Abstract
Adults with Down syndrome (DS) are at risk for developing Alzheimer disease (AD). While plasma Aβ is known to be elevated in DS, its relationship to cognitive functioning is unknown. To assess this relationship, samples from two groups of subjects were used. In the first group, nondemented adults with DS were compared to: 1) a group of young and old individuals without DS and 2) to a group of patients with AD. Compared to these controls, there were significantly higher levels of plasma Aβ in nondemented adults with DS while AD patients showed lower levels of plasma Aβ. A larger second group included demented and nondemented adults with DS, in order to test the hypothesis that plasma Aβ may vary as a function of dementia and ApoE genotype. Plasma Aβ levels alone did not dissociate DS adults with and without dementia. However, in demented adults with DS, ApoE4 was associated with higher Aβ40 but not Aβ42. After controlling for level of intellectual disability (mild, moderate, profound) and the presence or absence of dementia, there was an improved prediction of neuropsychological scores by plasma Aβ. In summary, plasma Aβ can help predict cognitive function in adults with DS independently of the presence or absence of dementia.
Keywords: apolipoprotein E, neuropsychology, Trisomy 21
Introduction
Middle-aged individuals with Down syndrome (DS) develop Alzheimer disease (AD) pathology [1–4] and as such, are at high risk for the development of dementia [5, 6]. Clinical signs of dementia are more commonly observed when individuals are over 50 years of age [5, 7–9]. By 40 years of age, however, all individuals with DS have neuropathological changes including senile plaques and neurofibrillary tangles (NFT) consistent with AD [2, 3, 10]. Senile plaques contain the β-amyloid peptide (Aβ) that is derived from a longer precursor protein, β-amyloid precursor protein (APP), the gene for which is on chromosome 21 (21q21.2). DS is a chromosomal disorder caused by triplication of all or part of the 21st chromosome (trisomy 21). The presence of extra genetic material leads to overexpression of the involved genes and the phenotype of DS is thought to be a consequence of this genetic imbalance. APP gene triplication in DS has been demonstrated to yield excess protein product [11] in peripheral samples (e.g. lymphocytes) and in brain [11, 12]. APP is cleaved by a combination of beta- and gamma-secretase enzyme activity to produce the longer more toxic 42 amino acid long peptide (Aβ42) or the shorter, more soluble 40 amino acid peptide (Aβ40) [13]. In DS, plasma Aβ40 and Aβ42 are increased in comparison to those without DS [14–17].
The rationale for studying plasma Aβ in DS is twofold. First, given the progressive age-dependent nature of AD neuropathology accumulation, we can test hypotheses aimed at early detection of disease by examining adults in middle age prior to the development of clinical signs of dementia. Second, identifying biomarkers of AD for DS may lead to the development of new clinical outcome measures and biomarkers for the testing of therapeutics that may slow or halt AD in DS. The relationship of plasma Aβ to age is not clear. Although plasma Aβ concentrations are higher in DS it is not clear whether levels increase with age or actually decrease as peripheral Aβ is sequestered into plaques, a hypothesis that has been advanced for measures of Aβ in cerebrospinal fluid from patients with AD in the general population.[18]. Despite life-long overexpression of APP in brain and in peripheral lymphocytes [11, 12], brain Aβ does not typically begin to deposit until after 30 years [2]. The initial deposits of Aβ are followed by an acceleration phase that includes NFT and full-blown AD pathology by the age of 40 years [19]. Thus, age-dependent changes in plasma Aβ may also not be linear if they reflect brain deposition [20].
Consistent with a non-linear age associated accumulation of Aβ in the brain, it is not surprising that plasma levels of Aβ in several studies of subjects with DS variably report increases [15, 16], decreases [21] or stable levels of Aβ with age [22]. Interestingly, Matsubara and colleagues [21] reported that plasma Aβ is stable until age 50 years and then declines, a finding that is more consistent with significant AD pathology in brain at an age when adults with DS are at the highest risk for developing dementia. However, in a more direct test of differences in plasma Aβ, the presence or absence of dementia in DS is associated with either no differences [17] or increased Aβ42 but not Aβ40 [22]. The different outcomes in these two studies may be due to the relatively small sample sizes of demented individuals, the severity of dementia in individual subjects or to other possible differences in the groups of subjects studied. Plasma measures of Aβ in the general population (non-DS) have also been variable. Increases in plasma Aβ with age [23] and higher levels in individuals with AD have been reported although there can be overlap with nondemented elderly controls [24]. Longitudinal studies suggest that plasma Aβ may be elevated in presymptomatic stages of AD but may fall off as dementia develops [25].
In order to explore the relationship of plasma Aβ levels to age, dementia status, and cognitive functioning, we utilized 2 groups of samples. In the first study, we compared plasma Aβ levels in a group of nondemented adults with DS to a group of young or old non-DS individuals and to a group of patients with AD to confirm and extend previous reports of increased plasma Aβ in DS. This first study validated our plasma Aβ measures and allowed us to test the hypothesis that plasma Aβ would be reduced with AD and higher overall in DS when compared to controls. In a second, larger group including demented (DS+AD) and nondemented adults with DS (DS) with a wide range of ages, we tested the hypothesis that plasma Aβ may vary with dementia, with level of intellectual disability, with cognitive performance and with ApoE genotype (not available from our first study).
Materials and Methods
Subjects
Two groups were used in the current study and are summarized in Table 1. The first (Study 1) was described in previous publications [26, 27]. We screened over 40 people with DS between the ages of 34 and 55 identified from a University clinic and from local community care facilities. Individuals with an IQ<45 were not included in the study because subsequent cognitive testing would not be reliable. Seventeen DS subjects were included in this study (WAIS-III FSIQ range 47–70, mean=55.7, SD=6.3). DS subjects received an independent neurological evaluation and none were found to have dementia by DMS-IV criteria. For comparison, we also recruited 17 people with possible or probable AD (MMSE range 9–29, mean =22.6, SD=5.5) from University clinics and community groups; all met DSM-IV criteria for dementia of the Alzheimer's type. Eleven age and sex-matched normal controls for the DS group (MMSE range 27–30, mean=29.2, SD=1.0) and 12 age and sex-matched normal controls for the AD group (MMSE range 27–30, mean=28.5 SD=1.2) were also recruited. These subjects represented normal aging for the non-DS group.
