Abstract
Human endothelial progenitor cells (hEPCs) participate in neovascularization of ischemic tissues. Function and number of hEPCs decline in patients with cardiovascular disease, and therapeutic strategies to enhance hEPC function remain an important field of investigation. The Wnt signaling system, comprising 19 lipophilic proteins, regulates vascular patterning in the developing embryo. However, the effects of Wnts on hEPCs and the adult vasculature remain unclear. We demonstrate here that Wnt1 is expressed in a subset of endothelial cells lining the murine embryonic dorsal aorta and is reactivated in malignant angiosarcoma, suggesting a strong association of Wnt1 with angiogenesis. We investigate the effects of Wnt1 in enhancing hEPC function and blood flow to ischemic tissues and show that Wnt1 enhances the proliferative and angiogenic functions of hEPCs in a hepatocyte growth factor (HGF)-dependent manner. Injection of Wnt1-expressing hEPCs increases blood flow and capillary density in murine ischemic hindlimbs. Furthermore, injection of Wnt1 protein alone similarly increases blood flow and capillary density in ischemic hindlimbs, and this effect is associated with increased HGF expression in ischemic muscle. These findings demonstrate that Wnt1, a marker of neural crest cells and hitherto unknown angiogenic function, is a novel angiogenic protein that is expressed in developing endothelial cells, exerts salutary effects on postnatal hEPCs, and can be therapeutically deployed to increase blood flow and angiogenesis in ischemic tissues.—Gherghe, C. M., Duan, J., Gong, J., Rojas, M., Klauber-Demore, N., Majesky, M., Deb, A. Wnt1 is a proangiogenic molecule, enhances human endothelial progenitor function, and increases blood flow to ischemic limbs in a HGF-dependent manner.
Keywords: angiogenesis, vascular progenitor, paracrine
Peripheral arterial disease is a debilitating condition caused by atherosclerotic narrowing and reduction of arterial blood flow to the extremities. Arterial insufficiency induces skin and muscle damage and severely reduces functionality of ischemic limbs. Estimates of the prevalence of peripheral arterial disease vary widely, but it is thought to affect 10–15% of the population >65 yr of age in the United States (1). Strategies to induce new blood vessel formation and increase blood flow to ischemic regions hold great promise for the treatment of peripheral arterial disease.
New blood vessel formation in the adult has been traditionally thought to arise from proliferation of existing blood vessels (2), but a large body of literature suggests that endothelial progenitor cells (EPCs) contribute to formation of new blood vessels in postnatal life (3–5). Although no universal marker for EPCs is known to date, human EPCs (hEPCs) have been classified as “early” or “late” depending on the time of outgrowth from culture of peripheral blood or bone marrow mononuclear cells (6). Early-outgrowth EPCs appear within a few days of culture, secrete important growth factors, and are thought to exert paracrine effects, while late-outgrowth EPCs appear within a few weeks, form cobblestone colonies typical of endothelial cells, and form capillary tube-like structures in vitro. hEPCs participate in neovascularization of ischemic tissues, either by directly incorporating into new blood vessels or by stimulating new vessel growth by paracrine mechanisms (7–9). Patients with vascular disease or at risk of developing vascular disease exhibit decreased numbers as well as impaired function of EPCs (10). Genes playing a critical role during development and organogenesis can exert therapeutic effects on postnatal organ dysfunction (11, 12). The Wnt signaling system, comprising 19 closely related lipophilic proteins, plays a critical role in organogenesis, as well as postnatal processes, such as carcinogenesis and wound repair (13). Several Wnts, such as Wnt2b, play an important role in developing placental vasculature, and Wnt7b deficiency leads to severe defects in pulmonary vasculature, resulting in perinatal lethality (14). Canonical Wnt/β-catenin signaling promotes proliferation of human umbilical vein endothelial cells (HUVECs) in vitro (15). We investigated the role of Wnts in enhancing function of late-outgrowth hEPCs and angiogenesis in ischemic tissues. Surprisingly, we observed that Wnt1, a marker of neural crest cells with no hitherto known vascular function, is expressed in a subset of endothelial cells in the murine embryonic dorsal aorta and is reactivated in endothelium-rich portions of angiosarcomas, suggesting a strong association between Wnt1 and angiogenesis. These observations led us to investigate the effects of Wnt1 on hEPCs and angiogenesis in ischemic tissues. Here, we report the effects of Wnt1 in enhancing hEPC function and demonstrate the beneficial effects of injecting Wnt1-overexpressing hEPCs into ischemic murine hindlimbs. We show that Wnt1 enhances hEPC function in a hepatocyte growth factor (HGF)-dependent manner, and injection of Wnt1 protein alone into the ischemic limb increases HGF expression in ischemic tissues, induces new capillary formation, and improves blood flow. These observations demonstrate that Wnt1 is a novel angiogenic protein that enhances hEPC function and blood flow to ischemic limbs and form a rational underpinning for the therapeutic use of Wnt1 in enhancing blood flow in ischemic tissues.
