Abstract
We aimed to identify mechanisms by which apolipoprotein B-48 (apoB-48) could have an atherogenic role by simultaneously studying the metabolism of postprandial apoB-48 and apoB-100 lipoproteins. The kinetics of apoB-48 and apoB-100, each in four density subfractions of VLDL and intermediate density lipoprotein (IDL), were studied by stable isotope labeling in a constantly fed state with half-hourly administration of almond oil in five postmenopausal women. A non-steady-state, multicompartmental model was used. Despite a much lower production rate, VLDL and IDL apoB-48 shared a similar secretion pattern with apoB-100: both were directly secreted into all fractions with similar percentage mass distributions. Fractional catabolic rates (FCRs) of apoB-48 and apoB-100 were similar in VLDL and IDL. We identified a fast turnover compartment of light VLDL that had a residence time of <30 min for apoB-48 and apoB-100. Finally, a high secretion rate of apoB-48 was associated with a slow FCR of VLDL and IDL apoB-100. In conclusion, the intestine secretes a spectrum of apoB lipoproteins, similar to what the liver secretes, albeit with a much lower secretion rate. Once in plasma, intestinal and hepatic triglyceride-rich lipoproteins have similar rates of clearance and participate interactively in similar metabolic pathways, with high apoB-48 production inhibiting the clearance of apoB-100.
Supplementary key words: kinetics, stable isotopes, triglyceride-rich lipoproteins, apolipoprotein B-48, apolipoprotein B-100
Lipids in the postprandial state may be more closely related to the risk of coronary heart disease (CHD) than levels in the fasting state, and impaired clearance of postprandial lipoproteins may be an underlying risk factor for CHD. A hypothesis linking postprandial triglyceride-rich lipoproteins (TRLs), including chylomicron and remnants, to CHD was first proposed by Zilversmit (1). Subsequent studies found that TRL and remnants were associated with the progression of coronary lesions or carotid intima-media thickness (2–4). Other studies have not found a greater postprandial TG response (5) or higher postprandial apolipoprotein B (apoB) lipoproteins (6) in CHD patients, although there was a correlation between the severity of coronary stenosis and the postprandial response of smaller hepatic apoB lipoproteins. Finally, the postprandial VLDL apoB-48 concentration was higher in CHD patients than in controls in some studies (2, 7) but not in others (3, 8). This array of findings, although generally supportive of a relation between postprandial lipoproteins and atherosclerosis, suggests that further progress may depend on greater understanding of the complexity of postprandial apoB lipoprotein metabolism.
The kinetics of apoB-48-containing TRLs have been studied by a variety of methods. Nestel (9) and Grundy and Mok (10) first examined the metabolism of chylomicrons by labeling the triglyceride component and found that chylomicron triglyceride was cleared from blood within minutes in normal participants and that the clearance was delayed in CHD patients (9). By labeling chylomicrons with retinyl palmitate, a more stable component of the lipoprotein, several groups also observed the clearance of retinyl palmitate to be fast (half-time of 15–30 min) (11–13). Similar results were also observed when the protein components of chylomicrons were labeled by iodination (14–16). When TRL from a patient with severe chylomicronemia caused by lipoprotein lipase deficiency was used as a source of apoB-48 and apoB-100 to radioiodinate, Stalenhoef et al. (15) observed rapid clearance of the heterologous apoB-48 (within 15 min) and apoB-100 (within 30 min) in normal participants but a markedly decreased catabolism in the same chylomicronemic patients. However, when TRL apoB-48 and apoB-100 kinetics were studied by endogenous labeling with stable isotopes, the clearance of chylomicrons was found to be significantly slower. Using a primed-constant infusion of trideuterated leucine in normolipidemic or mildly hyperlipidemic participants provided with small hourly feeding, several studies found that TRL apoB-48 had similar fractional turnover rates to TRL apoB-100, with an average fractional catabolic rate (FCR) of four to six pools per day and an average residence time of 4–6 h (17–21).
Among other factors, the apparently conflicting results for TRL apoB-48 kinetics may be attributable to the heterogeneity of chylomicrons and their remnants. There is increasing evidence that apoB-48 is present on a heterogeneous group of lipoproteins of various sizes and densities (22). Most of the previous kinetic studies of apoB-48 examined chylomicrons or TRLs as a single lipoprotein compartment and thus could not provide a comprehensive assessment of metabolic pathways within intestinal apoB lipoprotein subfractions. Therefore, in this kinetic study, we isolated apoB-48 in three VLDL subfractions and intermediate density lipoprotein (IDL) in a constantly fed state and compared the kinetics with those of corresponding apoB-100 fractions. This design serves as a next logical step in elucidating the metabolism of chylomicrons and the interrelationships among intestinal and hepatic TRLs.
MATERIALS AND METHODS
Research participants
Five healthy normolipidemic postmenopausal women were recruited. The study was approved by the Human Subjects Committees at Harvard School of Public Health and Brigham and Women’s Hospital. All participants gave informed consent.
Tracer infusion protocol
After an overnight fast, participants ingested 27 g of almond oil every 30 min during the first hour (0, 30, and 60 min) and then 9 g every 30 min afterward until the end of the study to stimulate continuous chylomicron production. Phosphatidylcholine (40 mg) was added to each 1 g of oil to facilitate gastrointestinal absorption. At 3 h, participants received a priming dose of trideuterated leucine (4.3 μmol/kg), followed by a continuous infusion at 7.4 μmol/kg/h for 9 h. Total caloric intake during the study was ~2,270 kcal, with 70% from monounsaturated fatty acids, 20% from polyunsaturated fatty acids, and 10% from saturated fatty acids. No protein was given, so as not to dilute the intestinal precursor pool with unlabeled dietary leucine.
Isolation of lipoproteins
Sequential preparative ultracentrifugation was used to isolate plasma apoB-containing lipoprotein according to Svedberg units of flotation (Sf) into three VLDL subfractions and IDL. The four subfractions were very light TRL (Sf > 400; chylomicron or very light VLDL), light VLDL (Sf = 60–400), dense VLDL (Sf = 20–60), and IDL (Sf = 12–20; density = 1.006–1.025 mg/dl) (23). LDL was also isolated, but the tracer enrichments of LDL were very low, and for this reason LDL kinetics were not studied. It should be pointed out that in lieu of a formal nomenclature system, in this study the terms “VLDL” and “IDL,” which have been used exclusively to describe apoB-100 lipoproteins by some researchers, are extended to describe apoB-48 lipoproteins of corresponding densities as well. In particular, apoB-48-containing lipoprotein in the Sf > 400 fraction is termed “chylomicron,” whereas apoB-100 lipoprotein of Sf > 400 is termed “very light VLDL.”
