Abstract
The skin is a vital organ that plays a crucial role in defending us from pathogens. Multiple players from the innate and adaptive immune system are involved, such as neutrophils, dendritic cells, lymphocytes and antimicrobial peptides. Chronic inflammatory skin diseases can be mediated by inflammatory T cells and their interactions with other cells in the skin. Vitamin D is generated in the skin upon sun exposure and has a variety of effects. Vitamin D and its analogs have been used with success in treating mild to moderate T cell-mediated skin diseases, but how they mediate the beneficial effects is not well understood. In the recent years, emerging evidence is rising that vitamin D analogs and its modulation on the immune system plays a major role. It has been shown that vitamin D analogs can induce the generation of regulatory T cells, which are able to suppress proliferation and alter the function of inflammatory T cells. This may help explain the therapeutic effects that are observed and at the same time give hope that in combination with other therapy or used alone, vitamin D analogs may be helpful when treating more severe forms of the diseases.
Key words: vitamin D, skin diseases, treatment, T cells, dendritic cells, regulatory T cells
The skin is an important barrier between the external environment and our internal environment. It is exposed to chemical and physiological factors, changes in temperature, UV-irradiation from the sun and it is under constant attack from microbes and small agents. The skin is not only involved in heat regulation and one of its major roles is inhibiting pathogens from entering our tissues. The immune system plays a crucial role in the defenses, where both the innate and adaptive immune system are involved. When a pathogen infects the body, members in the first line of defense, which include neutrophils and antimicrobial peptides try to eliminate the pathogen. If this is not enough to eradicate the infection, other cells are called to the scene, the lymphocytes. Dendritic cells (DCs) in the tissues take up the pathogen and migrate to the lymph nodes draining the site of invasion. The DCs cut the pathogen into small pieces (antigens) and show antigens from the pathogen to T cells in the lymph nodes. If the T cells are activated, they can migrate to the site of infection.
Recirculation and Trafficking of Lymphocytes to Tissues
Lymphocytes circulate between blood, lymph nodes and tissues and back to the blood, and this recirculation is important both for immune surveillance as well as for cells responding to infections. The idea that cells recirculate was put forward when it was observed that there was a rapid turnover of T cells in the blood. It was suggested that lymphocytes migrate from the blood vessels into tissues and return to lymphoid tissues through the afferent lymph. By labeling lymphocytes from the thoracic duct of rats with 32P or 51Cr and injecting them back into the blood of the animal, it was demonstrated that these lymphocytes could be recovered in large amounts in the thoracic duct or intestinal lymph.1,2 Further experiments showed that this mechanism is quite selective and T cells migrated preferentially to the tissue of their origin.3 The observation that T cells preferentially migrate back to the tissue of origin or where the antigen came from was an important step towards understanding how recirculation occurs. This is an organized and efficient system that guides the T cells where they are needed the most at a particular time-point.
The interactions between T cells and the endothelium is a regulated process involving three or more sequential steps, starting with initial contact between lymphocytes and the endothelium to stable attachment and resulting in T cells infiltrating the tissue, where multiple receptors and ligands are involved. The initial tethering to the vascular endothelium is mediated by selectins and integrins, which enables the T cells to slow down, roll along the endothelium and sample the chemokines that are presented by the endothelial cells. Chemokines are small proteins that are presented by endothelial cells on the luminal surface and upon binding to T cells they trigger activation of integrins and increase affinity, which results in T cell arrest on the endothelial wall. If appropriate chemokines are present, the T cells attach more firmly to the endothelium. This leads eventually to transendothelial migration of the T cells into the underlying tissue where the lymphocytes are guided further by chemokines and other chemoattractants to find their specific location.4 Different selectins, chemokines and integrins are important for the infiltration into different tissues thus, lymphocytes have diverse combinations of these receptors. This generates specificity where the specific combination of receptors that is needed for T cell migration into lymph nodes is different from those needed to access tissues and the tissues themselves have their own specific combination.4
Naïve lymphocytes are not very efficient in mediating immune responses and are activated by DCs within secondary lymphoid tissues, such as lymph nodes and spleen. In lymph nodes draining the skin, T cells are activated by DCs that have migrated from the skin and upon activation, these T cells start to express specific homing molecules, which enable them to leave the blood circulation and infiltrate the skin. The molecules that are known to be important for the migration into the skin include the adhesion molecule cutaneous lymphocyte-associated antigen (CLA), which binds to E-selectin on the vascular endothelial cells, and the chemokine receptor, CCR4, which binds to “chemokine thymus and activation-regulated chemokine” (TARC/CCL17).5–8 Within the skin the T cells can respond to chemokines such as macrophage-derived chemokine (MDC/CCL22),9 which can be secreted by various cell types and the “epithelial cutaneous T cell-attracting chemokine” (CTACK/CCL27), which is primarily expressed by keratinocytes in the skin and attracts T cells expressing CCR10.10–12 Increased expression of CCL17 and CCL27 is observed in inflamed skin12,13 and blocking CCL17 and CCL22 (the known ligands for CCR4), significantly reduced the numbers of T cells in inflamed ears in a mouse model of contact hypersensitivity.14 In contrast, blocking CCL27 alone did not appear to reduce the recruitment of T cells into the skin.15 CCL28 (“mucosa-associated epithelial chemokine”/MEC) is also a ligand for CCR10 and increased levels of CCL28 are found in the mucosa.16 However, elevated levels of CCL28 have also been found in serum of patients with skin diseases where T cells play an important role such as psoriasis and atopic dermatitis.17
Antimicrobial peptides also play an important role in defending the skin. Initial it was believed that these peptides were the skin's antibiotics whose only function was to kill microbes.18 It is now clear that their function goes beyond that. In skin, many cell types can produce these peptides, including keratinocytes and mast cells. The two main types of antimicrobial peptides are cathelicidins and beta-defensins. The only known cathelicidin in humans is LL-37 (reviewed in ref. 18).
