Abstract
Although not often discussed, the ovaries of women with polycystic ovary syndrome (PCOS) show all the hallmarks of increased TGF-β activity, with increased amounts of fibrous tissue and collagen in the ovarian capsule or tunica albuginea and ovarian stroma. Recent studies suggest that PCOS could have fetal origins. Genetic studies of PCOS have also found linkage with a microsatellite located in intron 55 of the extracellular matrix protein fibrillin 3. Fibrillins regulate TGF-β bioactivity in tissues by binding latent TGF-β binding proteins. We therefore examined expression of fibrillins 1–3, latent TGF-β binding proteins 1–4, and TGF-β 1–3 in bovine and human fetal ovaries at different stages of gestation and in adult ovaries. We also immunolocalized fibrillins 1 and 3. The results indicate that TGF-β pathways operate during ovarian fetal development, but most important, we show fibrillin 3 is present in the stromal compartments of fetal ovaries and is highly expressed at a critical stage early in developing human and bovine fetal ovaries when stroma is expanding and follicles are forming. These changes in expression of fibrillin 3 in the fetal ovary could lead to a predisposition to develop PCOS in later life.—Hatzirodos, N., Bayne, R. A., Irving-Rodgers, H. F., Hummitzsch, K., Sabatier, L., Lee, S., Bonner, W., Gibson, M. A., Rainey, W. E., Carr, B. R., Mason, H. D., Reinhardt, D. P., Anderson, R. A., Rodgers, R. J. Linkage of regulators of TGF-β activity in the fetal ovary to polycystic ovary syndrome.
Keywords: latent transforming growth factor-β binding protein, fibrillin
Polycystic ovary syndrome (PCOS) is a major burden on health costs (1) and is the commonest endocrine condition affecting women of reproductive age in Western societies, with an estimated incidence of 5–7%. It is characterized by hyperandrogenemia and hirsutism, chronic anovulation, and polycystic ovaries (2–4). Affected women are also at increased risk of anovulatory infertility, obesity, hyperlipidemia, type II diabetes, and possibly cardiovascular disease (2–4).
The etiology of PCOS is not well understood, but there is increasing evidence that PCOS may have fetal origins. Androgenization of the fetus in sheep (5) and nonhuman primates (6) produces an adult PCOS phenotype, and treatment is more effective if administered earlier in gestation rather than later (6). Adult PCOS ovaries have increased numbers of primordial follicles (7) that develop during fetal life, and androgenization of a fetus also increases the primordial follicle pool in adults (8). Congenital adrenal hyperplasia in humans, which is usually not diagnosed and therefore left untreated until birth, also leads to symptoms of PCOS later in life (9, 10).
Familial studies have also demonstrated heritability of PCOS suggesting that there is a genetic predisposition to it (11). First-degree female relatives display strong association between the metabolic abnormalities of PCOS and hyperandrogenemia (12, 13), and a recent study demonstrated that the brothers of women with PCOS also have a strong association with metabolic abnormalities (14). Genomic familial linkage studies have identified two regions linked to PCOS, one of which is close to the follistatin gene (15). Follistatin is a secreted protein that can bind both to the transforming growth factor-β (TGF-β) superfamily member activin and to extracellular heparan sulfate proteoglycans. Another genetic marker, the dinucleotide repeat microsatellite marker D19S884, shows familial linkage association with PCOS (16). This microsatellite maps to intron 55 of the fibrillin 3 gene canvassed as a potential PCOS susceptibility gene (14, 16). Interestingly both fibrillin 3 and follistatin share common TB (TGF-β binding) domains (17), and both fibrillin 3 and follistatin regulate the activity of members of the TGF-β superfamily.
Fibrillins are extracellular matrix glycoproteins forming connective tissue microfibrils associated with elastin fibers or extracellular microfilaments (18, 19). Fibrillins 1 and 2 also bind latent TGF-β binding proteins (LTBPs), of which there are 4 family members. Fibrillin 3 was only discovered in 2001, and hence much of its activity or roles can only be inferred from our knowledge of fibrillins 1 and 2. LTBP1, LTBP3, and LTBP4 can form covalent disulfide complexes with some of the propeptide TGF-βs while these are being processed within cells. These complexes are then secreted as the large latent TGF-β complexes. LTBP2 does not form such complexes. LTBP1 and LTBP3 can bind any of the 3 propeptides of TGF-β1, TGF-β2, or TGF-β3. LTBP4 can bind only propeptide TGF-β1 and then less efficiently than LTBP1 and LTBP3. Secreted LTBPs with or without associated propeptide TGF-βs can associate extracellularly with fibrillins at their N termini. LTBP2 can dissociate LTBP1 from fibrillin 1 (20) and so can also be involved in the regulation of the TGF-β bioactivity. Thus, fibrillins and LTBPs regulate the local bioavailability and action of TGF-β in a tissue.
It is well known that in both normal and fibrotic tissues, TGF-β stimulates fibroblast function, including production and deposition of collagen (21–29). In addition, in the fibrosis of many organs, TGF-β activity is enhanced (26, 28, 30). In the PCOS ovary, all stromal compartments are altered. The ovaries have 50% more ovarian capsule or tunica albuginea, containing more collagen (31). The thickness of the cortical stromal is increased by one-third, and the subcortical stroma, whether deep cortical or medullary, by 5-fold (31). Even the specialized stromal theca interna layer that develops around antral follicles behaves differently in PCOS, with elevated capacity to produce steroid hormones (32, 33).
Working on the hypothesis that fibrillin 3 could be affecting the TGF-β bioactivity in the ovary and producing the PCOS ovary phenotype, we initiated studies in bovine and human adult ovaries (34, 35). We found that FBN1, FBN2, and FBN3 and LTBP1 and LTBP2 are expressed in adult human and bovine ovaries (34, 35). However, the expression of FBN3 was very low in both normal and PCOS human adult ovaries and in adult bovine ovaries (34, 35). A more recent study, however, has localized fibrillin 3 to the perifollicular stroma associated with some primordial and primary follicles in adult ovaries (36). Since fibrillin 3 has been observed in other fetal tissues (37, 38), we have considered here the possibility that FBN3 is expressed in the fetal ovary, especially at a time when the essential structures of the ovary, including the primordial follicles and stroma, are forming. In addition, there is increasing evidence that PCOS may have fetal origins. We therefore examined expression of all TGF-βs, fibrillins, and LTBP family members in both human and bovine ovaries throughout gestation and immunolocalized fibrillins 1 and 3.