MATERIALS AND METHODS
EPC isolation and culture
Blood (100 ml) was collected from healthy subjects. Blood collection from human patients was approved by the Institutional Review Board of the University of North Carolina–Chapel Hill. Late-outgrowth EPCs were isolated as described previously (16). Briefly, the collected blood was initially mixed (1:2 ratio) with HBSS containing 0.5 mM EDTA and 0.1% BSA, and the mixed blood (35 ml) carefully layered on top of 15 ml Ficoll Paque (GE Healthcare, New York, NY, USA). Mononuclear cells were isolated by density gradient centrifugation by spinning the prepared blood samples at 400 g for 30 min at room temperature. The mononuclear layer was collected with a 1-ml Pasteur pipette and washed twice with EGM-2 medium (Lonza, Walkersville, MD, USA) containing 10% FBS. Cells were then plated on 6-well plates (20×106 cells/well) in complete EGM-2 medium containing 10% FBS and incubated at 37°C and 5% CO2. Prior to plating, plates were coated with type 1 rat tail collagen (BD Biosciences, Franklin Lakes, NJ, USA). Medium was initially replaced after 72 h, then every other day.
EPC characterization
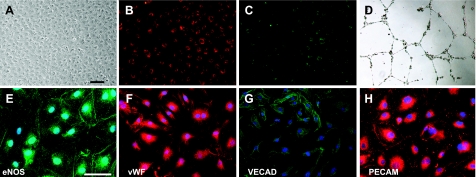
Typical cobblestone-appearing colonies were observed within 2–3 wk of culture of peripheral blood mononuclear cells. Cells were further passaged, and all the experiments were performed using cells between passages 5 and 9. EPCs were characterized as double-positive for acetylated low-density lipoprotein (Alexa 594 Dil AcLDL; Invitrogen, Carlsbad, CA, USA) and isolectin GS-IB4 (Alexa 488-labeled; Invitrogen) uptake. Cells were first incubated with DiI-acLDL (10 μg/ml) for 4 h and subsequently with isolectin (2.5 μg/ml) for 1 h. Phenotype of EPCs was further confirmed by immunofluorescence staining using typical endothelial cell antibodies, such as endothelial nitric oxide synthase (anti-eNOS; BD Biosciences), von Willebrand factor (anti-vWF; Santa Cruz, Santa Cruz, CA, USA), vascular endothelial cadherin (VECAD; anti-VE-cadherin; Abcam, Cambridge, MA, USA), and platelet/endothelial cell adhesion molecule (anti-PECAM; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Cells were stained using the primary antibody at 1:40 dilution for 1 h at room temperature, followed by the use of appropriate secondary at a dilution of 1:2000 in a similar manner. Primary antibody was omitted in negative controls.
Proliferation assay
EPCs (5×103) were seeded on rat tail type 1 collagen-precoated 48-well plates in EGM-2 medium with 10% FBS and were allowed to attach overnight. Subsequently, EPCs were washed with PBS and serum starved for 8 h in EGM-2 without serum. Medium was then removed, and the cells were supplemented with EGM-2 (2% FBS) with or without Wnt 1 protein (AbD Serotec, Raleigh, NC, USA) at 5 or 25 nM. Cells grown with EGM-2 (10% FBS) served as positive controls. Proliferation was assayed at 72 h using the CyQuant cellular proliferation assay kit (Invitrogen), according to the manufacturer's instructions. Fluorescence was measured using a Wallac Victor 1420 multilabel counter (PerkinElmer, Waltham, MA, USA).
Matrigel tube formation assay
Human EPCs (3.5×104) were plated on matrigel (BD Biosciences) in 24-well plates in EGM-2 (2% FBS) with or without Wnt1, with cells grown with EGM-2 (10% FBS) serving as positive controls. For inhibition of canonical pathway, human Dkk-1 protein (R&D Systems, Minneapolis, MN, USA) was added at a concentration of 50 nM at the same time with Wnt1. All the tubes formed in each well were counted 24 h after addition of Wnt1 or Wnt1 and Dkk-1. For silencing HGF expression, 50 nM scrambled siRNA or anti-HGF siRNA (sc-37111; Santa Cruz Biotechnology) was added at the same time with Wnt1, and tube analysis on matrigel was performed in a similar fashion. Capillary density was determined by 2 independent investigators masked to the control or experimental groups.
Real-time PCR
Total RNA was extracted using the SV total RNA isolation system (Promega, Fitchburg, WI, USA), and cDNA was synthesized using the Reverse Transcription System (Promega), according to the manufacturer's instructions, and quantitative PCR reactions were performed on a Bio-Rad IQ5 Detection System (Bio-Rad, Richmond, CA, USA).
