Abstract
Nebulin is a giant protein expressed at high levels in skeletal muscle. Mutations in the nebulin gene (NEB) lead to muscle weakness and various congenital myopathies. Despite increasing clinical and scientific interest, the pathogenesis of weakness remains unknown. The present study, therefore, aims at unraveling the underlying molecular mechanisms. Hence, we recorded and analyzed the mechanics as well as the X-ray diffraction patterns of human membrane-permeabilized single muscle fibers expressing nebulin mutations. Results demonstrated that, during contraction, the cycling rate of myosin heads attaching to actin is dramatically perturbed, causing a reduction in the fraction of myosin-actin interactions in the strong binding state. This phenomenon prevents complete thin-filament activation, more especially proper and full tropomyosin movement, further limiting additional binding of myosin cross-bridges. At the cell level, this reduces the force-generating capacity and, overall, provokes muscle weakness. To reverse such a negative cascade of events, future potential therapeutic interventions should, therefore, focus on the triggering component, the altered myosin cross-bridge cycling kinetics.—Ochala, J., Lehtokari, V.-L., Iwamoto, H., Li, M., Feng, H.-Z., Jin, J. P., Yagi, N., Wallgren-Pettersson, C. Pénisson-Besnier, I., Larsson, L. Disrupted myosin cross-bridge cycling kinetics triggers muscle weakness in nebulin-related myopathy.
Keywords: congenital myopathy, tropomyosin activation
Nebulin is a giant protein (molecular mass of 700–800 kDa) highly expressed in skeletal muscle (1). It spans along the length of the thin filament with the C terminus anchored in the Z-disk region and the N terminus directed toward the thin-filament pointed end (2, 3). Nebulin plays various important structural roles; it stabilizes the thin filament (3, 4), laterally links myofibrils at the Z disk, and may regulate Z-disk width (5). In addition, nebulin is involved in muscle contraction by directly modulating myosin-actin interactions and cycling kinetics (6, 7). This role is of great importance and would potentially explain why patients with nebulin-related myopathies usually suffer from muscle weakness. However, to date, experimentally based correlations are scarce (7–9).
Nebulin mutations lead to various myopathies, including nemaline myopathy (10), distal myopathy (11), and core-rod myopathy (12). Deletion of exon 55 in the nebulin gene (NEB) results in muscle weakness and nemaline myopathy. At the molecular level, it provokes a partial nebulin deficiency and thin-filament dysregulation via a length reduction (9). During contraction, this limits the overlap between thin and thick filaments, decreasing the fraction of potential myosin heads strongly binding to actin and subsequently reducing fiber force production (9). This cascade of events has been seen as the major contributor of muscle weakness (9). Nevertheless, deletion of exon 55 also induces a dramatic change in the rate of myosin attachment and detachment to/from actin (8). Unfortunately, this intriguing alteration has been underappreciated and, here, we hypothesized that this particular alteration is a key determinant of weakness in nebulin-related myopathies. Consequently, we aimed at investigating this by recording and analyzing the mechanics and X-ray diffraction patterns of human membrane-permeabilized single muscle fibers expressing nebulin mutations and compared them with control cells.
MATERIALS AND METHODS
Subjects
The study was conducted on samples from a 46-yr-old patient diagnosed with the probably most common of the congenital myopathies, nemaline myopathy. His parents were healthy, and he had 2 similarly affected brothers. The clinical picture was compatible with the typical form of nemaline myopathy in other respects, with a generalized muscle weakness of grade 4 on the Medical Research Council scale in most muscle groups, but as a rare feature, he had normal strength in his ankle dorsiflexors. Seven healthy subjects between 26 and 67 yr old, with no history of neuromuscular disease, served as controls. Informed consent was obtained from the patient and the control subjects enrolled in the present study. The local Uppsala University Ethics Committee approved the protocol, and the experiments were carried out according to the guidelines of the Declaration of Helsinki.
Muscle biopsies and membrane permeabilization
An open muscle biopsy of the deltoid muscle was performed in the patient while he was under local anesthesia. Percutaneous conchotome muscle biopsy specimens were obtained from control tibialis anterior or vastus lateralis muscles, while the control patients were under local anesthesia. The biopsy specimens were separated into 3 portions. One was fixed in 2.5% glutaraldehyde. Another was frozen in liquid-nitrogen-chilled propane and stored at −160°C. The final piece was placed in relaxing solution at 4°C. Bundles of ∼50 fibers were dissected free and then tied with surgical silk to glass capillary tubes at slightly stretched lengths. They were then treated with skinning solution (relaxing solution containing glycerol; 50:50 v/v) for 24 h at 4°C, after which they were transferred to −20°C. In addition, the muscle bundles were treated with sucrose, a cryoprotectant, within 1–2 wk for long-term storage (13). They were detached from the capillary tubes and snap frozen in liquid nitrogen-chilled propane and stored at −160°C.
Characterization of the mutations
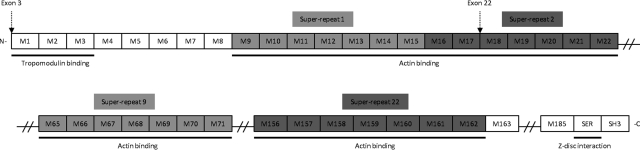
We first sequenced the encoding exons and exon-intron boundaries of ACTA1, TPM2, and TPM3 using genomic DNA of the index patient (Sequencher 4.7 software; Gene Codes Corp., Ann Arbor, MI, USA). This direct sequencing did not reveal any mutation. Then, we performed a haplotype analysis for NEB locus in the family (father, mother, 3 affected siblings, and 1 healthy sibling) using the fluorescent-labeled microsatellite markers D2S2324, D2S2277, D2S2275, D2S2236, and D2S2299. The results were analyzed by GeneMapper 4.0 software (Applied Biosystems, Foster City, CA, USA) and showed a strong positive indication of heterozygous linkage to the nebulin locus (2q21). To analyze the nebulin gene for mutations (ref. seq. NG_009382.1), we performed RT-PCR using a frozen muscle biopsy specimen sample from the patient. Total RNA was extracted from the biopsy using the RNeasy Mini kit (Qiagen Sciences, Valencia, CA, USA) including DNase treatment. The RNA was reverse-transcribed using M-MLV reverse transcriptase (Promega, Madison, WI, USA) and random hexamers (Promega). PCR was performed on cDNA using 35 RT-PCR primer pairs and Phusion High Fidelity DNA polymerase (Finnzymes, Espoo, Finland). The alternatively spliced exons (exons 63–66, 143, 144, 82–105, and 167–177) were sequenced directly using genomic DNA. We identified compound heterozygous splice-site mutations, one leading to skipping of exon 3 and the other to skipping of exon 22 (Fig. 1). The mutation leading to skipping of exon 3 is a duplication of the thymine in intron 3 (g.6357dupT, c.36+2dupT; ref. seq. NG_009382.1) and was inherited from the patient's father. The mutation inherited from the patient's mother is a point mutation (g.47420A>C, c.2106+3A>C) in intron 22 disrupting the splice signal of exon 22. None of these mutations were seen in any of the 200 control chromosomes studied.