MATERIALS AND METHODS
Tissues
Fetal bovine ovaries were obtained from the fetuses of pregnant Bos taurus cows (n=29) at slaughter at a local abattoir and transported on ice to the laboratory. Crown-rump length was measured to estimate gestational age (39). All ovaries were weighed after excision and then frozen on dry ice before storage at −80°C before RNA extraction. For mRNA analyses adult bovine ovaries were from a previous study of fibrillins and LTBPs (40, 41) of 23-mo-old female offspring whose mothers had been fed high-protein diets in the first and second trimesters (n=8) as described previously (40, 41). Adult bovine ovaries for immunohistochemistry were obtained from a local abattoir.
Human first- and second-trimester ovaries were obtained following medical termination of pregnancy for social reasons, as described previously (42). Maternal informed consent was obtained, and the study was approved by the Lothian Research Ethics Committee (Edinburgh, UK). Gestation was determined by ultrasound scan and subsequent direct measurement of foot length. For RNA analyses, ovaries were removed, snap-frozen, and stored at −80°C. The sex of first-trimester ovaries was confirmed by PCR for the male-specific gene SRY (43). For gestational analysis of expression, samples were grouped into 3 developmental stages: first trimester (8–11 wk; germ cell proliferation), early second trimester (14–16 wk; some initiation of meiosis), and late second trimester (17–21 wk; widespread meiosis, follicle formation). Due to limitations of the amounts of RNA from each fetal ovary, the expression analysis was performed in 2 batches of samples. In the first batch of samples, TGFB1, TGFB2, TGFB3, LTBP3, and LTBP4 were examined; in the second batch, the FBN genes and LTBP1 and LTBP2 were examined. In total, 29 samples, 11 first-trimester samples and 9 from each of the early and late second-trimester groups, were used in this study, with 5–6 samples analyzed for each gene at each gestation age. For immunohistochemistry, ovaries (n=4) and fetal lung as a control tissue were collected and fixed in Bouin's solution and processed into paraffin by standard methods.
Collection of adult human ovarian tissues (n=6) from patients undergoing procedures for benign gynecological conditions was approved by the Institution Review Boards of the University of Texas Southwestern Medical Center, St. George's University of London, and the University of Adelaide. Informed written consent was obtained from all patients before collection. Patient tissues that satisfied ≥3 criteria of PCOS as described previously (35, 44) were not included in this analysis. Tissues were either stored in RNAlater (Ambion, Austin, TX, USA) at −20°C or snap-frozen in liquid nitrogen and stored at −80°C before RNA isolation.
Gene expression analyses
To conduct RT-PCR, primers were designed against published mRNA sequences using Primer Express software (Applied Biosystems, Foster City, CA, USA); the primer sequences for human and bovine FBN1, FBN2, FBN3, LTBP1, LTBP2, LTBP3, LTBP4, TGFB1, TGFB2, and TGFB3 mRNA are shown in Supplemental Table S1. PCR products for each primer set were sequenced (3730 DNA analyzer; Applied Biosystems) to confirm the specificity of amplification for each respective gene.
For bovine samples, quantitative real-time RT-PCR was carried out using a Corbett Rotor Gene 6000 (Qiagen, Hilden, Germany) with 2 μl of diluted cDNA in a SYBR Green Master Mix (Applied Biosystems, Foster City, CA, USA). Samples were amplified in duplicate with an initial denaturation at 95°C for 10 min, followed by 40 cycles of 2-step amplification at 95°C for 15 s and 60°C for 60 s, followed by melt-curve analysis. To generate a standard curve for each PCR assay, DNA standards for each target sequence were prepared by subcloning the PCR products of the corresponding target sequence into pCR2.1-TOPO vector (Invitrogen, Mt. Waverley, VIC, Australia). The plasmid DNA was isolated and quantified using a Nanodrop spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA). Concentrations were calculated from absorbance at 260 nm. Plasmid DNA was serially diluted over 4 logs to establish a standard curve in a range, determined for each sample, which bounded the Ct values obtained for samples (between 1 ng/ml and 100 ag/ml). Concentration of each target was generated from the Ct and standard curve, and was normalized to the concentration of 18S ribosomal RNA in each sample (calculated by the Ct and standard curve for 18S). In each PCR reaction, melt curves were analyzed to confirm that the correct DNA had been amplified.
For human fetal ovaries, RNA was extracted using RNeasy Micro (first-trimester ovaries) or Mini kits (second-trimester ovaries) using on-column DNase digestion (Qiagen). The Superscript VILO cDNA synthesis kit (Invitrogen) was used to generate first-strand complementary DNA from 1 μg of fetal human RNA according to the manufacturer's protocol. For adult human ovaries, total RNA was prepared from ∼100 mg wet weight of stored cortical stromal tissues using 1 ml of Trizol reagent (Invitrogen) and 200 μl of chloroform. Total RNA (5 μg) was treated with DNase 1 (Ambion), and complementary DNA was synthesized from 1 μg of DNase-treated RNA using 200 U Superscript III reverse transcriptase (Invitrogen) and 500 ng random hexamers (Geneworks, Thebarton, SA, Australia).
Quantitative RT-PCR was performed using Power SYBR Green Master Mix (Applied Biosystems) and the ABI7500 Fast system with SDS2.01 software (Applied Biosystems) under standard conditions using the default 2-step PCR protocol with dissociation curve analysis. Standard curves for products of each gene transcript were performed using cDNA dilutions (1:5 to 1:10,000) generated from a 19-wk-old human fetal ovary RNA. Melt curves were analyzed to confirm specific products, and standard curves yielded slopes approaching 3.324 with R2 values close to 0.99, allowing quantification using the 2−ΔCt method. Relative comparisons were made between the target genes and the 18S ribosomal RNA gene. Gene expression for both human and bovine target sequences was subsequently expressed as femtomoles of target sequence mRNA per nanomole of 18S ribosomal RNA.
Statistical analyses
All statistical calculations were performed using SPSS version 9.2 (SAS Institute, Cary, NC, USA) and Microsoft Office Excel 2007 (Microsoft, Redmond, WA, USA). Data that were not normally distributed were log transformed when necessary, as indicated in Results. Comparisons of gene expression between groups were analyzed by 1-way ANOVA with post hoc Tukey's test. Results are presented as means ± se, and a value of P < 0.05 was considered significant. To test for associations between expression levels of each gene in fetal ovaries, correlation coefficients were calculated (Tables 1–3). Spearman correlation coefficients were used, as many of the data were not normally distributed. P values were corrected for multiple comparisons using the stepdown Sidak method. Due to limited yields of RNA from the human ovaries relative to the large number of genes analyzed, 2 different batches of samples were used, with only 5 samples common to both batches. Hence, the correlation analyses were conducted separately for each batch (Tables 2 and 3).
Table 1.