Table 1.
Subject demographics.
| Study 1 | Study 2 | |||||
|---|---|---|---|---|---|---|
| Young Controls | Aged Controls | Probable AD | Nondemented DS | Nondemented DS | Demented DS | |
| Sample Size | 11 | 12 | 17 | 17 | 26 | 52 |
| Gender (M/F) | 5/6 | 7/5 | 15/2 | 9/8 | 17/9 | 26/26 |
| Mean Age in years (SE) | 46.5 (2.0) | 74.2 (1.3) | 75.3 (1.8) | 44.1 (1.4) | 45.1(1.9) | 53.3(0.7) |
| Range of Ages in years | 39–56 | 66–83 | 61–91 | 37–54 | 26.5–60.2 | 41.4–63.2 |
| Plasma Aβ1-40 Mean in pmol (SE) | 45.5 (2.8) | 57.7 (4.7) | 51.2 (3.0) | 75.7 (6.6) | 63.7(4.74) | 66.9 (3.17) |
| Plasma Aβ1-42 Mean in pmol (SE) | 4.4 (0.45) | 6.2 (0.6) | 4.0 (0.3) | 7.3 (1.0) | 4.57 (.36) | 5.28 (.53) |
| Plasma Ratio Aβ1-42/Aβ1-40 | 0.10 (0.01) | 0.11 (0.01) | 0.08 (0.01) | 0.10 (0.01) | 0.09 (0.02) | 0.08 (0.01) |
The second group (Study 2) was part of an ongoing longitudinal study of aging in DS. Baseline blood samples were acquired from 52 demented (DS+AD) and 26 nondemented adults with DS (total=78) ranging from 26.5 to 63.2 years of age enrolled in the UCI-ADRC study of aging and a clinical trial. For these subjects, Brief Praxis Test (BPT) [28] scores were available for 61 subjects, 60 subjects had Severe Impairment Battery (SIB) [29] data, and Dementia Questionnaire for Persons with Mental Retardation (DMR)[30, 31] scores were available for 71 subjects. The DMR provides two components reflecting Social (DMRSoc) and Cognitive (DMRCog) function. The level of intellectual disability was established from case file reviews of historical full-scale IQ assessments and subjects were categorized as being mild, moderate or profoundly impaired.
All subjects or their legally-authorized representative gave informed consent in accord with procedures required and approved by the University of California, Irvine Institutional Review Board.
ApoE Genotyping
DNA was isolated from 100ul aliquots of whole blood on an automated nucleic acid purification system (MagNA Pure, Roche Diagnostics). Screening for the three common APOE alleles (E2, E3 and E4) was achieved using PCR-RFLP (polymerase chain reaction - restricted fragment length polymorphism) analysis [32, 33].
Blood collection and plasma (pretreatment)
Plasma samples were collected in EDTA tubes, centrifuged at 4000 rpm, aliquoted and frozen at −80°C until use. A concentrated solution of protease inhibitor cocktail was prepared by dissolving 1 tablet of protease inhibitor cocktail (PIC Complete Mini Roche Cat# 11836153001) in 2 ml PBS. Twenty μl of PIC solution was added to 480 μl plasma. To block cross-reaction of unidentified components in human plasma with mouse IgG in the enzyme-linked immunosorbent assay, the plasma was precleared using methods described previously [23]. Briefly, preclearing was performed by diluting 200 μL of each plasma sample with 350 μL of antigen coating buffer (ACB - 20mM phosphate, 400mM sodium chloride, 2mM EDTA, 0.4% Block Ace; (Dainippon Pharmaceutical, Osaka, Japan), 0.2% bovine serum albumin, 0.0765% 3-with two of these -1-propanesulfonate (CHAPS), pH 7.2), and 50 μL of the Chrompure Mouse IgG, whole molecule – agarose (Jackson Immnoresearch lab, West Grove, PA). After incubation for 2 hours at 4°C, the beads were removed by centrifugation for 1 min at 6000 rpm at 4°C. The plasma was added onto the plate as is after this preparation (final dilution ~ 1:3). Thus, plasma Aβ measures reflect “free Aβ”.
Sandwich Aβ ELISA
Aβ enzyme-linked immunosorbent assays were conducted on the day the plasma was thawed using commercial kits (Human β Amyloid (1-42) ELISA kit Wako Cat# 298-62401 and Human β Amyloid (1-40) ELISA Kit Wako Cat# 298-62301). The 96-well plates were coated with a monoclonal antibody BAN50, against human Aβ (1–16) as a capture antibody for the N-terminal portion of human Aβ 1-42. Captured Aβ from the human plasma sample was detected by a C-terminal specific antibody HRP-conjugated BC05 (Fab' fragment) for Aβ 1-42 and BA27 for Aβ 1-40. TMB substrate was added and the absorbance was read at 450 nm on a BioTek Synergy HT micro plate reader.
Data Analysis
Descriptive statistics, statistical analyses, and graphics were executed using SYSTAT (Systat Software, Inc, San Jose, California). A one-way ANOVA was used to compare average plasma Aβ and the ratio of Aβ42:Aβ40 for four groups (Young controls – YC, Old controls – OC, probable AD – AD and nondemented DS – DS) of subjects in Group 1. Two pairwise mean comparisons defined a priori (with Bonferroni adjustments to probabilities) were carried out for each Aβ measure. Correlations were computed to compare the relation of the Aβ measures against age. To compare Aβ means of DS subjects and controls, two-sample t-tests were used. In Study 2, two-way ANOVAS with diagnosis and level of cognitive function as factors and an Aβ measure or a cognitive score as the dependent variable were used to assess the impact of the factors on the variability of each outcome. Further, two linear multiple regression models were fit to each cognitive/functional score in the analyses of data from Group 2. In the first, dementia diagnosis and level of intellectual disability were predictors. In the second, Aβ42 or Aβ40 was included as a third predictor. An alpha level less than .05 was used to determine statistical significance.