Figure 1.
Schematic structure of human nebulin. Nebulin has 183 exons encoding various regions. Exon 3 partly encodes the N-terminal region of nebulin. Exon 22 encodes a central region that is arranged into 22 superrepeats (modules M9-M162; more precisely, it encodes modules M17 and M18 of the second superrepeat.
Histopathological analyses
Cryosections (10 μm) were processed using standard histological and histochemical techniques (14). Frozen sections (3 μm thick) were incubated with a mouse monoclonal anti α-actinin antibody (dilution 1:80; Novocastra, Newcastle upon Tyne, UK) and processed with Techmate 500 automated immunostainer (Dako, Carpinteria, CA, USA) and DAB detection (Fig. 2). Briefly, a predominance of type I fibers was observed in the muscle sample from the patient. Moreover, cells were more rounded than polygonal and were smaller than normal with a mild variability in size. Myonuclei were in their correct positions, some of them seeming enlarged. Nemaline bodies (rods) were present in nearly all the fibers and mostly clustered under the sarcolemma.
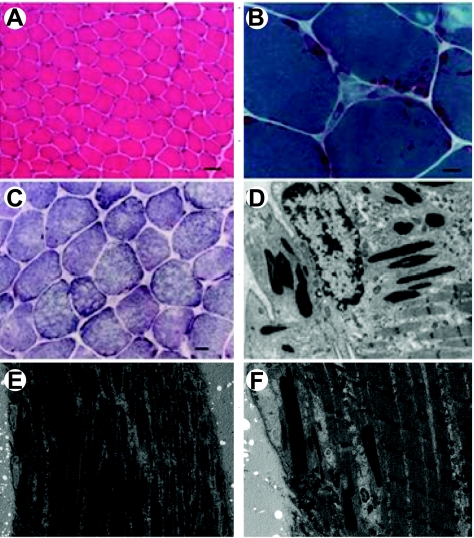
Figure 2.
Histopathological abnormalities in the patient. A) Hematoxylin and eosin stain shows a mild fiber size variability and an overall reduced diameter. B) Gomori trichrome demonstrates nemaline rods. C) Some fibers exhibit small focal areas lacking succinate dehydrogenase staining. D) Electron micrograph shows subsarcolemmal rods in the vicinity of a myonucleus. View: ×10,000. E, F) Electron micrographs show that sarcomeric structure is preserved. View: ×3000 (E); ×10,000 (F). Scale bars = 50 μm (A); 10 μm (B); 20 μm (C).
In addition, 1 piece of the biopsy was processed for electron microscopy according to standard procedures (14) and analysis confirmed no sarcomeric disorganization but the presence of nemaline bodies in all the fibers examined (Fig. 2). Neither intranuclear rods nor minicores were seen, although with oxidative enzyme staining, many fibers exhibited small and poorly defined areas lacking oxidative activity.
Mechanical recordings and analyses
On the day of experiment, bundles were desucrosed and transferred to a relaxing solution, and single fibers were dissected. A fiber 1 to 2 mm long was left between connectors leading to a force transducer (model 400A; Aurora Scientific, Aurora, ON, Canada) and a lever arm system (model 308B; Aurora Scientific) (15, 16). The 2 extremities of the fiber were tightly attached as described previously (15). The total compliance of the attachment system was carefully checked and remained constant for all fibers tested (5±0.5% of the fiber length). The apparatus was mounted on the stage of an inverted microscope (model IX70; Olympus, Center Valley, PA, USA). The sarcomere length was set to 2.70–2.80 μm and controlled during the experiment using a high-speed video analysis system (model 901A HVSLl; Aurora Scientific). The diameter of the fiber between the connectors was measured through the microscope at ×320 with an image analysis system prior to the mechanical experiments. Fiber depth was measured by recording the vertical displacement of the microscope nosepiece while focusing on the top and bottom surfaces of the fiber. The focusing control of the microscope was used as a micrometer. Diameter and depth were measured at 3 different locations along the length of each fiber, and the mean was considered as representative of cell dimensions. Cross-sectional area (CSA) was calculated from the diameter and depth, assuming an elliptical circumference, and was corrected for the 20% swelling that is known to occur during skinning (15). At 15°C, immediately preceding each activation, the fiber was immersed for 10–20 s in the preactivating solution.
Force
Force was calculated as the difference between the steady-state isometric force in activating solutions and the resting force measured in the same segment while in the relaxing solution. Force was adjusted for CSA, i.e., specific force. The time to steady-state isometric force was activation related and fiber dependent but usually lasted a few seconds.
Force-sarcomere length relationship
Each fiber was set at various sarcomere lengths from 2.20 to 3.20 μm, and the maximal force generated at pCa 4.5 was determined.
Force-pCa relationship
Each fiber was exposed to different solutions, with pCa varying from 9.0 to 4.5. Force was normalized to maximal force at pCa 4.5, allowing the construction of relative force-pCa curve. To determine the midpoint (termed pCa50) and the Hill coefficient (nH) of the curves, data were fitted (SigmaPlot 5.0 and Origin 6.1 Professional software; Jandel Scientific, San Rafael, CA, USA) using a 3-parameter Hill equation in the form X = [Ca2+]nH/([Ca50]nH + [Ca2+]nH), where X is the relative force and −log [Ca50] is the midpoint pCa50.
Stiffness
Once steady-state isometric force was reached, small-amplitude sinusoidal changes in length (ΔL; ±0.2% of fiber length) were applied at 500 Hz at one end of the fiber (ref. 17 and Fig. 3). The resultant force response (ΔF) was measured, and the mean of 20 consecutive readings of ΔL and ΔF was used to determine stiffness. The actual elastic modulus (E) was calculated as the difference between E in activating solutions and resting E measured in the same segment in the relaxing solution. E was determined as follows: E = (ΔF/ΔL) × (fiber length/CSA) (ref. 18). As for force measurements, stiffness was calculated in solutions with various pCa values (9.0–4.5). This allowed the construction of relative stiffness-pCa and stiffness-force relationships.
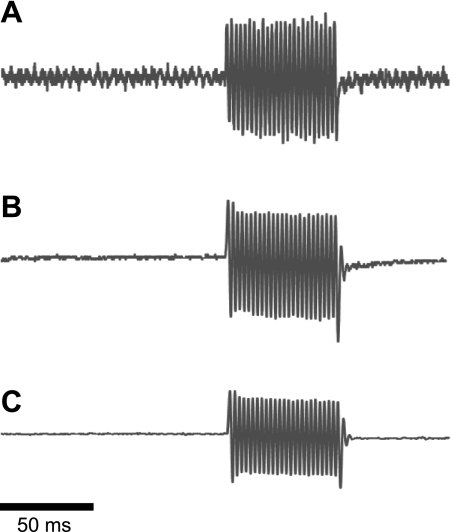
Figure 3.