Spearman correlation coefficients in mRNA expression levels of FBNs, LTBPs, and TGFBs in bovine fetal ovaries
| FBN1 | FBN2 | FBN3 | LTBP1 | LTBP2 | LTBP3 | LTBP4 | TGFB1 | TGFB2 | TGFB3 | |
|---|---|---|---|---|---|---|---|---|---|---|
| FBN1 | 1.000 | |||||||||
| FBN2 | 0.401 (0.5393) | 1.000 | ||||||||
| FBN3 | −0.054 (0.9998) | 0.466 (0.3123) | 1.000 | |||||||
| LTBP1 | 0.682 (0.0024) | 0.584 (0.0379) | 0.226 (0.9669) | 1.000 | ||||||
| LTBP2 | 0.863 (<0.0001) | 0.247 (0.9496) | −0.336 (0.7601) | 0.567 (0.0554) | 1.000 | |||||
| LTBP3 | 0.217 (0.9669) | −0.319 (0.8097) | −0.432 (0.4456) | −0.083 (0.9998) | 0.461 (0.3273) | 1.000 | ||||
| LTBP4 | 0.314 (0.8097) | −0.061 (0.9998) | −0.203 (0.9669) | 0.028 (0.9998) | 0.413 (0.5188) | 0.738 (0.0003) | 1.000 | |||
| TGFB1 | 0.756 (0.0001) | 0.528 (0.1199) | 0.436 (0.4377) | 0.629 (0.0119) | 0.528 (0.1199) | −0.015 (0.9998) | 0.086 (0.9998) | 1.000 | ||
| TGFB2 | 0.730 (0.0004) | 0.029 (0.9998) | −0.379 (0.5791) | 0.302 (0.8299) | 0.798 (<0.0001) | 0.413 (0.5188) | 0.425 (0.4720) | 0.389 (0.5791) | 1.000 | |
| TGFB3 | 0.712 (0.0009) | 0.405 (0.5324) | −0.065 (0.9998) | 0.390 (0.5791) | 0.655 (0.0057) | 0.223 (0.9669) | 0.390 (0.5791) | 0.502 (0.1830) | 0.770 (<0.0001) | 1.000 |
Spearman correlation coefficients (n=28; probability >|r| under H0:ρ=0) in mRNA expression levels of FBNs, LTBPs and TGFBs in bovine fetal ovaries. P values are in parenthesis and are corrected for multiple comparisons (45 separate P values) using the stepdown Sidak method.
Table 2.
Spearman correlation coefficients in mRNA expression levels of FBNs and LTBPs in human fetal ovaries
| FBN1 | FBN2 | FBN3 | LTBP1 | LTBP2 | |
|---|---|---|---|---|---|
| FBN1 | 1.000 | ||||
| FBN2 | 0.694 (0.0226) | 1.000 | |||
| FBN3 | 0.546 (0.1354) | 0.081 (0.8926) | 1.000 | ||
| LTBP1 | 0.603 (0.0779) | 0.115 (0.8926) | 0.839 (0.0005) | 1.000 | |
| LTBP2 | 0.330 (0.6160) | −0.177 (0.8847) | 0.636 (0.0555) | 0.744 (0.0085) | 1.000 |
Spearman correlation coefficients (n=16; probability >|r| under H0:ρ=0) in mRNA expression levels of FBNs and LTBPs in human fetal ovaries. P values are in parenthesis and are corrected for multiple comparisons (10 separate P values) using the stepdown Sidak method.
Table 3.
Spearman correlation coefficients in mRNA expression levels of LTBPs and TGFBs in human fetal ovaries
| LTBP3 | LTBP4 | TGFB1 | TGFB2 | TGFB3 | |
|---|---|---|---|---|---|
| LTBP3 | 1.000 | ||||
| LTBP4 | 0.880 (<0.0001) | 1.000 | |||
| TGFB1 | 0.273 (0.7194) | 0.108 (0.8841) | 1.000 | ||
| TGFB2 | 0.884 (<0.0001) | 0.749 (0.0021) | 0.165 (0.8841) | 1.000 | |
| TGFB3 | 0.853 (<0.0001) | 0.696 (0.0067) | 0.133 (0.8841) | 0.884 (<0.0001) | 1.000 |
Spearman correlation coefficients (n=18; probability >|r| under H0:ρ=0) in mRNA expression levels of LTBPs and TGFBs in human fetal ovaries. P values are in parenthesis and are corrected for multiple comparisons (10 separate P values) using the stepdown Sidak method.
Immunohistochemistry
Portions or whole fetal ovaries were used for localization using an indirect immunofluorescence method or were stained by hematoxylin and eosin. Frozen tissue sections (10 μm) were cut and mounted on Superfrost glass slides (HD Scientific Supplies, Wetherill Park, NSW, Australia) and stored at −20°C until used. Sections were dried under vacuum for 5 min, fixed in 10% neutral buffered formalin for 5 min, and rinsed 3 times for 5 min in hypertonic PBS (10 mM sodium/potassium phosphate with 0.274 M NaCl and 5 mM KCl, pH 7.2) before treatment with blocking solution [10% normal donkey serum (D-9663; Sigma Chemical, St. Louis, MO, USA) in antibody diluent containing 0.55 M NaCl and 10 mM sodium phosphate, pH 7.1] for 30 min at room temperature. Paraffin embedded tissue sections were dewaxed (3×5 min xylene, 3×5 min 100% ethanol, and 3×5 min Millipore water; Millipore, Billerica, MA, USA) and subjected to antigen retrieval by treatment with either 10 μg/ml bacterial proteinase XXIV (Sigma Chemical) or 20 μg/ml proteinase K (Roche Diagnostics, Castle Hill, NSW Australia) for fibrillin 3 or fibrillin 1 or 3, respectively, in 50 mM Tris-HCl (pH 7.6) for 5 min at 37°C.