Immunological Effects of Vitamin D
Vitamin D3 is generated in the skin after exposure to UVB-rays from the sun where it is converted to its active form, 1,25(OH)2D3, through an enzymatic pathway.19 When UVB-rays from the sun hit the skin, 7-dehydrocholesterol is converted to pre-vitamin D3, which isomerizes quickly to a more stable vitamin D3. Vitamin D3 is converted to 25-hydroxyvitamin D3 (25(OH)D) by 25-hydroxylases, which are enzymes that are a part of the cytochrome P450 family. 25(OH)D3 is a circulating metabolite that is converted to the active form, 1,25-dihydroxyvitamin D3 (1,25(OH)2D3), by 1alpha-hydroxylase enzymes (Fig. 1).20 The conversion of vitamin D3 into its active form occurs mainly in the liver and kidneys, however, it has been shown that several other cell types are also capable of this conversion, including keratinocytes,21 macrophages22 and DCs,20 which can all be present in the skin.
Figure 1.
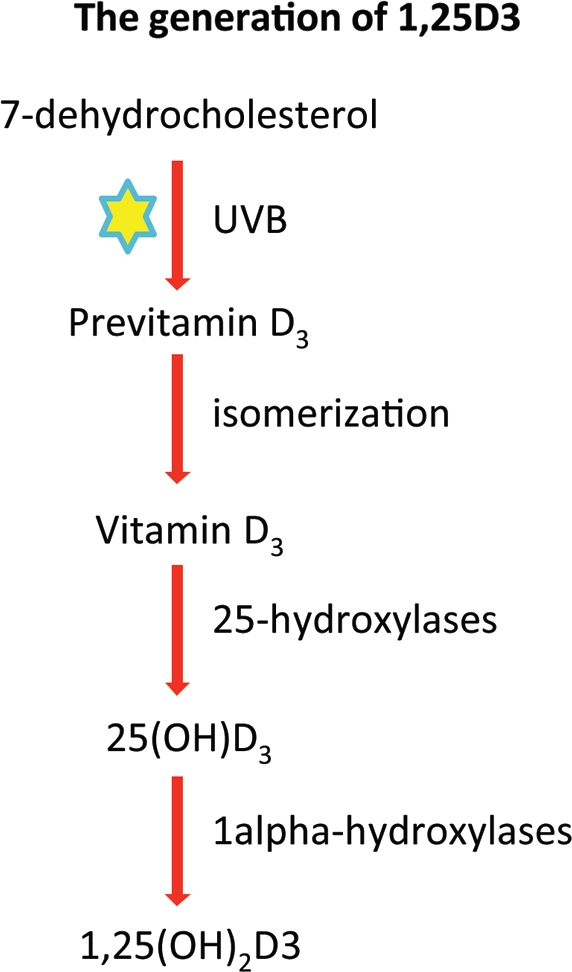
The generation of 1,25(OH)2D3. When UVB-rays from the sun hit the skin, 7-dehydrocholesterol is converted to pre-vitamin D3, which isomerizes quickly to a more stable vitamin D3. Vitamin D3 is rapidly converted to 25-hydroxyvitamin D (25(OH)D) by 25-hydroxylases, which are enzymes from the cytochrome P450 family. 25(OH)D is a circulating metabolite that is converted to the active form, 1,25(OH)2D3, by 1 alpha-hydroxylase enzymes.
The biological effects of 1,25(OH)2D3 are mediated through the vitamin D receptor (VDR), which is a member of the super-family of nuclear hormone receptors. The binding of 1,25(OH)2D3 to VDR leads to a conformational change in the VDR, and it binds to the retinoic X receptor (RXR), forming a heterodimer. This heterodimer translocates to the nucleus, where it can bind to a vitamin D response element and can either promote or prevent transcription of vitamin D-responsive genes in target cells.20 VDR is expressed in a variety of cells, such as bone, skin, intestines, kidneys and cells of the immune system. In skin, vitamin D3 is produced by keratinocytes and is known to regulate keratinocyte differentiation, inhibit keratinocyte proliferation and induce terminal differentiation of the keratinocytes.23 1,25(OH)2D3 has also been shown to have a variety of immunomodulatory effects, but VDR is expressed by activated B and T cells (both CD4+ and CD8+ T cells), monocytes, DCs and macrophages (reviewed in ref. 24). VDR expression is increased in activated T cells and binding of 1,25(OH)2D3 decreases the production of IFNgamma, IL-2, IL-17 and IL-22 from the T cells, while increasing the production of IL-4.24 This prevents or diminishes the expansion of proinflammatory Th1 and Th17 cells, which are major groups of inflammatory T cells. Thus, human T cells are affected directly by 1,25(OH)2D3.24,25
Dendritic Cells are important Regulators of T Cell Trafficking
One of the most important roles of DCs is the uptake of antigens and foreign substances and presenting them to T cells. The tissues where the DCs are derived from are important. Intestinal DCs are derived from lymph nodes that drain the intestines, and when DCs from these lymph nodes were used to activate T cells, the expression of gut-homing receptors was induced on the responding T cells.26,27 It was proposed that intestinal DCs are specialized in the induction of T cells that preferentially migrate to the gut. Specificity of DCs from these lymph nodes can, however, be altered and under different environmental conditions these DCs can induce the expression of skin-homing receptors on T cells.28,29 It is, therefore, not only the DCs but the local microenvironment that is crucial for the expression of homing receptors.
External environmental factors, including active metabolites of vitamins, have been shown to have significant effects on the homing of T cells. Vitamin A is mainly obtained through the diet, and it can be taken up, transported to the draining lymph nodes and processed by the local DCs and stromal cells.30–32 Vitamin A is metabolized in two steps to retinoic acid (RA), which is the most active from of the vitamin.20 Both DCs and stromal cells from the intestines had the enzymes needed to convert vitamin A to RA and were more effective than DCs from the peripheral lymph nodes (such as skin draining lymph nodes) at inducing the expression of the gut-homing receptors alpha4beta7 integrin and CCR9 on T cells.30–32 In contrast, the stromal cells in lymph nodes draining the skin do not appear to have these enzymes.