Measurement of tracer enrichment and apoB mass
After ultracentrifugation, lipoproteins were applied to 3–8% SDS-polyacrylamide gradient gels to isolate apoB. ApoB-48 and apoB-100 protein bands were excised and hydrolyzed (120°C, 24 h) in 6 N HCl under nitrogen. An internal standard, norleucine, was added to the mixture. The hydrolyzates were chilled for 20 min at −20°C and centrifuged at 3,000 rpm (5 min, 4°C). The clear supernatant containing free amino acids was separated from the precipitated polyacrylamide gels. Free amino acids were extracted using AG50W-X8 resin (Bio-Rad, Richmond, CA). The specimens were completely dried by heating at 60°C under nitrogen. Amino acids were converted to volatile heptafluorobutyric acid derivatives by heating with an acetyl chloride and propanol mixture at 110°C for 25 min, drying under nitrogen, and heating with heptafluorobutyric acid anhydride for 25 min at 60°C. The samples were analyzed on a 5890 gas chromatograph/5988A mass spectrometer (Hewlett-Packard, Palo Alto, CA) using negative chemical ionization and selective ion monitoring (24, 25). Isotopic enrichment and tracer-tracee ratio were calculated from ion current ratios using the method of Cobelli et al. (26). ApoB was quantitated by GC-MS from the leucine abundance peak calibrated in each subject using the internal standard norleucine. Plasma apoB mass (mg) was the plasma concentration of apoB protein multiplied by plasma volume. Plasma volume (liters) was assumed to be 4.5% of body weight (kg).
Model development and kinetic analysis
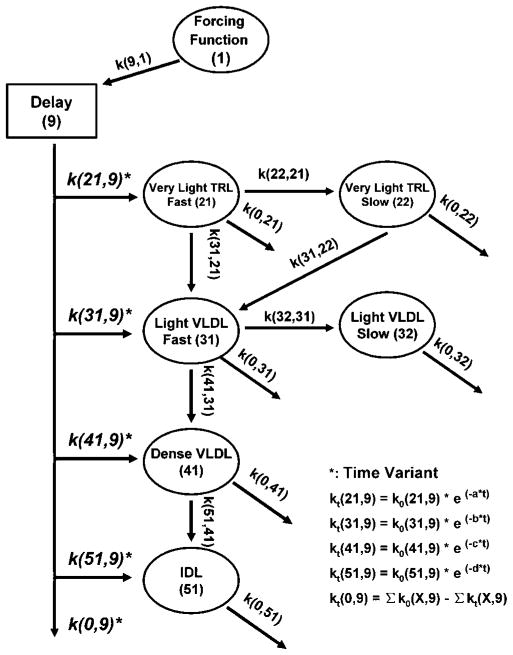
SAAM II software (version 1.2.1; SAAM Institute, Seattle, WA) was used to find the best fit model to the observed tracer and apoB mass data. Kinetics of apoB-48 and apoB-100 in chylomicron/very light VLDL, light VLDL, dense VLDL, and IDL fractions were described by a multicompartmental model (Fig. 1). This model provided for the direct secretion of apoB into all fractions for both apoB-48 and apoB-100 and allowed apoB to leave the plasma at any compartment; it was used for both apoB-48 and apoB-100 kinetic analysis. This model has similar structure to multicompartmental models of TRL metabolism used previously by us and other researchers (13, 24, 27). We also tested an alternative model that used very light VLDL as the sole precursor for light VLDL, dense VLDL, and IDL. This model could not fit the tracer-tracee or mass data for the three denser fractions as well as the first model, because their time of tracer appearance was too early and their masses were too large to come from very light VLDL.
Fig. 1.
Multicompartmental model for apolipoprotein B-48 (apoB-48) and apoB-100 metabolism. Compartment 1 represents a plasma leucine-forcing function. Compartment 9 is a delay compartment responsible for the production and secretion of apoB. Compartments 21–51 represent plasma pools of apoB. Rate constants leaving the delay compartment were made time-variant to account for the nonsteady state of apoB mass. See Materials and Methods for details. IDL, intermediate density lipoprotein; TRL, triglyceride-rich lipoprotein.
In Fig. 1, compartment 1 describes an amino acid-forcing function. Because labeling of intracellular and plasma leucine is not always similar (28), we used plateau values for the isotopic enrichments of dense VLDL apoB-48 and apoB-100 as the forcing functions to estimate the intracellular precursor pools. In subjects whose plateau was not reached for dense VLDL apoB enrichment at the end of the study, monoexponential regression was used to estimate the plateau value.
Compartment 9 is an intracellular delay compartment representing the synthesis of apoB in the liver for apoB-100 or in the intestine for apoB-48 and accounts for the time required for the synthesis and secretion of apoB into plasma. Compartments 21, 31, 41, and 51 represent plasma chylomicron/very light VLDL, light VLDL, dense VLDL, and IDL apoB, respectively. To obtain a good fit to chylomicron/very light VLDL and light VLDL apoB tracer data in the majority of subjects, slow turnover remnant compartments were required (compartments 22 and 32). The addition of these slow turnover compartments significantly improved the overall fitting of model to data by decreasing the Akaike information criterion and the Schwarz-Bayesian information criterion (P < 0.05 for both apoB-100 and apoB-48 models). Singular compartments were able to generate excellent fits to the tracer-tracee data for dense VLDL and IDL.