Primary antibodies used were mouse monoclonal antibodies raised against bovine fibrillin 1 (1:200 of ascites fluid; cat. no. MAB1919; Chemicon International, North Ryde, NSW, Australia) in combination with rabbit anti-mouse laminin 111 from the Engelbreth-Holm-Swarm tumor (1:100 dilution of 0.6 mg/ml; L 9393; Sigma Chemical). Fibrillin 3 antibody was a rabbit polyclonal antibody (4307-1) raised against the C-terminal half of human fibrillin 3 recombinantly expressed in human HEK293 cells and purified to homogeneity (38). The antiserum was preabsorbed with recombinant C-terminal half of fibrillin 2 and affinity purified using the fibrillin 3 C terminus to assure specificity to fibrillin 3 (38) and used at a final concentration of 33.2 μg/ml. Oct 3/4 antibody was a goat polyclonal antibody (N-19, sc-8628; Santa Cruz Biotechnology, Santa Cruz, CA, USA) and used at a final dilution of 1:100. For dual immunolocalization, secondary antibodies were donkey anti-rabbit IgG conjugated to fluorescein FITC (1:100; 711-096-152) and biotin-SP-conjugated AffiniPure donkey anti-mouse IgG (1:100; 715-066-151) followed by Cy3-conjugated streptavidin (1:100; 016-160-084) or donkey anti-rabbit IgG conjugated to Cy3 (1:100; 711-166-152) and biotin-SP-conjugated AffiniPure donkey anti-mouse IgG or donkey anti-goat IgG followed by fluorescein DTAF-conjugated streptavidin (1:100; 016-010-084). All secondary antibodies and conjugated streptavidins were purchased from Jackson ImmunoResearch Laboratories (West Grove, PA, USA). Sections were also treated with the nuclear stain DAPI solution (Molecular Probes, Eugene, OR, USA). Coverslips were attached with mounting medium for fluorescence (S3023; Dako, Carpinteria, CA, USA) and photographed with an Olympus BX50 microscope (Olympus, Tokyo, Japan) with an epifluorescence attachment and a Spot RT digital camera (Diagnostic Instruments, Sterling Heights, MI, USA).
RESULTS
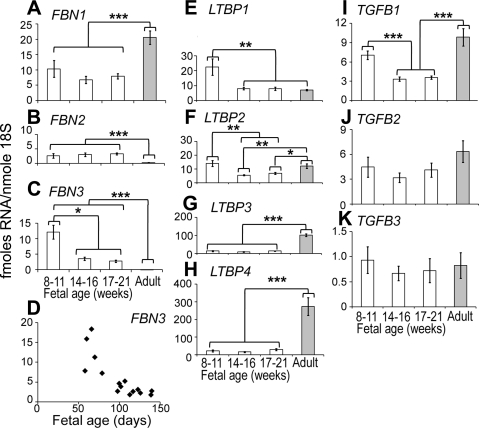
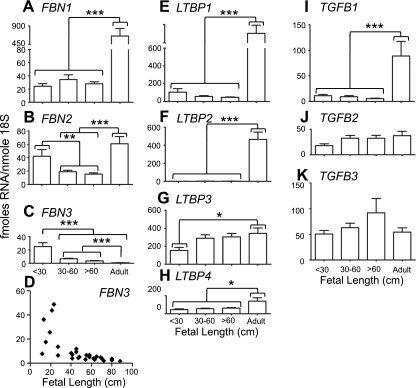
The most significant finding was that FBN3 was expressed highly in the first trimester in both human and bovine ovaries (Figs. 1 and 2). FBN3 expression then declined during gestation to very low levels and remained low in adult ovaries (Figs. 1C and 2C). The expression levels of FBN3 were as high as FBN1 and FBN2 in the first trimester, suggesting that FBN3 is a major fibrillin at this stage (Figs. 1 and 2). At this stage, stromal tissue is expanding as it penetrates into the developing ovary from the mesonephros. All other genes were expressed during fetal development (Figs. 1 and 2). LTBPs exhibited species differences in the adult compared to the fetal ovary, where LTBP3 and LTBP4 (Fig. 1E, F) were more highly expressed in adult human ovaries, and in bovine ovaries it was LTBP1 and LTBP2 (Fig. 2E, F).
Figure 1.
Expression levels of FBN1 (A), FBN2 (B), FBN3 (C, D), LTBP1 (E), LTBP2 (F), LTBP3 (G), LTBP4 (H), TGFB1 (I), TGFB2 (J), and TGFB3 (K) mRNA in whole fetal human ovary and adult tunica/cortical stroma. A–C, E–K) Data are means ± se (fmol/nmol 18S ribosomal RNA). For analysis, samples were grouped into 3 estimated gestational stages: first trimester (8–11 wk; germ cell proliferation), early second trimester (14–16 wk; some initiation of meiosis), and late second trimester (17–21 wk; widespread meiosis, follicle formation); n = 5–6/group. Data were analyzed by 1-way ANOVA with post hoc Tukey's test. Data for FBN2, FBN3, LTBP1, and LTBP2 were log transformed before analyses. *P < 0.05, **P < 0.01, ***P < 0.001, significant differences. D) Scatter plot of FBN3 expression vs. estimated gestational age.
Figure 2.
Expression levels of FBN1 (A), FBN2 (B), FBN3 (C, D), LTBP1 (E), LTBP2 (F), LTBP3 (G), LTBP4 (H), TGFB1 (I), TGFB2 (J), and TGFB3 (K) mRNA levels in bovine ovarian tissues. A–C, E–K) Data are means ± se (fmol/nmol 18S ribosomal RNA); n = 8, 11, 9, and 5–8 for whole ovaries from fetuses with crown-rump length of <30, 30–60, and >60 cm (estimated as <125, 125–194, and >194 d of gestation age, respectively, with bovine gestation being 282 d) and adult ovarian tunica/cortex, respectively. Data were analyzed by 1-way ANOVA with post hoc Tukey's test. Data for FBN1, FBN3, LTBP1, LTBP2 were log transformed before analyses. *P < 0.05, **P < 0.01, ***P < 0.001. D) Scatter plot of FBN3 expression levels vs. fetal crown-rump length.
The expression levels of a number of these genes were correlated with each other in fetal ovaries. The more significant correlations in bovine fetal ovaries were FBN1 with LTBP1 (P<0.01) and LTBP2 (P<0.0001) and with TGFB1 (P<0.0001), TGFB2 (P<0.001), and TGFB3 (P<0.001). TGFB2 correlated with LTBP2 (P<0.0001) and with TGFB3 (P<0.0001), and LTBP3 correlated with LTBP4 (P<0.001) (Table 1). Unfortunately not all genes could be measured on the same human fetal samples due to the limiting yields of RNA. Hence, 2 cohorts of samples were used, limiting the number of correlations that could be tested for. The more significant correlations in human fetal ovaries were FBN3 correlated with LTBP1 (P<0.0001); LTBP1 correlated with LTBP2 (P<0.001); LTBP3 correlated with LTBP4 (P<0.01), TGFB2 (P<0.0001), and TGFB3 (P<0.001); LTBP4 correlated with TGFB2 (P < 0.01) and TGFB3 (P<0.001); and TGFB2 correlated with TGFB3 (P<0.0001) (Tables 2 and 3).
