Abstract
Glucocorticoids (GCs) exert their biological and therapeutical actions through the GC receptor (GR), a ligand-dependent transcription factor. Synthetic GC derivatives are widely prescribed for treating numerous cutaneous inflammatory and immune diseases due to their great efficacy. However, chronic treatment with GCs produces adverse side-effects including skin atrophy, delayed wound healing, and in certain cases, GC resistance. The mechanisms underlying the therapeutic actions of the GR in skin have been extensively studied; in contrast, the role of GR as a modulator of epidermal development and homeostasis has received less attention. The ubiquitous functional inactivation of GR results in defective epidermal formation although the underlying mechanisms have not been fully characterized. The use of transcriptomic approaches both in vitro and in vivo allowed the identification of genes that are regulated by GR in developing and adult skin. A main goal to understand the role of GR in skin biology is to identify primary transcriptional targets as well as the signaling pathways mediating GR action. Furthermore, it will be important to decipher the contribution of GR in the different cellular compartments of the skin, including keratinocytes of the interfollicular epidermis and hair follicles, and their respective stem cell progenitors. Additionally, recent findings indicating that the skin acts as a true peripheral endocrine organ implies greater complexity than originally thought. The local production of GCs and other steroid hormones should be considered as a modulator of skin function under homeostatic and diseased conditions. Finally, studying GR function in skin should take into account that the mineralocorticoid receptor may also mediate GC actions and/or regulate transcription either by itself or in combination with GR. Addressing these issues should help to elucidate the mechanisms by which Gr contributes to establishment of a competent epidermal barrier and may also have implications in the context of dermatological treatments based on GC-analogs.
Key words: glucocorticoid receptor, skin, epidermis, hair follicle, keratinocyte, development, skin homeostasis, transcriptional regulation
Introduction
Glucocorticoids (GCs) are steroid hormones whose effects are mediated through the ubiquitously expressed intracellular GC receptor (GR), which belongs to the nuclear hormone receptor (NHR) superfamily.1,2 NHRs are ligand-dependent transcription factors that exert critical roles in skin function.3 In humans and mice, there is a single gene encoding for GR, called Nr3c1, composed by 9 exons.4,5 Nr3c1 undergoes both alternative splicing and alternative translational initiation, resulting in multiple GR protein isoforms. Alternative splicing generates multiple variants of which the most abundant and well studied are the GRα and GRβ. GRα is the predominant isoform and binds GCs whereas GRβ is expressed at much lower levels and is unable to bind endogenous or synthetic ligands.4–6 Increased ratios of GRβ/GRα have been shown to correlate with resistance to GCs.7 Moreover, GRβ is known to negatively affect gene regulation by ligand bound GRα.4–6 Alternative translation initation of GRα has been shown to result in eight protein isoforms which exhibit differential expression in tissues, regulate the expression of unique gene subsets and have different sensitivity to GCs.4 In this review, we will mainly focus on the classical GRα isoform, which we will refer to as GR.
GR has a modular structure with three functional domains.1,2,5 The amino-terminal transactivation domain contains an activating function that is hormone-independent (AF-1) and is required for GR association with the basal transcription machinery. The DNA Binding Domain (DBD) is composed of two zinc fingers, the first of which is the proximal P-box and is responsible for GR binding to specific DNA sequences in target genes known as glucocorticoid-response elements or GREs. The second zinc finger is the distal D-box and contains sequences important for receptor dimerization and nuclear translocation. The DBD is the most highly conserved domain throughout the steroid receptor family, which is particularly relevant for understanding the flexibility in the transcriptional regulation mediated by GR and other closely related nuclear receptors.1,2 The carboxy-terminal Ligand Binding Domain (LBD) binds to GCs and plays a critical role in the ligand-induced activation of GR. The LBD contains an additional activating function (AF-2), which is hormone-dependent and participates in interactions with other cofactors. A hinge region between the DBD and LBD confers structural flexibility allowing a single receptor dimer to interact with multiple GREs and it contains an additional nuclear localization signal.
Upon GC binding, GR dissociates from cytoplasmic complexes, dimerizes and translocates to the nucleus, where it can modulate gene transcription in a cell type-specific manner.1,2,5 Typical GREs were defined by similarity to the consensus sequence 5′-AGA ACA nnn TGT TCT-3′. However, it has been demonstrated that binding of GR monomers to GRE half-sites is sufficient to mediate GR-dependent transcription in many genes.8–10 GR can also regulate gene expression through interference with other transcription factors, such as NFκB, AP-1 or STATs, without direct binding to DNA.11–13 These protein-protein interactions can take place on regulatory sequences that do not contain GREs (tethering mechanism), as well as on DNA sequences that have both GREs and responsive elements of transcription factors that interact with GR (so-called composite elements). The mechanisms through which GR regulates gene expression are classically referred to as transactivation (TA; DNA-binding-dependent) and transrepression (TR; DNA-binding-independent). Classically, the TR function has been ascribed to the anti-inflammatory actions of GR whereas adverse side-effects have been linked to the TA function.11–13 TA and TR can be genetically dissected, as shown by the analysis of two mouse models: GR-/- mice, with total inactivation of GR,14,15 and GRdim/dim mice harboring a point mutation (A458T) in the D box DBD subdomain that renders GR defective in dimerizationdependent transactivation.16 GR-/- died perinatally due to the lack of alveolar surfactation whereas GRdim/dim mice survived.
Accumulating evidence indicates that the binomial TA vs. TR paradigm can no longer be sustained.17,18 First, it was reported that neither A458T nor other dimerization-defective mutants are globally TA-deficient, since the transcriptional potential of GR is dependent on the particular context of given GRE.8–10 Finally, GRE-dependent transcription has been shown to be essential for the anti-inflammatory actions mediated by GR, for instance, through the induction of genes such as Mkp-1 and Ikba.17–19
Post-translational modifications including phosphorylation, sumoylation and ubiquitination provide another level of control in the regulation of gene expression by the GR. Although it is known that these modifications alter GC signaling,5 their exact pathophysiological consequences remain to be characterized. On the other hand, the so-called non-genomic actions of the receptor have been well studied and involve GR interference with the phosphatidylinositol-3-kinase (PI3K) signaling pathway and its effector kinase AKT, a key regulator of cell proliferation and survival. This interference takes place with rapid kinetics, is independent of transcription, and plays a crucial role in the therapeutic actions of GCs in several tissues, including the skin.20,21
Given the prevalence of skin diseases and the great efficacy of using synthetic GC derivatives for treating these disorders, the study of GR has been of great interest. The knowledge regarding the mechanisms underlying GR therapeutical actions has been the topic of excellent reviews in references 7, 11–13, 17 and 18. However, the study of the role of GR as a modulator of the development of the epidermis and other stratified epithelia has received less attention. In this review, we mostly refer to the current knowledge regarding GR and skin homeostasis based on experimental mouse models. The generation and characterization of several genetically modified mice contributing to the understanding of GR function in developing and adult skin is summarized in Table 1.
Table 1.