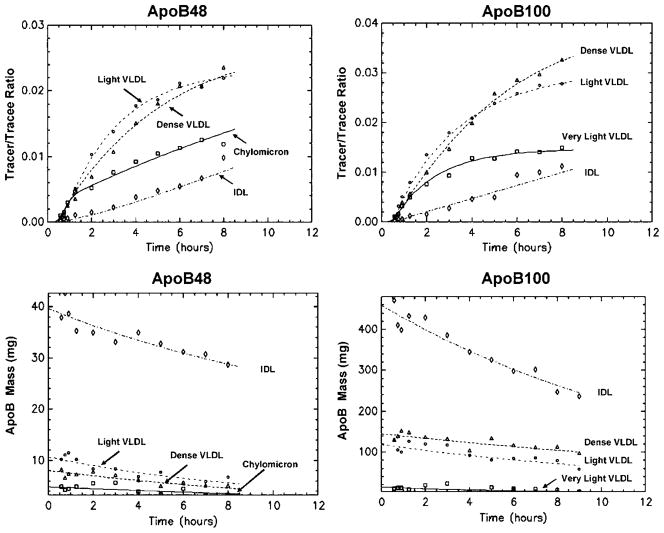
We noticed that during the course of the study, mean apoB-48 and apoB-100 concentrations decreased by 25–45% in some fractions (Fig. 2). We attributed this decrease in apoB production to two sources: 1) some participants experienced difficulty ingesting all of the allotted almond oil during later hours of the study; and 2) the feeding of 108 g of almond oil during 3 h before the start of tracer infusion might have generated high postprandial peak levels of TRL apoB-48 and apoB-100 that could not be sustained by the subsequent half-hourly intake of 9.0 g of almond oil.
Fig. 2.
Leucine tracer-tracee ratios and apoB masses in apoB-48 and apoB-100 lipoproteins. Observed values are given as symbols, and model-predicted values are given as lines. The X axis represents the time after infusion. Data points and curve fitting are based on the average enrichment and apoB mass of five participants.
To test the hypothesis that decreased synthesis could explain the decrease in apoB-48 and apoB-100 mass levels, we allowed the production of apoB to vary over time. Rate constants k(21,9), k(31,9), k(41,9), and k(51,9), which control for the organ secretion of apoB into chylomicron/very light VLDL, light VLDL, dense VLDL, and IDL fractions, respectively, were described as the product of their initial values at the beginning of the infusion and an exponential function (Fig. 1). Variables that determine the shape of exponential function (a, b, c, and d in Fig. 1 functions) were solved by SAAM II. Similar modeling techniques have been used previously by Malmstrom et al. (29–31) to successfully account for the nonsteady state of VLDL apoB-100 mass in participants undergoing glucose clamp tests. Using this approach, we obtained an excellent fit for both the leucine tracer data and the changes in apoB-48 and apoB-100 mass (Fig. 2). The results show that consistent with the decrease in apoB mass, secretion rate constants k(21,9)–k(51,9) decreased with time in a log-linear manner in all subjects. The average values for parameters a, b, c, and d were 0.034, 0.031, 0.023, and 0.086 for apoB-100 and 0.032, 0.052, 0.049, and 0.058 for apoB-48, respectively. The rate constant k(0,9) is a dummy variable, and its value is zero when there is no change in the sum of k(21,9)–k(51,9). When k(21, 9)–k (51,9) decrease with time in a non-steady-state situation, k(0,9) compensates for this and allows k(9,1) to remain constant. On the other hand, all of the other rate constants in the model, including the rate constants for clearance [k(0,21), k(0,22), … k(0,51)] and those for lipolysis [k(22,21), k(31,21), … k(51,41)], did not change with time. In addition, all of the FCRs also remained constant during the course of the study.
We also tested an alternative hypothesis, that the decrease in apoB mass was attributable to increased clearance, by keeping the production constant but allowing rate constants that were responsible for clearance [i.e., k(0,21)–k(0, 51)] to increase over time. In this setting, clearance rate constants were also described by exponential functions. This approach could not generate a satisfactory fit to either the enrichment or apoB mass data in three of the five participants, and the coefficients of variation of the model-derived parameters in all participants were higher than in the secretion-varying model. Finally, we allowed both secretion and clearance to be time-variant in the same model by applying exponential functions to not only k(21,9)–k(51,9) but also to k(0,21)–k(0, 51). The results showed that the rate constants for secretion changed much more with time than the rate constants for clearance. Thus, it seemed that although postprandial increases in lipoprotein clearance might contribute to some of the fluctuations of apoB mass, the decrease in production was the main factor responsible for the postprandial decrease of apoB mass. Above all, the central results of this study, the comparisons between apoB-48 and apoB-100 lipoprotein metabolism, were the same regardless of whether secretion, clearance, or both was made time-variant.
Statistical analysis
Data were analyzed using SAS software (SAS Institute, Cary, NC) and are presented as means ± SD. The Wilcoxon signed rank test was used for between-group comparisons, unless specified otherwise. Spearman correlation coefficients were also determined. P ≤ 0.05 was considered statistically significant.
RESULTS
The participants were postmenopausal women with an average age of 64 years and an average body mass index of 24 kg/m2. Their characteristics are shown in Table 1.
TABLE 1.
Baseline characteristics of the participants
| Participant | Age | Body Mass Index | Total Cholesterol | Triglyceride | LDL-Cholesterol | HDL-Cholesterol | ApoB |
|---|---|---|---|---|---|---|---|
| years | kg/m2 | mg/dl | |||||
| 1 | 62 | 27 | 179 | 90 | 78 | 73 | 59 |
| 2 | 64 | 23 | 181 | 81 | 102 | 63 | 104 |
| 3 | 64 | 22 | 222 | 61 | 128 | 82 | 70 |
| 4 | 64 | 22 | 262 | 104 | 170 | 71 | 74 |
| 5 | 66 | 27 | 235 | 140 | 159 | 48 | 95 |
| Mean | 64 | 24 | 216 | 95 | 127 | 67 | 80 |
| SD | 9 | 2 | 36 | 30 | 38 | 13 | 19 |
ApoB, apolipoprotein B.
Figure 2 shows the average tracer-tracee ratios of apoB leucine enrichment in VLDL and IDL subfractions. There was a delay from starting the infusion to the appearance of tracer in apoB. The average delay was 21 min for apoB-48 and 29 min for apoB-100. Figure 2 also shows the mass of apoB-48 and apoB-100. As discussed in Materials and Methods, apoB mass decreased in some fractions. The IDL fraction had the highest plasma mass (range, 27–43 mg) of apoB-48, followed by light VLDL (5–11 mg), dense VLDL (5–8 mg), and chylomicron (3–6 mg). This pattern of larger masses for smaller lipoproteins also held true for apoB-100: IDL also had the highest mass (237–470 mg), followed by dense VLDL (96–151 mg), light VLDL (60–130 mg), and very light VLDL (4–22 mg). As shown, pool sizes of apoB-100 VLDL and IDL were much higher than those for apoB-48 in all fractions. However, a relatively greater proportion of apoB-48 mass was found in chylomicrons compared with apoB-100 in very light VLDL (22% vs. 4%; P < 0.05).