Immunohistochemistry and immunofluorescence
Immunohistochemistry experiments were carried using anti-Wnt1 (Abcam) and anti-PECAM (Santa Cruz Biotechnology) antibodies. Sections were deparaffinized, subjected to antigen retrieval with citric acid, and incubated with Wnt1 or PECAM primary antibodies at 1:200 dilution. Tissues were then processed using secondary antibodies and reagents from Vectastain Kit (Vector Laboratories, Burlingame, CA, USA), according to the manufacturer's instructions. For double immunofluorescent staining, following deparaffinization, human tissue sections were incubated with anti-PECAM (overnight at 4°C) and anti-Wnt1 (30 min at room temperature) antibodies, followed by treatment with appropriate secondary antibodies. Sections were then analyzed and photographed using a Leica confocal microscope (Leica Microsystems, Wetzlar, Germany). For immunostaining of embryos, embryos were harvested and snap-frozen, and sections were fixed with acetone prior to immunostaining, using a similar procedure as above.
Retroviral infection
Retroviruses were generated using pRetroX-IRES-ZsGreen1 plasmid (Clontech, Mountain View, CA, USA), with or without the Wnt 1 gene, at the viral core facility at the University of North Carolina–Chapel Hill. EPCs were plated on 100-mm culture dishes and infected with retroviral particles (MOI 1:10) when they reached a density of 70–80%. Infected EPCs were sorted to a purity of >95% using a reflection sorter (iCyt Mission Technology, Champaign, IL, USA).
Western blot analysis
hEPCs were lysed, and cytosolic protein was isolated using the BioVision Cell Fractionation System (K25–50; BioVision, Inc., Mountain View, CA, USA), according to the manufacturer's instructions. Rabbit anti β-catenin antibodies (Cell Signaling Technology, Danvers, MA, USA) and an appropriate secondary antibody were used at 1:1000 dilution.
X-gal staining
Whole-mount embryos were fixed for 1 h at room temperature in 0.1 M phosphate buffer (pH 7.3), supplemented with 5 mM EDTA, pH 7.3, 2 mM MgCl2, and 0.2% glutaraldehyde. Embryos were then incubated overnight at 37°C in 0.1 M phosphate buffer (pH 7.3), supplemented with 2 mM MgCl2, 5 mM potassium ferrocyanide (K4Fe(CN)6·3H2O), 5 mM potassium ferricyanide (K3Fe(CN)6), and X-gal (5-bromo-4-chloro-3-indolyl-β-d-galactoside) to a final concentration of 1 mg/ml.
Animal model of hindlimb ischemia
All animal experiments were approved by the Institutional Animal Care and Use Committee at the University of North Carolina–Chapel Hill. We used 6-wk-old NOD/SCID mice (005557; Jackson Laboratories, Bar Harbor, ME, USA) for injection of hEPCs, while 6-wk-old C57BL/6 mice (Jackson Laboratories) were used for injection of Wnt1 alone after induction of hindlimb ischemia. Mice were anesthetized with 2% isoflurane and subjected to unilateral hindlimb ischemia. The femoral artery was carefully separated from the vein, ligated, and excised immediately distal to the bifurcation of the anterior epigastric and lateral caudal femoral arteries. The anterior epigastric artery was also ligated. Blood flow measurements were done under general anesthesia using a computer-assisted laser-Doppler perfusion image analyzer (Periscan PIM 3 blood perfusion imager; Perimed AB, Stockholm, Sweden) preoperatively and postoperatively, and at d 1, 3, and 7. Throughout the surgery and during laser-Doppler measurements, body temperature was maintained at 37.0 ± 0.5°C. PBS, Wnt 1, or EPCs (5×105; 100 μl final volume) was directly injected into the gastrocnemius muscle 15 min following femoral artery ligation. After 7 d, gastrocnemius muscles from 4 animals/group were isolated, fixed in 4% PFA for 30 min and 30% sucrose overnight, embedded in optimal cutting temperature (OCT) compound (Sakura Finetek, Torrance, CA, USA), and flash-frozen in liquid nitrogen. Capillary densities of both ischemic and nonischemic skeletal muscle tissues were analyzed by counterstaining with isolectin and determined by counting capillary vessels in 10 randomly selected microscopic fields. Investigators determining capillary counts, as well as assessing blood flow by laser-Doppler measurements, were masked to the groups.
Statistical analysis
All statistical correlations were calculated as standard t test. Bars in all figures represent means ± se.