Vitamin D has been associated with localization of T cells within the skin. DCs express the enzymes and are able to convert vitamin D3 to 1,25(OH)2D3.33 When 1,25(OH)2D3 was present during activation, the expression of CCR10 was induced on the responding T cells,33 which enables the T cells to migrate to the keratinocytes in the epidermal layers of the skin. The T cells themselves had one the of enzymes needed and were able to convert 25(OH)D3 to 1,25(OH)2D3 and their expression of CCR10 was increased. These results strengthen the role of micro-environmental factors in regulating T cell responses and thereby affecting immune responses.
T Cell Mediated Skin Diseases
Psoriasis and atopic dermatitis (AD) are chronic inflammatory skin diseases, where an important factor in the pathogenesis of the disease is increased infiltration of T cells into the skin. Psoriasis is characterized by raised, well-demarcated, erythematous plaques with silvery scales. In psoriatic lesions, keratinocytes hyperproliferate resulting in thickening of the epidermis with elongated rete ridges.34 There is increased infiltration of cells of the immune system into the dermal and epidermal layers of the skin. The inflammatory infiltrate consists mainly of DCs, macrophages and CD4+ T cells in the dermis and neutrophils and CD8+ T cells are dominating in the epidermis.34 In psoriasis, the CD4+ T cells in lesional skin are mostly of the Th1 and Th17 type, which produce IFNγ and IL-17,35–37 and recently a new T cell subpopulation has been described to be overexpressed in psoriasis, the Th22 cells.37,38 Plasmacytoid DCs (pDCs) are present in early psoriatic lesions, where they can become activated and secrete IFNα.39 In a xenograft model of human psoriasis, blocking IFNα signaling or inhibiting the ability of pDCs to produce IFNα prevented the development of psoriasis.39 pDCs can be activated through complexes of the antimicrobial peptide LL-37 and DNA binding to the Toll-like receptor 9 (TLR 9). pDCs do not normally respond to self-DNA but LL-37 was found to convert self-nucleic acid into a potent trigger of pDC activation by forming a complex with self-RNA and self-DNA. This triggers the activation of the pDCs through TLR9. The LL-37-self-DNA complexes signaled through the TLR9 and induced the release of IFNalpha from pDCs, which subsequently generated a T-cell response.40 LL-37 is normally not expressed in large amounts in healthy skin but it is overexpressed by keratinocytes in psoriatic skin. This could help explain why pDCs and subsequently T cells in psoriatic skin, can be chronically activated.
AD is a chronic inflammatory skin disease, where genetic, environmental and immunologic factors play a role in the pathogenesis of the disease. DCs also play a crucial role in the initiation and amplification of the immune response. The skin barrier in AD is impaired and this enables allergens and microbial antigens to come into close contact with DCs in the skin. Cross-linking of allergen-specific IgE molecules on skin DCs or binding of bacterial products induces a cascade of events resulting in increased numbers of activated circulating CD4+ and CD8+ T cells. Increased numbers of CD4+ T cells are also found in the dermis of patients with AD. In contrast to psoriasis, the T cells that are believed to initiate the acute phase of AD produce mainly IL-4, IL-5, IL-13 and IL-31.41,42 CCR8 (the receptor for CCL1) is implicated in the migration of T cells into the skin of patients with AD,43 and increased CCL1, CCL13, CCL17 and CCL18 expression was observed in patients with chronic AD compared with those with psoriasis. Furthermore, stronger expression of CCL1, CCL3, CCL4 and CCL11 mRNA was detected in patients with acute AD compared with chronic AD.13
It has been demonstrated that both CCL27 is strongly expressed in lesional skin of patients with AD and psoriasis.10 Furthermore, increased levels of CCL28 is found in serum of patients with AD and psoriasis.17 CCL27 and CCL28 are both ligands for CCR10+ T cells, which enables the T cells to migrate from the dermal to the keratinocytes in the epidermal layers of the skin.
Vitamin D as Treatment for Skin Diseases
Vitamin D3 derivatives and analogs are commonly used to treat mild to moderatecases of skin diseases such as psoriasis and atopic dermatitis.44 How they mediate their beneficial effects is not fully understood. Vitamin D analogs can be beneficial by balancing the hyperproliferation of the keratinocytes, and in recent years the role of vitamin D analogs in regulating the immune system is becoming more evident. Incorporating vitamin D with other treatments may also prove to be a candidate for a more effective targeted combination therapy in skin diseases although that needs further investigations.45
Vitamin D creams and oral supplements.
Vitamin D3 ointments and creams have been used for a long time as a treatment for psoriasis and shown beneficial effects in mild to moderate cases of the disease.45,46 Vitamin D can be obtained from the diet and by UV-induced synthesis in the skin as previously described. Food, however, is not a rich source of vitamin D but vitamin D can also be taken as a supplement.
The active metabolite, 1,25(OH)2D3 is known to induce keratinocyte to produce more of the antimicrobial peptide LL-37 and its antimicrobial activity is increased in vitro.47 Furthermore, psoriatic patients treated topically with 1,25(OH)2D3 (calcipotriol) had increased levels of LL-37 levels in lesional and non-lesional skin.48 Other studies have also shown that topical treatment with vitamin D analogues enhanced the upregulation of the antimicrobial protein hCAP18/LL-37,49 which was also rapidly upregulated upon skin injury,49 further demonstrating 1,25(OH)2D3 as a regulator of LL-37 production. Increased levels of the LL-37 protein have been detected in skin biopsies of patients with AD after supplementation with oral vitamin D.50 Patients with AD have lower levels of LL-37 compared to patients with psoriasis51 and this has raised the hope that by increasing the vitamin D3 metabolism or elevating vitamin D3 serum levels, it may be possible to restore an effective barrier in the skin of patients with AD. Not much is known about the effects of vitamin D on the production and secretion of either CCL27 or CCL28.