In all VLDL and IDL fractions examined, FCRs of apoB-100 were similar to those of apoB-48 (Table 2). Total VLDL apoB-48 had a FCR of 5.6 pools/day, corresponding to a residence time of 4.3 h, whereas total VLDL apoB-100 had a FCR of 7.0 pools/day, corresponding to a residence time of 3.4 h. We also noticed that the FCR of VLDL was significantly greater than that of IDL for both types of apoB, with an average residence time of 22–24 h.
TABLE 2.
FCRs, secretion rates, and pool sizes for apoB-100 and apoB-48 TRLs
| Lipoprotein Subclass | FCR
|
Secretion Rate
|
Pool Size
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| ApoB-100 | ApoB-48 | P | ApoB-100 | ApoB-48 | P | ApoB-100 | ApoB-48 | P | |
| pools/day | mg/kg/day | mg | |||||||
| Very light TRL (Sf > 400) | 8.2 ± 3.8 | 9.6 ± 4.7 | 0.6 | 1.6 ± 0.9 | 0.7 ± 0.4 | 0.1 | 7.2 ± 4.5 | 3.5 ± 0.9 | 0.2 |
| Light VLDL (Sf = 60–400) | 7.2 ± 2.6 | 9.7 ± 4.7 | 0.3 | 8.4 ± 5.6 | 0.4 ± 0.2 | 0.01 | 74 ± 37 | 6.8 ± 2.6 | 0.02 |
| Dense VLDL (Sf = 20–60) | 7.2 ± 3.1 | 8.9 ± 3.9 | 0.5 | 7.2 ± 3.3 | 0.3 ± 0.2 | 0.02 | 90 ± 43 | 5.4 ± 1.8 | 0.01 |
| Total VLDLa (Sf > 20) | 7.0 ± 2.3 | 5.6 ± 2.4 | 0.3 | 17.2 ± 9.1 | 1.4 ± 0.6 | 0.02 | 171 ± 102 | 16 ± 5.0 | 0.02 |
| Intermediate density lipoprotein (Sf = 12–20) | 1.0 ± 0.4 | 1.1 ± 0.5 | 0.8 | 4.7 ± 3.6 | 0.8 ± 0.6 | 0.03 | 287 ± 112 | 31 ± 6.2 | 0.03 |
Values shown are means ± SD of five participants at 4.5 h into the infusion. FCR, fractional catabolic rate; Sf, Svedberg units of flotation; TRL, triglyceride-rich lipoprotein.
Total VLDL includes all VLDL subfractions and is modeled as a single combined compartment. Its FCR may be lower than the FCRs of individual VLDL subfractions as a result of intercompartmental conversions.
The multicompartmental model had both fast and slow turnover pools for chylomicron/very light VLDL and light VLDL. Fast and slow turnover compartments (compartments 21 and 22) of chylomicron apoB-48 had FCRs of 55 ± 24 and 2.2 ± 1.1 pools/day, whereas the FCRs of the corresponding very light VLDL apoB-100 were 57 ± 22 and 1.4 ± 0.8 pools/day. For light VLDL, fast and slower turnover compartments (compartments 31 and 32) of apoB-48 had FCRs of 18 ± 7 and 1.6 ± 0.8 pools/day, whereas the FCRs of the corresponding apoB-100 pools were 15 ± 5 and 1.4 ± 0.8 pools/day. There was no statistically significant difference between apoB-48 and apoB-100 FCRs for subfractions of the same density.
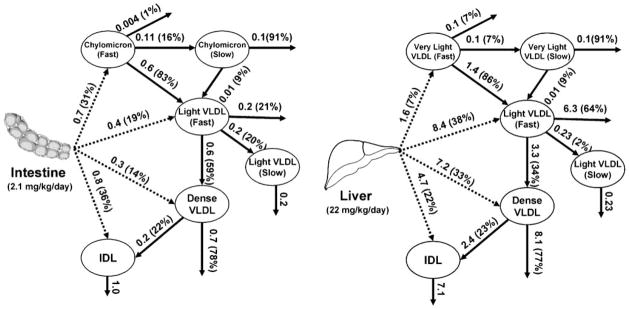
Because of the global decline of apoB mass during the course of the study, the secretion rates of apoB into various TRL fractions also decreased with time. At any given time, we found that apoB-100 had higher secretion rates than apoB-48: >20 times higher in light VLDL and dense VLDL fractions, ~5 times higher in IDL (P = 0.01–0.03), and ~2 times as high in the very light VLDL versus chylomicrons (P = 0.1) (Table 2). There was significant hepatic and intestinal production of apoB into the IDL fraction: on average, 4.7 mg/kg/day (22% of total production) for apoB-100 and 0.8 mg/kg/day (36%) for apoB-48. In the constantly fed state, on average, 2.1 mg/kg/day apoB-48 moved through the system, which was ~10% of apoB-100 flux (22 mg/kg/day) (Fig. 3). The secretion pattern was similar between intestinal and hepatic TRLs, with direct organ production of TRLs into all subfractions and significant production of IDL-sized lipoproteins.
Fig. 3.
ApoB-48 and apoB-100 flux during constant feeding. Flux (numbers above arrows, in mg/kg/day) indicates the amount of apoB through each metabolic pathway. The percentages in parentheses indicate the proportions of apoB from the originating compartment going through a particular pathway. Because of the nonsteady state of the fluxes and pool sizes, the values given are averages over the 9 h study.
Our results showed substantive conversions from chylomicron/very light VLDL to light VLDL in both apoB-48 and apoB-100 as a result of lipolysis. More than 80% of apoB-48 or apoB-100 mass in chylomicron/very light VLDL transferred to light VLDL. For light VLDL, lipolytic conversion to dense VLDL also played a role in apoB-48 and apoB-100 metabolism: 59% of apoB-48 and 34% of apoB-100 were converted to dense VLDL. For dense VLDL, lipolysis to IDL was a minor pathway constituting 22–23% of flux. For apoB-48 and apoB-100, flux shifted from delipidation to clearance as the density of the particles increased. Only 14% of VLDL apoB-48 or apoB-100 that was secreted was eventually transferred to IDL.