Summary of the skin phenotypes of genetically modified mice with GR gain- and loss-of-function
| Genetically modified mouse model | Spatio-temporal expression pattern of the transgene | Embryonic skin phenotype | Adult skin phenotype | References |
| K5-GR | KC-specific E13.5-adulthood |
epidermal atrophy reduced HF number dysplasic HFs |
alopecia impaired HF proliferation/differentiation |
46, 48 |
| K5-GR-TR (P493R/A494S) | KC-specific E13.5-adulthood |
NO | alopecia impaired HF proliferation/differentiation |
59 |
| GR-/- | all cell types constitutive |
impaired SC formation increased KC apoptosis |
-- perinatal death |
14, 15, 38 |
| GRdim/dim (A458T) | all cell types constitutive |
NO | NO | 16, 38 |
| K14-cre-ERT2//GRloxP/loxP | KC-specific conditional knock-out induced in adult age |
-- | thickened skin reduced KC differentiation increased dermal inflammation |
36 |
Abbreviations: GR, glucocorticoid receptor; K, keratin; KC, keratinocyte; HF, hair follicle.
Epidermal development takes place during embryogenesis through a complex coordinated process by which a single-layered epithelium derived from the embryonic (E) ectoderm (E10.5) gives rise to a differentiated stratified epithelium.22–24 This process requires a correct balance between proliferation, differentiation and programmed cell death. The epidermis acts as a barrier preserving the organism from dehydration, infection, uncontrolled thermoregulation and environmental damage. In normal epidermis, only keratinocytes in the basal layer (BL) and hair follicles (HF) are able to proliferate.25 During differentiation basal keratinocytes cease to proliferate, lose adherence to the basement membrane and migrate to outer layers called spinous, granular and stratum corneum.22,23 Complex changes in gene expression culminate in the conversion of viable keratinocytes into the dead, flattened squames of the stratum corneum. Alterations in these processes during fetal development may lead to a disturbed epidermal barrier, which can cause skin disorders of keratinization and cornification ranging from mild epithelial defects to death.26 Even mild defects in skin development may have consequences in the adult age leading to increased susceptibility to skin infections and inflammation.24,26 There are multiple signaling pathways regulating skin development including, among others, nuclear factor kappaB (NFκB), mitogen activated protein kinases (MAPKs), the epithelial-specific transcription factor p63 and Notch, which also regulate skin homeostasis in the adult animal.27 The epidermis maintains its capacity for self-renewal throughout life to ensure the reparation of the epithelial damage upon injury or infection. The homeostasis of this tissue is possible due to the epithelial stem cells, which are able to form the different cell lineages in the adult tissue and can be found in several compartments including the interfollicular epidermis, HF bulge and sebaceous glands.27 The mature HF consists of an outer root sheath contiguous with the basal layer of the epidermis, an inner root sheath serving as the channel from which the hair exits the skin surface, and the hair shaft itself.25,27 The HF along with the sebaceous glands and the arrector pili muscle form the pilosebaceous unit. Remarkably, this mini-organ undergoes cyclic transformations and regeneration during its entire life-time and is tightly controlled by hormone action, including GCs. During the process of HF morphogenesis a new hair forms and replaces the hair that degenerated and was released, through stages of rapid growth (anagen), apoptosis-driven regression (catagen) and quiescence (telogen).25
The Skin as a Peripheral Endocrine Organ
GC synthesis and release is subjected to a tight neuro-endocrine control through the hypothalamic-pituitary-adrenal (HPA) axis.28 Under stress, the hypothalamus produces corticotropin-releasing hormone (CRH) that stimulates the release of adrenocorticotropic hormone (ACTH) from the pituitary gland. Subsequently, the adrenal cortex synthesizes GCs that are released into the bloodstream and distributed throughout the organism to maintain tissue homeostasis.28
It is well established that the skin synthesizes vitamin D, retinoids, estrogens and progesterone, hormones that have a profound impact on skin pathophysiology.3 It was also shown that the skin is also able to synthesize cholesterol, the precursor of steroid hormones, as well as different components along the GC synthetic pathway such as CRH and ACTH.29,30 The observation that HFs can synthesize and secrete cortisol and modulate the production of other hormones by feedback mechanisms demonstrated that the skin itself acts as a peripheral endocrine organ.31 Very recently, it was reported that epidermal keratinocytes can synthesize de novo cortisol, both in vitro and in vivo, as well as the enzymes steroid 11b-hydroxylase (CYP11B1) and 11β-hydroxysteroid dehydrogenase 2 (11βHSD2).32 These enzymes catalyze the interconversion of active cortisol and inactive cortisone in humans (corticosterone and dehydrocorticosterone in rodents), thus controlling the biological availability of active hormone.29,30
A main consequence of these findings is that GC action can be locally modified at the pre-receptor level through changes in the expression of these enzymes. Additionally, cortisol production can be triggered by other signals such as IL-1β, a key cytokine in epidermal injury, through CYP11B1 induction by keratinocytes.32 Conversely, the inhibition of cortisol synthesis during wound healing increases IL-1β production, thus representing a feed-back mechanism to modulate GC-mediated signaling. The existence of additional sources of steroid hormones represents a breakthrough for understanding GR action and, in a broader sense, nuclear hormone receptor function in skin. It also has important therapeutical consequences since local hormone production must be taken into consideration in the context of dermatological treatments based on GC-analogs.
GR is Required for Epidermal Barrier Formation
A major use of GCs in the clinic, when a premature birth is anticipated, is to accelerate alveolar surfactation and favor lung maturation, thus preventing the lethal respiratory syndrome.24 In this case, it is obvious that the benefit-risk ratio justifies this treatment. Although the impact of GCs in the skin maturation of premature infants is not well characterized, classical reports using murine models support a positive role of GCs in epidermal maturation. It was shown that GCs accelerated epidermal barrier formation when injected in utero to pregnant rats.33 Conversely, CRH-deficient newborn mice, which exhibited GC deficiency, had delayed maturation of the stratum corneum, which was fully rescued by GC supplementation.34 More recently, a study reported that the exposure of wild type (WT) early embryos (E15.5) to dexamethasone (Dex) in utero accelerated epidermal barrier formation by approximately 12 h relative to controls, being evident at E16.5.35 This was preceded by significant changes in the transcriptional profile of Dex-treated relative to untreated skin only at E15.5, when the skin is still immature. Some of the genes identified as upregulated included the keratinocyte differentiation marker filaggrin as well as other genes mapping to the epidermal differentiation complex, a cluster encoding proteins of the outer epidermal layers. In contrast, when the embryo was exposed to the ligand at later developmental stages, only minor transcriptional changes were detected. Based on these data, the authors postulated that GC actions were crucial for epidermal barrier acquisition in a critical developmental window (E15 to E15.5), prior to the observed accelerated barrier formation.35 This developmental window fits with the endocrine anomalies described in GR-/- mice, featuring increased plasmatic ACTH and corticosterone levels that indicate that the HPA axis is active around E15.14 The timing of GC synthesis is in agreement with the expression pattern of GR in skin during mouse development; Nr3c1 mRNA peaks at the transition E14.5 to E16.5, correlating with the mRNA changes of the GR-regulated genes identified during skin development.36 This is in agreement with previous studies reporting GR mRNA upregulation in fetal rat epidermis in late gestation.37
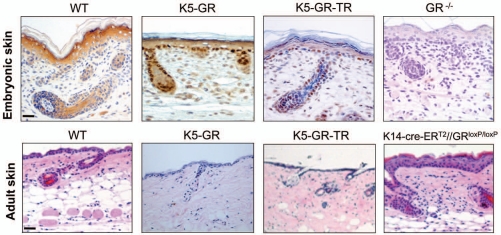
Recently, the histopathological and molecular characterization of mice with general ablation of GR has shed light into the physiological role of this transcription factor during epidermal development.36,38 The epidermis of GR-/- E18.5 embryos exhibited an immature appearance with abnormal proliferating cells in the suprabasal layers and increased apoptosis throughout all epidermal layers. This, along with impaired keratinocyte differentiation resulted in incomplete epidermal stratification and a defective skin barrier.38 The number of anagenic growing HFs was unchanged in GR-/- E18.5 mice. However, the reduced expression of keratin K6, normally expressed at the inner root sheath but not interfollicular epidermis, is suggestive of altered HF differentiation. The skin phenotype of GR-/- embryos, together with other mouse models with GR gain- and loss-of-function in this tissue, is illustrated in Figure 1.
