoa061267. [DOI] [PubMed] [Google Scholar]
- 3.Ricordi C, Lacy PE, Scharp DW. Automated islet isolation from human pancreas. Diabetes. 1989;38:140–142. doi: 10.2337/diab.38.1.s140. [DOI] [PubMed] [Google Scholar]
- 4.Antonioli B, Fermo I, Cainarca S, Marzorati S, Nano R, Baldissera M, et al. Characterization of collagenase blend enzymes for human islet transplantation. Transplantation. 2007;84:1568–1575. doi: 10.1097/01.tp.0000295719.88525.60. [DOI] [PubMed] [Google Scholar]
- 5.Faradji RN, Monroy K, Messinger S, Pileggi A, Froud T, Baidal DA, et al. Simple measures to monitor beta-cell mass and assess islet graft dysfunction. Am J Transplant. 2007;7:303–308. doi: 10.1111/j.1600-6143.2006.01620.x. [DOI] [PubMed] [Google Scholar]
- 6.Pileggi A, Alejandro R, Ricordi C. Clinical islet transplantation. Minerva Endocrinol. 2006;31:219–232. [PubMed] [Google Scholar]
- 7.Hering BJ, Kandaswamy R, Ansite JD, Eckman PM, Nakano M, Sawada T, et al. Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA. 2005;293:830–835. doi: 10.1001/jama.293.7.830. [DOI] [PubMed] [Google Scholar]
- 8.Tan J, Yang S, Cai J, Guo J, Huang L, Wu Z, et al. Simultaneous islet-kidney transplantation in 7 patients of type 1 diabetes with end-stage renal disease using a glucocorticoid-free immunosuppressive regimen with alemtuzumab induction. Diabetes. 2008;57:2666–2671. doi: 10.2337/db08-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ashcroft SJ, Bassett JM, Randle PJ. Isolation of human pancreatic islets capable of releasing insulin and metabolising glucose in vitro. Lancet. 1971;1:888–889. doi: 10.1016/S0140-6736(71)92445-7. [DOI] [PubMed] [Google Scholar]
- 10.Hopcroft DW, Mason DR, Scott RS. Standardization of insulin secretion from pancreatic islets: validation of a DNA assay. Horm Metab Res. 1985;17:559–561. doi: 10.1055/s-2007-1013606. [DOI] [PubMed] [Google Scholar]
- 11.Norfleet WT, Pagliara AS, Haymond MW, Matschinsky F. Comparison of alpha- and beta-cell secretory responses in islets isolated with collagenase and in the isolated perfused pancreas of rats. Diabetes. 1975;24:961–970. doi: 10.2337/diabetes.24.11.961. [DOI] [PubMed] [Google Scholar]
- 12.Dufrane D, Nenquin M, Henquin JC. Nutrient control of insulin secretion in perifused adult pig islets. Diabetes Metab. 2007;33:430–438. doi: 10.1016/j.diabet.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Haber EP, Hirabara SM, Gomes AD, Curi R, Carpinelli AR, Carvalho CR. Palmitate modulates the early steps of insulin signalling pathway in pancreatic islets. FEBS Lett. 2003;544:185–188. doi: 10.1016/S0014-5793(03)00503-9. [DOI] [PubMed] [Google Scholar]
- 14.Henquin JC, Dufrane D, Nenquin M. Nutrient control of insulin secretion in isolated normal human islets. Diabetes. 2006;55:3470–3477. doi: 10.2337/db06-0868. [DOI] [PubMed] [Google Scholar]
- 15.Szkudelski T. The insulin-suppressive effect of resveratrol—an in vitro and in vivo phenomenon. Life Sci. 2008;82:430–435. doi: 10.1016/j.lfs.2007.12.008. [DOI] [PubMed] [Google Scholar]
- 16.Zawalich WS, Tesz GJ, Yamazaki H, Zawalich KC, Philbrick W. Dexamethasone suppresses phospholipase C activation and insulin secretion from isolated rat islets. Metabolism. 2006;55:35–42. doi: 10.1016/j.metabol.2005.06.023. [DOI] [PubMed] [Google Scholar]
- 17.Zawalich WS, Zawalich KC. Effects of protein kinase C inhibitors on insulin secretory responses from rodent pancreatic islets. Mol Cell Endocrinol. 2001;177:95–105. doi: 10.1016/S0303-7207(01)00422-1. [DOI] [PubMed] [Google Scholar]
- 18.Cabrera O, Jacques-Silva MC, Berman DM, Fachado A, Echeverri F, Poo R, et al. Automated, high-throughput assays for evaluation of human pancreatic islet function. Cell Transplant. 2008;16:1039–1048. doi: 10.3727/000000007783472408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nduaguibe CC, Bentsi-Barnes K, Mullen Y, Kandeel F, Al-Abdullah IH. Serine protease inhibitors suppress pancreatic endogenous proteases and modulate bacterial neutral proteases. Islets. 2010;2:200–206. doi: 10.4161/isl.2.3.11714. [DOI] [PubMed] [Google Scholar]
- 20.Bentsi-Barnes K, Kandeel F, Al-Abdullah IH. Evaluation of human islet-specific functional quality cultured on different gas-permeable membranes. Transplant Proc. 2008;40:401–402. doi: 10.1016/j.transproceed.2008.01.036. [DOI] [PubMed] [Google Scholar]
- 21.Henquin JC, Ravier MA, Nenquin M, Jonas JC, Gilon P. Hierarchy of the beta-cell signals controlling insulin secretion. Eur J Clin Invest. 2003;33:742–750. doi: 10.1046/j.1365-2362.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- 22.Srivastava S, Goren HJ. Insulin constitutively secreted by beta-cells is necessary for glucose-stimulated insulin secretion. Diabetes. 2003;52:2049–2056. doi: 10.2337/diabetes.52.8.2049. [DOI] [PubMed] [Google Scholar]