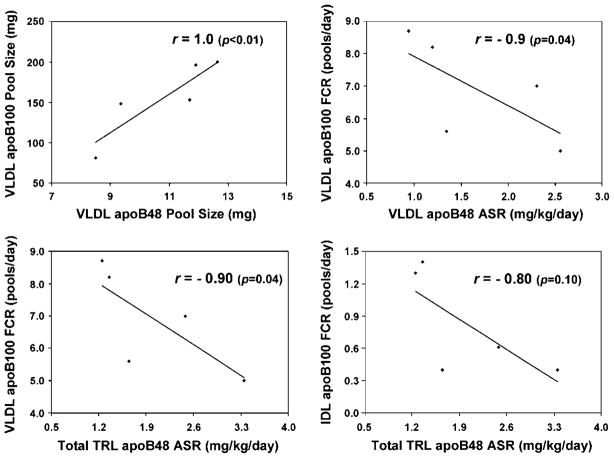
Pool sizes of intestinal and hepatic VLDL in the fed state were strongly correlated with fasting plasma TG (r = 0.9, P = 0.04) and were inversely correlated with fasting HDL cholesterol (r = −0.9, P = 0.04). ApoB-48 and apoB-100 pool sizes were strongly correlated in both VLDL (r = 1.0, P < 0.01) (Fig. 4, top left) and IDL (r = 0.80, P = 0.10). In addition, FCR of VLDL apoB-100 was inversely correlated with the secretion rate into plasma of VLDL apoB-48 (r = −0.90 P = 0.04) (Fig. 4, top right) and of total TRL (sum of VLDL and IDL) apoB-48 (r = −0.90, P = 0.04) (Fig. 4, bottom left). The FCR of IDL apoB-100 was also inversely correlated with the secretion rate of total TRL apoB-48 (r = −0.80 P = 0.10) (Fig. 4, bottom right).
Fig. 4.
Scatterplots of kinetic parameters of apoB-48 and apoB-100 TRLs. Spearman correlation coefficients (r) and P values are given. Data points represent kinetic parameters for five participants at the start of infusion. ASR, absolute secretion rate of apoB-48 from intestine into plasma.
DISCUSSION
In view of findings linking postprandial lipoprotein levels or responses to CHD, and some divergence among studies on the importance of intestinal apoB lipoproteins, we attempted to extend the understanding of postprandial apoB lipoprotein metabolism by studying detailed metabolic pathways of apoB-48 and apoB-100 lipoproteins during the ingestion of fat. Recently, we reported that postprandial apoB-48 is present in a surprisingly wide range of sizes, from chylomicrons to LDL (22). The key questions for this kinetic study were the secretion pattern of apoB-48, the predominant metabolic pathways of intestinal lipoproteins once in plasma, and the relation between apoB-48 and apoB-100 metabolism.
To stimulate constant chylomicron secretion, we provided study participants with half-hourly feeding of small amounts of almond oil after an initial large bolus. We found that under these conditions, apoB-48 and apoB-100 TRLs were both secreted into a wide spectrum of particle sizes and that, interestingly, more than one-third of total TRL apoB-48 is secreted as IDL. This suggests that the intestine is capable of secreting apoB-48 lipoproteins of various sizes in vivo in humans and that a significant percentage of smaller plasma apoB-48 lipoproteins are indeed nascent particles, instead of remnants of larger chylomicrons after lipolysis. This is consistent with results from animal experiments that intestinal enterocytes are capable of secreting chylomicrons of sizes from LDL to light VLDL (32). Nonetheless, we note that the postprandial apoB kinetics found in our study pertain to the specific dietary protocol used and may not entirely represent what happens during the usual dietary intake with discrete meals containing fat, carbohydrate, protein, and fiber.
This study examined simultaneously the kinetics of TRL apoB-48 and apoB-100 in multiple subfractions for the first time and found that although the production of apoB-100 is much higher than that of apoB-48 for all particle sizes, once in circulation, their metabolic pathways and FCRs become similar. In previous studies in which TRL apoB-48 has been examined as a single compartment, apoB-48 and apoB-100 had similar metabolic rates in plasma (17–21). Our results suggest that the lack of a ligand binding region in the LDL receptor does not seem to delay the clearance of apoB-48 TRL compared with apoB-100, implying the importance of other lipoprotein components in the clearance of TRL and remnants. In addition to apoE, a well-known ligand for hepatic receptors, we recently discovered that lipoprotein lipase (LPL) could also function like an apolipoprotein in humans by dissociating from capillary endothelium and attaching to circulating plasma TRLs in the postprandial state and play an important role in their catabolism (33). Chylomicrons with LPL could be removed from the circulation five to eight times faster than those without LPL.
Rather than studying apoB-48 TRL as a single lipoprotein fraction, we isolated from plasma the four density classes of TRLs: chylomicron, light VLDL, dense VLDL, and IDL. This allowed us to study the postprandial metabolism of chylomicrons and their remnants in detail. In previous stable isotope kinetics studies of apoB-48 that used a single apoB-48 TRL compartment (17–21), the residence time was long, between 4 and 6 h, which is much longer than the residence time of <30 min reported in some other kinetic studies using radioactive iodination or retinyl palmitate labeling methods (9–15). A number of possible explanations could account for the significant difference of TRL apoB-48 metabolism among these studies, including the influence of different types and amounts of caloric intake, the effect of in vitro labeling potentially causing the denaturation and rapid clearance of TRL by reticuloendothelial cells, and the reduced ability of in vivo labeling to detect fast turnover pools. The results of this study may help resolve the controversy. When examined as a single compartment, our data show that VLDL apoB-48 has an average residence time of 4.3 h, similar to previous studies using stable isotope labeling (17–21). However, when examined by multicompartmental modeling, we discover considerable kinetic heterogeneity among TRL apoB-48 subfractions. Not only does apoB-48 of VLDL density have significantly higher clearance rates compared with IDL apoB, but there are also fast and slower turnover compartments among large VLDL subfractions. Interestingly, the fast turnover pool of chylomicron apoB-48 has an average residence time of 26 min, similar to studies using radioiodination or retinyl palmitate labeling (12–15).
