Abstract
Topical glucocorticoids are highly anti-inflammatory effective but limited by their side effect potential, with skin atrophy being the most prominent one. Thus, determining the atrophogenic potential of novel compounds targeting the glucocorticoid receptor is important. Significant progress in the understanding of glucocorticoid receptor mediated molecular action has been made providing the basis for novel glucocorticoid receptor ligands with a potentially superior effect/side effect profile. Such compounds, however, need to be tested. The present gold standard for the reliable prediction of glucocorticoid induced skin atrophy are still in vivo models, however, in vitro models may replace them to some extent in the future. Indeed, advances in technologies to determine the atrophogenic potential of compounds in vitro has been made recently and promising novel test models like the human full thickness skin models are emerging. Their full predictive value, however, needs to be further evaluated. Currently, a screening approach starting with a combination of several in vitro test systems followed by subsequent testing of the most promising compounds in rodent models is recommended prior entering clinical studies with selected development compounds.
Key words: glucocorticoid, glucocorticoid receptor ligands, skin atrophy, skin model, in vitro assay, in vivo assay
Introduction
Topical glucocorticoids (GCs) belong to the most powerful and frequently used drugs for topical therapy of inflammatory skin diseases at all. Despite their great advantages in certain indications, undesired effects can evolve, in particular when used over longer periods or when applied improperly. Among those, skin atrophy is the most prominent one due to its irreversibility1 and frequency.2
Atrophy of the skin is characterized by a sincere loss in skin thickness and elasticity causing cutaneous transparency, increased fragility and telangiectatic surface.3–5 It is associated with an increased permeability and transepidermal water loss,6 which indicates a disturbed skin barrier function.7,8 Histopathologically, flat dermal-epidermal junctions,3 reduced epidermal thickness,5 reduced number of fibroblasts6,9 and a diminution of dermal collagen10,11 can be observed. Depletion of glycosaminoglycans e.g., hyaluronan is another potential feature of GC-induced skin atrophy.12,13
Topical GCs are classified according to their potencies from very strong to weak14—considering both their therapeutic and side effect potential. Efforts to identify topical GCs with predominantly beneficial effects led to the development of a GC specific therapeutic index (TIX). A TIX of 1–2 is defined as an equal relation of desired and adverse effects.15 Optimization of the benefit/risk ratio is a main challenge for the development of novel compounds targeting the GC receptor (GR).16,17 An always better understanding of the molecular mechanisms of GR-mediated effects and side effects triggered the discovery of novel GR-ligands with a putative superior profile. A particular attractive class of compounds are selective glucocorticid receptor agonists (SEGRAs) which are currently entering clinical development.18 For the development of novel optimized GR ligand predictive pre-clinical test systems can be very supportive to determine the atrophy risk.19 Skin atrophy can be modeled in vitro, in vivo and in humans. Classically, in vitro tests for skin atrophy assess proliferation of cutaneous cells,20 or collagen synthesis in primary human fibroblasts.21 Recently, epidermal thickness and collagen synthesis in three-dimensional full-thickness skin models (FTSM) were developed to determine GC ligand induced skin atrophy in vitro.22,23 In vivo, the hairless OFA hr/hr rat, however, is still the gold standard model for GC-induced skin atrophy in basic and pharmaceutical research.18,24 The purpose of this review is to summarize the current atrophy models and to highlight further perspectives.
Determining the Atrophogenic Potential in Pre-Clinical in vitro Models
The advantage of in vitro test systems in general is that they are fast, economical, and feasible with minimal amounts of compounds (Table 1). Consequently, they allow medium or even high throughput compound screening and are, thus, highly attractive for pharmaceutical industry, especially in early drug discovery. Those tests usually assess proliferation of keratinocytes and fibroblasts. Previous reports indicate that GCs might either favour or inhibit proliferation of fibroblasts, depending on the experimental model and on the working-group (reviewed in ref. 25). Discrepancies observed between in vitro experiments might be due to indirect effects of GCs on fibroblasts by affecting the synthesis or actions of various factors produced by other cell types. Yet, more recent studies show anti-proliferative effects of GCs on primary human fibroblasts20,26 and HaCaT cells, a human keratinocyte cell line,20 only. Beside their anti-proliferative effects in cutaneous cells, GCs also affect collagen metabolism. GCs inhibit collagen type I synthesis in primary human, collagen type I and III mRNA expression in mouse 3T3 fibroblasts and mRNA expression of matrix metalloproteinases 1, -2, -3 and 9 in primary human keratinocytes.21,22 The mRNA expression in the last two mentioned models (3T3 fibroblasts and human keratinocytes) is dose-dependently inhibited by GCs and the effects of different GCs correlate with their atrophogenic potential according o their topical GC class and to their TIX. Recently, the suppression of hyaluronan synthase 2 in human primary fibroblasts was also demonstrated to correlate with the atrophogenic potential of different GCs.27 The practical predictivity of a single monolayer cell culture test system, however, is not fully clear, yet.