Figure 1.
Histopathology of the embryonic and adul skin of genetically modified mice with GR gain- and loss-of-function. Histopathology of the embryonic (upper part) and adul skin (lower part) of genetically modified mice with GR gain- and loss-of-function. Upper part, immunostaining using an anti-GR specific antibody in paraffin-embedded E18.5 skin sections. Lower part, hematoxylin/eosin staining in paraffin-embedded adult skin sections. K5-GR, Keratinocyte-targeted expression of WT GR. K5-GR-TR, Keratinocyte-targeted expression of the mutant form of GR P493R/A494S. K14-cre-ERT2//GRloxP/loxP, Keratinocyte-specific inactivation of GR induced in the adult mouse.
At the molecular level, the transcriptomic profile of GR-/- embryonic skin showed differential expression of genes belonging to the functional category of ectoderm/epidermis development.36 Some of the major constituents of the cornified envelope including several genes encoding for the small proline rich proteins (Sprrs) and corneodesmosin (Cdsn), as well as genes involved in epidermal terminal differentiation (Ptgs1 and Bcl6) were strongly downregulated. Also, Krt77 and the epithelial-specific transcription factor Elf5, two genes expressed at relatively high levels during earlier stages of epidermal development were highly upregulated in the GR-deficient skin. These gene expression changes with aberrant expression of epidermal differentiation markers has been reported in several mouse models with impaired barrier formation.22 The differential expression profile in GR-/- mouse skin also included genes involved in apoptosis and lipid metabolism, two processes with a major role in epidermal barrier formation.36
Previous findings reported the changes in gene expression after Dex treatment in cultured human keratinocytes.39 In this work, Stojadinovic and coworkers showed that Dex treatment upregulated genes involved in stratum corneum formation, such as transglutaminase 1, filaggrin and Cdsn. Also, GCs inhibited involucrin expression specifically in the lower suprabasal layers as well as that of Jagged and AP2-γ, two genes that promote early keratinocyte differentiation. The authors suggested that GCs may have a dual effect on epidermal differentiation by promoting late differentiation while simultaneously inhibiting the early stages.39 Besides, they also demonstrated that GCs induce the expression of anti-apoptotic genes and repress pro-apoptotic genes.
In vitro studies using cultured mouse primary keratinocytes (MPKs) isolated from GR-/- mice demonstrated that keratinocyte proliferation and apoptosis was regulated in a cell-autonomous manner.38 However, although epidermal differentiation was severely impaired in vivo, cultured GR-/- MPKs were able to differentiate in the presence of high calcium, a classical model of in vitro keratinocyte differentiation.38 Besides, the gene expression changes detected in cultured keratinocytes upon Dex treatment showed some discrepancies relative to GR-/- MPKs.36,39 These differences may be explained by culture conditions as well as by the Dex dose and kinetics.35,36,39 Although the use of cultured cells provides relevant information, it seems reasonable to assume that the in vitro studies using MPKs do not fully recapitulate skin physiology. The generation of a mouse model with keratinocyte-restricted constitutive GR inactivation will allow for study of the epithelial contribution of GR in skin development and disease.
It has been suggested that GR acts in conjunction with the transcriptional regulator Krüppel-like factor 4 (KLF4) to coordinately regulate epidermal barrier formation.35 This study used E15.5 WT mice as well as embryos with either KLF4 inactivation (KLF4-/-) or keratinocyte-specific KLF4 overexpression (K5-KLF4 mice) to analyze the coordinated actions of GCs and KLF4. The comparison of the skin transcriptomic profile of these mouse models showed significant overlap of genes regulated by GR and KLF4. Although the authors reported that Klf4 was not induced upon Dex treatment in vivo, it was reported to be induced by Dex in human cultured keratinocytes; this controversy is likely due to the different experimental models.35,39
Some issues need to be taken into account for interpretation of the gene expression data obtained in GR-/- embryonic skin. First, the skin phenotype of the total GR knock-out may be due to the lack of GR in other cell types besides keratinocytes. Second, GR-/- mice have increased circulating hormone levels that might contribute to the observed skin phenotype. This raises a very interesting issue, the possibility that the elevated levels of GCs in GR-/- embryos may signal through the closely related mineralocorticoid receptor (MR) since it also binds GCs with high affinity. This is biologically relevant as both receptors recognize the same GREs and GR can heterodimerize with MR. These issues open the question of whether the lack of GR along with increased GC levels could result in increased MR signaling; should this be the case, MR would contribute to the observed GR-/- skin phenotype (see below).
Transcriptional and Non-Transcriptional Functions of GR in Skin Development
The histopathological analysis of GRdim/dim mouse skin revealed no differences relative to WT mice in either epidermal or HF formation.38 In addition, no changes in the expression and/or localization of several markers of epidermal differentiation were detected. This led to the postulation that the DNA-binding-independent actions of GR were sufficient to mediate epidermal and HF development during embryogenesis. However, since most genes in their natural context contain non-conventional GRE sites and/or composite elements, it is also possible that these genes are transcriptionally regulated in the GRdim/dim mice through direct binding of GR monomers to DNA.10
Recent data have demonstrated that most GR binding sites (GBS) are distributed evenly through the genome, which explains the difficulty of identifying GR transcriptional targets by conventional approaches searching for consensus GBS sequences in proximal promoters.10,40,41 The low overlap of GC-regulated genes among different cell types found by transcriptomic approaches supports that the contribution of cell-type specific transcription factors and coregulators is crucial to modulate GR-mediated transcription. The identification of primary GR-target genes in skin has been so far limited to conventional chromatin immunoprecipitation (ChIP) assays. This approach allowed identifying FK506-binding protein 51 (Fkbp51) and defensin beta1 (Defb1) as primary GR-target genes in keratinocytes.36 However, for those genes that did not show GR bound to candidate regulatory sequences, such as Krt77 and Elf5, it can not be ruled out that GR binds to other genomic sequences. Future advances rely on high throughput techniques of ChIP combined with either promoter array tiling (ChIP-chip) or parallel whole-genome sequencing (ChIP-seq),42 allowing the mapping of GBSs, which will eventually allow the identification of GR primary target genes in skin.