Ultraviolet B (UVB) irradiation and vitamin D production.
Treatment with UVB irradiation is a common treatment and is known to have therapeutic effects when treating psoriasis and to some extent AD. UVB affects the skin directly by inducing the differentiation of keratinocytes.52 It has also been postulated that the beneficial effects of UVB are in part due to their effects on T cells, such as decreased expression of skin homing molecules,53 decreased production of pro-inflammatory cytokines,54,55 and/or apoptosis of T cells.56 The beneficial effects of UVB irradiation may also be in part due to increased vitamin D production. Several studies have demonstrated that keratinocytes are fully functional at generating vitamin D during UVB irradiation. Cultured keratinocytes were shown to generate both vitamin D3 and 1,25(OH)2D3 (calcitriol) from 7-dehydrocholesterol after UVB-irradiation and LL-37 production was also increased.57 Using a different model (a skin-equivalent model), it was also demonstrated that during irradiation with UVB, previtamin D3 is generated from 7-dehydrocholesterol, which is converted to vitamin D3, followed by the formation of 25(OH)D3 (calcidiol) and finally the generation 1,25(OH)2D3 (calcitriol), In contrast, non-irradiated cultures and irradiated cultures without keratinocytes did not produce calcitriol. The amount of calcitriol that was generated was not only dependent on the concentration of 7-dehydrocholesterol but also on the UVB doses used. The results indicate that in the presence of physiologic concentrations of 7-dehydrocholesterol, keratinocytes may be a source of biologically active 1,25(OH)2D3 when irradiated with therapeutic doses of ultraviolet B.21
Another study showed that at onset of disease, patients with psoriasis or AD had vitamin D levels that could indicate insufficiency of vitamin D, but treatment with narrowband UVB irradiation significantly increased the levels in the serum of these individuals. The disease severity score (Psoriasis Area and Severity Index) improved significantly but no correlation was found to the increase of serum calcidiol. After narrowband UVB treatment, the production of the antimicrobial peptide LL-37 was increased, while human beta-defensin 2 expression was decreased in both psoriatic and AD lesions.58
It may be contradictory that topical 1,25(OH)2D3 applications are beneficial in psoriasis as such treatment has been reported to irritate the skin.59 One could expect that it would by difficult to use vitamin D analogs when treating skin diseases, yet, vitamin D3 analogs have been used to treat psoriasis for a long time successfully. Also, both vitamin D3 analogs and UVB-treatment, which are common effective treatments in psoriasis, increase the production of LL-37 from keratinocytes,48 and this should make psoriasis worse as it activates the pDCs,39,40 followed by increased production of IFNalpha. However, vitamin D3 analogs have been found to decrease inflammation in skin48 and in part reverse the changes observed within lesional skin. The reason for this is not clear, but it suggests that the proposed pathogenic role of LL-37 in skin diseases such as psoriasis, needs further investigation.
Vitamin D induces the generation of T regulatory cells in the skin.
The important role of DCs in T cell activation is highly relevant for T cell mediated skin diseases. Targeted immunotherapy and other treatments have been shown to reduce the numbers of DCs in patients with psoriasis with beneficial effects indicating an important role of these cells in the pathogenesis of psoriasis.
1,25(OH)2D3 is known to have immunosuppressive effects as previously described and one of their effects is to inhibit maturation of DCs.60–62 In the presence of 1,25(OH)2D3, the expression of co-stimulatory molecules and maturation markers is decreased on DCs (reviewed in ref. 24) making them less effective as stimulators of T cells. Furthermore, 1,25(OH)2D3 has also been shown to induce the generation of regulatory T cells.63–65 Several different types of regulatory T cells have been described, which have the capacity to inhibit a diversity of immune responses and maintain immunologic tolerance in the periphery.66 Many cell types are known to be able to inhibit immune responses by production of anti-inflammatory cytokines, but a lot of attention has been given to T cells called regulatory T cells. These regulatory T cells are of specific interest in the context of T cell mediated skin diseases because of their ability to suppress proliferation and expansion of antigen-specific T cells. Several regulatory T cells exist, but one the most studied cells today are CD4+ T cells that are CD25+CD127low expressing cells. These cells are thought to play an important role in maintaining peripheral tolerance and inhibiting immune responses to self antigens.
It was shown that 1,25(OH)2D3 selectively induced myeloid DCs to become tolerogenic DCs, which were able to suppress T cell activity and decrease their production of IFNγ.64 In contrast, these effects were not seen for the pDCs. But, as 1,25(OH)2D3 is known to prevent maturation of DCs, treatment with 1,25(OH)2D3 could prevent the maturation of the pDCs, making them very weak activators of T cells. Most in vivo data on the effects of 1,25(OH)2D3 on T regulatory cells comes from animal studies. In mice, it was demonstrated that topically applied 1,25(OH)2D3 enhanced the suppressive capacity of CD4+CD25+ cells from the draining lymph nodes.67,68 The vitamin D analog, TX527 induced the generation of CD4+ CD25highCD127low regulatory T cells with functional capacity to suppress activation and proliferation of effector T cells. Furthermore, these regulatory T cells had increased expression of homing molecules making them capable of entering inflamed sites, where they can suppress the inflammatory T cells.69 It has also been shown that immunization through 1,25(OH)2D3 treated skin induced the generation of CD4+CD25+ regulatory T cells, which were able to prevent the proliferation of antigen-specific CD8+ T cells and their production of IFNγ.67 When the effects of topical application of 1,25(OH)2D3 were compared with those of UVB irradiation, CD4+ cells from skin-draining lymph nodes of either 1,25(OH)2D3-treated or UVB-irradiated mice could suppress antigen-specific immune responses when transferred adoptively into naive mice. Also, these cells had increased capacity to suppress immune responses in both in vitro and in vivo assay systems.68
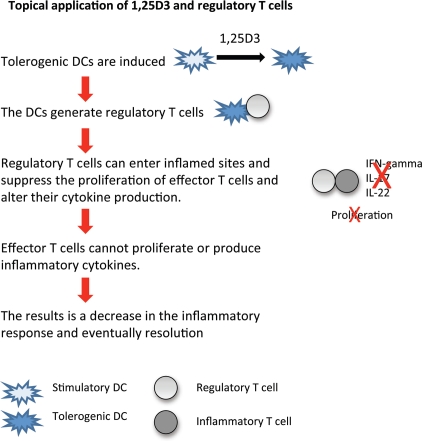
Thus, vitamin D analogs may help the immune system in targeting and inhibiting specifically the function and proliferation of inflammatory T cells at inflamed sites, such as the skin (Fig. 2).