Table 1.
Characteristics of models for determination of glucocorticoid induced skin atrophy (modified after ref. 51)
| In vitro monolayer cell cultures | In vitro full-thickness skin models | In vivo models | In patients | |
| Pro |
|
|
|
|
| Con |
|
|
|
|
The three-dimensional growing human FTSM has been introduced more recently to keep cells under more physiological conditions compared to classical monolayer cell cultures. The structure of FTSM closely parallels human skin.28 They offer characteristics that are much closer to the in vivo situation of the skin in comparison to monolayer cell culture systems such as stratification, homeostasis, expression and location of specific differentiation markers.29,30 It has been shown, that the following parameters of GC-induced skin atrophy can be detected in FTSM: epidermal thinning, reduced proliferation of keratinocytes in stratum basale, inhibition of collagen type I and III and MMP-1 and -3 synthesis.22,23 In addition to their undesired effects, the beneficial effects of GCs may be determined within the same experimental model by measurement of stimulus-induced interleukin-6 and -8 secretion into medium.23 The benefit of skin tissue models compared to monolayer cell systems or animal experiments are (1) the human origin of the cells, (2) the warranted interactions of keratinocytes and fibroblasts and (3) the reduced variability of read out parameters (Table 1). It may be postulated that these facts result in a higher predictivity of these test systems for the clinical situation. This is supported by the findings that skin equivalents are already proven alternatives to traditional animal testing, like dermal corrosion and skin irritation tests.31
Taken together, there are promising development for in vitro test systems for GC-induced skin atrophy, their fully predictive potential, however, needs to be further determined.
Determining the Atrophogenic Potential in Pre-Clinical in vivo Models
Many pre-clinical animal models are described to determine the atrophogenic potential of GCs.19 Atrophogenicity has been evaluated in mice,32,33 in rats,34,35 in pigs36,37 and in dogs.3 Castor and Baker showed as early as 1950 that topical application of hydrocortisone to rat skin caused dermal thinning.38 Overall the rat seems to be the best suited species. In the past, rats were shaved for atrophy assessment;32,39 however, due to the unreliability of haired skin responses,40,41 the use of hairless animals is the preferred now. Indeed, the current gold standard for pre-clinical testing of GR ligands is the hairless OFA hr/hr rat.34 Here, hairless rats are daily treated over 19 days. Our recent statistical studies, however, showed that the treatment duration of hairless rat skin atrophy models might be reduced to 5 days, which would be beneficial for animal protection and economical reasons (Schoepe et al., in preparation).
As a major overall parameter, skin thickness is determined over time using different methods either by counting the cell layers in skin biopsies42 or are measured with a special gauge.32,34 Detection of skin-breaking strength of the GC-treated area is additionally used parameter.34 Biochemical and histological analyses on skin biopsies are also performed. Further tissue parameters can be assessed in biopsies of GC-treated skin, e.g., regression of sebaceous glands, size and number of horn-filled cysts, subcutaneous fat and muscular layers.33,42 On the molecular level, various proteins like glucosaminoglycan, fibronectin and collagens may be used as indicators of GC effects.39,43 Beside the direct parameters of skin atrophy, systemic effects of topically administered compounds can also be measured such as weight loss of body, thymus, spleen and adrenal glands.34,35,39
The pros of in vivo pre-clinical tests compared to clinical tests are the reduced variability, higher cost-efficiency and in particular ethical aspects (Table 1). An evident con of many in vivo models is, however, their still limited predictivity for the human situation. Anti-inflammatory activities of GCs differ in rats and humans,44 and, since anti-inflammatory potencies and atrophogenic potential usually closely correlate in the classical GCs, the relative dermal atrophogenic potencies are also expected to vary between the species.39 Compared to humans, the hairless rat seems to be more sensitive to atrophy following topical GC treatment. Anatomical differences of rodent and human skin such as the high number of skin appendages and the absence of a papillary dermis in rodents clearly might influence pharmacokinetics.32 Therefore, absorption of topically administered compounds in rodent skin can exceed human skin by ten times.