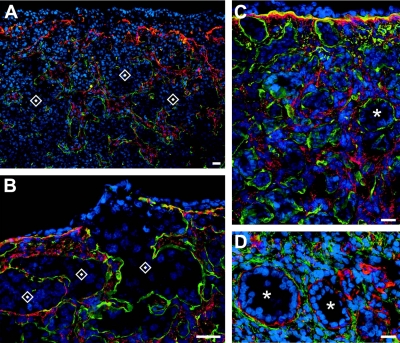
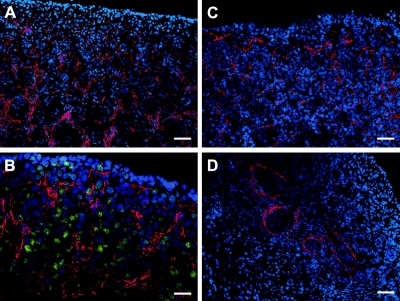
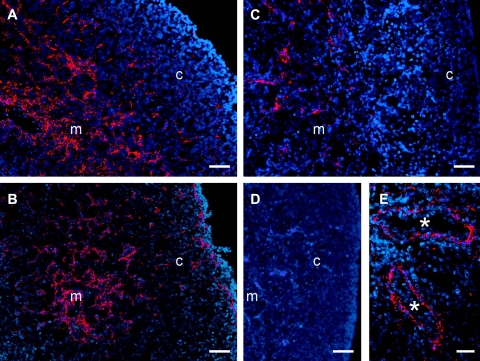
We also immunolocalized fibrillins 1 and 3. For fibrillin 1, ovaries from 12 bovine fetuses of 13- to 80-cm crown-rump length, estimated at 86–241 d of gestation, were examined. Fibrillin 1 localized to fibers in the fetal ovarian stroma. During development, cells in stroma expand into and penetrate between the nests of oogonia or ovigerous cords (Fig. 3A, B). Stromal cells also penetrate underneath the surface epithelium of the ovary as it develops (Figs. 3B, C) and at later stages of ovarian development between the newly formed follicles (Figs. 3C, D). For localization of fibrillin 3, bovine ovaries from 17 fetuses of a size range from 11.5- to 104-cm crown-rump length, estimated as 82–296 d of gestation, and 8 adult ovaries were examined. In humans, 4 fetal ovaries at 10, 12, 14, and 19 wk of gestation were examined. In early fetal ovarian development in bovine (Fig. 4) and human ovaries (Figs. 5A–C) fibrillin 3 localized to fibers in the stroma between ovigerous cords or nests of oogonia, easily visualized as immunopositive for Oct3/4 (Fig. 4B). Staining was also observed in the cells around terminal bronchioles in fetal lung (Fig. 5E), as shown previously (38). Staining for fibrillin 3 was more intense in first-trimester ovaries and fainter or not detectable at later stages (Figs. 4 and 5). Some bovine fetal ovaries of the second and third trimester showed thin fibers around cell clusters in the inner cortex, whereas the remaining cortex was negative. Fibrillin 3 was not detectable in bovine adult ovaries.
Figure 3.
Merged images of dual immunolocalization of fibrillin 1 (red in A–C; green in D) and the basal lamina components of laminin 111 (green in A–C; red in D) counterstained with DAPI (blue) in bovine ovaries of fetuses at crown-rump length of 13 (A), 50 (B), 54 (C), and 75 cm (D), estimated as 86, 171, 181, and 229 d of gestation, respectively. Scale bars = 20 μm. Asterisks indicate follicles; diamonds indicate nests of oogonia.
Figure 4.
A, C, D) Immunolocalization of fibrillin 3 (red) counterstained with DAPI (blue) in bovine ovaries of fetuses of crown-rump length 16 (A), 28 (C), and 50 cm (D), estimated as 93, 120, and 171 d of gestation, respectively. B) Merged images of immunolocalization of fibrillin 3 (red) and Oct 3/4 (green) counterstained with DAPI (blue) in bovine ovary at crown-rump length of 11.5 cm, estimated as 82 d of gestation. Scale bars = 50 μm (A, C, D); 25 μm (B).
Figure 5.
Immunolocalization of fibrillin-3 (red) counterstained with DAPI (blue) in human ovaries at 10 (A), 12 (B), 14 (C), and 19 (D) wk of gestation and in fetal lung at 18 wk of gestation (E). Scale bars = 50 μm (A–D); 25 μm (E). Asterisks indicate terminal bronchioles. m, medulla; c, cortex.
DISCUSSION
In this study, we examined the expression of FBN1, FBN2, FBN3, LTBP1, LTBP2, LTBP3, LTBP4, TGFB1, TGFB2, and TGFB3 in bovine and human fetal ovaries throughout gestation and in adult ovaries for comparison. We also immunolocalized fibrillins 1 and 3 to the expanding stroma of fetal ovaries. Given the familial linkage to PCOS of microsatellite D19S884, located within an intron in the fibrillin 3 gene, one of our most interesting findings was the pattern of expression of FBN3 during gestation.
In both fetal bovine and human ovaries, FBN3 was expressed highly in the first trimester and then declined dramatically and was undetectable in adult ovaries as observed here and also previously (34, 35). This is in agreement with findings in other tissues (37, 38). FBN2 was also highly expressed during gestation in both bovine and human ovaries, but was elevated further in bovine adult ovaries, unlike in human ovaries. FBN1 was expressed at about the same level as FBN3 in the first trimester in both species, but unlike FBN3, the levels of FBN1 remained constant throughout gestation and were about a fifth of those observed in adult ovaries. These results suggest a major role for fibrillins, including FBN3, in the first trimester when oogonia are replicating and the stromal tissue is expanding during fetal development. What regulates their expression in ovaries is not known, but in other tissues, IGF-1, TGF-β (45), sex steroid hormones (46–48), and relaxin (49) have been observed to do so.
Fibrillin 1 has been localized in adult bovine ovaries (34), adult human ovaries (36), and now in bovine fetal ovaries. In adult bovine ovaries, fibrillin 1 staining was intense in the ovarian tunica albuginea immediately adjacent to the surface epithelium and less intense in the underlying layers of the tunica and in the stroma of the ovarian cortex (34), where follicles are located. Fibrillin 1 staining was slightly more intense in the stroma immediately surrounding primordial and primary follicles and in antral follicles, and fibrillin 1 localized to the theca interna and was less fibrillar in appearance than in the stroma in the adult bovine ovary (34). In human ovaries, similar patterns of staining to bovine ovaries have been observed (36). In bovine fetal ovaries, as observed here, fibrillin 1 was present at the early stages examined in the stroma between the nests of oogonia or ovigerous cords. At later stages of fetal development, fibrillin 1 was still present in the stroma surrounding primordial and growing follicles. Thus, it is clear that fibrillin 1 is a major component of extracellular matrix of ovarian stromal compartments throughout ovarian development.