Although we could not physically isolate the fast turnover pool of very light TRLs, this pool does not seem to be an artifact because its addition significantly increased the fit of the model to the data and its existence in chylomicrons was also identified using a different labeling technique with oral retinyl palmitate (13). Several previous kinetic studies conducted in the fasting state using either exogenous radioiodine labeling or endogenous stable isotope labeling have identified fast and slow turnover components of VLDL apoB-100 (24, 34, 35). Specific apolipoproteins on chylomicrons, such as apoE, apoC-II, and lipoprotein lipase, could accelerate their metabolism. Nonetheless, we caution that the inference by kinetic analysis of fast chylomicron or VLDL pools is limited by the inherent complexities of kinetic tracer studies, the relatively small numbers of study participants, and the specific laboratory techniques used to separate lipoprotein subfractions. The exact origin and nature of these fast and slow turnover pools need to be studied further.
This study also revealed a positive correlation in secretion rates and plasma levels between intestinal TRL and hepatic TRL, similar to what Welty et al. observed (18). These results indicate that in addition to competition for clearance, increased intestinal production of chylomicrons may lead to the enhanced delivery of exogenous lipids to liver, which will result in an increased production of hepatic lipoproteins.
The findings of this study add support to the hypothesis proposed by Welty et al. (18) and Brunzell and colleagues (36) that intestinal and hepatic TRLs may compete for a common, saturable metabolic pathway postprandially. Welty et al. (18) reported an inverse correlation between TRL apoB-48 production rate and VLDL apoB-100 FCR, and the current study confirms their results and extends them to additional TRL fractions. This demonstrates that the accumulation of chylomicron remnants may contribute to the increase of apoB-100 TRL and remnants in the postprandial state by competing for clearance pathways. This is particularly important considering the relatively low plasma level of chylomicrons compared with VLDL apoB-100 in these healthy participants. It has been demonstrated that apoB-48 TRLs have higher affinity for lipoprotein lipase than apoB-100 TRLs (37), and there are also reports that compared with VLDL and remnants, chylomicron remnants have significantly higher binding affinity to perfused rat liver (38) and to human hepatoma cells (39). Thus, apoB-48 TRL and remnants may play a disproportionally more important role in postprandial lipoprotein metabolism than what their apparent plasma concentrations suggest.
The decline of apoB mass during the course of the study is probably a result of the dietary regime. Non-steady-state modeling revealed that the decline of secretion rates was the main mechanism. Interestingly, we also noticed slight heterogeneity in the slope of decline among VLDL and IDL secretion rates. In particular, the decrease in the secretion rate of apoB-48 IDL was less pronounced compared with that in VLDL fractions, resulting in IDL representing 27% of total apoB-48 secretion at 9 h after infusion, compared with only 17% at the start of infusion. This may indicate a shift in the secretion pattern of the intestine from large, buoyant, triglyceride-rich particles after the initial large bolus of almond oil to smaller, denser particles when the study participants ingested small amounts of the oil every 30 min.
We noticed that IDL in our study had a slower turnover rate compared with some previous studies (40). Judging from its relatively large pool size, this is probably attributable to the fact that a high density cutoff point of 1.025 mg/dl was used. Some light LDL particles with longer residence time might be isolated together with IDL.
One limitation of this study is that the enrichment of trideuterated leucine was too low to measure in the LDL fraction of apoB-48 and apoB-100. ApoB-48 in LDL constitutes approximately one-third of total plasma apoB-48 mass after a fat load (22). Lack of an LDL compartment makes it difficult to study the metabolic pathways of IDL apoB-48 in more detail. Because IDL is secreted by the intestine, we suspect that LDL is too. Giving a larger dose of isotope for a longer duration would be an approach to increase the likelihood of achieving enough tracer enrichment in apoB-48 in LDL-sized particles.
In conclusion, this study extends to intestinal TRLs the sophistication in kinetic analysis that has been accorded previously to hepatic lipoproteins by separating intestinal TRLs into four density fractions with distinct metabolism: chylomicrons, light VLDL, dense VLDL, and IDL. This allowed us to investigate the metabolism of apoB-48 and apoB-100 subfractions simultaneously, in parallel and with the same kinetic model. The results show that despite their apparent differences in protein structure and origin, similar density fractions of apoB-48 and apoB-100 TRLs not only have similar FCRs and flux patterns but are also interconnected and interdependent in the fed state. Production of intestinal apoB lipoproteins in response to dietary fat leads to 1) enhanced production of hepatic lipoproteins through increased substrate delivery in the form of chylomicron remnants, and 2) delay in the clearance of hepatic TRLs as a result of competition for the same lipolytic and clearance pathways. Therefore, considering their low plasma levels and similar metabolism to hepatic apoB lipoproteins, we conclude that the atherogenicity of intestinal TRLs may be related more to their effect on the production and clearance of hepatic lipoproteins than to a direct effect on the arterial wall.
Acknowledgments
The authors are grateful to the volunteers for participating in this study. The authors also express thanks to Helena Judge Ellis for her technical and managerial assistance. This work was supported by Grant HL-069376 from the National Heart, Lung, and Blood Institute, National Institutes of Health.