Original recordings for stiffness calculation. A) Length signal. B) Force signal for a fiber from a control. C) Force signal for a cell from the patient. Scale bar = 50 ms.
Apparent rate constant of force redevelopment
At pCa 4.5, once steady-state isometric force was reached, a slack by 20% of the original fiber length was introduced within 1–2 ms at one end of the fiber, resulting in a rapid reduction of force to near zero. This was followed by a brief period of unloaded shortening (20 ms), after which the preparation was quickly restretched to its original length, and the force recovered to its original steady-state value. As described previously (19), the apparent rate constant of force redevelopment (ktr) was estimated by linear transformation of the half-time of force redevelopment (t1/2) as follows: ktr = 0.693/t1/2 (ref. 20).
Unloaded shortening velocity
At pCa 4.5, once steady-state isometric force was reached, 9 slacks of various amplitudes were rapidly introduced (within 1–2 ms) at one end of the fiber (21). Slacks were applied at different amplitudes, ranging from 7 to 13% of the fiber length. The fiber was reextended between releases while relaxed to minimize changes in sarcomere length. During the slack test, the time required to take up the imposed release was measured from the onset of the length step to the beginning of the tension redevelopment. A straight line including four or more data points was fitted to a plot of release length vs. time, using least-squares regression. The slope of the line divided by the fiber segment length was recorded as the maximum unloaded shortening velocity (V0) for that fiber segment.
For all the contractile measurements, strict acceptance criteria were applied. First, the sarcomere length was checked during the experiments, using a high-speed video analysis system (model 901A HVSL; Aurora Scientific). A muscle fiber was accepted and included in the analyses if the sarcomere length of a single muscle fiber changed by <0.10 μm between relaxation and maximum activation, maximal force changed by <10% from first to final activation, and r of the slope (plot of release length vs. time) for V0 calculation was >0.96.
After the mechanical measurements, each fiber was placed in urea buffer (120 g urea, 38 g thiourea, 70 ml H20, 25 g mixed bed resin, 2.89 g dithiothreitol, 1.51 g Trizma base, 7.5 g SDS, and 0.004% bromphenol blue) in a plastic microcentrifuge tube and stored at −160°C.
In vitro motility assay
The unregulated actin used was purified from rabbit skeletal muscle and was fluorescently labeled with rhodamine-phalloidin (Molecular Probes, Eugene, OR, USA). The single fiber in vitro motility system has been described in detail elsewhere (22, 23). In brief, a short muscle fiber segment was placed on a glass slide between 2 strips of grease, and a nitrocellulose-coated coverslip was placed on top, creating a flow cell with a volume of −5 μl. Myosin was extracted from the fiber segment through addition of a high-salt buffer (0.5 M KCl; 25 mM HEPES, pH 7.6; 4 mM MgCl2; 4 mM EGTA; 2 mM ATP; and 1% 2-mercaptoethanol). After 30 min incubation on ice, a low-salt buffer (25 mM KCl; 25 mM HEPES, pH 7.6; 4 mM MgCl2; 1 mM EGTA; and 1% 2-mercaptoethanol) was applied, followed by BSA (1 mg/ml). Nonfunctional myosin molecules were blocked with fragmentized F-actin, and rhodamine-phalloidin-labeled actin filaments were subsequently infused into the flow cell, followed by motility buffer (2 mM ATP, 0.1 mg/ml glucose oxidase, 23 μg/ml catalase, 2.5 mg/ml glucose, and 0.4% methyl cellulose in low-salt buffer) to initiate movement. The pH of the buffers was adjusted with KOH, and the final ionic strength of the motility buffer was 71 mM. The flow cell was placed on the stage of an inverted epifluorescence microscope (model IX 70; Olympus) and thermostatically controlled at 25°C. Actin movements were filmed with an image-intensified SIT camera (SIT 66; Dage-MTI, Michigan City, IN, USA) and recorded on tape with a videocassette recorder. From each single-fiber preparation, 20 actin filaments moving at constant speed in an oriented motion were selected for speed analysis. Recordings and analysis were only performed from preparations in which >90% of the filaments moved bidirectionally. A filament was tracked from the center of mass, and the speed was calculated from 20 frames at an acquisition rate of 5 or 1 frames/s, depending on the fiber type, using an image-analysis package (Image-Pro Plus 6.0, Media Cybernetics, Bethesda, MD, USA). The mean speed of the 20 filaments was calculated and was taken as representative for the muscle fiber (Vf).
Protein expression
The myosin heavy chain isoform composition of fibers was determined by 6% SDS-PAGE. The acrylamide concentration was 4% (w/v) in the stacking gel and 6% in the running gel, and the gel matrix included 30% glycerol. Sample loads were kept small (equivalent to ∼0.05 mm of fiber segment) to improve the resolution of the myosin heavy chain bands (types I, IIa, and IIx). Electrophoresis was performed at 120 V for 24 h with a Tris-glycine electrode buffer (pH 8.3) at 15°C (SE 600 vertical slab gel unit; Hoefer Scientific Instruments, Holliston, MA, USA). The gels were silver stained and subsequently scanned in a soft laser densitometer (Molecular Dynamics, Sunnyvale, CA, USA) with a high spatial resolution (50-μm pixel spacing) and 4096 optical density levels.
Quantification of myosin heavy chain, actin, and myosin light chain contents of fibers was determined by 12% SDS-PAGE. The acrylamide concentration was 4% (w/v) in the stacking gel and 12% in the running gel, and the gel matrix included 10% glycerol. The gels were stained with Coomassie blue (0.5 g brilliant blue, 225 ml MeOH, 225 ml distilled H20, and 50 ml acetic acid). The contents were then calculated from the densitometric scanning (see above).
For nebulin quantification, cryosections were immediately transferred into cold SDS-PAGE sample buffer, homogenized by pipetting, and heated at 80°C for 5 min. As described previously (24), high-molecular-weight muscle proteins were examined by SDS-PAGE using 2–12% gradient gels with acrylamide:bis-acrylamide ratio of 180:1. The Bio-Rad minigels (Bio-Rad, Richmond, CA, USA) were run at 15 mA, and the protein bands resolved were visualized with Coomassie blue R250 staining. Densitometry quantification of the nebulin bands was performed on images scanned at 600 dpi using Scion Image software (Scion Corp., Frederick, MD, USA). Three copies of the SDS-gel were analyzed, and the level of nebulin was normalized to the total protein in the muscle sample. The amounts of sample loading were normalized and adjusted to avoid oversaturation of the protein bands. Protein bands in duplicate gels were electrically transferred to nitrocellulose membranes using a Bio-Rad semidry electrotransfer apparatus for Western blot analysis. To enhance the transfer of high- molecular-weight proteins, a 3-buffer system was used (24). The cathode buffer contained 25 mM Tris-base, 191 mM glycine, and 20% methanol at pH 8.3. The first anode buffer contained 25 mM Tris-base at pH 10.8, and the second anode buffer contained 300 mM Tris-base and 0.05% SDS at pH 10.8. The transfer was done at 4.4 mA/cm2 for 30 min. The membrane was incubated with a mixture of monoclonal antibodies against mouse nebulin and chicken nebulette (25) in TBS containing 0.1% BSA at 4°C overnight. After high-stringency washes with TBS containing 0.5% Triton X-100 and 0.05% SDS, the membrane was incubated with alkaline phosphatase-labeled goat anti-mouse IgG second antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) in TBS-BSA and washed again as above. The blots were then developed in 5-bromo-4-chloro-3-indolylphosphate nitro blue tetrazolium substrate solution to reveal the nebulin bands.