Determining the Atrophogenic Potential in Clinical Models
The ultimate atrophogenicity of GCs can finally be determined in human clinical studies only. Skin atrophy is potentially irreversible and obtaining skin biopsies represents an invasive method with certain risks (scars, infection) (Table 1). Therefore, such studies are rarely done or at least compounds are applied to very small areas and non-invasive methods are used. There are many non-invasive methods to clinically determine a GCs' atrophogenic potential. The skin-blanching or vasoconstriction assay45 is a current model to rank classical topical GCs. This assay utilizes the localized vasoconstriction side effect after topical GCs application. The vasoconstriction may be quantified by a dermoscop,46 by a chromameter or by digital image analysis.47 Whereas this method is appropriate for the characterization of classical GCs, it may not be appropriate for novel GR agonists with dissociation between effects and side effects, since the assay just measures the potency of GCs. However, several methods exist to measure the thinning of the skin directly, e.g., confocal laser microscopy, micrometer screw gauge and ultrasound.6,48 Confocal laser scanning microscopy is an accurate instrument for three-dimensional reconstructions of the skin. A screw gauge measures the thickness of skin folds, but it may also include a varying amounts of subcutaneous tissue. The methodology of ultrasonography delivers a two-dimensional, cross-sectional view of the skin. 20 MHz sonography, or in particular skin sonography at large, is the experimental approach of choice so far to quantify cutaneous atrophy in the context of topical GC application to the skin. The methodology has originally been developed by Marks.49 These two methods delineate one thickness of skin as well as they allow the selective measuring of epidermal or dermal thickness without any other tissues. All of these methods are capable of detecting skin atrophy induced by a potent topical GC. For both research purposes and clinical evaluation, measurement of GC-induced skin barrier impairment is frequently applied. Here, evaporimetry is used to demonstrate transepidermal water loss. Stratum corneum lipid assessment is used to demonstrate reduction of ceramides, cholesterol or free fatty acids.6 All in all, measurements for atrophogenicity in humans are burdensome and time-consuming, sometimes requiring 6 weeks of occlusive exposure.50
Concluding Remarks
Insight into the molecular mechanisms of GR-mediated actions stimulated the development of novel GR ligands with an improved TIX. It seems particular promising to exploit different molecular mechanisms that are assumed to underlie anti-inflammation and side effects including atrophy, as by the concept of selective glucocorticoid receptor agonists (SEGRA).18 Further progress in understanding the molecular mechanisms of GC-induced skin atrophy and establishment and validation of novel models for determining the atrophogenic potential will further support such important developments.
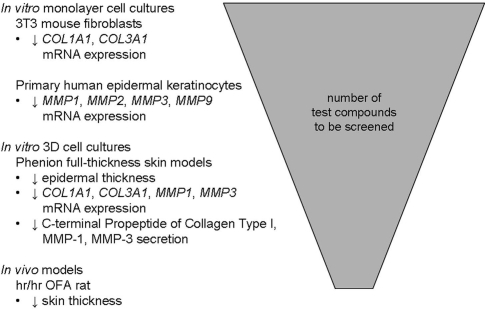
The effects of GCs on skin have been investigated for over 30 years and are showing a reduced proliferation of GR target cells, fibroblasts and keratinocytes and a disturbed metabolism of extracellular matrix proteins. Many test systems determine the atrophogenic potential of GCs using in vivo models such as measuring skin thickness in hairless rats, the current standard in pre-clinical tests. Indeed, they are quite predictive. The current research, however, focuses on development of novel human in vitro models as (1) the demand of reduction, refinement or replacement of animal experiments (3R concept) is high and as (2) the cells are of human origin. Promising in vitro models are described, whose data correlate with the atrophogenicity of GCs. These in vitro models deal classically with the GR-dependent regulation of collagen synthesis or other extracellular matrix proteins in monolayer fibroblast cultures. GC sensitive regulation of only one parameter per test system e.g., proliferation or ‘type I collagen’ was examined. It is possible that analyzing regulation of various extracellular matrix proteins gives better information about the atrophogenicity of GCs. First experiments suggest that skin equivalents will become a more predicitive future in vitro model for determining atrophogenicity of GCs and may to some extent replace animal models. In these three-dimensional tissue models, (1) parameters of skin atrophy such as epidermal thinning and proliferation can be determined, (2) various other molecular parameters can be verified in different cell types within one experiment and (3) and the undesired atrophogenic side effects of GCs can be determined beside the compounds' desired anti-inflammatory effects. A single highly predictive and easy-to-use in vitro skin atrophy test system that is fully predictable, however, has not been established yet. Based on our expiriences a combination of three different in vitro models based on 3T3 fibroblasts, human keratinocytes and FTSM with several readouts is recommended to determine atrophogenicity of GR ligands in vitro.22 Further experiments are needed to ultimately reduce this panel and to demonstrate the true predictability for the clinic. After identifying promising candidates in such in vitro test systems suited for screening, selected compounds should be tested in rodent animal and drug candidates finally in man (Fig. 1).