References
- 1.Zilversmit DB. Atherogenesis: a postprandial phenomenon. Circulation. 1979;60:473–485. doi: 10.1161/01.cir.60.3.473. [DOI] [PubMed] [Google Scholar]
- 2.Karpe F, Steiner G, Uffelman K, Olivecrona T, Hamsten A. Postprandial lipoproteins and progression of coronary atherosclerosis. Atherosclerosis. 1994;106:83–97. doi: 10.1016/0021-9150(94)90085-x. [DOI] [PubMed] [Google Scholar]
- 3.Boquist S, Ruotolo G, Tang R, Bjorkegren J, Bond MG, de Faire U, Karpe F, Hamsten A. Alimentary lipemia, post-prandial triglyceride-rich lipoproteins, and common carotid intima-media thickness in healthy, middle-aged men. Circulation. 1999;100:723–728. doi: 10.1161/01.cir.100.7.723. [DOI] [PubMed] [Google Scholar]
- 4.Hodis HN, Mack WJ, Dunn M, Liu C, Selzer RH, Krauss RM. Intermediate-density lipoproteins and progression of carotid arterial wall intima-media thickness. Circulation. 1997;95:2022–2026. doi: 10.1161/01.cir.95.8.2022. [DOI] [PubMed] [Google Scholar]
- 5.Schaefer EJ, Audelin MC, McNamara JR, Shah PK, Tayler T, Daly JA, Augustin JL, Seman LJ, Rubenstein JJ. Comparison of fasting and postprandial plasma lipoproteins in subjects with and without coronary heart disease. Am J Cardiol. 2001;88:1129–1133. doi: 10.1016/s0002-9149(01)02047-1. [DOI] [PubMed] [Google Scholar]
- 6.Mero N, Malmstrom R, Steiner G, Taskinen MR, Syvanne M. Postprandial metabolism of apolipoprotein B-48-and B-100-containing particles in type 2 diabetes mellitus: relations to angiographically verified severity of coronary artery disease. Atherosclerosis. 2000;150:167–177. doi: 10.1016/s0021-9150(99)00364-0. [DOI] [PubMed] [Google Scholar]
- 7.Bjorkegren J, Boquist S, Samnegard A, Lundman P, Tornvall P, Ericsson CG, Hamsten A. Accumulation of apolipoprotein C-I-rich and cholesterol-rich VLDL remnants during exaggerated postprandial triglyceridemia in normolipidemic patients with coronary artery disease. Circulation. 2000;101:227–230. doi: 10.1161/01.cir.101.3.227. [DOI] [PubMed] [Google Scholar]
- 8.Karpe F, Hellenius ML, Hamsten A. Differences in postprandial concentrations of very-low-density lipoprotein and chylomicron remnants between normotriglyceridemic and hyper-triglyceridemic men with and without coronary heart disease. Metabolism. 1999;48:301–307. doi: 10.1016/s0026-0495(99)90076-8. [DOI] [PubMed] [Google Scholar]
- 9.Nestel PJ. Relationship between plasma triglycerides and removal of chylomicrons. J Clin Invest. 1964;43:943–949. doi: 10.1172/JCI104980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grundy SM, Mok HY. Chylomicron clearance in normal and hyperlipidemic man. Metabolism. 1976;25:1225–1239. doi: 10.1016/s0026-0495(76)80006-6. [DOI] [PubMed] [Google Scholar]
- 11.Berr F, Kern F., Jr Plasma clearance of chylomicrons labeled with retinyl palmitate in healthy human subjects. J Lipid Res. 1984;25:805–812. [PubMed] [Google Scholar]
- 12.Cortner JA, Coates PM, Le NA, Cryer DR, Ragni MC, Faulkner A, Langer T. Kinetics of chylomicron remnant clearance in normal and in hyperlipoproteinemic subjects. J Lipid Res. 1987;28:195–206. [PubMed] [Google Scholar]
- 13.Karpe F, Olivecrona T, Hamsten A, Hultin M. Chylomicron/chylomicron remnant turnover in humans: evidence for margination of chylomicrons and poor conversion of larger to smaller chylomicron remnants. J Lipid Res. 1997;38:949–961. [PubMed] [Google Scholar]
- 14.Schaefer EJ, Jenkins LL, Brewer HB., Jr Human chylomicron apolipoprotein metabolism. Biochem Biophys Res Commun. 1978;80:405–412. doi: 10.1016/0006-291x(78)90691-5. [DOI] [PubMed] [Google Scholar]
- 15.Stalenhoef AF, Malloy MJ, Kane JP, Havel RJ. Metabolism of apolipoproteins B-48 and B-100 of triglyceride-rich lipoproteins in normal and lipoprotein lipase-deficient humans. Proc Natl Acad Sci USA. 1984;81:1839–1843. doi: 10.1073/pnas.81.6.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaefer EJ, Gregg RE, Ghiselli G, Forte TM, Ordovas JM, Zech LA, Brewer HB., Jr Familial apolipoprotein E deficiency. J Clin Invest. 1986;78:1206–1219. doi: 10.1172/JCI112704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lichtenstein AH, Hachey DL, Millar JS, Jenner JL, Booth L, Ordovas J, Schaefer EJ. Measurement of human apolipoprotein B-48 and B-100 kinetics in triglyceride-rich lipoproteins using [5,5,5-2H3]leucine. J Lipid Res. 1992;33:907–914. [PubMed] [Google Scholar]
- 18.Welty FK, Lichtenstein AH, Barrett PH, Dolnikowski GG, Schaefer EJ. Human apolipoprotein (apo) B-48 and apoB-100 kinetics with stable isotopes. Arterioscler Thromb Vasc Biol. 1999;19:2966–2974. doi: 10.1161/01.atv.19.12.2966. [DOI] [PubMed] [Google Scholar]
- 19.Welty FK, Lichtenstein AH, Barrett PH, Jenner JL, Dolnikowski GG, Schaefer EJ. Effects of apoE genotype on apoB-48 and apoB-100 kinetics with stable isotopes in humans. Arterioscler Thromb Vasc Biol. 2000;20:1807–1810. doi: 10.1161/01.atv.20.7.1807. [DOI] [PubMed] [Google Scholar]
- 20.Batista MC, Welty FK, Diffenderfer MR, Sarnak MJ, Schaefer EJ, Lamon-Fava S, Asztalos BF, Dolnikowski GG, Brousseau ME, Marsh JB. Apolipoprotein A-I, B-100, and B-48 metabolism in subjects with chronic kidney disease, obesity, and the metabolic syndrome. Metabolism. 2004;53:1255–1261. doi: 10.1016/j.metabol.2004.05.001. [DOI] [PubMed] [Google Scholar]
- 21.Welty FK, Lichtenstein AH, Barrett PH, Dolnikowski GG, Schaefer EJ. Interrelationships between human apolipoprotein A-I and apolipoproteins B-48 and B-100 kinetics using stable isotopes. Arterioscler Thromb Vasc Biol. 2004;24:1703–1707. doi: 10.1161/01.ATV.0000137975.14996.df. [DOI] [PubMed] [Google Scholar]