X-ray diffraction recordings and analyses
At 2 to 3 d before X-ray recordings, bundles were desucrosed and transferred to a relaxing solution, and single fibers were dissected. Arrays of ∼30 fibers were set up (26–30). For each fiber, both ends were clamped to half-split gold meshes for electron microscopy (width 3 mm), which had been glued to precision-machined ceramic chips (width 3 mm) designed to fit to a specimen chamber. The arrays were then transferred to the skinning solution and stored at −20°C.
On the day of X-ray recordings, arrays were placed in a plastic dish containing a preactivating solution and washed thoroughly to remove the glycerol. They were then transferred to the specimen chamber, capable of manual length adjustment and force measurement (force transducer, AE801; Memscap, Bernin, France), also filled with the preactivating solution. Mean sarcomere length was measured and set to 2.70 or >3.60 μm. Subsequently, for arrays at a sarcomere length equal to 2.70 μm, X-ray diffraction patterns were recorded at 15°C, first in the preactivating solution and then in the activating solution (pCa 4.5) when maximal steady-state isometric force was reached. It should be mentioned that the activating solution was supplied to the chamber by using a remote-controlled pump. For arrays at a sarcomere length >3.60 μm, the protocol was identical except that preactivating and activating solutions were replaced by low-EGTA rigor and calcium-rigor solutions with 2,3-butanedione monoxime (to prevent major sarcomere inhomogeneities).
For each array, ∼20–30 diffraction patterns were recorded for each solution at the BL45XU beamline of SPring-8 (Japan Synchrotron Radiation Research Institute, Hyogo, Japan) and were analyzed as described previously (26–29). The wavelength was 0.09 nm, and the specimen-to-detector distance was 2 m. As a detector, a cooled CCD camera (C4880; 1000×1018 pixels; Hamamatsu Photonics, Hamamatsu, Japan) was used in combination with an image intensifier (VP5445; Hamamatsu Photonics). To minimize radiation damage, the exposure time was kept low (2 s) and the specimen chamber was moved along the fiber axis at a constant speed of 100 mm/s by using a pulse-motor-driven z stage (Sigma-Koki, Hidaka, Japan). Because of this movement, the total photons absorbed by the array were reduced (31). Moreover, we placed an aluminum plate (thickness 0.35–0.5 mm) upstream of the specimen chamber. The beam flux was estimated to be between 2.7 × 1011 and 4.0 × 1011 photons/s after attenuation, and the beam size at the sample position was 0.2 mm (vertical) and 0.3 mm (horizontal). Following X-ray recordings, background scattering was subtracted, and reflection intensities were determined as described elsewhere (26–29, 32).
Solutions
Relaxing and activating solutions contained the following (in mM): 4 Mg-ATP, 1 free Mg2+, 20 imidazole, 7 EGTA, 14.5 creatine phosphate, 324 U/ml creatine phosphokinase, 1000 U/ml catalase, and KCl to adjust the ionic strength to 180 mM and pH to 7.0. Dithiothreitol (DTT) was also added (1 mM). The preactivating solution was identical to the relaxing solution except that the EGTA concentration was reduced to 0.5 mM. The concentrations of free Ca2+ were 10−9 M (relaxing and preactivating solutions) and 10−6.3, 10−5.9, 10−5.5, 10−5.1, 10−4.9, and 10−4.5 M (activating solutions), expressed as pCa (i.e., −log [Ca2+]). Low-EGTA rigor and calcium-rigor solutions, for overstretch experiments (>3.60 μm), had similar compositions to preactivating and activating solutions except that mgATP, creatine phosphate, and creatine phosphokinase were absent and that 2,3-butanedione monoxime was included with a concentration of 20 mM.
Statistical analyses
Data are presented as means ± se. Sigma Stat software (Jandel Scientific) was used to generate descriptive statistics. Given the small numbers of hybrid (type I/IIa and IIa/IIx) and pure type IIa and IIx fibers observed in these experiments, comparisons were restricted to fibers expressing the type I myosin heavy chain isoform. The unpaired Student's t test was used, and in cases where the data did not meet the criteria of normality (P<0.05, Kolmogorov-Smirnov test), nonparametric Mann-Whitney rank-sum tests were performed. Otherwise, regressions were performed and relationships were considered significant different from 0 at P < 0.05. For X-ray data, 2-way ANOVAs [group (control − patient) × exposure (preactivation − activation)] were applied. In the event of differences, the post hoc Tukey test was used.
RESULTS
Single muscle fiber mechanics
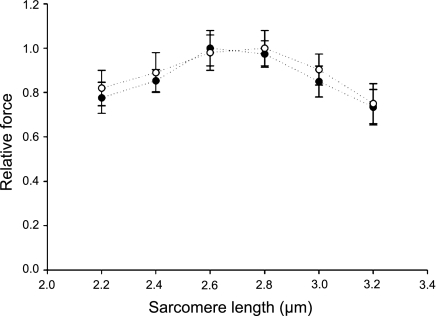
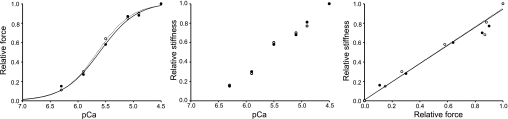
The single muscle fiber preparation allows direct measurements of contractility in cells with an intact myofilament lattice, but without the confounding effects of nerves, excitation-contraction coupling, fiber architecture, and intercellular connective tissue. Thus, after membrane permeabilization, fibers were isolated and individually mounted. Cells carrying nebulin mutations came from a patient with a skipping of exons 3 and 22 in the NEB gene, whereas control fibers originated from 7 different healthy individuals. The force-sarcomere length relationship (pCa 4.5) was first evaluated and not found significantly different between the patient and the controls (Fig. 4). This demonstrates that, in the patient, thin-thick filament overlap is preserved and cannot account for the weakness. However, specific force, E0, and ktr at saturating calcium concentration (pCa 4.5) were lower in the patient when compared with the controls. Mean specific force was 14.30 ± 1.10 N/cm2 in the patient (n=31) and 20.80 ± 0.70 N/cm2 in the controls (n=68; P<0.05). Mean stiffness was 1940 ± 120 N/cm2 (n=23) in the patient and 3100 ± 400 N/cm2 in the controls (n=20; P<0.05). Mean ktr was 8.80 ± 1.00 s−1 in the patient (n=12) and 20.90 ± 1.60 s−1 in the controls (n=21; P<0.05). A trend toward a slower V0 was also noticed at pCa 4.5 but not statistically significant. Mean V0 was 0.68 ± 0.15 muscle length (ML)/s in the patient (n=10) and 0.89 ± 0.07 ML/s in the controls (n=25). In addition, pCa50 and nH did not differ between the patient and the controls (Fig. 5). Mean pCa50 was 5.65 ± 0.15 in the patient (n=12) and 5.66 ± 0.06 in the controls (n=43); mean nH was 3.30 ± 0.32 in the patient (n=12) and 3.73 ± 0.55 in the controls (n=43).