Figure 2.
Hypothesis: Benefits of topical application of 1,25D3. When DCs are maturing in the presence of 1,25(OH)2D3 (1,25D3), they capability to fully mature is decreased and they become less efficient at taking up antigen and activating T cells. Instead they become tolerogenic and induce the generation of regulatory T cells, which suppress the proliferation of effector T cells and alter their cytokine production. These regulatory T cells have increased expression of homing receptors enabling them to enter sites of inflammation. The results are that the effector T cells (inflammatory cells) at inflamed sites are not effective at proliferating or producing inflammatory cytokines (decreased production of IL-17, IL-22 and IFNgamma). There is a decrease in the inflammatory response and the disease resolves slowly.
Conclusion
T cell mediated skin diseases affect millions of people around the globe. They can be very uncomfortable to those affected and have been reported to reduce the quality of life of those affected.70 Treatments are generally rather unspecific and do not always target the skin specifically. More and more is being done in developing more specific treatments that target the skin as an organ. Combination therapy also gives hope that less concentrations can be used of each of the drugs making them safer to use. Vitamin D and its analogs may prove to be ideal candidates for combination treatments and as we learn more about them and how they work, we can design the ideal combination to use that is effective but safe.
Acknowledgments
The author is supported with grants from Nordforsk, the Nordic Research Board (Nordic Stem Cell Mobility Program), the University of Iceland Research Fund and the Landspitali University Hospital Research Fund.
References
- 1.Gowans JL. The recirculation of lymphocytes from blood to lymph in the rat. J Physiol. 1959;146:54–69. doi: 10.1113/jphysiol.1959.sp006177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shorter RG, Bollman JL. Experimental transfusion of lymphocytes. Am J Physiol. 1960;198:1014–1018. doi: 10.1152/ajplegacy.1960.198.5.1014. [DOI] [PubMed] [Google Scholar]
- 3.Cahill RN, Poskitt DC, Frost DC, Trnka Z. Two distinct pools of recirculating T lymphocytes: migratory characteristics of nodal and intestinal T lymphocytes. J Exp Med. 1977;145:420–428. doi: 10.1084/jem.145.2.420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Butcher EC. Leukocyte-endothelial cell recognition: three (or more) steps to specificity and diversity. Cell. 1991;67:1033–1036. doi: 10.1016/0092-8674(91)90279-8. [DOI] [PubMed] [Google Scholar]
- 5.Berg EL, Yoshino T, Rott LS, Robinson MK, Warnock RA, Kishimoto TK, et al. The cutaneous lymphocyte antigen is a skin lymphocyte homing receptor for the vascular lectin endothelial cell-leukocyte adhesion molecule 1. J Exp Med. 1991;174:1461–1466. doi: 10.1084/jem.174.6.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Picker LJ, Michie SA, Rott LS, Butcher EC. A unique phenotype of skin-associated lymphocytes in humans. Preferential expression of the HECA-452 epitope by benign and malignant T cells at cutaneous sites. Am J Pathol. 1990;136:1053–1068. [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature. 1999;400:776–780. doi: 10.1038/23495. [DOI] [PubMed] [Google Scholar]
- 8.Campbell JJ, O'Connell DJ, Wurbel MA. Cutting edge: Chemokine receptor CCR4 is necessary for antigen-driven cutaneous accumulation of CD4 T cells under physiological conditions. J Immunol. 2007;178:3358–3362. doi: 10.4049/jimmunol.178.6.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pivarcsi A, Homey B. Chemokine networks in atopic dermatitis: traffic signals of disease. Curr Allergy Asthma Rep. 2005;5:284–290. doi: 10.1007/s11882-005-0068-y. [DOI] [PubMed] [Google Scholar]
- 10.Homey B, Alenius H, Müller A, Soto H, Bowman EP, Yuan W, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. 2002;8:157–165. doi: 10.1038/nm0202-157. [DOI] [PubMed] [Google Scholar]
- 11.Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC Chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. 2001;194:1541–1547. doi: 10.1084/jem.194.10.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang V, Lonsdorf AS, Fang L, Kakinuma T, Lee VC, Cha E, et al. Cutting edge: Rapid accumulation of epidermal CCL27 in skin-draining lymph nodes following topical application of a contact sensitizer recruits CCR10-expressing T cells. J Immunol. 2008;180:6462–6466. doi: 10.4049/jimmunol.180.10.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gros E, Bussmann C, Bieber T, Forster I, Novak N. Expression of chemokines and chemokine receptors in lesional and nonlesional upper skin of patients with atopic dermatitis. J Allergy Clin Immunol. 2009;124:753–760. doi: 10.1016/j.jaci.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Nakagami Y, Kawashima K, Yonekubo K, Etori M, Jojima T, Miyazaki S, et al. Novel CC chemokine receptor 4 antagonist RS-1154 inhibits ovalbumin-induced ear swelling in mice. Eur J Pharmacol. 2009;624:38–44. doi: 10.1016/j.ejphar.2009.09.058. [DOI] [PubMed] [Google Scholar]