Results
Within each group of individuals, we first tested for gender differences in plasma Aβ to determine if this variable should be included as a covariate in subsequent analyses. No significant gender differences were observed (p>0.05).
A Comparison of DS to non-DS Controls – Study 1
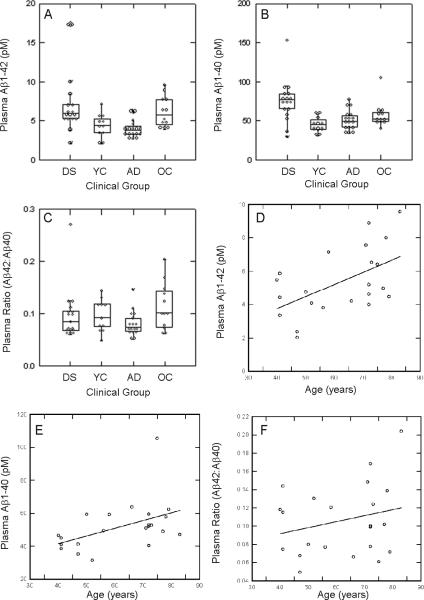
To confirm that individuals with DS have higher overall levels of plasma Aβ we compared all 4 groups of subjects (young nondemented DS, young non-DS, AD patients and aged-non-DS controls) in a one-way ANOVA from the first study and found significant differences in plasma Aβ42 (F(3,53)=5.5 p=0.002), Aβ40 (F(3,53)=7.7 p<.0005) but not for the ratio of Aβ42:Aβ40 (F(3,53=1.73 p<.20)(Fig. 1A, B, C). Nondemented individuals with DS had 1.66 fold higher levels of plasma Aβ and adjusted probabilities of tests of pairwise differences in means were significant relative to age-matched controls (for Aβ42, p=0.012 and for Aβ40 p<0.005). Further, subjects with AD had lower levels of plasma Aβ42 (p=0.056) but not Aβ40 (p=0.696) compared to age matched controls while the Aβ42:Aβ40 ratio was higher in older controls compared to AD cases (p=0.028). Thus, as described in previous reports, we observed significantly higher levels of plasma Aβ in adults with DS and lower levels of plasma Aβ in AD patients relative to age-matched controls.
Figure 1.
Individual and group differences in plasma Aβ in Study 1. (A) Plasma Aβ1-42 was significantly higher in nondemented adults with DS relative to similarly aged young controls (YC). Patients with AD had lower plasma Aβ1-42 than nondemented adults with DS. (B) Plasma Aβ1-40 was highest in nondemented adults with DS relative to young controls (YC), old controls (OC) and patients with AD. (C) No differences across groups were noted in the ratio of Aβ42:Aβ40. Higher amounts of plasma Aβ1-42 (D) and Aβ1-40 (E) are associated with increased age in individuals without DS. However, the ratio of Aβ42:Aβ40 was stable across age (F). In the box plots, horizontal lines represent the median, the bottom line is the 25th percentile and the top line is the 75th percentile. Individual data points are shown. Lines indicate significant differences in post hoc Bonferroni comparisons at the p<.05 level. Lines represent a linear regression.
Effects of Age on Plasma Aβ in DS and Controls – Study 1
We next examined age effects on plasma Aβ in non-DS cases (n=11 young and n=12 old, total age range between 39–83 years). Figure 1 (D, E) shows that there is a significant correlation between plasma Aβ42 and age (r=0.53 p=0.01) and a weaker correlation with plasma Aβ40 (r=0.43 p=0.042). The correlation with the ratio of Aβ42:Aβ40 was not significant (Figure 1F). These correlations remained significant when one outlier with very high plasma Aβ1-40 was removed. Therefore, both plasma Aβ40 and Aβ42 appear to increase with age when DS is not present. In contrast, in the DS group, similar age and plasma Aβ correlation analyses found no significant associations with age.
Effects of Age on Plasma Aβ in DS – Study 2
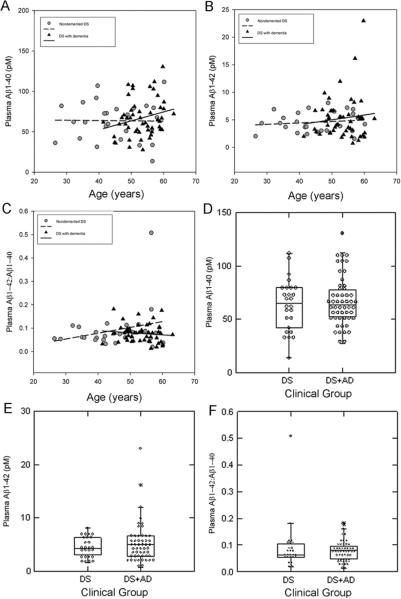
Plasma was subsequently collected from a newly recruited second, larger cohort of subjects with DS that included groups of individuals that were either demented or nondemented. This group also included a wider range of ages of adults with DS to extend and replicate the results of the first study with a subset of demented individuals. As seen in Study 1, there were no significant correlations in DS adults between age and plasma Aβ40 (r=−0.015 p=0.94, n=26), Aβ42 (r=0.14 p=0.51 n=26) and the ratio of Aβ42:Aβ40 (r=0.26 p=0.19 n=26)(Figure 2A, B, C) A similar lack of association was observed in DS+AD (Aβ40 – r=0.24 p=0.09 n=52; Aβ42 – r=0.11 p=0.42 n=52; Aβ42:Aβ40 – r=−0.12, p=0.40) (Figure 2A, B, C).
Figure 2.
Plasma Aβ as a function of age in DS subjects with and without dementia. The correlation between age and plasma Aβ1-42 (A), Aβ1-40 (B) and the ratio of Aβ42:Aβ40 (C) in individuals with DS (with dementia - triangle or without dementia – circle) was not significant. (D) In demented adults with DS, plasma Aβ1-42 was not different from those without dementia. No differences were observed in plasma Aβ1-40 (E) nor the ratio of Aβ42:Aβ40 (F) when comparing demented vs nondemented adults. Lines represent linear regressions. The horizontal line in the middle of each box is the median, the bottom of the box is the 25th percentile and the top is the 75th percentile and individual data points are shown.