Further evidence supports the idea that the mechanisms by which GR regulates epidermal formation are mediated through its DNA-binding-independent actions. On one hand, the activity of the MAPK ERK was constitutively increased in GR-/- skin and MPKs relative to WT, suggesting that GR modulates skin homeostasis, at least partially, by antagonizing ERK function.38 Supporting this, a selective ERK pharmacological inhibitor partially reversed the increased apoptosis of GR-/- MPKs. GR-/- skin showed alterations in the activity of caspase-14, a non-apoptotic epidermal-specific caspase that normally induces the formation of mature filaggrin and its degradation into free amino acids, thus providing a competent stratum corneum.43 The lack of GR caused deficient processing of caspase-14 into active caspase-14 although no changes were detected in its transcript levels.38
GR Regulates Epithelial Morphogenesis
The control of a precise spatiotemporal expression pattern of GR and its ligand is crucial for normal embryo development.44 Under normal circumstances, the embryo is protected from excess GCs by the activity of 11βHSD2 which is present at high levels in the placenta. If the mechanisms of control fail, the consequences are deleterious, as evidenced by the teratogenic effects of GCs, which include reduced birth weight, increased HPA axis reactivity, hyperglycemia and hypertension.44 Although the precise mechanisms underlying these GC effects are not fully understood, they correlate with altered expression levels and activity of GR in the affected tissues. Moreover, excess GCs can also elicit epigenetic changes in the GR-target gene promoters.45
Consistent with the teratogenicity of high levels of GCs, our previous work demonstrated that GR overexpression in epithelial basal cells by means of the keratinocyte-specific K5 promoter (K5-GR mice) affected all ectoderm-derived epithelia.46–48 K5 regulatory sequences drive transgene expression following a well defined expression pattern in the basal cells of stratified epithelia starting around E13.5.49 K5-GR mice exhibited abnormal morphogenesis of the epidermis, hair, teeth and exocrine glands.46,48 In fact, the phenotype of these mice recapitulates the triad of clinical signs that define the human syndrome hypohidrotic ectodermal dysplasia (HED).50 The severity of the epithelial phenotypes correlated with transgene expression levels ranging from epidermal hypoplasia and underdeveloped, dysplastic hair follicles to a complete absence of all epidermal layers (Fig. 1). Other epidermal appendages such as vibrissae and eyebrows also appeared underdeveloped.46 The fact that higher GR dosage elicits more severe phenotypes underlines the requirement of a balanced GR-mediated signaling during epithelial morphogenesis. A common feature of HED patients also found in several mouse models for this disease is defective IκB kinase (IKK)/NFκB signaling.50 K5-GR mice showed decreased IKKα and IKKγ expression and reduced NFκB activity in tooth epithelial cells.48 This is in agreement with the known antagonism between GR and the IKK/NFκB pathway in many cell types including keratinocytes.11,21
The developing skin of K5-GR mice showed a reduced proliferation rate of the keratinocytes (approximately 30%) from E16.5 to the postnatal (P) age.46 The growth inhibitory effect of GR is in agreement with the known anti-mitotic effects of topically applied GCs in skin in vivo51 as well as in keratinocyte cell lines.52 It is indeed remarkable that the overexpression of MR under the K5 promoter (K5-MR mice) 53 causes a phenotype highly similar to that of K5-GR mice. K5-MR mice featured atrophic skin, a reduced number of hair follicles, increased keratinocyte apoptosis and premature epidermal barrier formation.53 These alterations led to perinatal death, as occurred with the highest expressing K5-GR transgenic line. The epidermal maturation of K5-GR embryos has not been tested by permeability assays, however, immunostaining showed increased expression of the keratinocyte differentiation marker loricrin (unpublished results). Moreover, the enzyme 3-hydroxy-3-methylglutaryl-CoA synthase (Hgmcs2), specifically involved in steroid and lipid biosynthesis, was upregulated in these mice, suggesting an accelerated epidermal barrier formation.54 Hmgcs2 is a transcriptional target of other NHRs.55 It is not clear whether MR overexpression accelerates epidermal maturation by inducing keratinocyte differentiation and/or by changing the levels and/or composition of epidermal lipids.
The phenotypical coincidences between K5-GR and K5-MR transgenic mouse models include an eyelid-open-at birth (EOB) phenotype but exclude alterations in vibrissae, teeth or palate.46,53 Unexpectedly, GR-/- embryos also showed an EOB phenotype.56 Eyelid epithelial defects were due, at least in part, to the lack of antagonism between GR and epidermal growth factor receptor (EGFR) signaling, causing sustained activation of ERK and the upregulation of K6 in E18.5 embryos. In vivo K6 induction in the epithelial cells of GR-/- embryos is likely mediated through AP-1 sites in its promoter.57 Conversely, GR inhibited keratinocyte migration in vitro by interfering with EGFR-mediated signaling in the eyelid epithelium.56 Although the mechanisms mediating the EOB phenotype in K5-MR have not been addressed, it could be speculated that MR and GR use similar mechanisms to regulate eyelid epithelial development. Altogether, and as it has been recently suggested, it seems relevant to further study the role of MR in the cutaneous biology.58
The examination of a mouse model expressing the GR P493R/A494S (GR-TR) mutant under the control of K5 promoter (K5-GR-TR) has provided important results.59 GR-TR is a transactivation-defective mutant that retains the transrepression properties. The comparison of the effects of GR vs. GR-TR in the epidermis and other stratified epithelia in vivo is feasible since both transgenes use the same regulatory sequences and are expressed at similar protein levels. Contrary to K5-GR mice, the skin architecture of K5-GR-TR mice in the perinatal period showed no significant changes (Fig. 1). Furthermore, the levels and pattern of expression of markers of proliferation or differentiation of the epidermis or HFs at this stage were also unchanged.59 However, K5-GR-TR embryos exhibited an EOB phenotype almost identical to K5-GR and K5-MR mice.47,53,59 This indicates that the mechanisms by which GR regulates epithelial development are tissue-specific. Moreover, while the overexpression of GR-TR did not disturb epidermal development, it caused severe alterations in the adult epidermis and HFs. These temporal-specific actions have also been described for other nuclear hormone receptors such as the retinoid X receptor α and the vitamin D receptor, which also affected specifically HF function in the adult age.3
GR Mechanisms Modulating Adult Skin Homeostasis
The adverse reactions that accompany GC therapy upon prolonged treatments have been well characterized and include skin atrophy, delayed wound healing and more rarely GC resistance.51 In addition, short-term topical and systemic GC treatment to adult mouse skin disturbed epidermal barrier competence, an effect also induced by psychological stress, likely due to the increased production of endogenous GCs.60,61 Skin atrophy is one of the most common GC-associated side-effects and consists in the reduction in skin thickness and elasticity as well as increased fragility. Together, these features result in impaired skin barrier function. In the adult age, K5-GR and K5-GR-TR mice showed a similar skin phenotype featuring epidermal thinning and a significant reduction in the number of hair follicles (25% and 50%, respectively)48,59 (Fig. 1). These effects reproduce the skin atrophy and occasional alopecia observed after prolonged treatments with GC analogs in human patients.51