- 15.Parham M, Yi-Yang Yvonne L, Nicole B, Khusru A, Thomas MZ. CCR4 and CCR10 ligands play additive roles in mouse contact hypersensitivity. Exper Dermatol. 2008;17:30–34. doi: 10.1111/j.1600-0625.2007.00630.x. [DOI] [PubMed] [Google Scholar]
- 16.Pan J, Kunkel EJ, Gosslar U, Lazarus N, Langdon P, Broadwell K, et al. Cutting edge: A novel chemokine ligand for CCR10 and CCR3 expressed by epithelial cells in mucosal tissues. J Immunol. 2000;165:2943–2949. doi: 10.4049/jimmunol.165.6.2943. [DOI] [PubMed] [Google Scholar]
- 17.Kagami S, Kakinuma T, Saeki H, Tsunemi Y, Fujita H, Sasaki K, et al. Increased serum CCL28 levels in patients with atopic dermatitis, psoriasis sulgaris and bullous pemphigoid. J Investig Dermatol. 2005;124:1088–1090. doi: 10.1111/j.0022-202X.2005.23700.x. [DOI] [PubMed] [Google Scholar]
- 18.Schauber J, Gallo RL. The vitamin D pathway: a new target for control of the skin's immune response? Exper Dermatol. 2008;17:633–639. doi: 10.1111/j.1600-0625.2008.00768.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prosser DE, Jones G. Enzymes involved in the activation and inactivation of vitamin D. Trends Biochem Sci. 2004;29:664–673. doi: 10.1016/j.tibs.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 20.Mora JR, Iwata M, von Andrian UH. Vitamin effects on the immune system: vitamins A and D take centre stage. Nat Rev Immunol. 2008;8:685–698. doi: 10.1038/nri2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehmann B, Rudolph T, Pietzsch J, Meurer M. Conversion of vitamin D3 to 1,25-dihydroxyvitamin D3 in human skin equivalents. Exper Dermatol. 2000;9:97–103. doi: 10.1034/j.1600-0625.2000.009002097.x. [DOI] [PubMed] [Google Scholar]
- 22.Liu PT, Stenger S, Li H, Wenzel L, Tan BH, Krutzik S, et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- 23.Kragballe K, Wildfang IL. Calcipotriol (MC 903), a novel vitamin D3 analogue stimulates terminal differentiation and inhibits proliferation of cultured human keratinocytes. Arch Dermatol Res. 1990;282:164–167. doi: 10.1007/BF00372616. [DOI] [PubMed] [Google Scholar]
- 24.Baeke F, Korf H, Overbergh L, van Etten E, Verstuyf A, Gysemans C, et al. Human T lymphocytes are direct targets of 1,25-dihydroxyvitamin D3 in the immune system. J Steroid Biochem Mol Biol. 2010;121:221–227. doi: 10.1016/j.jsbmb.2010.03.037. [DOI] [PubMed] [Google Scholar]
- 25.Ikeda U, Wakita D, Ohkuri T, Chamoto K, Kitamura H, Iwakura Y, et al. 1[alpha],25-Dihydroxyvitamin D3 and all-trans retinoic acid synergistically inhibit the differentiation and expansion of Th17 cells. Immunol Lett. 2010;134:7–16. doi: 10.1016/j.imlet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 26.Stagg AJ, Kamm MA, Knight SC. Intestinal dendritic cells increase T cell expression of alpha4beta7 integrin. Eur J Immunol. 2002;32:1445–1454. doi: 10.1002/1521-4141(200205)32:5<1445::AID-IMMU1445>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 27.Mora JR, Bono MR, Manjunath N, Weninger W, Cavanagh LL, Rosemblatt M, et al. Selective imprinting of gut-homing T cells by Peyer's patch dendritic cells. Nature. 2003;424:88–93. doi: 10.1038/nature01726. [DOI] [PubMed] [Google Scholar]
- 28.Dudda JC, Lembo A, Bachtanian E, Huehn J, Siewert C, Hamann A, et al. Dendritic cells govern induction and reprogramming of polarized tissue-selective homing receptor patterns of T cells: important roles for soluble factors and tissue microenvironments. Eur J Immunol. 2005;35:1056–1065. doi: 10.1002/eji.200425817. [DOI] [PubMed] [Google Scholar]
- 29.Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, von Andrian UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. 2005;201:303–316. doi: 10.1084/jem.20041645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwata M, Hirakiyama A, Eshima Y, Kagechika H, Kato C, Song SY. Retinoic acid imprints gut-homing specificity on T cells. Immunity. 2004;21:527–538. doi: 10.1016/j.immuni.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Molenaar R, Greuter M, van der Marel AP, Roozendaal R, Martin SF, Edele F, et al. Lymph node stromal cells support dendritic cell-induced gut-homing of T cells. J Immunol. 2009;183:6395–6402. doi: 10.4049/jimmunol.0900311. [DOI] [PubMed] [Google Scholar]
- 32.Hammerschmidt SI, Ahrendt M, Bode U, et al. Stromal mesenteric lymph node cells are essential for the generation of gut-homing T cells in vivo. J Exp Med. 2008;205:2483–2490. doi: 10.1084/jem.20080039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, et al. DCs metabolize sunlight-induced vitamin D3 to ‘program’ T cell attraction to the epidermal chemokine CCL27. Nat Immunol. 2007;8:285–293. doi: 10.1038/ni1433. [DOI] [PubMed] [Google Scholar]
- 34.Valdimarsson H, Bake BS, Jonsdotdr I, Fry L. Psoriasis: a disease of abnormal Keratinocyte proliferation induced by T lymphocytes. Immunology Today. 1986;7:256–259. doi: 10.1016/0167-5699(86)90005-8. [DOI] [PubMed] [Google Scholar]