Figure 4.
Force-sarcomere length relationships of cells from the patient (n=12, ●) and from controls (n=15, ○). Values are presented as means ± se.
Figure 5.
Typical force-pCa, stiffness-pCa, and stiffness-force relationships in fibers from a control subject (○) and from the patient (●).
In an attempt to test whether the above contractile deregulation was directly due to the mutations or to a secondary modification of the myosin molecule, the single muscle fiber in vitro motility assay was used in which the motor protein is extracted from muscle fiber segments 1 to 2 mm long and the interactions with unregulated thin filaments are measured (23). The in vitro motility speed Vf of actin filaments propelled by myosin differed (P<0.05) between the patient (0.48 ± 0.02 μm/s; n=9) and the controls (0.58±0.02 μm/s; n=17), suggesting that, in addition to mutation-related modifications, a secondary alteration of myosin molecules contributes to the above dysfunction. It should be noticed that the Vf values measured here were slightly lower when compared with values in the literature. Some differences in the experimental protocol related to the myosin extraction technique (fiber vs. frozen tissue), the cover slide type (mica vs. glass), and motility analysis (bidirectional linear movement vs. curvilinear movement) may potentially explain the smaller Vf data.
Protein expression
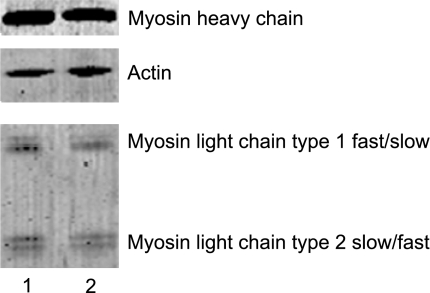
We evaluated the myosin heavy chain, myosin light chain, and actin relative contents. Myosin heavy chain to actin ratio was 1.69 ± 0.08 in the patient (n=29) and 1.53 ± 0.10 in the controls (n=35; Fig. 6). This finding suggests that there is no preferential loss of the motor protein and, therefore, such a loss cannot account for the changes. Nevertheless, a myosin light chain isoform difference appeared between the patient and the controls (Fig. 6). The type 1 essential myosin light chain isoform composition had a slower profile (Table 1). This phenomenon likely contributes to the contractile deregulation and modification of in vitro Vf.
Figure 6.
Electrophoretic analysis of myosin and actin on a 12% SDS-PAGE gel. Lane 1, patient cell; lane 2, control fiber.
Table 1.
Myosin light chain isoform composition
| Group | MyLC type 1fast | MyLC type 1slow | MyLC type 2slow | MyLC type 2fast |
|---|---|---|---|---|
| Control | 0.18 ± 0.02 | 0.29 ± 0.02 | 0.49 ± 0.02 | 0.04 ± 0.02 |
| Patient | 0.09 ± 0.02* | 0.44 ± 0.04* | 0.41 ± 0.04 | 0.06 ± 0.02 |
Values are normalized to total myosin light chain content and appear as means ± se. MyLC, myosin light chain.
P < 0.05 vs. control.
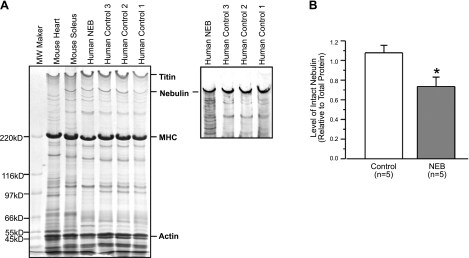
The nebulin relative content was also estimated, and we found that the ratio of nebulin to total protein was reduced in the patient (Fig. 7). Nebulin translation begins at exon 3 and skipping of that exon may mean that translation in the patient begins somewhere later (the next possible ATG is at the end of exon 5). This phenomenon likely contributes to the partial nebulin deficiency in the patient.
Figure 7.
Electrophoretic and Western blot analysis of nebulin. A) Gradient (2–12%) SDS-PAGE gel shows lower level of nebulin in the patient muscle (NEB) as compared with 3 control samples. Western blot detected multiple degradation bands of nebulin in the patient muscle sample. B) Densitometry quantification of the SDS-gel using Scion Image software was performed for 1 patient muscle and 3 control muscle samples with 3 duplicated SDS gels. Level of intact nebulin relative to total protein was significantly lower in the patient sample as compared with the controls. While the present study reports the first case of the nebulin mutation, multiple runs of the SDS gel and Western blot were examined by densitometry to validate the technical reliability of nebulin quantification. Values are shown as means ± se. *P < 0.05 vs. control.
X-ray diffraction pattern
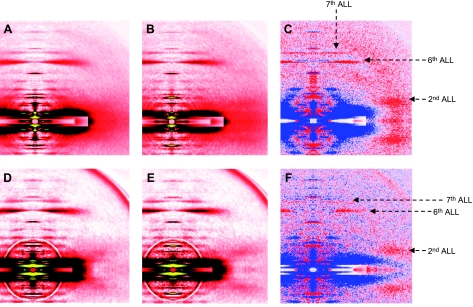
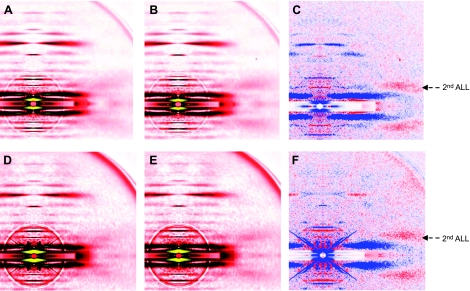
To further investigate the underlying molecular mechanisms responsible for the contractile deregulation, we performed X-ray experiments and monitored the intensity changes in tropomyosin [far off-meridional part of the second actin layer line (ALL) at 1/19 nm−1] and actin (6th and 7th ALL at 1/5.9 and 1/5.1 nm−1), in single fibers during activation under various conditions. The diffraction patterns are shown in Figs. 8 and 9. All the intensities are summarized in Table 2. At a sarcomere length considered as optimal (2.70 μm) where there is a full thin-thick filament overlap, the reflection of the second ALL was enhanced in controls on addition of calcium. The sixth and seventh ALLs were also intensified. In the patient, the reflection of the second ALL was also enhanced during activation but to a lesser extent when compared with the controls. The intensities of the sixth and seventh ALLs, however, did not differ on activation. In addition, a separate number of cells were overstretched beyond the thin-thick filament overlap (sarcomere length >3.60 μm). Interestingly, on addition of calcium, the second ALL reflection was increased similarly in the controls as in the patient. Apart from the evaluation of ALL intensity changes, equatorial reflections (1,0 and 1,1 intensities) were also analyzed and the ratio I1,1 to I1,0 was calculated for fibers set at the optimal sarcomere length (2.70 μm). In the controls, this ratio increased on addition of calcium (0.639±0.036 in preactivating solution vs. 1.505±0.073 in activating solution). In the patient, a weaker augmentation was observed (0.590±0.029 in preactivating solution vs. 1.008±0.089 in activating solution).