RESULTS
Wnt1 is expressed in the developing vasculature and reactivated in vascular tumors in adult life
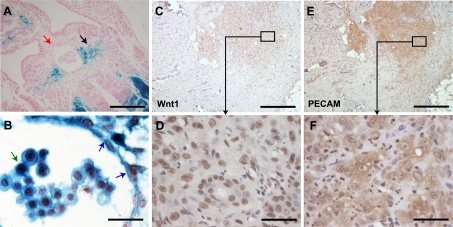
We crossed Wnt1 Cre-expressing mice with the lineage reporter Rosa26RlacZ mice and examined the aorto-gonado-mesonephric (AGM) region of 10.5 days postfertilization (dpf) Wnt1Cre/R26RlacZ embryos (Fig. 1A). Cells expressing Wnt1 anytime during embryonic development will express lacZ, and we observed strong lacZ expression in a subset of endothelial cells lining the dorsal aorta, as well as in presumably hematopoietic cells residing in the lumen of the aorta (Fig. 1B). Double immunostaining with endothelial and Wnt1 antibodies confirmed that endothelial cells lining the dorsal aorta expressed Wnt1 (Supplemental Fig S1A). Wnt1 was originally described as a mammary tumor oncogene, but the expression of Wnt1 in tumor vasculature is unknown (17). We examined human skin angiosarcomas and observed that highly undifferentiated regions of the tumor stained for Wnt1. Staining of parallel sections (7 μM apart) demonstrated that regions of the tumor richly staining for Wnt1 (Fig. 1C, D) also expressed the endothelial marker PECAM (Fig. 1E, F). Furthermore, double immunostaining of tumor-rich regions confirmed that endothelial cells identified by PECAM expression also expressed Wnt1 (Supplemental Fig S1B). The expression of Wnt1 in the embryonic endothelium, as well as its reactivation in the endothelium of angiosarcomas, suggests a strong association of Wnt1 with angiogenesis. These observations led us to determine whether Wnt1 exerts physiological effects on postnatal hEPCs and whether this molecule could be therapeutically deployed to enhance blood flow in ischemic tissues.
Figure 1.
Wnt1 is expressed in developing endothelium and reactivated in human skin angiosarcomas. A) Xgal staining of the AGM region in 10.5 dpf Wnt1Cre/R26RlacZ embryos shows lacZ expression in peridorsal mesenchyme (black arrow; red arrow indicates dorsal aorta) B) Higher magnification demonstrates Xgal staining of endothelial cells (blue arrows) and of cells of hematopoietic origin (green arrow). C–F) Immunohistochemistry for Wnt1 (C, E) and PECAM (D, F) in adjacent sections (7 μm apart) of human skin angiosarcoma demonstrates expression of Wnt1 and PECAM in undifferentiated regions of the tumor. Panels E and F show higher-magnification views of boxed areas in C and D, respectively. Scale bars: 200 μm (A); 20 μm (B); 500 μm (C, E); 40 μm (D, F).
Wnt1 significantly enhances hEPC proliferation and angiogenesis in vitro
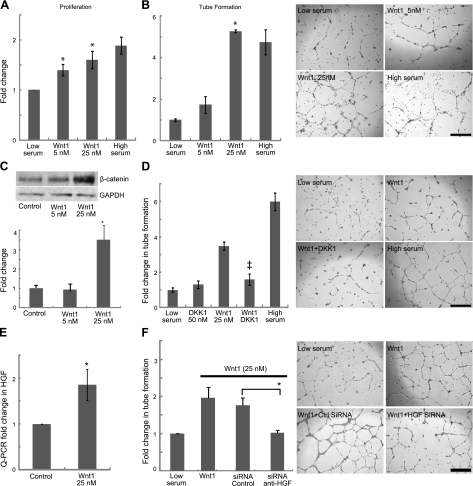
We isolated hEPCs from peripheral blood of healthy subjects and confirmed their endothelial phenotype (Fig. 2). Wnt1 was not expressed by hEPCs (Supplemental Fig. S2A). We treated hEPCs grown under low-serum conditions with human Wnt1 recombinant protein to determine effects on proliferation and angiogenesis. Human EPCs grown under high-serum conditions served as positive controls. Wnt1 significantly enhanced hEPC proliferation by ∼2-fold (Fig. 3A) in a concentration-dependent manner and dramatically induced tube formation of hEPCs within 24 h compared to untreated controls (Fig. 3B). We were surprised to observe that the tube formation of hEPCs under low-serum conditions in the presence of Wnt1 was equivalent to hEPCs grown with high serum (10%) complementation. We subsequently interrogated the downstream mechanisms of Wnt1 effects on hEPC. Wnt1 is known to exert its effects through β-catenin-dependent and -independent pathways (18). Human EPCs were grown under low-serum conditions with or without Wnt1 for 24 h, followed by analysis of cytoplasmic β-catenin levels. Western blotting showed a concentration-dependent increase in cytoplasmic β-catenin levels in hEPCs treated with Wnt1 (Fig. 3C). We next inhibited the canonical pathway using the specific canonical pathway inhibitor Dickoppf-1 (Dkk-1; ref. 13) to determine the effects of this pathway in mediating Wnt1 tube formation of hEPCs. Wnt1 increased matrigel tube formation by almost 4-fold, and the concomitant addition of Dkk-1 reduced tube formation by almost 50% (Fig. 3D). Dkk-1 in the absence of Wnt1 did not affect tube formation (Fig. 3D). These studies demonstrate that Wnt1 exerts proangiogenic effects on hEPC predominantly via the canonical β-catenin pathway. To explore whether Wnt1 might exert proangiogenic effects via up-regulation of angiogenic cytokines, we screened for changes in expression of several angiogenic molecules following Wnt1 treatment of hEPC (Supplemental Fig S2B) and observed a significant increase in hepatocyte growth factor (HGF) expression (Fig. 3E). We next transfected hEPCS with siRNA targeting HGF (Supplemental Fig S3A) and observed a 50% decrease in tube formation following HGF silencing in Wnt1-treated hEPCs (Fig. 3F), suggesting a critical role of HGF in Wnt1-mediated angiogenesis. Human EPCs treated with scrambled siRNA controls did not exhibit any changes in tube formation (Fig. 3F).