GCs are known inhibitors of keratin gene expression including K5/K14 and K6/K16, which are markers of keratinocyte proliferation under physiological (K5/K14) and pathological (K6/K16) circumstances.57 This inhibition mainly occurs by two independent mechanisms, both mediated through its transrepression function. One of these mechanisms requires the binding of GR monomers to GREs at the keratin promoters whereas the other involves protein-protein interactions between GR and AP-1, suppressing AP-1-mediated induction.62 In both cases, GR monomers are sufficient to mediate these actions. GR/AP-1 cross-talk is very relevant in skin since AP-1 is crucial for many processes in skin physiopathology including epithelial development, skin inflammation and cancer.63 This antagonism has been demonstrated in vivo in several transgenic mouse models. The adult skin of K5-GR and K5-GR-TR mice showed reduced expression of K5.46,59 Furthermore, the expression of K6, a marker of hyper-proliferative skin diseases, which is induced upon treatment with the phorbol ester PMA, was strongly and similarly repressed in mice overexpressing either GR or GR-TR.59 In GRdim/dim adult mouse skin, the PMA-induced expression of two AP-1-target genes encoding for matrix metalloproteinases, Mmp-3 and Mmp-13, was efficiently inhibited.64 However, the scenario is rather complex since, as reported for other cell types, the formation of several AP-1 hetero- and homo-dimeric complexes may result in differential regulation of AP-1-target genes.63 This might explain the observed upregulation of the AP-1 target gene Mmp-11 gene, which also contains AP-1 binding sites in its promoter, in K5-GR mouse skin.54
Based on the possibility to dissociate the transactivation and transrepression functions of GR, several non-steroidal selective GR agonists or SEGRAs have been developed.51 The parameters used to determine whether SEGRAs have a better therapeutical index relative to classical GC treatments include skin atrophy, reduced inflammation and suppression of the HPA axis activity. Usually, the profile of SEGRAs is tested by using in vivo65 and, more recently, in vitro66 models. Some of these novel compounds offer promising approaches for the treatment of skin diseases.51 The experimental data using GR mouse models suggest further complexity regarding the dissociation of the GR functions; however, this does not necessarily recapitulate the clinical situation. A comparative study of the responses of K5-GR and K5-GR-TR mice to acute and chronic PMA revealed that GR-TR can efficiently repress IL-1β and Mmp-3 transcription (to a similar extent than GR) whereas GR-TR only weakly repressed IL-6 and TNFα transcripts.59 These data illustrate that the GR-TR mediated repression occurs in a gene-specific manner. Moreover, it reinforces the idea that transactivation by the GR is indeed relevant for the GC anti-inflammatory actions.
The use of of gain- and loss-of function approaches should contribute to a better understanding of the functional role of GR. Until recently, this was not feasible given the perinatal lethality of the GR-/- mice.14,15 The generation of a conditional knock-out mouse model with keratinocyte-restricted GR inactivation inducible in adult animals (K14-cre-ERT2//GRloxP/loxP mice) has demonstrated the epithelial contribution of GR in adult skin homeostasis.36 K14-cre-ERT2//GRloxP/loxP mice featured thickened skin with increased keratinocyte proliferation and inflammation, as well as impaired epidermal differentiation, demonstrating that GR function in keratinocytes is required for proper skin homeostasis (Fig. 1). Many of the genes identified as GR-regulated during skin development were similarly controlled in the adult tissue, including Cdsn, Sprr2d, Defb1 and Fkbp51. However, other genes such as Krt77 and Elf5 were regulated by GR exclusively during embryonic development. Altogether, these data indicate that GR regulates common and differential gene subsets in embryonic and adult skin. It will be important to study the role of Fkbp51 and Defb1 genes, which were also identified as primary GR transcriptional targets.36 Fkbp51 expression has been described in adult follicular stem cells67 as well as in epidermal keratinocytes.36 The antimicrobial peptide defensin β1 (Defb1) is related to the innate immune response and epithelial defense. Importantly, the decreased Defb1 mRNA expression and reduced terminal differentiation observed in the embryonic GR-/- and the adult K14-cre-ERT2//GRloxP/loxP skin suggest a role for this gene in keratinocyte differentiation in vivo.36
The prolonged use of GCs may result in the development of GC-resistance in several allergic and inflammatory diseases7 although this phenomenon is rare in dermatology. In skin, the GC-induced resistance can produce a paradoxical effect by which GCs lead to hyperproliferation instead of growth inhibition. It was postulated that GC-induced desensitization could be due to differential responses in distinct keratinocyte subpopulations.68 Chebotaev and coworkers reported that topical chronic GC treatment of mouse adult skin significantly decreased GR expression in interfollicular epidermal keratinocytes but not in the hair follicle bulge keratinocytes. These results suggest that the increased sensitivity of bulge keratinocytes to the antiproliferative effect of GCs is due to the contribution of the epidermal stem cells located in this compartment.68 The examination of additional stem cell populations located in the IFE and sebaceous glands would add to this conclusion.27
Despite its relevance, the mechanisms underlying GR function in HFs are not well characterized. The overexpression of GR and GR-TR in basal keratinocytes resulted in a reduced number of HFs as well as impairment in HF proliferation and differentiation.46,54,59 The atrophic HFs were often accompanied by hyperplasic sebaceous glands, a common response secondary to alopecia. Besides, K5-MR adult mice generated by conditional postnatal expression of MR to overcome the perinatal lethality also showed a severe skin phenotype. The skin of these mice featured dysplasic HFs leading to cysts and progressive alopecia.53 The formation of cysts usually occurs when the HFs are irreversibly damaged, as reported in other mouse models with NHR inactivation such as retinoid X receptor α conditional epidermal knock-out and vitamin D receptor-deficient mice; both of these mouse models also have progressive alopecia.3
At the molecular level, the analysis of the gene expression profile of K5-GR adult skin supported a role for GR in HF differentiation.54 Among the identified genes, there was a large subset of hair keratin intermediate filament (krt) and hair keratin-associated protein (krtap) genes, as well as several hox genes. This is clinically relevant since dysregulation of krt, krtaps and hox genes can cause hair disorders.69 KRTAPs are essential to form a rigid and resistant hair shaft through their extensive disulfide bond cross-linking with abundant cysteine residues of hair keratins. Hox genes, besides playing a major role in HF development and cycling, have been involved in transcriptional regulation of krtaps.70
Conclusions and Perspectives
Several transcriptomic analyses have reported that in keratinocytes, GR regulates the expression of a variety of genes, including those involved in apoptosis, cellular adhesion, lipid metabolism and formation of the stratum corneum. Based on these data, it is particularly relevant to perform functional studies that help to dissect the exact mechanisms by which GR regulates keratinocyte terminal differentiation and epidermal barrier formation. Alterations in these processes during embryogenesis can result in increased susceptibility of adult individuals to inflammatory skin diseases such as psoriasis and atopic dermatitis. Therefore, studying the mechanisms of GR function during development and identifying GR primary targets will be useful for designing therapies based on GC analogs. A major question that remains unanswered is whether the epithelial contribution of GR is required for normal skin development as well as to mediate the therapeutical actions of GCs in different skin diseases. In order to answer these questions, it is necessary to study a mouse model with constitutive GR inactivation restricted to keratinocytes.