- 23.Boffa DJ, Waka J, Thomas D, Suh S, Curran K, Sharma VK, et al. Measurement of apoptosis of intact human islets by confocal optical sectioning and stereologic analysis of YO-PRO-1-stained islets. Transplantation. 2005;79:842–845. doi: 10.1097/01.TP.0000155175.24802.73. [DOI] [PubMed] [Google Scholar]
- 24.Brissova M, Fowler MJ, Nicholson WE, Chu A, Hirshberg B, Harlan DM, et al. Assessment of human pancreatic islet architecture and composition by laser scanning confocal microscopy. J Histochem Cytochem. 2005;53:1087–1097. doi: 10.1369/jhc.5C6684.2005. [DOI] [PubMed] [Google Scholar]
- 25.Fraker C, Timmins MR, Guarino RD, Haaland PD, Ichii H, Molano D, et al. The use of the BD oxygen biosensor system to assess isolated human islets of langerhans: oxygen consumption as a potential measure of islet potency. Cell Transplant. 2006;15:745–758. doi: 10.3727/000000006783981440. [DOI] [PubMed] [Google Scholar]
- 26.Ichii H, Inverardi L, Pileggi A, Molano RD, Cabrera O, Caicedo A, et al. A novel method for the assessment of cellular composition and beta-cell viability in human islet preparations. Am J Transplant. 2005;5:1635–1645. doi: 10.1111/j.1600-6143.2005.00913.x. [DOI] [PubMed] [Google Scholar]
- 27.Papas KK, Colton CK, Nelson RA, Rozak PR, Avgoustiniatos ES, Scott WE, 3rd, et al. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplant. 2007;7:707–713. doi: 10.1111/j.1600-6143.2006.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vandewalle B, Douillard C, Kerr Conte J, Gmyr V, Riachi R, D'Herbomez M, et al. Human pancreatic islet quality control: easy assessment of metabolic functions. Exp Clin Endocrinol Diabetes. 1999;107:214–219. doi: 10.1055/s-0029-1212101. [DOI] [PubMed] [Google Scholar]
- 29.Caiazzo R, Gmyr V, Kremer B, Hubert T, Soudan B, Lukowiak B, et al. Quantitative in vivo islet potency assay in normoglycemic nude mice correlates with primary graft function after clinical transplantation. Transplantation. 2008;86:360–363. doi: 10.1097/TP.0b013e31817ef846. [DOI] [PubMed] [Google Scholar]
- 30.Sabek OM, Fraga DW, Minoru O, McClaren JL, Gaber AO. Assessment of human islet viability using various mouse models. Transplant Proc. 2005;37:3415–3416. doi: 10.1016/j.transproceed.2005.09.049. [DOI] [PubMed] [Google Scholar]
- 31.Weber DJ. FDA regulation of allogeneic islets as a biological product. Cell Biochem Biophys. 2004;40:19–22. doi: 10.1385/CBB:40:3S:019. [DOI] [PubMed] [Google Scholar]
- 32.Loganathan G, Dawra RK, Pugazhenthi S, Wiseman AC, Sanders MA, Saluja AK, et al. Culture of impure human islet fractions in the presence of alpha-1 antitrypsin prevents insulin cleavage and improves islet recovery. Transplant Proc. 2010;42:2055–2057. doi: 10.1016/j.transproceed.2010.05.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eizirik DL, Korbutt GS, Hellerstrom C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. J Clin Invest. 1992;90:1263–1268. doi: 10.1172/OCI115989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cabrera O, Berman DM, Kenyon NS, Ricordi C, Berggren PO, Caicedo A. The unique cytoarchitecture of human pancreatic islets has implications for islet cell function. Proc Natl Acad Sci USA. 2006;103:2334–2339. doi: 10.1073/pnas.0510790103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.De Marinis YZ, Zhang E, Amisten S, Taneera J, Renstrom E, Rorsman P, et al. Enhancement of glucagon secretion in mouse and human pancreatic alpha cells by protein kinase C (PKC) involves intracellular trafficking of PKCalpha and PKCdelta. Diabetologia. 2010;53:717–729. doi: 10.1007/s00125-009-1635-x. [DOI] [PubMed] [Google Scholar]
- 36.Ricordi C, Fraker C, Szust J, Al-Abdullah I, Poggioli R, Kirlew T, et al. Improved human islet isolation outcome from marginal donors following addition of oxygenated perfluorocarbon to the cold-storage solution. Transplantation. 2003;75:1524–1527. doi: 10.1097/01.TP.0000058813.95063.7A. [DOI] [PubMed] [Google Scholar]
- 37.Latif ZA, Noel J, Alejandro R. A simple method of staining fresh and cultured islets. Transplantation. 1988;45:827–830. doi: 10.1097/00007890-198804000-00038. [DOI] [PubMed] [Google Scholar]
- 38.Ricordi C, Gray DW, Hering BJ, Kaufman DB, Warnock GL, Kneteman NM, et al. Islet isolation assessment in man and large animals. Acta Diabetol Lat. 1990;27:185–195. doi: 10.1007/BF02581331. [DOI] [PubMed] [Google Scholar]
- 39.Gray DW, Morris PJ. The use of fluorescein diacetate and ethidium bromide as a viability stain for isolated islets of Langerhans. Stain Technol. 1987;62:373–381. doi: 10.3109/10520298709108028. [DOI] [PubMed] [Google Scholar]