- 35.Teunissen MB, Koomen CW, de Waal Malefyt R, Wierenga EA, Bos JD. Interleukin-17 and interferon-gamma synergize in the enhancement of proinflammatory cytokine production by human keratinocytes. J Invest Dermatol. 1998;111:645–649. doi: 10.1046/j.1523-1747.1998.00347.x. [DOI] [PubMed] [Google Scholar]
- 36.Kryczek I, Bruce AT, Gudjonsson JE, Johnston A, Aphale A, Vatan L, et al. Induction of IL-17+ T cell trafficking and development by IFN-gamma: mechanism and pathological relevance in psoriasis. J Immunol. 2008;181:4733–4741. doi: 10.4049/jimmunol.181.7.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kagami S, Rizzo HL, Lee JJ, Koguchi Y, Blauvelt A. Circulating Th17, Th22, and Th1 cells are increased in psoriasis. J Invest Dermatol. 2010;130:1373–1383. doi: 10.1038/jid.2009.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Res PC, Piskin G, de Boer OJ, van der Loos CM, Teeling P, Bos JD, et al. Overrepresentation of IL-17A and IL-22 producing CD8 T cells in lesional skin suggests their involvement in the pathogenesis of psoriasis. PLoS ONE. 2010;5:14108. doi: 10.1371/journal.pone.0014108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nestle FO, Conrad C, Tun-Kyi A, Homey B, Gombert M, Boyman O, et al. Plasmacytoid predendritic cells initiate psoriasis through interferon-{alpha} production. J Exp Med. 2005;202:135–143. doi: 10.1084/jem.20050500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lande R, Gregorio J, Facchinetti V, Chatterjee B, Wang YH, Homey B, et al. Plasmacytoid dendritic cells sense self-DNA coupled with antimicrobial peptide. Nature. 2007;449:564–569. doi: 10.1038/nature06116. [DOI] [PubMed] [Google Scholar]
- 41.Neis MM, Peters B, Dreuw A, Wenzel J, Bieber T, Mauch C, et al. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol. 2006;118:930–937. doi: 10.1016/j.jaci.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 42.Bilsborough J, Leung DY, Maurer M, Howell M, Boguniewicz M, Yao L, et al. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. 2006;117:418–425. doi: 10.1016/j.jaci.2005.10.046. [DOI] [PubMed] [Google Scholar]
- 43.Gombert M, Dieu-Nosjean MC, Winterberg F, Bünemann E, Kubitza RC, Da Cunha L, et al. CCL1-CCR8 interactions: an axis mediating the recruitment of T cells and Langerhans-type dendritic cells to sites of atopic skin inflammation. J Immunol. 2005;174:5082–5091. doi: 10.4049/jimmunol.174.8.5082. [DOI] [PubMed] [Google Scholar]
- 44.Norris D. Mechanisms of action of topical therapies and the rationale for combination therapy. J Am Acad Dermatol. 2005;53:17–25. doi: 10.1016/j.jaad.2005.04.027. [DOI] [PubMed] [Google Scholar]
- 45.Kircik L. Efficacy and safety of topical calcitriol 3 microg/g ointment, a new topical therapy for chronic plaque psoriasis. J Drugs Dermatol. 2009;8:9–16. [PubMed] [Google Scholar]
- 46.Abramovits W. Calcitriol 3 microg/g ointment: an effective and safe addition to the armamentarium in topical psoriasis therapy. J Drugs Dermatol. 2009;8:17–22. [PubMed] [Google Scholar]
- 47.White JH. Vitamin D as an inducer of cathelicidin antimicrobial peptide expression: Past, present and future. J Steroid Biochem Mol Biol. 2010;121:234–238. doi: 10.1016/j.jsbmb.2010.03.034. [DOI] [PubMed] [Google Scholar]
- 48.Peric M, Koglin S, Dombrowski Y, Gross K, Bradac E, Büchau A, et al. Vitamin D analogs differentially control antimicrobial peptide/“alarmin” expression in psoriasis. PLoS ONE. 2009;4:6340. doi: 10.1371/journal.pone.0006340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Heilborn JD, Weber G, Grönberg A, Dieterich C, Ståhle M. Topical treatment with the vitamin D analogue calcipotriol enhances the upregulation of the antimicrobial protein hCAP18/LL-37 during wounding in human skin in vivo. Exper Dermatol. 2010;19:332–338. doi: 10.1111/j.1600-0625.2009.00997.x. [DOI] [PubMed] [Google Scholar]
- 50.Hata TR, Kotol P, Jackson M, Nguyen M, Paik A, Udall D, et al. Administration of oral vitamin D induces cathelicidin production in atopic individuals. J Allergy Clin Immunol. 2008;122:829–831. doi: 10.1016/j.jaci.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Howell MD, Novak N, Bieber T, et al. Interleukin-10 Downregulates Anti-Microbial Peptide Expression in Atopic Dermatitis. J Investig Dermatol. 2005;125:738–745. doi: 10.1111/j.0022-202X.2005.23776.x. [DOI] [PubMed] [Google Scholar]
- 52.Matsui MS, Wang N, DeLeo VA. Ultraviolet radiation B induces differentiation and protein kinase C in normal human epidermal keratinocytes. Photodermatol Photoimmunol Photomed. 1996;12:103–108. doi: 10.1111/j.1600-0781.1996.tb00185.x. [DOI] [PubMed] [Google Scholar]
- 53.Sigmundsdottir H, Gudjonsson JE, Valdimarsson H. The effects of ultraviolet B treatment on the expression of adhesion molecules by circulating T lymphocytes in psoriasis. Br J Dermatol. 2003;148:996–1000. doi: 10.1046/j.1365-2133.2003.05318.x. [DOI] [PubMed] [Google Scholar]