Figure 2.
Characterization of human peripheral blood-derived late outgrowth EPCs. A–D) Typical cobblestone-like colony of late-outgrowth hEPCs (A) that uptakes both acetylated low-density lipoprotein (B) and isolectin GS-IB4 (C), and forms tubes on matrigel (D). E–H) Human EPCs stain for common endothelial markers, such as eNOS (E), vWF (F), VECAD (G), and PECAM (H). Scale bars = 100 μm (A–50 μm (E–H).
Figure 3.
Wnt1 effects on hEPC. A) Wnt1 effects on hEPC proliferation (n=5). *P < 0.05 vs. low serum. B) Matrigel tube formation (n=3) and representative images. *P < 0.05 vs. low serum. C) Western blot for β-catenin on Wnt1-treated hEPC with corresponding densitometry analysis. D) DKK1 (50 nM) effects on Wnt1 matrigel tube formation (n=3) and representative images. ‡P < 0.05 vs. Wnt1 (25 nM). E) Expression of HGF in hEPC treated with Wnt1 compared to BSA-treated control. F) Inhibition of Wnt1-mediated tube formation via siRNA against HGF (n=5) and representative images. *P < 0.05. Scale bars = 500 μM.
Wnt1-overexpressing EPCs enhance blood flow recovery in a hindlimb ischemia mouse model
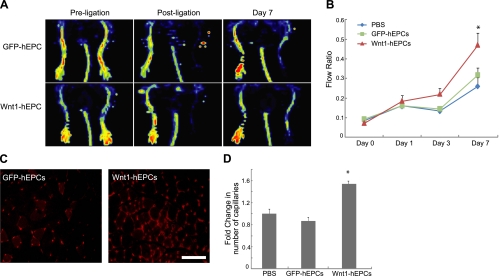
Given our observations on the beneficial effects of Wnt1 in enhancing hEPC function, we next investigated whether administration of Wnt1-overexpressing EPCs could increase angiogenesis and improve blood flow to ischemic hindlimbs. There is a paucity of studies determining the effects of late outgrowth EPC in improving blood flow, but late-outgrowth EPCs have been used as vehicles for gene delivery or for targeting tumor angiogenesis in vivo (19, 20). We infected hEPCs with retroviruses containing Wnt1 coexpressing ZsGreen fluorescent protein (Wnt1-hEPCs) or control vector carrying ZsGreen alone (GFP-hEPCs). Cells were then sorted for GFP using flow cytometry to obtain a purity of >95% (Supplemental Fig. S3B). We induced unilateral hindlimb ischemia via femoral artery ligation and excision in NOD/SCID mice and confirmed successful interruption of distal blood flow by laser-Doppler techniques (Fig. 4A). We then injected PBS, GFP-hEPC, or Wnt1-hEPC (5×105 cells) directly into the gastrocnemius muscle distal to the site of femoral artery ligature. We quantified the recovery of blood flow in the pedal area at 1, 3, and 7 d and expressed it as the ratio of blood flow between the ischemic and contralateral uninjured limb. Injection of Wnt1-hEPCs resulted in an almost 2-fold significant increase in blood flow at 7 d following injury compared to injection of GFP-hEPCs (Fig. 4A, B). Staining for capillaries with isolectin showed a 2-fold increase in capillary formation in muscle injected with Wnt1-hEPCs (Fig. 4C, D). When we examined whether injected hEPCs incorporated into new capillaries in ischemic tissues, we did not observe any differences in the number of GFP-hEPC and Wnt1-hEPC-injected cells that also stained with isolectin (data not shown), suggestive of a paracrine effect rather than direct incorporation into vasculature.