Figure 1.
Proposed screening cascade—throughput of novel test compounds in atrophy models. (Fig. modified from ref. 51).
Abbreviations
- FTSM
full-thickness skin models
- GC
glucocorticoid
- GR
glucocorticoid receptor
- SEGRA
selective glucocorticoid receptor agonist
- TIX
therapeutic index
References
- 1.Sterry W, Asadullah K. Topical glucocorticoid therapy in dermatology. Ernst Schering Res Found Workshop. 2002:39–54. doi: 10.1007/978-3-662-04660-9_3. [DOI] [PubMed] [Google Scholar]
- 2.Schäcke H, Döcke WD, Asadullah K. Mechanisms involved in the side effects of glucocorticoids. Pharmacol Ther. 2002;96:23–43. doi: 10.1016/s0163-7258(02)00297-8. [DOI] [PubMed] [Google Scholar]
- 3.Kimura T, Doi K. Dorsal skin reactions of hairless dogs to topical treatment with corticosteroids. Toxicol Pathol. 1999;27:528–535. doi: 10.1177/019262339902700506. [DOI] [PubMed] [Google Scholar]
- 4.Booth BA, Tan EM, Oikarinen A, Uitto J. Steroid-induced dermal atrophy: effects of glucocorticosteroids on collagen metabolism in human skin fibroblast cultures. Int J Dermatol. 1982;21:333–337. doi: 10.1111/j.1365-4362.1982.tb03140.x. [DOI] [PubMed] [Google Scholar]
- 5.Mills CM, Marks R. Side effects of topical glucocorticoids. Curr Probl Dermatol. 1993;21:122–131. doi: 10.1159/000422371. [DOI] [PubMed] [Google Scholar]
- 6.Kolbe L, Kligman AM, Schreiner V, Stoudemayer T. Corticosteroid-induced atrophy and barrier impairment measured by non-invasive methods in human skin. Skin Res Technol. 2001;7:73–77. doi: 10.1034/j.1600-0846.2001.70203.x. [DOI] [PubMed] [Google Scholar]
- 7.Kao JS, Fluhr JW, Man MQ, Fowler AJ, Hachem JP, Crumrine D, et al. Short-term glucocorticoid treatment compromises both permeability barrier homeostasis and stratum corneum integrity: inhibition of epidermal lipid synthesis accounts for functional abnormalities. J Invest Dermatol. 2003;120:456–464. doi: 10.1046/j.1523-1747.2003.12053.x. [DOI] [PubMed] [Google Scholar]
- 8.Sheu HM, Yu HS, Sheen TC. Histochemical and ultrastructural studies on cutaneous side effects due to topical corticosteroids. I. Changes in epidermis. Derm Sinica. 1989;7:143–153. [Google Scholar]
- 9.Saarni H, Hopsu-Havu VK. The decrease of hyaluronate synthesis by anti-inflammatory steroids in vitro. Br J Dermatol. 1978;98:445–449. doi: 10.1111/j.1365-2133.1978.tb06539.x. [DOI] [PubMed] [Google Scholar]
- 10.Cutroneo KR, Rokowski R, Counts DF. Glucocorticoids and collagen synthesis: comparison of in vivo and cell culture studies. Coll Relat Res. 1981;1:557–568. doi: 10.1016/s0174-173x(81)80037-4. [DOI] [PubMed] [Google Scholar]
- 11.Nuutinen P, Autio P, Hurskainen T, Oikarinen A. Glucocorticoid action on skin collagen: overview on clinical significance and consequences. J Eur Acad Dermatol Venereol. 2001;15:361–362. [PubMed] [Google Scholar]
- 12.Gebhardt C, Averbeck M, Diedenhofen N, Willenberg A, Anderegg U, Sleeman JP, et al. Dermal hyaluronan is rapidly reduced by topical treatment with glucocorticoids. J Invest Dermatol. 2010;130:141–149. doi: 10.1038/jid.2009.210. [DOI] [PubMed] [Google Scholar]