We also immunolocalized fibrillin 3 in bovine and human fetal ovaries. Fibrillin 3 appeared as microfibrils and was localized to the stromal areas between the nests of oogonia or ovigerous cords. The staining was more intense at the earlier stages, in agreement with the observed high levels of mRNA for FBN3, and staining was less intense or undetectable at later stages of fetal ovarian development. Fibrillin 3 has been localized in adult human and PCOS ovaries by others, and they observed staining in stroma at or near some of the primordial and primary follicles in some specimens (36). We have not been able to confirm this finding; however, the levels of mRNA for fibrillin 3 are low in adult stroma, and we did not detect any fibrillin 3 in bovine adult ovaries. Fibrillin 2 has also been localized in adult human ovaries (36) to arterioles in the ovarian cortex and outer aspect of the theca externa of antral follicles; in particular, staining was intense in regressing follicles at their periphery where larger blood vessels were present (36).
Fibrillins sequester or bind LTBPs. In the current study, the levels of LTBP1 and LTBP2 were significantly lower in bovine fetal ovaries than in the tunica/stroma of adult ovaries. No immunostaining has been observed by us in bovine fetal ovaries using similar methods and antibodies, as reported previously for localization of LTBP1 and LTBP2 in adult bovine ovaries (34). In adult bovine ovaries, LTBP1 localizes to the ovarian tunica albuginea immediately below the surface epithelium, to the ovarian stroma again as fibrillar structures and as punctuate staining around primordial to preantral follicles, and to the theca externa adjacent to the theca interna as fibrils (34). LTBP2 localizes to the ovarian tunica albuginea immediately below the surface epithelium and to the theca externa of antral follicles distal to where the LTBP1 localizes (34). The levels of expression of LTBP3 in bovine fetal ovaries were high and similar to adult ovarian tunica/stroma, except for the first trimester, when they were 2-fold lower. LTBP4 levels were significantly lower at all fetal ages. Thus, in bovine fetal ovaries, the predominant LTBP is LTBP3, which has the capability of associating with all 3 TGF-βs and the fibrillins.
In human fetal ovaries, the expression patterns of LTBPs were different from those of the bovine. The levels of LTBP3 and LTBP4 were substantially lower relative to the adult and to bovine fetal ovaries, suggesting that they might have less important roles in the human fetal ovary. The mRNA level of LTBP2 was as high in the human ovary as in the bovine fetal ovary, and that of LTBP1 was lower. However, both were similar to or as high as adult human tunica/stroma, unlike in the bovine, where the adult levels were substantially elevated compared to bovine fetal ovaries. Thus, in human fetal ovaries, LTBP1 and LTBP2 would appear to be more important. Since LTBP2 can dissociate LTBP1 from fibrillin 1 (20), the regulation of TGF-β bioactivity could be more complex in the human than the bovine fetal ovary.
The levels of expression of TGFBs were generally relatively constant during fetal development in both bovine and human ovaries. However, in the bovine fetal ovaries, the levels of TGFB1 were low compared to adult ovaries and to the levels of TGFB2 and TGFB3. In the human fetal ovaries, TGFB1 and TGFB2 were much higher than TGFB3 and not substantially different from levels in adult tunica/stroma, except in the second trimester in humans, when LTBP1 was about a third the level of that of the adult ovary. Thus, again there is a species difference in expression patterns during fetal ovarian development.
The expression levels of a number of these genes were correlated with each other in fetal ovaries. The more significant correlations in bovine fetal ovaries were FBN1 with LTBP1 and LTBP2 and with TGFB1, TGFB2, and TGFB3; TGFB2 with LTBP2; TGFB2 with TGFB3; and LTBP3 with LTBP4. In adult bovine ovarian tunica/stroma, fewer significant positive correlations in expression levels have been observed (34). The more significant correlations in human fetal ovaries were FBN3 with LTBP1; LTBP1 with LTBP2; LTBP3 with LTBP4; both LTBP3 and LTBP4 with both TGFB2 and TGFB3; and TGFB2 with TGFB3. In adult human ovarian tissues, FBN1 expression was observed to be correlated with FBN2 and LTBP1, and FBN2 correlated with LTBP1 (35). Thus, our results suggest that there is a degree of coordinated regulation among some of these genes in both humans and bovines.
In summary, our findings suggest that fibrillin 3 could be involved in the etiology of PCOS by exerting its action during development of the fetal ovary, particularly early in development as the stroma expands and oogonia undergo mitosis. Since fibrillins are stromal matrices and since the ovarian stromal compartments are altered in women with PCOS (31), fibrillin 3 expression in the developing fetal ovary, via the activity of TGF-β to regulate stromal formation and function, could predispose an individual to PCOS in later life. This study therefore heralds a role for fibrillins and LTBPs in regulating the activity of TGF-βs in the development of the stromal compartments of the normal and PCOS ovary. It also links previous genetic data implicating fibrillin 3 with a physiological mechanism, in line with animal models and clinical observations, of a fetal predisposition to development of PCOS in adulthood.
Supplementary Material
Acknowledgments
The authors thank Mr. Thomas Sullivan for assistance with the statistical analyses and T&R Pastoral for donation of bovine ovaries.