Figure 4.
Wnt1-overexpressing hEPCs enhance blood flow recovery in ischemic limb. A) Doppler analysis of NOD/SCID mice preischemia, postischemia, and at 7 d following injection of Wnt1-hEPCs or GFP-hEPCs. B) Blood flow recovery in mice injected with PBS (n=7), GFP-hEPCs (n=12), and Wnt1-hEPCs (n=12). *P < 0.05 vs. GFP-hEPCs. C) Representative capillary staining with isolectin (GS-IB4) in ischemic tissues injected with GFP-hEPCs or Wnt1-hEPCs. Scale bar = 100 μm. D) Capillary count analysis in ischemic tissues (n=4 mice/group). *P < 0.05 vs. PBS control.
Wnt 1 protein significantly enhances blood flow recovery in a murine hindlimb ischemia mouse model
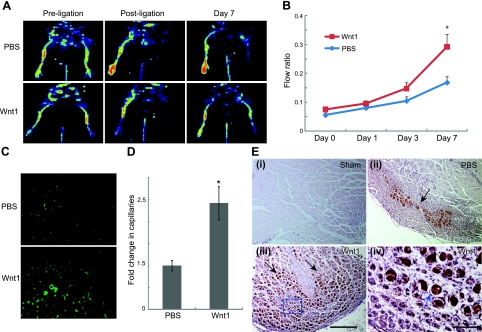
The improvement of blood flow following injection of Wnt1-hEPCs associated with a lack of incorporation into vasculature suggests that the mechanism of benefit is primarily secondary to paracrine mechanisms. We thus subsequently investigated whether injection of Wnt 1 protein alone increases blood flow to ischemic tissues. Following induction and confirmation of hindlimb ischemia by laser-Doppler scanning (Fig. 5A), we injected Wnt1 protein (1 μg/animal) or PBS directly into the gastrocnemius muscle. By 7 d, Wnt1 injected mice had significantly higher blood flow compared to PBS controls (Fig. 5A, B). The 2-fold increase in blood flow recovery was associated with higher number of capillaries in Wnt1 injected hindlimbs (Fig. 5C, D). As Wnt1 effects on hEPCs were mediated by HGF, we determined whether HGF was increased in Wnt1-injected ischemic hindlimbs. Analysis of hindlimb sections injected with PBS following injury demonstrated focal up-regulation of HGF in the region of injury compared to sham-injured muscle (Fig. 5Ei, ii), consistent with prior reports of ischemia inducing HGF expression (21). However, animals that received Wnt1 in ischemic tissues showed dramatic expression of HGF in the region of injury (Fig. 5Eiii, iv). These observations support our in vitro data of the critical roles of HGF in mediating Wnt1 effects on angiogenesis.
Figure 5.
Wnt1 protein enhances blood flow recovery in ischemic limb. A) Doppler analysis of C57BL/6 mice preischemia, immediately after femoral artery ligation, and at 7 d after ligation in PBS- and Wnt1-injected animals. B) Blood flow recovery in PBS (n=8) and Wnt1 (n=12) treatment groups. C) Representative section showing capillaries in ischemic tissues of PBS- and Wnt1-injected animals. D) Fold change in number of capillaries (stained with isolectin) of Wnt1- vs. PBS-injected animals (n=5) E) i–iii) Immunohistochemistry for HGF (black arrows) in sham-injured muscle (i) and muscle subjected to ischemic injury injected with PBS (ii) or Wnt1 (iii). iv) Higher-magnification view of boxed area in panel iii (blue arrow). Scale bars = 500 μm (Eiii); 100 μm (Eiv). *P < 0.05.
DISCUSSION
In this report, we describe the unexpected proangiogenic effects of Wnt1. Wnt1 is expressed in the developing central nervous system and is a marker of neural crest cells. Overexpression of Wnt1/β-catenin induces HUVEC proliferation in vitro (15, 22), but vasculogenic functions of Wnt1 have not been described in the developing embryo or postnatal life. We show that Wnt1 is expressed in a subset of endothelial cells lining the embryonic dorsal aorta. Although a portion of the aortic arch is derived from Wnt1-expressing neural crest cells, hEPCs do not express Wnt1, suggesting that Wnt1 is transiently expressed or that Wnt1-expressing endothelial cells observed in the dorsal aorta either migrate, die, or remain below the detection threshold of our assay. However, Wnt1 expression is dramatically reactivated in endothelial cells of malignant angiosarcomas. The expression of Wnt1 in embryonic as well as tumor endothelium suggests a strong association between Wnt1 and angiogenesis. Genes expressed during organ development can exert important physiological effects on the postnatal organ (11, 12). The angiogenic effects of canonical Wnt signaling have been reported previously, but as the specific effects of Wnt1 on angiogenesis are unknown, we investigated the functions of Wnt1 on hEPC function and angiogenesis.