- 22.Campos H, Khoo C, Sacks FM. Diurnal and acute patterns of postprandial apolipoprotein B-48 in VLDL, IDL, and LDL from normolipidemic humans. Atherosclerosis. 2005;181:345–351. doi: 10.1016/j.atherosclerosis.2004.12.045. [DOI] [PubMed] [Google Scholar]
- 23.Lindgren F, Jensen L, Hatch F. The isolation and quantitative analysis of serum lipoproteins. In: Nelson G, editor. Blood Lipids and Lipoproteins: Quantitation, Composition and Metabolism. John Wiley and Sons; New York: 1972. pp. 181–274. [Google Scholar]
- 24.Campos H, Walsh BW, Judge H, Sacks FM. Effect of estrogen on very low density lipoprotein and low density lipoprotein subclass metabolism in postmenopausal women. J Clin Endocrinol Metab. 1997;82:3955–3963. doi: 10.1210/jcem.82.12.4437. [DOI] [PubMed] [Google Scholar]
- 25.Walsh BW, Li H, Sacks FM. Effects of postmenopausal hormone replacement with oral and transdermal estrogen on high density lipoprotein metabolism. J Lipid Res. 1994;35:2083–2093. [PubMed] [Google Scholar]
- 26.Cobelli C, Toffolo G, Bier DM, Nosadini R. Models to interpret kinetic data in stable isotope tracer studies. Am J Physiol. 1987;253:E551–E564. doi: 10.1152/ajpendo.1987.253.5.E551. [DOI] [PubMed] [Google Scholar]
- 27.Millar JS, Maugeais C, Ikewaki K, Kolansky DM, Barrett PH, Budreck EC, Boston RC, Tada N, Mochizuki S, Defesche JC, et al. Complete deficiency of the low-density lipoprotein receptor is associated with increased apolipoprotein B-100 production. Arterioscler Thromb Vasc Biol. 2005;25:560–565. doi: 10.1161/01.ATV.0000155323.18856.a2. [DOI] [PubMed] [Google Scholar]
- 28.Fisher WR, Venkatakrishnan V, Fisher ES, Stacpoole PW, Zech LA. The 3H-leucine tracer: its use in kinetic studies of plasma lipoproteins. Metabolism. 1997;46:333–342. doi: 10.1016/s0026-0495(97)90262-6. [DOI] [PubMed] [Google Scholar]
- 29.Malmstrom R, Packard CJ, Watson TD, Rannikko S, Caslake M, Bedford D, Stewart P, Yki-Jarvinen H, Shepherd J, Taskinen MR. Metabolic basis of hypotriglyceridemic effects of insulin in normal men. Arterioscler Thromb Vasc Biol. 1997;17:1454–1464. doi: 10.1161/01.atv.17.7.1454. [DOI] [PubMed] [Google Scholar]
- 30.Malmstrom R, Packard CJ, Caslake M, Bedford D, Stewart P, Yki-Jarvinen H, Shepherd J, Taskinen MR. Defective regulation of triglyceride metabolism by insulin in the liver in NIDDM. Diabetologia. 1997;40:454–462. doi: 10.1007/s001250050700. [DOI] [PubMed] [Google Scholar]
- 31.Malmstrom R, Packard CJ, Caslake M, Bedford D, Stewart P, Yki-Jarvinen H, Shepherd J, Taskinen MR. Effects of insulin and acipimox on VLDL1 and VLDL2 apolipoprotein B production in normal subjects. Diabetes. 1998;47:779–787. doi: 10.2337/diabetes.47.5.779. [DOI] [PubMed] [Google Scholar]
- 32.Cartwright IJ, Higgins JA. Direct evidence for a two-step assembly of apoB48-containing lipoproteins in the lumen of the smooth endoplasmic reticulum of rabbit enterocytes. J Biol Chem. 2001;276:48048–48057. doi: 10.1074/jbc.M104229200. [DOI] [PubMed] [Google Scholar]
- 33.Zheng C, Murdoch SJ, Brunzell JD, Sacks FM. Lipoprotein lipase bound to apolipoprotein B lipoproteins accelerates clearance of postprandial lipoproteins in humans. Arterioscler Thromb Vasc Biol. 2006;26:891–896. doi: 10.1161/01.ATV.0000203512.01007.3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beltz WF, Kesaniemi YA, Howard BV, Grundy SM. Development of an integrated model for analysis of the kinetics of apolipoprotein B in plasma very low density lipoproteins, intermediate density lipoproteins, and low density lipoproteins. J Clin Invest. 1985;76:575–585. doi: 10.1172/JCI112009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zech LA, Grundy SM, Steinberg D, Berman M. Kinetic model for production and metabolism of very low density lipoprotein triglycerides. Evidence for a slow production pathway and results for normolipidemic subjects. J Clin Invest. 1979;63:1262–1273. doi: 10.1172/JCI109421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunzell JD, Hazzard WR, Porte D, Jr, Bierman EL. Evidence for a common, saturable, triglyceride removal mechanism for chylomicrons and very low density lipoproteins in man. J Clin Invest. 1973;52:1578–1585. doi: 10.1172/JCI107334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van Beek AP, van Barlingen HH, de Ruijter-Heijstek FC, Jansen H, Erkelens DW, Dallinga-Thie GM, de Bruin TW. Preferential clearance of apoB-48-containing lipoproteins after heparin-induced lipolysis is modulated by lipoprotein lipase activity. J Lipid Res. 1998;39:322–332. [PubMed] [Google Scholar]
- 38.Noel SP, Dupras R. The kinetic parameters of the uptake of very-low-density lipoprotein remnant cholesteryl esters by perfused rat livers. Biochim Biophys Acta. 1983;754:117–125. doi: 10.1016/0005-2760(83)90152-2. [DOI] [PubMed] [Google Scholar]
- 39.Krempler F, Kostner GM, Friedl W, Paulweber B, Bauer H, Sandhofer F. Lipoprotein binding to cultured human hepatoma cells. J Clin Invest. 1987;80:401–408. doi: 10.1172/JCI113086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Marsh JB, Welty FK, Lichtenstein AH, Lamon-Fava S, Schaefer EJ. Apolipoprotein B metabolism in humans: studies with stable isotope-labeled amino acid precursors. Atherosclerosis. 2002;162:227–244. doi: 10.1016/s0021-9150(01)00709-2. [DOI] [PubMed] [Google Scholar]