Effects of ApoE on Plasma Aβ in DS in Study 2
We next tested for differences in plasma Aβ as a function of ApoE genotype, which could not be tested in Study 1 as DNA was not available. ApoE genotype was available for 72 of the 76 subjects in Study 2. Table 2 shows the ApoE genotype of individuals in each of our subject groups as a function of clinical status (DS vs. DS+AD). The proportions of individuals from the entire group falling into E2 (2/2, 2/3), E3 (3/3) and E4 (2/4, 3/4, 4/4) genotypes was 16.6, 33.3 and 50.0% respectively. A univariate ANOVA comparing DS to DS+AD and those with and without an ApoE4 allele on the level of plasma Aβ shows no differences overall. We also noted that the group of DS+AD had the highest representation of subjects with an ApoE4 allele (20/60, 33.3%) compared to those without dementia (6/33 – 18.2%). Thus, we re-examined the effect of ApoE genotype on individuals with DS+AD alone and found that plasma Aβ40 was significantly higher with the presence of ApoE4 (t(48)=2.21 p=0.03) but not Aβ42 (t(48)<1 p=n.s.). A similar analysis that included only nondemented DS samples did not show ApoE4 associated plasma Aβ differences.
Table 2.
Effect of ApoE genotype and presence of dementia on Plasma Aβ in DS
| Group | N | Demented | At least one E4 allele | Aβ1-40 | Aβ1-42 | Ratio Aβ1-42/Aβ1-40 |
|---|---|---|---|---|---|---|
| 1 | 15 | − | − | 64.72(6.6) | 4.57(0.50) | 0.098 (0.017) |
| 2 | 7 | − | + | 61.39(9.28) | 4.14(0.54) | 0.0786 (0.024) |
| 3 | 33 | + | − | 61.75(4.03) | 4.97(0.72) | 0.0781 (0.011) |
| 4 | 17 | + | + | 76.51(5.07) | 5.75(0.79) | 0.0754 (0.016) |
Dementia, Level of Intellectual Disability, Current Cognitive Function, and Plasma Aβ in DS – Study 2
In the AD subjects without DS from Study 1, we observed lower plasma Aβ levels than similarly aged nondemented controls in a cross-sectional comparison. Thus, we hypothesized that DS+ AD may have lower plasma Aβ than DS subjects. As shown in Figure 2, there were no significant differences in plasma Aβ40 (t(76)= −0.58 p=0.57), Aβ42 (t(76)=−1.10 p=0.27) or the ratio of Aβ42:Aβ40 (t(76)=0.90 p=0.37) between DS and DS+AD in study 2 (Figure 2, C, D, E). However, individual variability in plasma Aβ was observed; differences in level of intellectual disability or dementia severity could be related to this variability.
We first addressed the concern that the level of intellectual disability may be related to plasma Aβ level. A two-way ANOVA including level of intellectual disability (mild, moderate, profound) and dementia diagnosis (nondemented, demented) suggests that average plasma Aβ40 and plasma Aβ42 do not differ due to level of intellectual disability (for Aβ40, F(1,70)<1 p=n.s., Aβ42, F(1,70)<1 p=n.s., and Aβ42:Aβ40, F(1,70)<1 p=n.s.) nor dementia status.
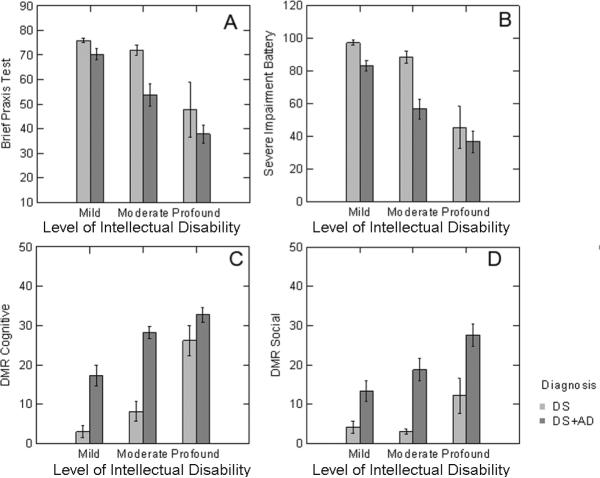
We next assessed the relationship between plasma Aβ, the level of intellectual disability and presence of dementia on neuropsychological/functional test scores. Figure 3 shows the interaction between level of intellectual disability and the presence of dementia in DS. First, in parallel to the assessment of the relation of level of intellectual disability and diagnosis to plasma Aβ, a two-way ANOVA was used to assess how these factors contribute to the variability of BPT, the SIB and the DMRSoc and DMRCog subscales. A diagnosis of dementia (F(1,55)=9.08 p=0.004) and more profound cognitive impairment (F(2,55)=21.32 p<0.0005) both contribute to lower mean BPT test scores (Figure 3A). Similar outcomes were noted in the SIB with the presence of dementia (F(1,54)=12.84 p=0.001) and profound level of intellectual disability (F(1,54)=29.94 p<0.0005) leading to lower mean scores (Figure 3B). On the DMR tests of social and cognitive function, higher scores were associated with presence of dementia (DMRCog – F(1,65)=50.1 p<0.0005); DMRSoc – F(1,65)=26.9 p<0.0005) and the level of intellectual disability (DMRCog – F(2, 65)=32.3 p<0.0005); DMRSoc – F(2,65)=6.52 p=0.003) (Figure 3C,D).
Figure 3.
Association between level of intellectual disability, presence of dementia and neuropsychological function. DS adults with dementia scored lower on a direct measure of function in the brief praxis test (A) and in the severe impairment battery (B) with level of intellectual disability also contributing to lower scores. Informant based measures on the DMR cognitive (C) and social (D) subtests revealed higher scores were observed in DS adults with dementia as compared to without dementia. Further, more profound levels of intellectual disability also contributed to higher DMR scores. Bars represent means and error bars, standard errors of the mean.