- 54.Sigmundsdottir H, Johnston A, Gudjonsson J, Valdimarsson H. Narrowband UVB irradiation decreases the production of pro-inflammatory cytokines by stimulated T cells. Arch Dermatol Res. 2005;297:39–42. doi: 10.1007/s00403-005-0565-9. [DOI] [PubMed] [Google Scholar]
- 55.Walters IB, Ozawa M, Cardinale I, Gilleaudeau P, Trepicchio WL, Bliss J, et al. Narrowband (312-nm) UV-B suppresses interferon gamma and interleukin (IL) 12 and increases IL-4 transcripts: differential regulation of cytokines at the single-cell level. Arch Dermatol. 2003;139:155–161. doi: 10.1001/archderm.139.2.155. [DOI] [PubMed] [Google Scholar]
- 56.Ozawa M, Ferenczi K, Kikuchi T, Cardinale I, Austin LM, Coven TR, et al. 312-nanometer ultraviolet B light (narrow-band UVB) induces apoptosis of T cells within psoriatic lesions. J Exp Med. 1999;189:711–718. doi: 10.1084/jem.189.4.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Peric M, Lehmann B, Vashina G, Dombrowski Y, Koglin S, Meurer M, et al. UV-B-triggered induction of vitamin D3 metabolism differentially affects antimicrobial pep-tide expression in keratinocytes. J Allergy Clin Immunol. 2010;125:746–749. doi: 10.1016/j.jaci.2009.12.933. [DOI] [PubMed] [Google Scholar]
- 58.Vähävihu K, Ala-Houhala M, Peric M, Karisola P, Kautiainen H, Hasan T, et al. Narrowband ultraviolet B treatment improves vitamin D balance and alters antimicrobial peptide expression in skin lesions of psoriasis and atopic dermatitis. Br J Dermatol. 2010;163:321–328. doi: 10.1111/j.1365-2133.2010.09767.x. [DOI] [PubMed] [Google Scholar]
- 59.Guilhou JJ. The therapeutic effects of vitamin D3 and its analogues in psoriasis. Exper Opin Invest Drugs. 1998;7:77–84. doi: 10.1517/13543784.7.1.77. [DOI] [PubMed] [Google Scholar]
- 60.Penna G, Adorini L. 1 Alpha,25-dihydroxyvitamin D3 inhibits differentiation, maturation, activation, and survival of dendritic cells leading to impaired alloreactive T cell activation. J Immunol. 2000;164:2405–2411. doi: 10.4049/jimmunol.164.5.2405. [DOI] [PubMed] [Google Scholar]
- 61.Berer A, Stöckl J, Majdic O, Wagner T, Kollars M, Lechner K, et al. 1,25-Dihydroxyvitamin D3 inhibits dendritic cell differentiation and maturation in vitro. Exper Hematol. 2000;28:575–583. doi: 10.1016/s0301-472x(00)00143-0. [DOI] [PubMed] [Google Scholar]
- 62.Pedersen AW, Holmstrøm K, Jensen SS, Fuchs D, Rasmussen S, Kvistborg P, et al. Phenotypic and functional markers for 1α,25-dihydroxyvitamin D3-modified regulatory dendritic cells. Clin Exper Immunol. 2009;157:48–59. doi: 10.1111/j.1365-2249.2009.03961.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gregori S, Casorati M, Amuchastegui S, Smiroldo S, Davalli AM, Adorini L. Regulatory T cells induced by 1 alpha,25-dihydroxyvitamin D3 and mycophenolate mofetil treatment mediate transplantation tolerance. J Immunol. 2001;167:1945–1953. doi: 10.4049/jimmunol.167.4.1945. [DOI] [PubMed] [Google Scholar]
- 64.Penna G, Amuchastegui S, Giarratana N, Daniel KC, Vulcano M, Sozzani S, et al. 1,25-Dihydroxyvitamin D3 selectively modulates tolerogenic properties in myeloid but not plasmacytoid dendritic cells. J Immunol. 2007;178:145–153. doi: 10.4049/jimmunol.178.1.145. [DOI] [PubMed] [Google Scholar]
- 65.Penna G, Amuchastegui S, Laverny G, Adorini L. Vitamin D receptor agonists in the treatment of autoimmune diseases: selective targeting of myeloid but not plasmacytoid dendritic cells. J Bone Miner Res. 2007;22:69–73. doi: 10.1359/jbmr.07s217. [DOI] [PubMed] [Google Scholar]
- 66.von Andrian UH, Chambers JD, McEvoy LM, Bargatze RF, Arfors K, Butcher EC. Two-step model of leukocyteendothelial cell interaction in inflammation: distinct roles for LECAM-1 and the leukocyte beta 2 integrins in vivo. Proc Natl Acad Sci USA. 1991;88:7538–7542. doi: 10.1073/pnas.88.17.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ghoreishi M, Bach P, Obst J, Komba M, Fleet JC, Dutz JP. Expansion of antigen-specific regulatory T cells with the topical vitamin d analog calcipotriol. J Immunol. 2009;182:6071–6078. doi: 10.4049/jimmunol.0804064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gorman S, Kuritzky LA, Judge MA, Dixon KM, McGlade JP, Mason RS, et al. Topically applied 1,25-dihydroxyvitamin D3 enhances the suppressive activity of CD4+CD25+ cells in the draining lymph nodes. J Immunol. 2007;179:6273–6283. doi: 10.4049/jimmunol.179.9.6273. [DOI] [PubMed] [Google Scholar]
- 69.Baeke F, Korf H, Overbergh L, Verstuyf A, Thorrez L, Van Lommel L, et al. The vitamin D analog, TX527, promotes a human CD4+CD25highCD127low regulatory T cell profile and induces a migratory signature specific for homing to sites of inflammation. J Immunol. 2011;186:132–142. doi: 10.4049/jimmunol.1000695. [DOI] [PubMed] [Google Scholar]
- 70.Gelfand JM, Feldman SR, Stern RS, Thomas J, Rolstad T, Margolis DJ. Determinants of quality of life in patients with psoriasis: A study from the US population. J Amer Acad Dermatol. 2004;51:704–708. doi: 10.1016/j.jaad.2004.04.014. [DOI] [PubMed] [Google Scholar]