Pang and colleagues investigate the mechanism by which Bax and Bak permeabilize the mitochondrial outer membrane by expressing Bax and Bak in bacteria. Bax and Bak demonstrate holin behavior and can replace holin during bacterial lysis by forming holes on the bacterial membrane, allowing the escape of the cell wall-degrading endolysin. In addition, the authors show for the first time that structural elements of Bax control the size of the functional pore.
Keywords: mitochondrial outer membrane permeabilization (MOMP), Bax and Bak, holin, bacteriophage, bacterial plasma membrane holes, bacterial lysis
Abstract
The mechanism of Bax/Bak-dependent mitochondrial outer membrane permeabilization (MOMP), a central apoptotic event primarily controlled by the Bcl-2 family proteins, remains not well understood. Here, we express active Bax/Bak in bacteria, the putative origin of mitochondria, and examine their functional similarities to the λ bacteriophage (λ) holin. As critical effectors for bacterial lysis, holin oligomers form membrane lesions, through which endolysin, a muralytic enzyme, escapes the cytoplasm to attack the cell wall at the end of the infection cycle. We found that active Bax/Bak, but not any other Bcl-2 family protein, displays holin behavior, causing bacterial lysis by releasing endolysin in an oligomerization-dependent manner. Strikingly, replacing the holin gene with active alleles of Bax/Bak results in plaque-forming phages. Furthermore, we provide evidence that active Bax produces large membrane holes, the size of which is controlled by structural elements of Bax. Notably, lysis by active Bax is inhibited by Bcl-xL, and the lysis activity of the wild-type Bax is stimulated by a BH3-only protein. Together, these results mechanistically link MOMP to holin-mediated hole formation in the bacterial plasma membrane.
In response to most apoptotic stimuli, the cell undergoes mitochondrial outer membrane permeabilization (MOMP), releasing cytochrome c and other mitochondrial proteins into the cytosol to initiate the caspase cascade (Newmeyer and Ferguson-Miller 2003; Danial and Korsmeyer 2004; Jiang and Wang 2004). As a central event during apoptosis, MOMP is primarily controlled by the Bcl-2 family proteins, which include the anti-apoptotic (e.g., Bcl-2 and Bcl-xL), the multi-domain proapoptotic (e.g., Bax and Bak), and the proapoptotic BH3-only (e.g., Bik, Bim, and Puma) proteins. While the anti-apoptotic proteins are believed to inhibit MOMP by suppressing the function of proapoptotic Bcl-2 family proteins, the BH3-only proteins positively regulate MOMP by either neutralizing the anti-apoptotic proteins or directly triggering Bax/Bak activation (Galonek and Hardwick 2006; Kim et al. 2006; Willis et al. 2007; Chipuk and Green 2008; Lovell et al. 2008). Genetic analysis of mice doubly deficient for Bax and Bak established the essential role of these two proteins in mitochondria-dependent apoptosis (Wei et al. 2001). During apoptosis, both Bax and Bak become activated and form homo-oligomers in the outer membrane of mitochondria (OMM) (Nechushtan et al. 1999; Antonsson et al. 2001; Kim et al. 2009). These homo-oligomers are believed to cause pore formation on the OMM and function as effectors for MOMP, as they have been demonstrated to permeabilize liposomes and outer membrane vesicles in vitro (Kuwana et al. 2002; Lovell et al. 2008). However, the physical makeup (lipidic or proteinaceous), molecular composition, shape, and size of the putative pores remain unclear (Antignani and Youle 2006; Tait and Green 2010).
As single-cell organisms, bacteria share many similarities with mitochondria, including their sizes, membranes, genomes, and ribosomes, etc., and have been widely considered the origin of mitochondria during an endo-symbiotic evolution process (Dyall et al. 2004). Of importance, like MOMP, the bacterial plasma membrane becomes permeabilized and allows the release of functional proteins during bacteriophage-mediated lysis (Wang et al. 2000; Bayles 2007). In canonical phage lysis, the holin, a small membrane protein, is one of two phage-encoded effectors essential for host lysis (Altman et al. 1983; Wang et al. 2000). The other effector, endolysin, is a soluble cell wall-degrading enzyme (Bienkowska-Szewczyk et al. 1981). During the latent period of bacteriophage infection, the holin accumulates in the cytoplasmic membrane until under a genetically programmed schedule, forms homo-oligomers, and produces membrane holes large enough for the nonspecific passage of endolysin (Wang et al. 2000; Bayles 2007; Rice and Bayles 2008). Once across the cell membrane, the endolysin degrades the cell wall, causing an explosive disruption of the cell due to osmotic forces (Grundling et al. 2001). The similarities between the strategies used in MOMP during mammalian apoptosis and holin-mediated bacterial cell death have been noted earlier (Bayles 2007). In this study, we explore the functional link between active Bax/Bak and the holin, characterize the Bax/Bak-mediated lesions in the bacterial membrane, and investigate the involvement of Bax helices in the control of the membrane pores.