Funding to support this research was obtained from the National Health and Medical Research Council of Australia, the University of Adelaide, the Clive and Vera Ramaciotti Foundation, the Wellcome Trust, the National Institute of Health, and the Medical Research Council UK. N.H., H.F.I-R., K.H., S.L., W.B., M.A.G., and R.J.R. planned the experiments, conducted RT-PCR, immunohistochemistry, and statistical analysis, and wrote the manuscript. R.A.B. and R.A.A. conducted RT-PCR and provided human fetal ovarian samples and reviewed the manuscript. W.E.R., B.R.C., and H.D.M. provided human adult ovarian samples and RNA and reviewed the manuscript. L.S. and D.P.R. expressed recombinant fibrillins, made antisera to FBN3 and adsorbed it against FBN2, established methods for immunostaining, and reviewed the manuscript.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Azziz R., Marin C., Hoq L., Badamgarav E., Song P. (2005) Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J. Clin. Endocrinol. Metab. 90, 4650–4658 [DOI] [PubMed] [Google Scholar]
- 2. Padmanabhan V. (2009) Polycystic ovary syndrome–“a riddle wrapped in a mystery inside an enigma.” J. Clin. Endocrinol. Metab. 94, 1883–1885 [DOI] [PubMed] [Google Scholar]
- 3. Norman R. J., Dewailly D., Legro R. S., Hickey T. E. (2007) Polycystic ovary syndrome. Lancet 370, 685–697 [DOI] [PubMed] [Google Scholar]
- 4. Diamanti-Kandarakis E., Kandarakis H., Legro R. S. (2006) The role of genes and environment in the etiology of PCOS. Endocrine 30, 19–26 [DOI] [PubMed] [Google Scholar]
- 5. Veiga-Lopez A., Ye W., Phillips D. J., Herkimer C., Knight P. G., Padmanabhan V. (2008) Developmental programming: deficits in reproductive hormone dynamics and ovulatory outcomes in prenatal, testosterone-treated sheep. Biol. Reprod. 78, 636–647 [DOI] [PubMed] [Google Scholar]
- 6. Abbott D. H., Barnett D. K., Bruns C. M., Dumesic D. A. (2005) Androgen excess fetal programming of female reproduction: a developmental aetiology for polycystic ovary syndrome? Hum. Reprod. Update 11, 357–374 [DOI] [PubMed] [Google Scholar]
- 7. Webber L. J., Stubbs S., Stark J., Trew G. H., Margara R., Hardy K., Franks S. (2003) Formation and early development of follicles in the polycystic ovary. Lancet 362, 1017–1021 [DOI] [PubMed] [Google Scholar]
- 8. Forsdike R. A., Hardy K., Bull L., Stark J., Webber L. J., Stubbs S., Robinson J. E., Franks S. (2007) Disordered follicle development in ovaries of prenatally androgenized ewes. J. Endocrinol. 192, 421–428 [DOI] [PubMed] [Google Scholar]
- 9. Hague W. M., Adams J., Rodda C., Brook C. G., de Bruyn R., Grant D. B., Jacobs H. S. (1990) The prevalence of polycystic ovaries in patients with congenital adrenal hyperplasia and their close relatives. Clin. Endocrinol. (Oxf.) 33, 501–510 [DOI] [PubMed] [Google Scholar]
- 10. Barnes R. B., Rosenfield R. L., Ehrmann D. A., Cara J. F., Cuttler L., Levitsky L. L., Rosenthal I. M. (1994) Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J. Clin. Endocrinol. Metab. 79, 1328–1333 [DOI] [PubMed] [Google Scholar]
- 11. Amato P., Simpson J. L. (2004) The genetics of polycystic ovary syndrome. Best Pract. Res. Clin. Obstet. Gynaecol. 18, 707–718 [DOI] [PubMed] [Google Scholar]
- 12. Legro R. S., Bentley-Lewis R., Driscoll D., Wang S. C., Dunaif A. (2002) Insulin resistance in the sisters of women with polycystic ovary syndrome: association with hyperandrogenemia rather than menstrual irregularity. J. Clin. Endocrinol. Metab. 87, 2128–2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Yildiz B. O., Yarali H., Oguz H., Bayraktar M. (2003) Glucose intolerance, insulin resistance, and hyperandrogenemia in first degree relatives of women with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 88, 2031–2036 [DOI] [PubMed] [Google Scholar]
- 14. Urbanek M., Sam S., Legro R. S., Dunaif A. (2007) Identification of a polycystic ovary syndrome susceptibility variant in fibrillin-3 and association with a metabolic phenotype. J. Clin. Endocrinol. Metab. 92, 4191–4198 [DOI] [PubMed] [Google Scholar]
- 15. Urbanek M., Legro R. S., Driscoll D. A., Azziz R., Ehrmann D. A., Norman R. J., Strauss J. F., 3rd, Spielman R. S., Dunaif A. (1999) Thirty-seven candidate genes for polycystic ovary syndrome: strongest evidence for linkage is with follistatin. Proc. Natl. Acad. Sci. U. S. A. 96, 8573–8578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Stewart D. R., Dombroski B. A., Urbanek M., Ankener W., Ewens K. G., Wood J. R., Legro R. S., Strauss J. F., 3rd, Dunaif A., Spielman R. S. (2006) Fine mapping of genetic susceptibility to polycystic ovary syndrome on chromosome 19p13.2 and tests for regulatory activity. J. Clin. Endocrinol. Metab. 91, 4112–4117 [DOI] [PubMed] [Google Scholar]
- 17. Thompson T. B., Lerch T. F., Cook R. W., Woodruff T. K., Jardetzky T. S. (2005) The structure of the follistatin:activin complex reveals antagonism of both type I and type II receptor binding. Dev. Cell 9, 535–543 [DOI] [PubMed] [Google Scholar]
- 18. Ramirez F., Pereira L. (1999) The fibrillins. Int. J. Biochem. Cell Biol. 31, 255–259 [DOI] [PubMed] [Google Scholar]
- 19. Kielty C. M., Sherratt M. J., Shuttleworth C. A. (2002) Elastic fibres. J. Cell Sci. 115, 2817–2828 [DOI] [PubMed] [Google Scholar]
- 20. Hirani R., Hanssen E., Gibson M. A. (2007) LTBP-2 specifically interacts with the amino-terminal region of fibrillin-1 and competes with LTBP-1 for binding to this microfibrillar protein. Matrix Biol. 26, 213–223 [DOI] [PubMed] [Google Scholar]
- 21. LeClair R., Lindner V. (2007) The role of collagen triple helix repeat containing 1 in injured arteries, collagen expression, and transforming growth factor beta signaling. Trends Cardiovasc. Med. 17, 202–205 [DOI] [PubMed] [Google Scholar]
- 22. Christner P. J., Ayitey S. (2006) Extracellular matrix containing mutated fibrillin-1 (Fbn1) down regulates Col1a1, Col1a2, Col3a1, Col5a1, and Col5a2 mRNA levels in Tsk/+ and Tsk/Tsk embryonic fibroblasts. Amino Acids 30, 445–451 [DOI] [PubMed] [Google Scholar]
- 23. Verrecchia F., Mauviel A. (2004) TGF-beta and TNF-alpha: antagonistic cytokines controlling type I collagen gene expression. Cell. Signal. 16, 873–880 [DOI] [PubMed] [Google Scholar]
- 24. Govinden R., Bhoola K. D. (2003) Genealogy, expression, and cellular function of transforming growth factor-beta. Pharmacol. Ther. 98, 257–265 [DOI] [PubMed] [Google Scholar]