Figure 8.
X-ray diffraction patterns from human controls (A–C) and from the patient (D–F). Cells are set at an optimal sarcomere length (2.70 μm) in preactivating (A, D) and activating (B, E) solutions. Differences in intensity profiles (C, F) show enhanced (red) and weakened (blue) areas after calcium addition.
Figure 9.
X-ray diffraction patterns from human controls (A–C) and from the patient (D–F) show overstretched fibers (> 3.60 μm), in low-EGTA rigor (A, D) and calcium-rigor (B, E) solutions. Differences in intensity profiles (C, F) show enhanced (red) and weakened (blue) areas after calcium addition.
Table 2.
Thin-filament-based intensity reflections
| Group | SL (μm) | Condition | Second ALL | Sixth ALL | Seventh ALL |
|---|---|---|---|---|---|
| Control | 2.70 | Preactivation | 0.047 ± 0.007 | 1 | 0.323 ± 0.015 |
| Activation | 0.186 ± 0.009* | 1.120 ± 0.028* | 0.392 ± 0.031* | ||
| >3.60 | Preactivation | 0.074 ± 0.005 | 1 | 0.252 ± 0.003 | |
| Activation | 0.150 ± 0.002* | 0.946 ± 0.006* | 0.236 ± 0.002 | ||
| Patient | 2.70 | Preactivation | 0.033 ± 0.007 | 1 | 0.403 ± 0.015 |
| Activation | 0.118 ± 0.008*,# | 1.019 ± 0.012# | 0.407 ± 0.013 | ||
| >3.60 | Preactivation | 0.043 ± 0.010 | 1 | 0.359 ± 0.011 | |
| Activation | 0.129 ± 0.007* | 1.004 ± 0.020 | 0.357 ± 0.016 |
Values are normalized to total intensity of sixth ALL in absence of calcium. All values are means ± se. SL, sarcomere length.
P <0.05 vs. preactivation for same group (control or patient).
P <0.05 vs. control for same condition (preactivation or activation).
DISCUSSION
Understanding the pathogenesis of muscle weakness in nebulin-related myopathies is primordial to establish potential therapeutic strategies. In the present study, we proved that, in the presence of nebulin mutations, the cycling kinetics of myosin head is severely disrupted during contraction, triggering a cascade of defects and ultimately causing weakness.
Altered myosin cross-bridge cycling rate
We observed a loss in specific force. The combined smaller fiber stiffness (E0) and I1,1 to I1,0 ratio at pCa 4.5, as well as preserved force-stiffness relationship (Fig. 5) suggest that the lower force-generating capacity is predominantly caused by a reduction in the fraction of myosin heads strongly bound to actin rather than a decrease in the force per myosin cross-bridge. This finding is in line with previous studies (6, 8, 9, 33) but is not caused by a thin-filament length reduction and subsequent insufficient thick-thin-filament overlap during contraction since the force-sarcomere length relationship was maintained in the presence of nebulin mutations (Fig. 4). Alternatively, a more plausible underlying mechanism is an alteration of myosin cross-bridge kinetics. The apparent rate constant of force redevelopment ktr was dramatically reduced by 58% with nebulin mutations. Similarly, a trend toward a slower maximum unloaded shortening velocity V0 and a lower in vitro motility speed Vf (−17%) were noticed. The ktr reflects the myosin cross-bridge cycle turnover rate and according to the 2-state cross-bridge model, it is proportional to fapp + gapp, with fapp being the rate constant for attachment and gapp the rate constant for detachment (19, 34). V0 and Vf, on the other hand, are primarily determined by the enzymatic properties of the myosin heads (35, 36) and V0 has gapp as rate-limiting step (21). Hence, here, a slight modification of gapp may occur, but this phenomenon cannot solely explain the large change in ktr. Therefore, a dramatic reduction in fapp must happen in the presence of nebulin mutations. This slower attachment rate suggests a shorter time spent by each myosin molecule in a strongly bound force-producing conformation, limiting the fraction of active myosin cross-bridges. The underlying mechanisms remain unclear but may directly involve the mutations. In fact, skipping of exon 3 may provoke a partial nebulin deficiency in the patient. This may disturb nebulin affinity for other thin-filament proteins and also locally affect the binding of myosin heads to the thin filament. Skipping of exon 22 may alter the central portion of nebulin, which is known to directly interact with actin monomers and myosin heads (37). Therefore, skipping of this exon may directly modify nebulin's interaction with myosin molecules (Fig. 1). Other secondary mechanisms are also likely to occur. Indeed, as Vf was altered in the presence of nebulin mutations, we propose that, in addition to possible direct effects of the mutations, some enzymatic properties of the myosin heads are disturbed and this is probably related to the slower profile of the essential of light chain isoforms (refs. 38, 39 and Fig. 6). To our knowledge, this myosin light chain perturbation has never been reported in nebulin-related myopathies and may be of great importance when considering potential therapeutic approaches such as myosin activators (40).
Incomplete thin-filament activation
In addition to an alteration of myosin cross-bridge cycling kinetics, other phenomena may underlie the loss in force-generating capacity and in the fraction of myosin heads strongly bound to actin in the presence of nebulin mutations. The process of myosin head attachment to actin is controlled by the activation of thin-filament proteins including tropomyosin. Such activation is triggered by calcium as well as by myosin cross-bridges (41). According to the steric blocking model (41, 42), in the absence of calcium, the position of tropomyosin on the thin filament essentially blocks the attachment of myosin heads to actin. When calcium is added, troponin undergoes conformational changes, allowing tropomyosin to move on the thin filament, partially exposing myosin binding sites on actin. Full tropomyosin activation and complete exposure of myosin binding sites on actin occur when myosin cross-bridges further displaces tropomyosin. Various studies, using X-ray diffraction, have verified this double azimuthal movements of tropomyosin away from its resting position by showing an intensification of the far off-meridional part of the second ALL in activated muscles (43). Here, in the presence of nebulin mutations, a weaker intensity change of this ALL was observed during activation (Fig. 8). Consequently, tropomyosin displacement over the surface of actin is partially inhibited, provoking a nonexposure of all myosin binding sites and blocking the attachment of some myosin heads. To evaluate the origin of the tropomyosin incomplete activation and to distinguish between calcium and myosin cross-bridge contributions, fibers were overstretched beyond thin-thick filament overlap and activated (26, 30). Results emphasized that, under this condition, the second ALL intensification is similar between fibers carrying nebulin mutations and the control cells (Fig. 9). This proves that the incomplete tropomyosin activation is solely myosin cross-bridge-related. Then, even though studies show that some nebulin regions directly bind to tropomyosin (44), skipping of exons 3 and 22 in the NEB gene is unlikely to perturb tropomyosin during calcium activation. On the other hand, the inefficient turnover rate of the myosin heads, observed in the present study is undoubtedly responsible, at least in part, for tropomyosin's incomplete activation.