- 13.Kaya G, Tran C, Sorg O, Hotz R, Grand D, Carraux P, et al. Hyaluronate fragments reverse skin atrophy by a CD44-dependent mechanism. PLoS Med. 2006;3:493. doi: 10.1371/journal.pmed.0030493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Niedner R. Glukokortikosteroide in der Dermatologie: Kontrollierter Einsatz erforderlich. Dtsch Ärztebl. 1996;93:2868–2872. (Ger). [Google Scholar]
- 15.Luger T, Loske KD, Elsner P, Kapp A, Kerscher M, Korting HC, et al. Topische Dermatotherapie mit Glukokortikoiden Therapeutischer Index. JDDG. 2004;2:629–634. doi: 10.1046/j.1439-0353.2004.03626.x. (Ger). [DOI] [PubMed] [Google Scholar]
- 16.Korting HC, Unholzer A, Sch□fer-Korting M, Tausch I, Gassmueller J, Nietsch KH. Different skin thinning potential of equipotent medium-strength glucocorticoids. Skin Pharmacol Appl Skin Physiol. 2002;15:85–91. doi: 10.1159/000049394. [DOI] [PubMed] [Google Scholar]
- 17.Löwenberg M, Stahn C, Hommes DW, Buttgereit F. Novel insights into mechanisms of glucocorticoid action and the development of new glucocorticoid receptor ligands. Steroids. 2008;73:1025–1029. doi: 10.1016/j.steroids.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 18.Schäcke H, Zollner TM, Döcke WD, Rehwinkel H, Jaroch S, Skuballa W, et al. Characterization of ZK 245186, a novel, selective glucocorticoid receptor agonist for the topical treatment of inflammatory skin diseases. Br J Pharmacol. 2009;158:1088–1103. doi: 10.1111/j.1476-5381.2009.00238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schoepe S, Schäcke H, May E, Asadullah K. Glucocorticoid therapy-induced skin atrophy. Exp Dermatol. 2006;15:406–420. doi: 10.1111/j.0906-6705.2006.00435.x. [DOI] [PubMed] [Google Scholar]
- 20.Wach F, Bosserhoff A, Kurzidym U, Nowok K, Landthaler M, Hein R. Effects of mometasone furoate on human keratinocytes and fibroblasts in vitro. Skin Pharmacol Appl Skin Physiol. 1998;11:43–51. doi: 10.1159/000029807. [DOI] [PubMed] [Google Scholar]
- 21.Ponec M, De Haas C, Bachra BN, Polano MK. Effects of glucocorticosteroids on cultured human skin fibroblasts. III. Transient inhibition of cell proliferation in the early growth stages and reduced susceptibility in later growth stages. Arch Dermatol Res. 1979;265:219–227. doi: 10.1007/BF00407888. [DOI] [PubMed] [Google Scholar]
- 22.Schoepe S, Schäcke H, Bernd A, Zöller N, Asadullah K. Identification of novel in vitro test systems for the determination of glucocorticoid receptor ligand-induced skin atrophy. Skin Pharmacol Physiol. 2010;23:139–151. doi: 10.1159/000270386. [DOI] [PubMed] [Google Scholar]
- 23.Zöller NN, Kippenberger S, Thaci D, Mewes K, Spiegel M, Sattler A, et al. Evaluation of beneficial and adverse effects of glucocorticoids on a newly developed full-thickness skin model. Toxicol In Vitro. 2008;22:747–759. doi: 10.1016/j.tiv.2007.11.022. [DOI] [PubMed] [Google Scholar]
- 24.Mirshahpanah P, Döcke WD, Merbold U, Asadullah K, Röse L, Schäcke H, et al. Superior nuclear receptor selectivity and therapeutic index of methylprednisolone aceponate versus mometasone furoate. Exp Dermatol. 2007;16:753–761. doi: 10.1111/j.1600-0625.2007.00597.x. [DOI] [PubMed] [Google Scholar]