Recently, several additional GR isoforms have been identified, although no information of their expression and activity has been reported in the skin. The tissue-specific expression patterns and post-translational regulatory mechanisms for these isoforms add further complexity to the understanding of GR signaling. GRβ has been postulated as a dominant negative form of GRα-dependent transcription that could be responsible for GC resistance in inflammatory diseases. Considering the wide use of the GCs in clinical practice, the variable outcome of GC treatment among patients, and the phenomenon of GC resistance, it is a main goal to determine the underlying causes for the differential GC responses in order to design and adjust the GC-based treatments to achieve best efficacy with minimal adverse effects.
Additionally, given the interactions among different nuclear hormone receptors and the prescription of combined hormone analogs for treating skin diseases, future work should be aimed at deciphering the complex interactions between different hormone signaling pathways. Supported by recent findings, it seems particularly relevant to analyze a possible cross-talk between GR and MR in keratinocytes, which may have consequences in GC-based therapies.
Acknowledgments
We acknowledge the grant of the Spanish Ministery (SAF2008-00540). We are indebted to Lisa M. Sevilla and José Leon for critical reading of the manuscript.
References
- 1.Nicolaides NC, Galataa Z, Kinob T, Chrousosa GP, Charmandari E. The human glucocorticoid receptor: Molecular basis of biologic function. Steroids. 2010;75:1–12. doi: 10.1016/j.steroids.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanisic V, Lonard DM, O'Malley BW. Modulation of steroid hormone receptor activity. Prog Brain Res. 2010;181:153–176. doi: 10.1016/S0079-6123(08)81009-6. [DOI] [PubMed] [Google Scholar]
- 3.Schmuth M, Watson RE, Deplewski D, Dubrac S, Zouboulis CC, Grifiths CE. Nuclear hormone receptors in human skin. Horm Metab Res. 2007;39:96–105. doi: 10.1055/s-2007-961808. [DOI] [PubMed] [Google Scholar]
- 4.Lu NZ, Cidlowski JA. Glucocorticoid receptor isoforms generate transcription specificity. Trends Cell Biol. 2006;16:301–307. doi: 10.1016/j.tcb.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Revollo JR, Cidlowski JA. Mechanisms generating diversity in glucocorticoid receptor signaling. Ann NY Acad Sci. 2009;1179:167–178. doi: 10.1111/j.1749-6632.2009.04986.x. [DOI] [PubMed] [Google Scholar]
- 6.Hinds TD, Jr, Ramakrishnan S, Cash HA, Stechschulte LA, Heinrich G, Najjar SM, Sanchez ER. Discovery of glucocorticoid receptor-β in mice with a role in metabolism. Mol Endocrinol. 2010;24:1715–1727. doi: 10.1210/me.2009-0411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barnes PJ, Adcock IM. Glucocorticoid resistance in inflammatory diseases. Lancet. 2009;373:1905–1917. doi: 10.1016/S0140-6736(09)60326-3. [DOI] [PubMed] [Google Scholar]
- 8.Adams M, Meijer OC, Wang J, Bhargava A, Pearce D. Homodimerization of the glucocorticoid receptor is not essential for response element binding: activation of the phenylethanolamine n-methyltransferase gene by dimerization-defective mutants. Mol Endocrinol. 2003;17:2583–2592. doi: 10.1210/me.2002-0305. [DOI] [PubMed] [Google Scholar]
- 9.Rogatsky I, Wang JC, Derynck MK, Nonaka DF, Khodabakhsh DB, Haqq CM, et al. Target-specific utilization of transcriptional regulatory surfaces by the glucocorticoid receptor. Proc Natl Acad Sci USA. 2003;100:13845–13850. doi: 10.1073/pnas.2336092100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.So A, Chaivorapol C, Bolton EC, Li H, Yamamoto KR. Determinants of cell- and gene-specific transcriptional regulation by the glucocorticoid receptor. PLoS Genet. 2007;23:927–938. doi: 10.1371/journal.pgen.0030094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Bosscher K, Vanden Berghe W, Haegeman G. The interplay between the glucocorticoid receptor and nuclear factorκB or activator protein-1: Molecular mechanisms for gene repression. Endocr Rev. 2003;24:488–522. doi: 10.1210/er.2002-0006. [DOI] [PubMed] [Google Scholar]
- 12.Kassel O, Herrlich P. Cross-talk between the glucocorticoid receptor and other transcription factors: molecular aspects. Mol Cell Endocrinol. 2007;275:13–29. doi: 10.1016/j.mce.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 13.Davies L, Karthikeyan N, Lynch JT, Sial E, Gkourtsa A, Demonacos C, et al. Cross talk of signaling pathways in the regulation of the glucocorticoid receptor function. Mol Endocrinol. 2009;22:1331–1344. doi: 10.1210/me.2007-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cole TJ, Blendy AP, Monaghan K, Schmid W, Aguzzi A, Fantuzzi G, et al. Targeted disruption of the glucocorticoid receptor gene blocks adrenergic chromaffin cell development and severely retards lung maturation. Genes Dev. 1995;9:1608–1621. doi: 10.1101/gad.9.13.1608. [DOI] [PubMed] [Google Scholar]
- 15.Tronche F, Kellendonk C, Kretz O, Gass P, Anlag K, Orban PC, et al. Disruption of the glucocorticoid receptor gene in the nervous system results in reduced anxiety. Nat Genet. 1999;23:99–103. doi: 10.1038/12703. [DOI] [PubMed] [Google Scholar]
- 16.Reichardt HM, Kaestner KH, Tuckermann J, Kretz O, Wessely O, Bock R, et al. DNA binding of the glucocorticoid receptor is not essential for survival. Cell. 1998;93:531–541. doi: 10.1016/s0092-8674(00)81183-6. [DOI] [PubMed] [Google Scholar]
- 17.Clark A. Anti-inflammatory functions of glucocorticoid-induced genes. Mol Cell Endocrinol. 2007;275:79–97. doi: 10.1016/j.mce.2007.04.013. [DOI] [PubMed] [Google Scholar]
- 18.De Bosscher K, Haegeman G. Latest perspectives on antiinflammatory actions of glucocorticoids. Mol Endocrinol. 2009;23:281–291. doi: 10.1210/me.2008-0283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tuckermann JP, Kleiman A, Moriggl R, Spanbroek R, Neumann A, Illing A, et al. Macrophages and neutrophils are the targets for immune suppression by glucocorticoids in contact allergy. J Clin Invest. 2007;117:1381–1390. doi: 10.1172/JCI28034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Losel R, Wehling M. Nongenomic actions of steroid hormones. Nat Rev Mol Cell Biol. 2003;4:46–56. doi: 10.1038/nrm1009. [DOI] [PubMed] [Google Scholar]