Unaffected calcium sensitivity of contraction
Specific force was similarly affected at all calcium concentrations (pCa 9.0–4.5), as attested by the maintained calcium sensitivity of force production (pCa50) and Hill coefficient (Fig. 5). This finding is in line with what was found in newly born knockout mice (complete lack of nebulin; ref. 6) but differs from data obtained in 10-d-old knockout rodents and in humans where the exon 55 of the NEB gene is deleted (partial nebulin deficiency; refs. 7, 9). The underlying mechanisms for this discrepancy between studies remain unknown and need further investigation.
Molecular basis of weakness and conclusion
Combining all the results, the molecular mechanisms underlying muscle weakness in presence of nebulin mutations are unraveled. That is, the genetic defects together with a secondary myosin essential light chain isoform perturbation deregulate the cycling rate of myosin heads, decreasing the fraction of myosin cross-bridges in the strong binding state. This modulation induces incomplete tropomyosin activation, blocking the exposure of additional myosin binding sites on actin and subsequently hindering the attachment of further myosin cross-bridges. Overall, this leads to a limited cell force production and, thus, to muscle weakness. The nemaline rods are unlikely to contribute to the cascade of events leading to an inability to generate force. According to the literature, these rods are secondary phenomena of the disease and often appear after muscle weakness (45).
As the present study is carried out in 1 patient with nebulin mutations, it may not totally be excluded that other specific mutations in the large NEB gene lead to other particular pathophysiological mechanisms. Nevertheless, according to the present results, in nebulin-related myopathies where a change in cross-bridge cycling kinetics is seen, interventions that would primarily restore the dysfunction may potentially become therapeutic strategies for the future.
Acknowledgments
The authors thank Dr. P. Marcorelles for the electron microscopy study and Y. Hedström, C. Dumez, and I. Viau for excellent technical assistance. The authors also thank Dr. K. Pelin for helpful comments on the manuscript. X-ray experiments were performed under the approval of the SPring-8 Proposal Review Committee (2009B1918).
This study was supported by grants from the Swedish Research Council, Association Française contre les Myopathies, Tore Nilson Stiftelse, Stiftelsen Apotekare Hedbergs Fond för Medicinsk Forskning, and Rektors Resebidrag från Wallenbergstiftelsen to J.O.; the U.S. National Institutes of Health (AR-048816) to J.-P.J.; the Academy of Finland, Association Française contre les Myopathies, Sigrid Jusélius Foundation, Finska Läkaresällskapet, and Medicinska Understödsföreningen Liv och Hälsa to C.W.-P.; and the Swedish Research Council (8651), Association Française contre les Myopathies, Swedish Cancer Foundation, European Commission (MyoAge, Fp7 CT-223756), King Gustaf V and Queen Victoria's Foundation, and Thuréus Foundation to L.L.
REFERENCES
- 1. Wang K. (1996) Titin/connectin and nebulin: giant protein rulers of muscle structure and function. Adv. Biophys. 33, 123–134 [PubMed] [Google Scholar]
- 2. Wang K., Wright J. (1988) Architecture of the sarcomere matrix of skeletal muscle: immunoelectron microscopic evidence that suggests a set of parallel inextensible nebulin filaments anchored at the Z line. J. Cell Biol. 107, 2199–2212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Castillo A., Nowak R., Littlefield K. P., Fowler V. M., Littlefield R. S. (2009) A nebulin ruler does not dictate thin filament lengths. Biophys. J. 96, 1856–1865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pappas C. T., Krieg P. A., Gregorio C. C. Nebulin regulates actin filament lengths by a stabilization mechanism. J. Cell Biol. 189, 859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tonino P., Pappas C. T., Hudson B. D., Labeit S., Gregorio C. C., Granzier H. Reduced myofibrillar connectivity and increased Z-disk width in nebulin-deficient skeletal muscle. J. Cell Sci. 123, 384–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bang M. L., Caremani M., Brunello E., Littlefield R., Lieber R. L., Chen J., Lombardi V., Linari M. (2009) Nebulin plays a direct role in promoting strong actin-myosin interactions. FASEB J. 23, 4117–4125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chandra M., Mamidi R., Ford S., Hidalgo C., Witt C., Ottenheijm C., Labeit S., Granzier H. (2009) Nebulin alters cross-bridge cycling kinetics and increases thin filament activation: a novel mechanism for increasing tension and reducing tension cost. J. Biol. Chem. 284, 30889–30896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ottenheijm C. A., Hooijman P., Dechene E. T., Stienen G. J., Beggs A. H., Granzier H. (2010) Altered myofilament function depresses force generation in patients with nebulin-based nemaline myopathy (NEM2). J. Struct. Biol. 170, 334–343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ottenheijm C. A., Witt C. C., Stienen G. J., Labeit S., Beggs A. H., Granzier H. (2009) Thin filament length dysregulation contributes to muscle weakness in nemaline myopathy patients with nebulin deficiency. Hum. Mol. Genet. 18, 2359–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lehtokari V. L., Pelin K., Sandbacka M., Ranta S., Donner K., Muntoni F., Sewry C., Angelini C., Bushby K., Van den Bergh P., Iannaccone S., Laing N. G., Wallgren-Pettersson C. (2006) Identification of 45 novel mutations in the nebulin gene associated with autosomal recessive nemaline myopathy. Hum. Mutat. 27, 946–956 [DOI] [PubMed] [Google Scholar]
- 11. Wallgren-Pettersson C., Lehtokari V. L., Kalimo H., Paetau A., Nuutinen E., Hackman P., Sewry C., Pelin K., Udd B. (2007) Distal myopathy caused by homozygous missense mutations in the nebulin gene. Brain 130, 1465–1476 [DOI] [PubMed] [Google Scholar]
- 12. Romero N. B., Lehtokari V. L., Quijano-Roy S., Monnier N., Claeys K. G., Carlier R. Y., Pellegrini N., Orlikowski D., Barois A., Laing N. G., Lunardi J., Fardeau M., Pelin K., Wallgren-Pettersson C. (2009) Core-rod myopathy caused by mutations in the nebulin gene. Neurology 73, 1159–1161 [DOI] [PubMed] [Google Scholar]
- 13. Frontera W. R., Larsson L. (1997) Contractile studies of single human skeletal muscle fibers: a comparison of different muscles, permeabilization procedures, and storage techniques. Muscle Nerve 20, 948–952 [DOI] [PubMed] [Google Scholar]