We observed that Wnt1 dramatically increased proliferation and matrigel tube formation of hEPCs in a concentration-dependent manner via the canonical β-catenin pathway. Injection of Wnt1 overexpressing hEPCs increased blood flow to ischemic limbs by almost 3-fold, and improvement in blood flow was associated with a higher number of capillaries in Wnt1-injected ischemic tissue. Human EPCs can contribute to new blood vessel formation either by direct incorporation into vasculature or indirectly by exerting proangiogenic paracrine effects. In our study, Wnt1 overexpressing hEPCs did not incorporate into existing capillary tubes, suggesting a paracrine mechanism of action. Human EPCs are known to express growth factors and angiogenic cytokines that mediate beneficial paracrine effects (8, 9, 23), and a preliminary screening of cytokines demonstrated increased HGF expression in Wnt1-treated hEPCs. HGF is a potent growth factor that exerts trophic effects on a variety of cell types, and Wnt signaling is associated with increased HGF activity in colonic cancer stem cells (24). Silencing of HGF expression dramatically reduced the proangiogenic effects of Wnt1 on hEPC, suggesting that Wnt1 overexpressing hEPCs were increasing capillary formation and blood flow via HGF-dependent paracrine mechanisms. In this regard, Wnt1 injection alone into ischemic hindlimbs dramatically increased blood flow, and again this effect was associated with increased HGF expression and capillary formation in ischemic tissue. Increased capillary formation in ischemic tissues of animals injected with Wnt1 protein could be secondary to proliferative and proangiogenic effects of Wnt1 on native endothelium or on EPCs recruited to the site of injury.
In summary, we demonstrate a novel function of Wnt1 as a proangiogenic molecule. Wnt1 is expressed in a subset of endothelial cells of the dorsal aorta and reactivated in the endothelium of angiosarcomas. Wnt1 enhances function of hEPCs and increases blood flow to ischemic extremities, likely by paracrine mechanisms mediated by HGF. Our observations suggest a potential therapeutic role of Wnt1 in enhancing hEPC function prior to cell-based therapy or for increasing blood flow to ischemic extremities.
Supplementary Material
Acknowledgments
The authors thank the Mouse Histology Core (Virus and Flow Cytometry Core, University of North Carolina–Chapel Hill) for assistance with techniques. The authors thank Jackie Kylander and Taylor Kopple (Mouse Cardiovascular Core, University of North Carolina–Chapel Hill) for assistance with laser-Doppler scanning and Dr. Gobinda Sarkar (Mayo Clinic, Rochester, MN, USA) for critique of the manuscript.
This work was supported by grants from the National Institutes of Health and Ellison Medical Foundation to A.D.
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1. Becker G. J., McClenny T. E., Kovacs M. E., Raabe R. D., Katzen B. T. (2002) The importance of increasing public and physician awarness of peripheral arterial disease. J. Vasc. Interv. Radiol. 13, 7–11 [DOI] [PubMed] [Google Scholar]
- 2. Conway E. M., Collen D., Carmeliet P. (2001) Molecular mechanisms of blood vessel growth. Cardiovasc. Res. 49, 507–521 [DOI] [PubMed] [Google Scholar]
- 3. Asahara T., Takahashi T., Masuda H., Kalka C., Chen D., Iwaguro H. (1999) VEGF contributes to postnatal neovascularization by mobilizing bone marrow-derived endothelial progenitor cells. EMBO J. 18, 3964–3972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Takahashi C., Kalka C., Masuda H., Chen M., Silver M., Kearney M. (1999) Ischemia and cytokine induced mobilization of bone marrow derived endothelial progenitor cells for neovascularization. Nat. Med. 5, 434–438 [DOI] [PubMed] [Google Scholar]
- 5. Tepper O. M., Capla J. M., Galiano R. D., Ceradini D. J., Callaghan M. J., Kleinmann M. E. (2004) Adult vasculogenesis occurs through the in situ recruitment, proliferation and tubulization of circulating bone marrow derived cells. Blood 105, 1068–1077 [DOI] [PubMed] [Google Scholar]
- 6. Deb A., Patterson C. (2010) Hard luck stories: the reality of endothelial progenitor cells continues to fall short of the promise. Circulation 121, 850–852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Asahara T., Murohara T., Sullivan A., Silver M., van der Zee R., Li T., Witzenbichler B., Schatteman G., Isner J. M. (1997) Isolation of putative progenitor endothelial cells for angiogenesis. Science 275, 964–967 [DOI] [PubMed] [Google Scholar]