- 25. Khan R., Sheppard R. (2006) Fibrosis in heart disease: understanding the role of transforming growth factor-beta in cardiomyopathy, valvular disease and arrhythmia. Immunology 118, 10–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chapman H. A. (2004) Disorders of lung matrix remodeling. J. Clin. Invest. 113, 148–157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hetzel M., Bachem M., Anders D., Trischler G., Faehling M. (2005) Different effects of growth factors on proliferation and matrix production of normal and fibrotic human lung fibroblasts. Lung 183, 225–237 [DOI] [PubMed] [Google Scholar]
- 28. Kisseleva T., Brenner D. A. (2007) Role of hepatic stellate cells in fibrogenesis and the reversal of fibrosis. J. Gastroenterol. Hepatol. 22(Suppl. 1), S73–S78 [DOI] [PubMed] [Google Scholar]
- 29. Prud'homme G. J. (2007) Pathobiology of transforming growth factor beta in cancer, fibrosis and immunologic disease, and therapeutic considerations. Lab. Invest. 87, 1077–1091 [DOI] [PubMed] [Google Scholar]
- 30. Bottinger E. P. (2007) TGF-beta in renal injury and disease. Semin. Nephrol. 27, 309–320 [DOI] [PubMed] [Google Scholar]
- 31. Hughesdon P. E. (1982) Morphology and morphogenesis of the Stein-Leventhal ovary and of so-called “hyperthecosis.” Obstet. Gynecol. Surv. 37, 59–77 [DOI] [PubMed] [Google Scholar]
- 32. Nelson V. L., Legro R. S., Strauss J. F., 3rd, McAllister J. M. (1999) Augmented androgen production is a stable steroidogenic phenotype of propagated theca cells from polycystic ovaries. Mol Endocrinol 13, 946–957 [DOI] [PubMed] [Google Scholar]
- 33. Nelson V. L., Qin K. N., Rosenfield R. L., Wood J. R., Penning T. M., Legro R. S., Strauss J. F., 3rd, McAllister J. M. (2001) The biochemical basis for increased testosterone production in theca cells propagated from patients with polycystic ovary syndrome. J. Clin. Endocrinol. Metab. 86, 5925–5933 [DOI] [PubMed] [Google Scholar]
- 34. Prodoehl M. J., Irving-Rodgers H. F., Bonner W., Sullivan T. M., Micke G. C., Gibson M. A., Perry V. E., Rodgers R. J. (2009) Fibrillins and latent TGFβ binding proteins in bovine ovaries of offspring following high or low protein diets during pregnancy of dams. Mol. Cell. Endocrinol. 307, 133–144 [DOI] [PubMed] [Google Scholar]
- 35. Prodoehl M. J., Hatzirodos N., Irving-Rodgers H. F., Zhao Z. Z., Painter J. N., Hickey T. E., Gibson M. A., Rainey W. E., Carr B. R., Mason H. D., Norman R. J., Montgomery G. W., Rodgers R. J. (2009) Genetic and gene expression analyses of the polycystic ovary syndrome candidate gene fibrillin-3 and other fibrillin family members in human ovaries. Mol. Hum. Reprod. 15, 829–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jordan C. D., Bohling S. D., Charbonneau N. L., Sakai L. Y. (2010) Fibrillins in adult human ovary and polycystic ovary syndrome: is fibrillin-3 affected in PCOS? J. Histochem. Cytochem. 58, 903–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Corson G. M., Charbonneau N. L., Keene D. R., Sakai L. Y. (2004) Differential expression of fibrillin-3 adds to microfibril variety in human and avian, but not rodent, connective tissues. Genomics 83, 461–472 [DOI] [PubMed] [Google Scholar]
- 38. Sabatier L., Miosge N., Hubmacher D., Lin G., Davis E. C., Reinhardt D. P. (2010) Fibrillin-3 expression in human development. Matrix Biol. 21, 21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Russe I. (1983) Oogenesis in cattle and sheep. Bibl. Anat. 24, 77–92 [PubMed] [Google Scholar]
- 40. Prodoehl M. J., Irving-Rodgers H. F., Bonner W. M., Sullivan T. M., Micke G. C., Gibson M. A., Perry V. E., Rodgers R. J. (2009) Fibrillins and latent TGFbeta binding proteins in bovine ovaries of offspring following high or low protein diets during pregnancy of dams. Mol. Cell. Endocrinol. 307, 133–141 [DOI] [PubMed] [Google Scholar]
- 41. Sullivan T. M., Micke G. C., Greer R. M., Irving-Rodgers H. F., Rodgers R. J., Perry V. E. (2009) Dietary manipulation of Bos indicus x heifers during gestation affects the reproductive development of their heifer calves. Reprod. Fertil. Dev. 21, 773–784 [DOI] [PubMed] [Google Scholar]
- 42. Coutts S. M., Childs A. J., Fulton N., Collins C., Bayne R. A., McNeilly A. S., Anderson R. A. (2008) Activin signals via SMAD2/3 between germ and somatic cells in the human fetal ovary and regulates kit ligand expression. Dev. Biol. 314, 189–199 [DOI] [PubMed] [Google Scholar]
- 43. Friel A., Houghton J. A., Glennon M., Lavery R., Smith T., Nolan A., Maher M. (2002) A preliminary report on the implication of RT-PCR detection of DAZ, RBMY1, USP9Y and protamine-2 mRNA in testicular biopsy samples from azoospermic men. Int. J. Androl. 25, 59–64 [DOI] [PubMed] [Google Scholar]
- 44. Mason H. D., Willis D. S., Beard R. W., Winston R. M., Margara R., Franks S. (1994) Estradiol production by granulosa cells of normal and polycystic ovaries: relationship to menstrual cycle history and concentrations of gonadotropins and sex steroids in follicular fluid. J. Clin. Endocrinol. Metab. 79, 1355–1360 [DOI] [PubMed] [Google Scholar]
- 45. Kenney M. C., Zorapapel N., Atilano S., Chwa M., Ljubimov A., Brown D. (2003) Insulin-like growth factor-I (IGF-I) and transforming growth factor-beta (TGF-beta) modulate tenascin-C and fibrillin-1 in bullous keratopathy stromal cells in vitro. Exp. Eye Res. 77, 537–546 [DOI] [PubMed] [Google Scholar]
- 46. Wen Y., Polan M. L., Chen B. (2006) Do extracellular matrix protein expressions change with cyclic reproductive hormones in pelvic connective tissue from women with stress urinary incontinence? Hum. Reprod. 21, 1266–1273 [DOI] [PubMed] [Google Scholar]
- 47. Son E. D., Lee J. Y., Lee S., Kim M. S., Lee B. G., Chang I. S., Chung J. H. (2005) Topical application of 17beta-estradiol increases extracellular matrix protein synthesis by stimulating tgf-Beta signaling in aged human skin in vivo. J. Invest. Dermatol. 124, 1149–1161 [DOI] [PubMed] [Google Scholar]
- 48. Natoli A. K., Medley T. L., Ahimastos A. A., Drew B. G., Thearle D. J., Dilley R. J., Kingwell B. A. (2005) Sex steroids modulate human aortic smooth muscle cell matrix protein deposition and matrix metalloproteinase expression. Hypertension 46, 1129–1134 [DOI] [PubMed] [Google Scholar]
- 49. Samuel C. S., Sakai L. Y., Amento E. P. (2003) Relaxin regulates fibrillin 2, but not fibrillin-1, mRNA and protein expression by human dermal fibroblasts and murine fetal skin. Arch. Biochem. Biophys. 411, 47–55 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.