- 21.Leis H, Page A, Ramírez A, Bravo A, Segrelles C, Paramio J, et al. Glucocorticoid Receptor counteracts tumorigenic activity of Akt in skin through interference with the phosphatidylinositol-3-kinase (PI3K) signaling pathway. Mol Endocrinol. 2004;18:303–311. doi: 10.1210/me.2003-0350. [DOI] [PubMed] [Google Scholar]
- 22.Candi E, Schmidt R, Melino G. The cornified envelope: a model of cell death in the skin. Nature Rev Mol Cell Biol. 2005;6:328–340. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 23.Raj D, Brash DE, Grossman D. Keratinocyte apoptosis in epidermal development and disease. J Invest Dermatol. 2006;126:243–257. doi: 10.1038/sj.jid.5700008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Segre JA. Epidermal barrier formation and recovery in skin disorders. J Clin Invest. 2006;116:1150–1158. doi: 10.1172/JCI28521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schmidt-Ullrich R, Paus R. Molecular principles of HF induction and morphogenesis. BioEssays. 2005;27:247–261. doi: 10.1002/bies.20184. [DOI] [PubMed] [Google Scholar]
- 26.Elias PM. Therapeutic implications of a barrier-based pathogenesis of atopic dermatitis. Ann Dermatol. 2010;22:245–254. doi: 10.5021/ad.2010.22.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Blanpain C, Fuchs E. Epidermal homeostasis: a balancing act of stem cells in the skin. Nature Rev Mol Cell Biol. 2009;207:207–218. doi: 10.1038/nrm2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of GR function and tissue sensitivity to glucocorticoids. Endocr Rev. 1996;17:245–261. doi: 10.1210/edrv-17-3-245. [DOI] [PubMed] [Google Scholar]
- 29.Slominski A, Wortsman J, Paus R, Elias PM, Tobin DJ, Feingold KR. Skin as an endocrine organ: implications for its function. Drug Discov Today Dis Mech. 2008;5:137–144. doi: 10.1016/j.ddmec.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zouboulis CC. The skin as an endocrine organ. Dermatoendocrinol. 2009;1:250–252. doi: 10.4161/derm.1.5.9499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ito N, Ito T, Kromminga A, Bettermann A, Takigawa M, Kees F, et al. Human hair follicles display a functional equivalent of the hypothalamic-pituitary-adrenal axis and synthesize cortisol. Differentiation. 1997;62:21–31. doi: 10.1096/fj.04-1968fje. [DOI] [PubMed] [Google Scholar]
- 32.Vukelic S, Stojadinovic O, Pastar I, Rabach M, Krzyzanowska A, Lebrun E, et al. Cortisol synthesis in epidermis is induced by IL-1 and tissue injury. J Biol Chem. 2011;286:10265–10275. doi: 10.1074/jbc.M110.188268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aszterbaum M, Feingold KR, Menon GK, Williams ML. Glucocorticoids accelerate fetal maturation of the epidermal permeability barrier in the rat. J Clin Invest. 1993;91:2703–2708. doi: 10.1172/JCI116509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanley K, Feingold KR, Komuves LG, Ellias P, Muglia LJ, Majzoub JA, Williams ML. Glucocorticoid deficiency delays stratum corneum maturation in fetal mouse. J Invest Dermatol. 1998;11:440–444. doi: 10.1046/j.1523-1747.1998.00303.x. [DOI] [PubMed] [Google Scholar]
- 35.Patel S, Xi ZF, Seo EY, McGaughey D, Segre JA. Klf4 and corticosteroids activate an overlapping set of transcriptional targets to accelerate in utero epidermal barrier acquisition. Proc Natl Acad Sci USA. 2006;103:18668–18673. doi: 10.1073/pnas.0608658103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sevilla L, Bayo P, Latorre V, Sanchis Ana, Pérez Paloma. Glucocorticoid receptor regulates overlapping and differential gene subsets in developing and adult skin. Mol Endocrinol. 2010;24:2166–2178. doi: 10.1210/me.2010-0183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kitraki E, Kittas C, Stylianopoulou F. Glucocorticoid receptor gene expression during rat embryogenesis. An in situ hybridization study. Differentiation. 1997;62:21–31. doi: 10.1046/j.1432-0436.1997.6210021.x. [DOI] [PubMed] [Google Scholar]
- 38.Bayo P, Sanchis A, Bravo A, Cascallana JL, Buder K, Tuckermann J, et al. Glucocorticoid receptor is required for skin barrier competence. Endocrinology. 2008;149:1377–1388. doi: 10.1210/en.2007-0814. [DOI] [PubMed] [Google Scholar]
- 39.Stojadinovic O, Lee B, Vouthounis C, Vukelic S, Pastar I, Blumenberg M, et al. Novel genomic effects of glucocorticoids in epidermal keratinocytes. inhibition of apoptosis, interferon-γ pathway and wound healing along with promotion of terminal differentiation. J Biol Chem. 2007;282:4021–4034. doi: 10.1074/jbc.M606262200. [DOI] [PubMed] [Google Scholar]
- 40.So A, Cooper SB, Feldman BJ, Manuchehri M, Yamamoto KR. Conservation analysis predicts in vivo occupancy of glucocorticoid receptor-binding sequences at glucocorticoid-induced genes. Proc Natl Acad Sci USA. 2008;105:5745–5749. doi: 10.1073/pnas.0801551105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meijsing SH, Pufall MA, So A, Bates DL, Chen L, Yamamoto KR. DNA binding site sequence directs glucocorticoid receptor structure and activity. Science. 2009;24:407–410. doi: 10.1126/science.1164265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zecchini V, Mills IG. Putting chromatin immunoprecipitation into context. J Cell Biochem. 2009;107:19–29. doi: 10.1002/jcb.22080. [DOI] [PubMed] [Google Scholar]
- 43.Denecker G, Hoste E, Gilbert B, Hochepied T, Ovaere P, Lippens S, et al. Caspase-14 protects against epidermal UVB photodamage and water loss. Nat Cell Biol. 2007;9:666–674. doi: 10.1038/ncb1597. [DOI] [PubMed] [Google Scholar]
- 44.Seckl JR, Nyirenda MJ, Walker BR, Chapman KE. Glucocorticoids and fetal programming. Biochem Soc Trans. 1999;27:74–78. doi: 10.1042/bst0270074. [DOI] [PubMed] [Google Scholar]
- 45.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–289. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 46.Pérez P, Page A, Bravo A, del Río M, Gimenez-Conti I, Budunova I, et al. Altered skin development and impaired proliferative and inflammatory responses in transgenic mice overexpressing the glucocorticoid receptor. FASEB J. 2001;15:2030–2036. doi: 10.1096/fj.00-0772fje. [DOI] [PubMed] [Google Scholar]