We computed correlation coefficients (Pearson) between individual test scores and plasma Aβ40, Aβ42, and Aβ42:Aβ40 but found no significant associations. However, for the BPT, SIB, and DMRCog scale, more than half their variability is due to a dementia diagnosis and level of intellectual disability. Thus, we used a regression analysis to remove this variability and then to assess the association between plasma Aβ and the cognitive test scores. The extent of improvement for including plasma Aβ measures in predicting individual cognitive test scores was assessed using the percentage increase in R2 due to the addition of the Aβ measure and also the t statistic for that variable's contribution (Table 3).
Table 3.
Multiple R2 from Regression models
| Measure | Base Model | Add Aβ40 | Increase | Add Aβ42 | Increase |
|---|---|---|---|---|---|
| BPT | 0.347 | 0.402 | 5.5% | 0.418 | 7.1% |
| SIB | 0.471 | 0.502 | 3.1% | 0.491 | 2.0% |
| DMRCog | 0.699 | 0.717 | 1.8% | 0.726 | 2.7% |
| DMRSoc | 0.356 | 0.357 | 0.1% | 0.386 | 3.0% |
Increases in R2 indicate that plasma Aβ42 and Aβ40 are associated with BPT and SIB scores after removing the variability of diagnosis and pre-existing impairment from the score. For both DMR scales, including plasma Aβ42 levels significantly increased R2. Similar effects were observed with adding Aβ40 to the model except on DMRSoc. For most regression analyses, one subject with high Aβ levels was identified as an outlier because its Studentized residual was so extreme. The models were rerun after removing this case and in each regression, the statistical model predictions of cognitive performance were improved. Thus, taking into consideration pre-existing intellectual disability and dementia diagnosis we find that plasma Aβ can improve the statistical prediction of test performances.
Discussion
We describe the role of level of intellectual disability on plasma Aβ and how plasma Aβ (more often Aβ1-42 than Aβ1-40) may be useful for statistically predicting neuropsychological/functional test scores in adults with DS. Subjects in study 2 were evaluated on several direct tests of function (BPT, SIB) and an informant based measure (DMR). We first established that level of intellectual disability alone did not predict plasma Aβ. We found that higher plasma Aβ1-40 and Aβ1-42 was associated with lower BPT scores. However, lower BPT scores could have simply reflected level of intellectual disability as this was a single time point measure. Interestingly, individuals with more severe intellectual disability prior to the emergence of clinical dementia showed a stronger association between BPT scores and plasma Aβ1-42 than individuals with milder pre-dementia intellectual disability. However, plasma Aβ was not associated with scores on either the SIB or on the DMR tests but this may be due to smaller sample size as not all participants had these scores. The association between cognitive function and plasma Aβ in DS has been described in a few reports but none have observed a significant correlation [14, 34]. This lack of association may be due to the utilization of tests that may not be the most sensitive for dementia or cognitive decline in adults with DS. For example, the MMSE is confounded with low baseline intelligence [35]. Further, MMSE scores also do not predict plasma Aβ level in studies of sporadic AD [24, 36]. Thus, a novel finding in the current study is that individual variation in the amount of plasma Aβ in DS may be predicted on the basis of the extent of the existing cognitive impairment but is not due to differences in pre-dementia levels of intellectual disability.
The current study also shows consistent results with previous reports of significantly higher plasma Aβ40 and Aβ42 in DS relative to age-matched non-DS subjects [14–17, 22]. A more recent study has extended these results to children with DS [37]. In the current study, plasma Aβ40 and Aβ42 were increased ~1.6 fold in DS as compared to non-DS subjects, which is slightly higher than would be predicted based on a gene dosage effect of APP but is lower than previous publications [14, 17]. However, plasma Aβ levels and ratios are not linearly associated with age in DS nor are they decreased in DS with AD, which contrasts with our observations in a group of patients with sporadic AD and a previous report in DS [22, 34]..
We predicted that age-dependent accumulation of brain Aβ in DS may be reflected in peripheral measures of Aβ. But we also predicted, based on previous reports on the age of onset of brain Aβ that plasma Aβ changes with age in DS would also not be linear. However, no changes in plasma Aβ were observed between the ages of 30–40 years when diffuse Aβ is first visualized in DS brain or from 40–50 years when neuritic Aβ plaques develop. A drop in plasma Aβ42 and an increase in Aβ40 was observed in very old DS individuals (>60 years) in a previous study [21]. We and others have also detected significantly lower levels of plasma Aβ in sporadic AD [36]. However, despite higher levels of plasma Aβ in DS than non-DS subjects, early signs of AD neuropathology in DS cannot be detected by mean group differences in plasma Aβ. The lack of age association between plasma Aβ and the well-established age of onset of AD pathology in the brains of adults with DS [2] also confirms that plasma Aβ may not reflect brain Aβ as reported in sporadic AD [38].
A few studies comparing adults with DS with and without dementia using measures of plasma Aβ [17, 22] have been reported. However, previous studies have included small numbers of subjects with dementia. The current study included 52 adults with (DS+AD) and 24 (DS) without dementia. Overall, the DS+AD were older but when age is included as a covariate to compare differences in plasma Aβ, no differences were observed. Thus, cross-sectional or single time point measures of plasma Aβ may not differentiate DS from DS+AD and our results are consistent with a recent larger study [39]. However, it is possible that repeated measures or longitudinal assays can detect changes in plasma Aβ that may reflect progressive AD neuropathology as suggested in the non-DS population [25]. Further, a longer duration of dementia in DS is associated with higher plasma Aβ [40].
In the current study, Aβ40 was selectively higher in the group of DS+AD but not in DS, which contrasts with some previous studies [16, 37]. However, in another cohort of DS individuals found increased plasma Aβ1-42 in subjects who carry an E4 allele [22]. The current study and one by Schupf and colleagues [22] were different in two respects: (1) the current study had fewer subjects without the E4 allele and; (2) a larger number of demented subjects in the current study. The distribution of our subjects into ApoE genotypes was also slightly different from a previous report [41] but this may reflect an enrollment bias towards recruiting DS adults with dementia to the study. This may suggest that ApoE genotype may play a subtle role on plasma Aβ levels but varies from study to study. Indeed, results from our study that used two separate groups of individuals show significant differences in the amount of plasma Aβ even when all assays were run together and suggests that multiple factors may affect outcomes of peripheral assays [42]. For example, it is possible that the time of day the samples were collected or co-morbidities as well as medication history may influence steady-state levels of plasma Aβ measures [43, 44]. Additionally, recent reports suggest that the duration of dementia in DS might also be an important factor predicting plasma Aβ [40]. However, to our knowledge, there is limited information regarding the strength of association or correlation between plasma Aβ measures taken across time in DS and varies between 3–14% in longitudinal studies of non-DS individuals [45].