- 8. Rehman J., Li J., Orschell C. M., March K. L. (2003) Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107, 1164–1169 [DOI] [PubMed] [Google Scholar]
- 9. Gnecchi M., Zhang Z., Ni A., Dzau V. (2008) Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 103, 1204–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hill J. M., Zalos G., Halcox J. P. J., Schenke W. H., Waclawiw M. A., Quyyumi A. A., Finkel T. (2003) Circulating endothelial progenitor cells, vascular function, and cardiovascular risk. N. Engl. J. Med. 348, 593–600 [DOI] [PubMed] [Google Scholar]
- 11. Bock-Marquette I., Saxena A., White M. D., Michael DiMaio J., Srivastava D. (2004) Thymosin β4 activates integrin-linked kinase and promotes cardiac cell migration, survival and cardiac repair. Nature 432, 466–472 [DOI] [PubMed] [Google Scholar]
- 12. Kusano K. F., Pola R., Murayama T., Curry C., Kawamoto A., Iwakura A., Shintani S., Ii M., Asai J., Tkebuchava T., Thorne T., Takenaka H., Aikawa R., Goukassian D., von Samson P., Hamada H., Yoon Y. S., Silver M., Eaton E., Ma H., Heyd L., Kearney M., Munger W., Porter J. A., Kishore R., Losordo D. W. (2005) Sonic hedgehog myocardial gene therapy: tissue repair through transient reconstitution of embryonic signaling. Nat. Med. 11, 1197–1204 [DOI] [PubMed] [Google Scholar]
- 13. Gordon M. D., Nusse R. (2006) Wnt signaling: multiple pathways, multiple receptors, and multiple transcription factors. J. Biol. Chem. 281, 22429–22433 [DOI] [PubMed] [Google Scholar]
- 14. Monkley S. J., Delaney S. J., Pennisi D. J., Christiansen J. H., Wainwright B. J. (1996) Targeted disruption of the Wnt2 gene results in placentation defects. Development 122, 3343–3353 [DOI] [PubMed] [Google Scholar]
- 15. Masckauchan H. T. N., Shawber C. J., Funahashi Y., Li C., Kitajewski J. (2005) Wnt/b-catenin signaling induces proliferation, survival and interleukin-8 in human endothelial cells. Angiogenesis 8, 43–51 [DOI] [PubMed] [Google Scholar]
- 16. Lin Y., Weisdorf D. J., Solovey A., Hebbel R. P. (2000) Origins of circulating endothelial cells and endothelial outgrowth from blood. J. Clin. Invest. 105, 71–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Nusse R., Brown A., Papkoff J., Scambler P., Shackleford G., McMahon A., Moon R., Varmus H. (1991) A new nomenclature for int-1 and related genes: the Wnt gene family. Cell 64, 231 [DOI] [PubMed] [Google Scholar]
- 18. You L., He B., Uematsu K., Xu Z., Mazieres J., Lee A., McCormick F., Jablons D. M. (2004) Inhibition of Wnt-1 signaling induces apoptosis in beta-catenin-deficient mesothelioma cells. Cancer Res. 64, 3474–3478 [DOI] [PubMed] [Google Scholar]
- 19. Matsui H., Shibata M., Brown B., Labelle A., Hegadorn C., Andrews C., Hebbel R. P., Galipeau J., Hough C., Lillicrap D. (2007) Ex vivo gene therapy for hemophilia A that enhances safe delivery and sustained in vitro factor VIII expression from lentivirally engineered endothelial progenitors. Stem Cells 25, 2660–2669 [DOI] [PubMed] [Google Scholar]
- 20. Dudek A. Z., Bodempudi V., Welsh B. W., Janiski P., Griffin R. J., Milbauer L. C., Hebbel R. P. (2007) Systemic inhibition of tumor angiogenesis by endothelial cell-based gene therapy. Br. J. Cancer 97, 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ono K., Matsumori A., Shioi T., Furukawa Y., Sasayama S. (1997) Enhanced expression of hepatocyte growth factor/c-Met by myocardial ischemia and reprerfusion in a rat model. Circulation 95, 2471–2472 [DOI] [PubMed] [Google Scholar]
- 22. Zerlin M., Julius M. A., Kitajewski J. (2008) Wnt/Frizzled signaling in angiogenesis. Angiogenesis 11, 63–69 [DOI] [PubMed] [Google Scholar]
- 23. Dzau V. J., Gnecchi M., Pachori A. S., Morello F., Melo L. G. (2005) Therapeutic potential of endothelial progenitor cells in cardiovascular diseases. Hypertension 46, 7–18 [DOI] [PubMed] [Google Scholar]
- 24. Vermeulen L., De Sousa E. M. F., van der Heijden M., Cameron K., de Jong J. H., Borovski T., Tuynman J. B., Todaro M., Merz C., Rodermond H., Sprick M. R., Kemper K., Richel D. J., Stassi G., Medema J. P. (2010) Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 12, 468–476 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.