- 47.Cascallana JL, Bravo A, Page A, Budunova I, Slaga TJ, Jorcano JL, Pérez P. Disruption of eyelid and cornea development by targeted overexpression of the glucocorticoid receptor. Int J Dev Biol. 2003;47:59–64. [PubMed] [Google Scholar]
- 48.Cascallana JL, Bravo A, Donet E, Leis H, Lara MMa F, Paramio JM, et al. Ectoderm-targeted overexpression of the glucocorticoid receptor induces hypohidrotic ectodermal dysplasia. Endocrinology. 2005;146:2629–2638. doi: 10.1210/en.2004-1246. [DOI] [PubMed] [Google Scholar]
- 49.Ramírez A, Page A, Gandarillas A, Zanet J, Pibre S, Vidal M, et al. A keratin K5Cre transgenic line appropriate for tissue-specific or generalized Cre-mediated recombination. Genesis. 2004;39:52–57. doi: 10.1002/gene.20025. [DOI] [PubMed] [Google Scholar]
- 50.Smahi A, Courtois G, Rabia SH, Doffinger R, Bodemer C, Munnich A, et al. The NFκB signalling pathway in human diseases: from incontinentia pigmenti to ectodermal dysplasias and immune-deficiency syndromes. Hum Mol Genet. 2002;11:2371–2375. doi: 10.1093/hmg/11.20.2371. [DOI] [PubMed] [Google Scholar]
- 51.Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 52.Budunova IV, Carbajal S, Kang H, Viaje A, Slaga TJ. Altered glucocorticoid receptor expression and function during mouse skin carcinogenesis. Mol Carcinogen. 1997;18:177–185. [PubMed] [Google Scholar]
- 53.Marie YS, Toulon A, Paus R, Maubec E, Cherfa A, Grossin M, et al. Targeted skin overexpression of the mineralocorticoid receptor in mice causes epidermal atrophy, premature skin barrier formation, eye abnormalities and alopecia. Am J Pathol. 2007;171:846–860. doi: 10.2353/ajpath.2007.060991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Donet E, Bayo P, Calvo E, Labrie F, Pérez P. Identification of novel glucocorticoid receptor-regulated genes involved in epidermal homeostasis and hair follicle differentiation. J Steroid Biochem Mol Biol. 2007;108:8–16. doi: 10.1016/j.jsbmb.2007.05.033. [DOI] [PubMed] [Google Scholar]
- 55.Calleja C, Messadde N, Chapellie RB, Yang H, Krezel W, Li M, et al. Genetic and pharmacological evidence that a retinoic acid cannot be the RXR-activating ligand in mouse epidermis keratinocytes. Genes Dev. 2006;20:1525–1538. doi: 10.1101/gad.368706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sanchis A, Bayo P, Sevilla L, Pérez P. Glucocorticoid receptor antagonizes EGFR function to regulate eyelid development. Int J Dev Biol. 2010;54:1473–1480. doi: 10.1387/ijdb.103071as. [DOI] [PubMed] [Google Scholar]
- 57.Ramot Y, Paus R, Tiede S, Zlotogorski A. Endocrine controls of keratin expression. BioEssays. 2009;31:389–399. doi: 10.1002/bies.200800121. [DOI] [PubMed] [Google Scholar]
- 58.Farman N, Maubec E, Poeggeler B, Klatte JE, Jaisser F, Paus R. The mineralocorticoid receptor as a novel player in skin biology: beyond the renal horizon? Exp Dermatol. 2010;19:100–107. doi: 10.1111/j.1600-0625.2009.01011.x. [DOI] [PubMed] [Google Scholar]
- 59.Donet E, Bosch P, Sanchis A, Bayo P, Ramírez A, Cascallana JL, et al. Transrepression function of the glucocorticoid receptor regulates eyelid development and keratinocyte proliferation but is not sufficient to prevent skin chronic inflammation. Mol Endocrinol. 2008;22:799–812. doi: 10.1210/me.2007-0284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kao JS, Fluhr JW, Man MQ, Fowler AJ, Hachem JP, Crumrine D, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456–464. doi: 10.1046/j.1523-1747.2003.12053.x. [DOI] [PubMed] [Google Scholar]
- 61.Choi EH, Demerjian M, Crumrine D, Brown BE, Mauro T, Elias PM, Feingold KR. Glucocorticoid blockade reverses psychological stress-induced abnormalities in epidermal structure and function. FASEB J. 2005;19:1332–1334. doi: 10.1152/ajpregu.00010.2006. [DOI] [PubMed] [Google Scholar]
- 62.Radoja N, Komine M, Jho SH, Blumenberg M, Tomic-Canic M. Novel mechanism of steroid action in skin through glucocorticoid receptor monomers. Mol Cell Biol. 2000;20:4328–4339. doi: 10.1128/mcb.20.12.4328-4339.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Angel P, Szabowski A, Schorpp-Kistner M. Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene. 2001;20:2413–2423. doi: 10.1038/sj.onc.1204380. [DOI] [PubMed] [Google Scholar]
- 64.Tuckermann JP, Reichardt HM, Arribas R, Richter KH, Schütz G, Angel P. The DNA-binding-independent function of the glucocorticoid receptor mediates repression of AP-1-dependent genes in skin. J Cell Biol. 1999;147:1365–1370. doi: 10.1083/jcb.147.7.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Schäcke H, Schottelius A, Döcke WD, Strehlke P, Jaroch S, Schmees N, et al. Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects. Proc Natl Acad Sci USA. 2004;101:227–232. doi: 10.1073/pnas.0300372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Schoepe S, Schäcke H, Bernd A, Zöller N, Asadullah K. Identification of novel in vitro test systems for the determination of glucocorticoid receptor ligand-induced skin atrophy. Skin Pharmacol Physiol. 2010;23:139–151. doi: 10.1159/000270386. [DOI] [PubMed] [Google Scholar]
- 67.Chebotaev D, Yemelyanov A, Zhu L, Lavker RM, Budunova I. The tumor suppressor effect of the glucocorticoid receptor in skin is mediated via its effect on follicular epithelial stem cells. Oncogene. 2007;26:3060–3068. doi: 10.1038/sj.onc.1210108. [DOI] [PubMed] [Google Scholar]
- 68.Chebotaev DV, Yemelyanov AY, Lavker RM, Budunova IV. Epithelial cells in the hair follicle bulge do not contribute to epidermal regeneration after glucocorticoidinduced cutaneous atrophy. J Invest Dermatol. 2007;127:2749–2758. doi: 10.1038/sj.jid.5700992. [DOI] [PubMed] [Google Scholar]
- 69.Schweizer J, Bowden PE, Coulombe PA, Langbein L, Lane EB, Magin TM, et al. New consensus nomenclature for mammalian keratins. J Cell Biol. 2006:174169–174174. doi: 10.1083/jcb.200603161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tkatchenko AV, Visconti RP, Shang L, Papenbrock T, Pruett ND, Ito T, et al. Overexpression of Hoxc13 in differentiating keratinocytes results in downregulation of a novel hair keratin gene cluster and alopecia. Development. 2001;128:1547–1558. doi: 10.1242/dev.128.9.1547. [DOI] [PubMed] [Google Scholar]