- 25.Durant S, Duval D, Homo-Delarche F. Factors involved in the control of fibroblast proliferation by glucocorticoids: a review. Endocr Rev. 1986;7:254–269. doi: 10.1210/edrv-7-3-254. [DOI] [PubMed] [Google Scholar]
- 26.Lange K, Kleuser B, Gysler A, Bader M, Maia C, Scheidereit C, et al. Cutaneous inflammation and proliferation in vitro: differential effects and mode of action of topical glucocorticoids. Skin Pharmacol Appl Skin Physiol. 2000;13:93–103. doi: 10.1159/000029913. [DOI] [PubMed] [Google Scholar]
- 27.Averbeck M, Gebhardt C, Anderegg U, Simon JC. Suppression of hyaluronan synthase 2 expression reflects the atrophogenic potential of glucocorticoids. Exp Dermatol. 2010;19:757–759. doi: 10.1111/j.1600-0625.2010.01099.x. [DOI] [PubMed] [Google Scholar]
- 28.Ackermann K, Borgia SL, Korting HC, Mewes KR, Schafer-Korting M. The Phenion full-thickness skin model for percutaneous absorption testing. Skin Pharmacol Physiol. 2010;23:105–112. doi: 10.1159/000265681. [DOI] [PubMed] [Google Scholar]
- 29.Kurzen H, Henrich C, Booken D, Poenitz N, Gratchev A, Klemke CD, et al. Functional characterization of the epidermal cholinergic system in vitro. J Invest Dermatol. 2006;126:2458–2472. doi: 10.1038/sj.jid.5700443. [DOI] [PubMed] [Google Scholar]
- 30.Mewes KR, Raus M, Bernd A, Zöller NN, Sattler A, Graf R. Elastin expression in a newly developed full-thickness skin equivalent. Skin Pharmacol Physiol. 2007;20:85–95. doi: 10.1159/000097655. [DOI] [PubMed] [Google Scholar]
- 31.Tierschutzbericht. Tierschutzbericht der Bundesregierung. Bundesministerium für Verbraucherschutz, Ernährung und Landwirtschaft; 2005. pp. 69–70. (Ger). [Google Scholar]
- 32.Kirby JD, Munro DD. Steroid-induced atrophy in an animal and human model. Br J Dermatol. 1976;94:111–119. doi: 10.1111/j.1365-2133.1976.tb02279.x. [DOI] [PubMed] [Google Scholar]
- 33.Sheu HM, Lee JY, Kuo KW, Tsai JC. Permeability barrier abnormality of hairless mouse epidermis after topical corticosteroid: characterization of stratum corneum lipids by ruthenium tetroxide staining and high-performance thin-layer chromatography. J Dermatol. 1998;25:281–289. doi: 10.1111/j.1346-8138.1998.tb02399.x. [DOI] [PubMed] [Google Scholar]
- 34.Schäcke H, Schottelius A, Döcke WD, Strehlke P, Jaroch S, Schmees N, et al. Dissociation of transactivation from transrepression by a selective glucocorticoid receptor agonist leads to separation of therapeutic effects from side effects. Proc Natl Acad Sci USA. 2004;101:227–232. doi: 10.1073/pnas.0300372101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smith JG, Jr, Wehr RF, Chalker DK. Corticosteroid-induced cutaneous atrophy and telangiectasia. Experimental production associated with weight loss in rats. Arch Dermatol. 1976;112:1115–1117. [PubMed] [Google Scholar]
- 36.Lavker RM, Dong G, Zheng PS, Murphy GF. Hairless micropig skin. A novel model for studies of cutaneous biology. Am J Pathol. 1991;138:687–697. [PMC free article] [PubMed] [Google Scholar]
- 37.Winter GD, Wilson L. The effect of clobetasone butyrate and other topical steroids on skin thickness of the domestic pig. Br J Dermatol. 1976;94:545–550. doi: 10.1111/j.1365-2133.1976.tb05144.x. [DOI] [PubMed] [Google Scholar]