An additional consideration with Aβ measures is potential binding to other plasma proteins such as albumin [46] or apolipoprotein J [47]. In addition anti-Aβ antibodies can be measured in the plasma of DS blood [48] and may also reduce detection of Aβ. Through pre-clearing our samples, Aβ attached to these proteins and possibly existing antibodies was most likely reduced leaving free Aβ that was measured by ELISA. The source of plasma Aβ may also include larger contributions from peripheral tissues than that reflecting brain (e.g. [49]). However, all plasma samples were treated equivalently during processing suggesting that despite these possible variables contributing to Aβ level, it still provides useful information with respect to cognition in DS.
In summary, we observe that the amount of plasma Aβ can improve the prediction of neuropsychological test scores reflecting cognitive function while taking into account the level of pre-existing intellectual disability and presence or absence of dementia. As an extension of previous reports using a larger sample size, we find no differences in a single time point measure of plasma Aβ between adults with DS with or without dementia. However, in DS with AD, those with an ApoE4 allele show significantly higher plasma Aβ40. We are currently following individuals from the second study to determine if changes in clinical measures or in plasma Aβ levels might predict conversion to AD or worsening of dementia.
Acknowledgements
This project was supported by grant UCI ADRC AG16573 (ITL), HD-37427 (RJH) from NICHD and, in part, by AG21912 (ITL), the “My Brother Joey Neuroscience Fund” (ITL) and DHHS, Eunice Kennedy Shriver National Institute of child Health & Human Development R01 HD064993 (EH, FAS). The authors also thank the subjects and families for participating in the study. Disclosure Statement: The authors have no actual or potential conflicts of interest.
References
- [1].Mann DMA. The pathological association between Down syndrome and Alzheimer disease. Mech. Ageing and Develop. 1988;43:99–136. doi: 10.1016/0047-6374(88)90041-3. [DOI] [PubMed] [Google Scholar]
- [2].Mann DMA, Esiri MM. The pattern of acquisition of plaques and tangles in the brains of patients under 50 years of age with Down's syndrome. J. Neurol. Sci. 1989;89:169–179. doi: 10.1016/0022-510x(89)90019-1. [DOI] [PubMed] [Google Scholar]
- [3].Wisniewski K, Wisniewski H, Wen G. Occurrence of neuropathological changes and dementia of Alzheimer's disease in Down's syndrome. Ann. Neurol. 1985;17:278–282. doi: 10.1002/ana.410170310. [DOI] [PubMed] [Google Scholar]
- [4].Hof PR, Bouras C, Perl DP, Sparks DL, Mehta N, Morrison JH. Age-related distribution of neuropathologic changes in the cerebral cortex of patients with Down's syndrome. Arch. Neurol. 1995;52:379–391. doi: 10.1001/archneur.1995.00540280065020. [DOI] [PubMed] [Google Scholar]
- [5].Lai F, Williams MD. A prospective study of Alzheimer Disease in Down Syndrome. Archives of Neurology. 1989;46:849–853. doi: 10.1001/archneur.1989.00520440031017. [DOI] [PubMed] [Google Scholar]
- [6].Lott IT, Head E. Down syndrome and Alzheimer's disease: A link between development and aging. Ment Retard Dev Disabil Res Rev. 2001;7:172–178. doi: 10.1002/mrdd.1025. [DOI] [PubMed] [Google Scholar]
- [7].Tyrrell J, Cosgrave M, McCarron M, McPherson J, Calvert J, Kelly A, McLaughlin M, Gill M, Lawlor BA. Dementia in people with Down's syndrome. Int J Geriatr Psychiatry. 2001;16:1168–1174. doi: 10.1002/gps.502. [DOI] [PubMed] [Google Scholar]
- [8].Prasher VP, Filer A. Behavioural disturbance in people with Down's syndrome and dementia. J Intellect Disabil Res. 1995;39:432–436. doi: 10.1111/j.1365-2788.1995.tb00547.x. [DOI] [PubMed] [Google Scholar]
- [9].Bush A, Beail N. Risk factors for dementia in people with down syndrome: issues in assessment and diagnosis. Am J Ment Retard. 2004;109:83–97. doi: 10.1352/0895-8017(2004)109<83:RFFDIP>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- [10].Wisniewski K, Howe J, Williams G, Wisniewski HM. Precocious aging and dementia in patients with Down's syndrome. Biological Psychiatry. 1978;13:619–627. [PubMed] [Google Scholar]
- [11].Rumble B, Retallack R, Hilbich C, Simms G, Multhaup G, Martins R, Hockey A, Montgomery P, Beyreuther K, Masters CL. Amyloid A4 and its precursor in Down's syndrome and Alzheimer's disease. New Engl. J. Med. 1989;320:1446–1462. doi: 10.1056/NEJM198906013202203. [DOI] [PubMed] [Google Scholar]
- [12].Pallister C, Jung SS, Shaw I, Nalbantoglu J, Gauthier S, Cashman NR. Lymphocyte content of amyloid precursor protein is increased in Down's syndrome and aging. Neurobiol Aging. 1997;18:97–103. doi: 10.1016/s0197-4580(96)00207-2. [DOI] [PubMed] [Google Scholar]