- 14. Penisson-Besnier I., Monnier N., Toutain A., Dubas F., Laing N. (2007) A second pedigree with autosomal dominant nemaline myopathy caused by TPM3 mutation: a clinical and pathological study. Neuromuscul. Disord. 17, 330–337 [DOI] [PubMed] [Google Scholar]
- 15. Moss R. L. (1979) Sarcomere length-tension relations of frog skinned muscle fibres during calcium activation at short lengths. J. Physiol. 292, 177–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larsson L., Moss R. L. (1993) Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J. Physiol. 472, 595–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Martyn D. A., Smith L., Kreutziger K. L., Xu S., Yu L. C., Regnier M. (2007) The effects of force inhibition by sodium vanadate on cross-bridge binding, force redevelopment, and Ca2+ activation in cardiac muscle. Biophys. J. 92, 4379–4390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. McDonald K. S., Fitts R. H. (1995) Effect of hindlimb unloading on rat soleus fiber force, stiffness, and calcium sensitivity. J. Appl. Physiol. 79, 1796–1802 [DOI] [PubMed] [Google Scholar]
- 19. Brenner B., Eisenberg E. (1986) Rate of force generation in muscle: correlation with actomyosin ATPase activity in solution. Proc. Natl. Acad. Sci. U. S. A. 83, 3542–3546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Regnier M., Martyn D. A., Chase P. B. (1998) Calcium regulation of tension redevelopment kinetics with 2-deoxy-ATP or low [ATP] in rabbit skeletal muscle. Biophys. J. 74, 2005–2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Edman K. A. (1979) The velocity of unloaded shortening and its relation to sarcomere length and isometric force in vertebrate muscle fibres. J. Physiol. 291, 143–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hook P., Larsson L. (2000) Actomyosin interactions in a novel single muscle fiber in vitro motility assay. J. Muscle Res. Cell. Motil. 21, 357–365 [DOI] [PubMed] [Google Scholar]
- 23. Hook P., Li X., Sleep J., Hughes S., Larsson L. (1999) In vitro motility speed of slow myosin extracted from single soleus fibres from young and old rats. J. Physiol. 520, 463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jin J. P. (1995) Cloned rat cardiac titin class I and class II motifs. Expression, purification, characterization, and interaction with F-actin. J. Biol. Chem. 270, 6908–6916 [PubMed] [Google Scholar]
- 25. Ogut O., Hossain M. M., Jin J. P. (2003) Interactions between nebulin-like motifs and thin filament regulatory proteins. J. Biol. Chem. 278, 3089–3097 [DOI] [PubMed] [Google Scholar]
- 26. Iwamoto H. (2009) Evidence for unique structural change of thin filaments upon calcium activation of insect flight muscle. J. Mol. Biol. 390, 99–111 [DOI] [PubMed] [Google Scholar]
- 27. Iwamoto H., Oiwa K., Suzuki T., Fujisawa T. (2001) X-ray diffraction evidence for the lack of stereospecific protein interactions in highly activated actomyosin complex. J. Mol. Biol. 305, 863–874 [DOI] [PubMed] [Google Scholar]
- 28. Iwamoto H., Oiwa K., Suzuki T., Fujisawa T. (2002) States of thin filament regulatory proteins as revealed by combined cross-linking/X-ray diffraction techniques. J. Mol. Biol. 317, 707–720 [DOI] [PubMed] [Google Scholar]
- 29. Iwamoto H., Wakayama J., Fujisawa T., Yagi N. (2003) Static and dynamic x-ray diffraction recordings from living mammalian and amphibian skeletal muscles. Biophys. J. 85, 2492–2506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ochala J., Iwamoto H., Larsson L., Yagi N. A myopathy-linked tropomyosin mutation severely alters thin filament conformational changes during activation. Proc. Natl. Acad. Sci. U. S. A. 107, 9807–9812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wakayama J., Tamura T., Yagi N., Iwamoto H. (2004) Structural transients of contractile proteins upon sudden ATP liberation in skeletal muscle fibers. Biophys. J. 87, 430–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yagi N. (2003) An x-ray diffraction study on early structural changes in skeletal muscle contraction. Biophys. J. 84, 1093–1102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Gokhin D. S., Bang M. L., Zhang J., Chen J., Lieber R. L. (2009) Reduced thin filament length in nebulin-knockout skeletal muscle alters isometric contractile properties. Am. J. Physiol. Cell Physiol. 296, C1123–C1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Huxley A. F. (1957) Muscle structure and theories of contraction. Prog. Biophys. Biophys. Chem. 7, 255–318 [PubMed] [Google Scholar]
- 35. Siemankowski R. F., White H. D. (1984) Kinetics of the interaction between actin, ADP, and cardiac myosin-S1. J. Biol. Chem. 259, 5045–5053 [PubMed] [Google Scholar]
- 36. Thedinga E., Karim N., Kraft T., Brenner B. (1999) A single-fiber in vitro motility assay. In vitro sliding velocity of F-actin vs. unloaded shortening velocity in skinned muscle fibers. J. Muscle Res. Cell. Motil. 20, 785–796 [DOI] [PubMed] [Google Scholar]
- 37. Root D. D., Wang K. (2001) High-affinity actin-binding nebulin fragments influence the actoS1 complex. Biochemistry 40, 1171–1186 [DOI] [PubMed] [Google Scholar]
- 38. Gordon A. M., Homsher E., Regnier M. (2000) Regulation of contraction in striated muscle. Physiol. Rev. 80, 853–924 [DOI] [PubMed] [Google Scholar]
- 39. Reiser P. J., Bicer S. (2006) Multiple isoforms of myosin light chain 1 in pig diaphragm slow fibers: Correlation with maximal shortening velocity and force generation. Arch. Biochem. Biophys. 456, 112–118 [DOI] [PubMed] [Google Scholar]
- 40. Solaro R. J. (2009) CK-1827452, a sarcomere-directed cardiac myosin activator for acute and chronic heart disease. IDrugs 12, 243–251 [PubMed] [Google Scholar]
- 41. Lehman W., Craig R. (2008) Tropomyosin and the steric mechanism of muscle regulation. Adv. Exp. Med. Biol. 644, 95–109 [DOI] [PubMed] [Google Scholar]
- 42. Huxley H. E. (1973) Structural changes in the actin- and myosin-containing filaments during contraction. Cold Spring Harbor Symp. Quant. Biol. 37, 361–376 [Google Scholar]
- 43. Parry D. A., Squire J. M. (1973) Structural role of tropomyosin in muscle regulation: analysis of the x-ray diffraction patterns from relaxed and contracting muscles. J. Mol. Biol. 75, 33–55 [DOI] [PubMed] [Google Scholar]
- 44. Wang K., Knipfer M., Huang Q. Q., van Heerden A., Hsu L. C., Gutierrez G., Quian X. L., Stedman H. (1996) Human skeletal muscle nebulin sequence encodes a blueprint for thin filament architecture. Sequence motifs and affinity profiles of tandem repeats and terminal SH3. J. Biol. Chem. 271, 4304–4314 [DOI] [PubMed] [Google Scholar]
- 45. Ochala J. (2008) Thin filament proteins mutations associated with skeletal myopathies: defective regulation of muscle contraction. J. Mol. Med. 86, 1197–1204 [DOI] [PubMed] [Google Scholar]