- 38.Castor CW, Baker BL. The local action of adrenocortical steroids on epidermis and connective tissue of the skin. Endocrinology. 1950;47:234–241. doi: 10.1210/endo-47-4-234. [DOI] [PubMed] [Google Scholar]
- 39.Young JM, Yoxall BE, Wagner BM. Corticosteroidinduced dermal atrophy in the rat. J Invest Dermatol. 1977;69:458–462. doi: 10.1111/1523-1747.ep12511301. [DOI] [PubMed] [Google Scholar]
- 40.Marks R. Methods for the assessment of skin atrophogenicity of topical corticosteroids. Dermatologica. 1976;152:117–126. doi: 10.1159/000257872. [DOI] [PubMed] [Google Scholar]
- 41.Young JM, Yoxall BE, Wagner BM. Topical betamethasone 17-valerate is an anticorticosteroid in the rat. 1. Dermal atrophy. Br J Dermatol. 1978;99:655–663. doi: 10.1111/j.1365-2133.1978.tb07060.x. [DOI] [PubMed] [Google Scholar]
- 42.Woodbury R, Kligman AM. The hairless mouse model for assaying the atrophogenicity of topical corticosteroids. Acta Derm Venereol. 1992;72:403–406. [PubMed] [Google Scholar]
- 43.Schwartz E, Mezick JA, Gendimenico GJ, Kligman LH. In vivo prevention of corticosteroid-induced skin atrophy by tretinoin in the hairless mouse is accompanied by modulation of collagen, glycosaminoglycans and fibronectin. J Invest Dermatol. 1994;102:241–246. doi: 10.1111/1523-1747.ep12371770. [DOI] [PubMed] [Google Scholar]
- 44.Child KJ, English AF, Gilbert HG, Hewitt A, Woollett EA. Vasoconstrictor and systemic activities of topical steroids. Arch Dermatol. 1968;97:407–410. [PubMed] [Google Scholar]
- 45.Hepburn DJ, Aeling JL, Weston WL. A reappraisal of topical steroid potency. Pediatr Dermatol. 1996;13:239–245. doi: 10.1111/j.1525-1470.1996.tb01211.x. [DOI] [PubMed] [Google Scholar]
- 46.Vázquez-López F, Marghoob AA. Dermoscopic assessment of long-term topical therapies with potent steroids in chronic psoriasis. J Am Acad Dermatol. 2004;51:811–813. doi: 10.1016/j.jaad.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 47.Smith EW, Haigh JM, Surber C. Quantification of corticosteroid-induced skin vasoconstriction: visual ranking, chromameter measurement or digital imaging analysis. Dermatology. 2002;205:3–10. doi: 10.1159/000063144. [DOI] [PubMed] [Google Scholar]
- 48.Newton JA, Whitaker J, Sohail S, Young MM, Harding SM, Black MM. A comparison of pulsed ultrasound, radiography and micrometer screw gauge in the measurement of skin thickness. Curr Med Res Opin. 1984;9:113–118. doi: 10.1185/03007998409109568. [DOI] [PubMed] [Google Scholar]
- 49.Tan CY, Marks R, Payne P. Comparison of xeroradiographic and ultrasound detection of corticosteroid induced dermal thinning. J Invest Dermatol. 1981;76:126–128. doi: 10.1111/1523-1747.ep12525463. [DOI] [PubMed] [Google Scholar]
- 50.Lehmann P, Zheng P, Lavker RM, Kligman AM. Corticosteroid atrophy in human skin. A study by light, scanning and transmission electron microscopy. J Invest Dermatol. 1983;81:169–176. doi: 10.1111/1523-1747.ep12543603. [DOI] [PubMed] [Google Scholar]
- 51.Schoepe S. In vitro test systems for glucocorticoidinduced skin atrophy: Investigations of in vitro test systems for the detection of glucocorticoid-induced skin atrophy as a tool in drug discovery. Saarbrücken: Suedwestdeutscher Verlag fuer Hochschulschriften; 2009. [Google Scholar]