- [13].Selkoe DJ. Normal and abnormal biology of the beta-amyloid precursor protein. Annu. Rev. Neurosci. 1994;17:489–517. doi: 10.1146/annurev.ne.17.030194.002421. [DOI] [PubMed] [Google Scholar]
- [14].Cavani S, Tamaoka A, Moretti A, Marinelli L, Angelini G, Di Stefano S, Piombo G, Cazzulo V, Matsuno S, Shoji S, Furiya Y, Zaccheo D, Dagna-Bricarelli F, Tabaton M, Mori H. Plasma levels of amyloid β 40 and 42 are independent from ApoE genotype and mental retardation in Down syndrome. American Journal of Medical Genetics. 2000;95:224–228. [PubMed] [Google Scholar]
- [15].Mehta PD, Dalton AJ, Mehta SP, Kim KS, Sersen EA, Wisniewski HM. Increased plasma amyloid β protein 1-42 levels in Down syndrome. Neuroscience Letters. 1998;241:13–16. doi: 10.1016/s0304-3940(97)00966-x. [DOI] [PubMed] [Google Scholar]
- [16].Mehta PD, Mehta SP, Fedor B, Patrick BA, Emmerling M, Dalton AJ. Plasma amyloid beta protein 1-42 levels are increased in old Down Syndrome but not in young Down Syndrome. Neurosci Lett. 2003;342:155–158. doi: 10.1016/s0304-3940(03)00275-1. [DOI] [PubMed] [Google Scholar]
- [17].Tokuda T, Fukushima T, Ikeda S, Sekijima Y, Shoji S, Yanagisawa N, Tamaoka A. Plasma levels of amyloid beta proteins Abeta1-40 and Abeta1-42(43) are elevated in Down's syndrome. Ann Neurol. 1997;41:271–273. doi: 10.1002/ana.410410220. [DOI] [PubMed] [Google Scholar]
- [18].Blennow K. Cerebrospinal fluid protein biomarkers for Alzheimer's disease. NeuroRx. 2004;1:213–225. doi: 10.1602/neurorx.1.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Head E, Lott IT. Down syndrome and beta-amyloid deposition. Curr Opin Neurol. 2004;17:95–100. doi: 10.1097/00019052-200404000-00003. [DOI] [PubMed] [Google Scholar]
- [20].Nistor M, Don M, Parekh M, Sarsoza F, Goodus M, Lopez GE, Kawas C, Leverenz J, Doran E, Lott IT, Hill M, Head E. Alpha- and beta-secretase activity as a function of age and beta-amyloid in Down syndrome and normal brain. Neurobiol Aging. 2007;28:1493–1506. doi: 10.1016/j.neurobiolaging.2006.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Matsubara E, Sekijima Y, Tokuda T, Urakami K, Amari M, Shizuka-Ikeda M, Tomidokoro Y, Ikeda M, Kawarabayashi T, Harigaya Y, Ikeda S, Murakami T, Abe K, Otomo E, Hirai S, Frangione B, Ghiso J, Shoji M. Soluble Abeta homeostasis in AD and DS: impairment of anti-amyloidogenic protection by lipoproteins. Neurobiol Aging. 2004;25:833–841. doi: 10.1016/j.neurobiolaging.2003.10.004. [DOI] [PubMed] [Google Scholar]
- [22].Schupf N, Patel B, Silverman W, Zigman WB, Zhong N, Tycko B, Mehta PD, Mayeux R. Elevated plasma amyloid beta-peptide 1-42 and onset of dementia in adults with Down syndrome. Neurosci Lett. 2001;301:199–203. doi: 10.1016/s0304-3940(01)01657-3. [DOI] [PubMed] [Google Scholar]
- [23].Fukumoto H, Tennis M, Locascio JJ, Hyman BT, Growdon JH, Irizarry MC. Age but not diagnosis is the main predictor of plasma amyloid beta-protein levels. Arch Neurol. 2003;60:958–964. doi: 10.1001/archneur.60.7.958. [DOI] [PubMed] [Google Scholar]
- [24].Mehta PD, Pirttila T, Mehta SP, Sersen EA, Aisen PS, Wisniewski HM. Plasma and cerebrospinal fluid levels of amyloid beta proteins 1-40 and 1-42 in Alzheimer disease. Arch Neurol. 2000;57:100–105. doi: 10.1001/archneur.57.1.100. [DOI] [PubMed] [Google Scholar]
- [25].Kawarabayashi T, Shoji M. Plasma biomarkers of Alzheimer's disease. Curr Opin Psychiatry. 2008;21:260–267. doi: 10.1097/YCO.0b013e3282fc989f. [DOI] [PubMed] [Google Scholar]
- [26].Haier RJ, Alkire MT, White NS, Uncapher MR, Head E, Lott IT, Cotman CW. Temporal cortex hypermetabolism in Down syndrome prior to the onset of dementia. Neurology. 2003;61:1673–1679. doi: 10.1212/01.wnl.0000098935.36984.25. [DOI] [PubMed] [Google Scholar]
- [27].Haier RJ, Head K, Head E, Lott IT. Neuroimaging of individuals with Down's syndrome at-risk for dementia: evidence for possible compensatory events. Neuroimage. 2008;39:1324–1332. doi: 10.1016/j.neuroimage.2007.09.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sano MC, Aisen PS, Dalton AJ, Andrews HF, Tsai W-Y, Consortium IDSAD. Assessment of aging individuals with Down syndrome in clinical trials: Preliminary results of baseline evaluations. Journal of Policy and Practice in Intellectual Disabilities. 2005;2:126–138. [Google Scholar]
- [29].Panisset M, Roudier M, Saxton J, Boller F. Severe impairment battery. A neuropsychological test for severely demented patients. Arch Neurol. 1994;51:41–45. doi: 10.1001/archneur.1994.00540130067012. [DOI] [PubMed] [Google Scholar]
- [30].Evenhuis HM. Evaluation of a screening instrument for dementia in ageing mentally retarded persons. J Intellect Disabil Res. 1992;36:337–347. doi: 10.1111/j.1365-2788.1992.tb00532.x. [DOI] [PubMed] [Google Scholar]
- [31].Evenhuis HM. Further evaluation of the Dementia Questionnaire for Persons with Mental Retardation (DMR) J. Intellect Disabil Res. 1996;40:369–373. doi: 10.1046/j.1365-2788.1996.786786.x. [DOI] [PubMed] [Google Scholar]
- [32].Black SC, Hewett S, Kotubi Y, Brunt RV, Reckless JP. Isoform patterns of apolipoprotein E in diabetes mellitus. Diabet Med. 1990;7:532–539. doi: 10.1111/j.1464-5491.1990.tb01437.x. [DOI] [PubMed] [Google Scholar]
- [33].Ossendorf M, Prellwitz W. Rapid and easy apoliprotein E genotyping using an improved PCR-RFLP technique. QiageNews. 2000;1:11–12. [Google Scholar]
- [34].Matsuoka Y, Andrews HF, Becker AG, Gray AJ, Mehta PD, Sano MC, Dalton AJ, Aisen PS. The relationship of plasma Abeta levels to dementia in aging individuals with down syndrome. Alzheimer Dis Assoc Disord. 2009;23:315–318. doi: 10.1097/WAD.0b013e3181aba61e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Lai F, Williams RS. A prospective study of Alzheimer disease in Down syndrome. Arch Neurol. 1989;46:849–853. doi: 10.1001/archneur.1989.00520440031017. [DOI] [PubMed] [Google Scholar]
- [36].Pesaresi M, Lovati C, Bertora P, Mailland E, Galimberti D, Scarpini E, Quadri P, Forloni G, Mariani C. Plasma levels of beta-amyloid (1–42) in Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006;27:904–905. doi: 10.1016/j.neurobiolaging.2006.03.004. [DOI] [PubMed] [Google Scholar]
- [37].Mehta PD, Capone G, Jewell A, Freedland RL. Increased amyloid beta protein levels in children and adolescents with Down syndrome. J Neurol Sci. 2007;254:22–27. doi: 10.1016/j.jns.2006.12.010. [DOI] [PubMed] [Google Scholar]
- [38].Freeman SH, Raju S, Hyman BT, Frosch MP, Irizarry MC. Plasma Aβ δοεσ νοτ ρεφλεχτ βραιν Aβ λεϖελσ. J Neuropathol Exp Neurol. 2007;66:264–271. doi: 10.1097/NEN.0b013e31803d3ae4. [DOI] [PubMed] [Google Scholar]
- [39].Jones EL, Hanney M, Francis PT, Ballard CG. Amyloid beta concentrations in older people with Down syndrome and dementia. Neurosci Lett. 2009;451:162–164. doi: 10.1016/j.neulet.2008.12.030. [DOI] [PubMed] [Google Scholar]
- [40].Prasher VP, Sajith SG, Mehta P, Zigman WB, Schupf N. Plasma beta-amyloid and duration of Alzheimer's disease in adults with Down syndrome. Int J Geriatr Psychiatry. 2009 doi: 10.1002/gps.2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schupf N, Kapell D, Lee JH, Zigman W, Canto B, Tycko B, Mayeux R. Onset of dementia is associated with apolipoprotein E epsilon4 in Down's syndrome. Ann Neurol. 1996;40:799–801. doi: 10.1002/ana.410400518. [DOI] [PubMed] [Google Scholar]
- [42].Schupf N, Sergievsky GH. Genetic and host factors for dementia in Down's syndrome. Br J Psychiatry. 2002;180:405–410. doi: 10.1192/bjp.180.5.405. [DOI] [PubMed] [Google Scholar]
- [43].Hoglund K, Wiklund O, Vanderstichele H, Eikenberg O, Vanmechelen E, Blennow K. Plasma levels of beta-amyloid(1–40), beta-amyloid(1–42), and total beta-amyloid remain unaffected in adult patients with hypercholesterolemia after treatment with statins. Arch Neurol. 2004;61:333–337. doi: 10.1001/archneur.61.3.333. [DOI] [PubMed] [Google Scholar]
- [44].Blasko I, Kemmler G, Krampla W, Jungwirth S, Wichart I, Jellinger K, Tragl KH, Fischer P. Plasma amyloid beta protein 42 in non-demented persons aged 75 years: effects of concomitant medication and medial temporal lobe atrophy. Neurobiol Aging. 2005;26:1135–1143. doi: 10.1016/j.neurobiolaging.2005.03.006. [DOI] [PubMed] [Google Scholar]
- [45].Mayeux R, Tang MX, Jacobs DM, Manly J, Bell K, Merchant C, Small SA, Stern Y, Wisniewski HM, Mehta PD. Plasma amyloid beta-peptide 1-42 and incipient Alzheimer's disease. Ann Neurol. 1999;46:412–416. doi: 10.1002/1531-8249(199909)46:3<412::aid-ana19>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- [46].Biere AL, Ostaszewski B, Stimson ER, Hyman BT, Maggio JE, Selkoe DJ. Amyloid beta-peptide is transported on lipoproteins and albumin in human plasma. J Biol Chem. 1996;271:32916–32922. doi: 10.1074/jbc.271.51.32916. [DOI] [PubMed] [Google Scholar]
- [47].Hammad SM, Ranganathan S, Loukinova E, Twal WO, Argraves WS. Interaction of apolipoprotein J-amyloid beta-peptide complex with low density lipoprotein receptor-related protein-2/megalin. A mechanism to prevent pathological accumulation of amyloid beta-peptide. J Biol Chem. 1997;272:18644–18649. doi: 10.1074/jbc.272.30.18644. [DOI] [PubMed] [Google Scholar]
- [48].Conti E, Galimberti G, Piazza F, Raggi ME, Ferrarese C. Increased Soluble APPalpha, Abeta 1-42, and Anti-Abeta 1-42 Antibodies in Plasma From Down Syndrome Patients. Alzheimer Dis Assoc Disord. 2009 doi: 10.1097/WAD.0b013e3181aba63a. [DOI] [PubMed] [Google Scholar]
- [49].Kuo YM, Kokjohn TA, Watson MD, Woods AS, Cotter RJ, Sue LI, Kalback WM, Emmerling MR, Beach TG, Roher AE. Elevated abeta42 in skeletal muscle of Alzheimer disease patients suggests peripheral alterations of AbetaPP metabolism. Am J Pathol. 2000;156:797–805. doi: 10.1016/s0002-9440(10)64947-4. [DOI] [PMC free article] [PubMed] [Google Scholar]