Results
An active mutant of Bax (miniBax) displayed holin-like behavior, causing rapid bacterial lysis
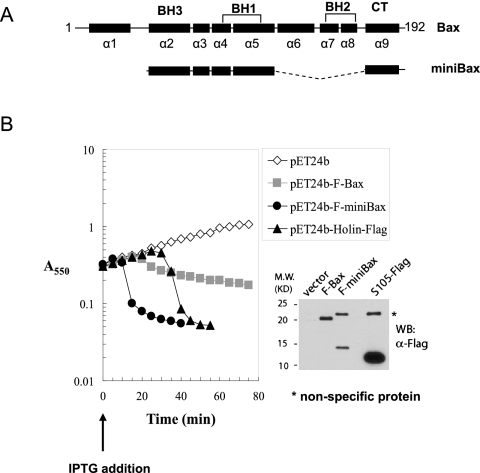
In our earlier study, a truncation mutant of Bax containing helices 2–5 and helix 9 was found to be constitutively active in inducing apoptosis in Bax/Bak double knockout (DKO) cells (George et al. 2007). We named this mutant “miniBax” (Fig. 1A) due to its much reduced size and the retained apoptotic activity in the DKO cells as compared with full-length Bax (George et al. 2007). Due to numerous failed attempts to construct a plasmid intended for expressing miniBax in bacterial extracts, we suspected that an unintended expression of miniBax might have an adverse effect on the growth of the competent Escherichia coli cells used in the ligation procedures, consistent with a toxic effect of Bax on bacteria reported earlier (Asoh et al. 1998). We therefore cloned the N-terminally Flag-tagged miniBax gene or a similarly tagged full-length Bax gene into a pET vector in which the expression of the target protein in E. coli is under the tight control of an isopropyl β-D-thiogalactopyranoside (IPTG)-inducible promoter. As shown in Figure 1B, with the empty vector as a negative control, the IPTG-induced expression of Bax initially caused a reduced growth rate, followed by a gradual decrease in A550 during the 75-min time-course experiment, indicative of a partial bacteriolytic activity. In contrast, the expression of miniBax caused a dramatic drop in optical density ∼10 min after IPTG induction (Fig. 1B). Strikingly, the culture became clear and viscous, highly reminiscent of bacterial lysis caused by phage infection. However, an involvement of contaminating bacteriophages in this “culture clearing” phenomenon is unlikely due to the dependence on IPTG-induced miniBax expression (Fig. 1B). In this system, it appears that miniBax is serving as a holin to release the T7 endolysin produced during the BL21(DE3)pLysS induction. In fact, expression of a C-terminally Flag-tagged S105, the phage λ holin, in the same context also caused a precipitous drop in A550, albeit at a later time (∼30 min) (Fig. 1B).
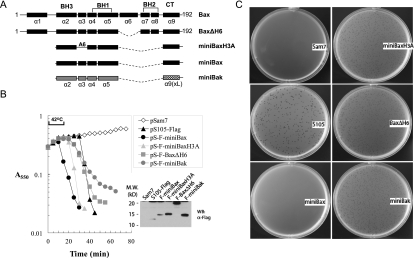
Figure 1.
An active Bax mutant displays a holin-like bacterial lysis activity. (A) Diagram of Bax and miniBax. (B) Behavior of miniBax, Bax, and S105 in the pET system. BL21(DE3)pLysS E. coli transformed with the pET24b vector or pET24b constructs expressing F-miniBax, F-Bax, or S105-Flag were grown to A550 ∼0.3. Following the addition of IPTG (0.3 mM), A550 was measured at 5-min intervals. After 1 h, the cultures were harvested and subjected to SDS-PAGE, followed by Western blot with an α-Flag antibody.
Functional replacement of holin S105 by miniBax during bacterial lysis
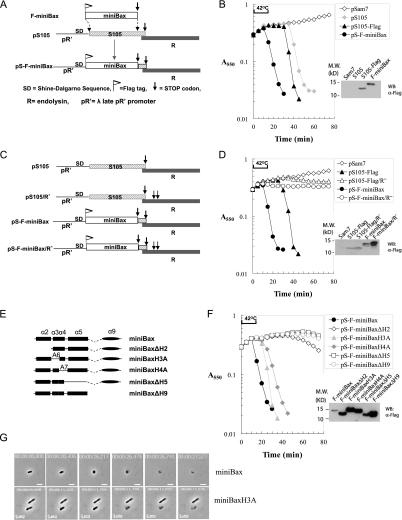
To explore the functional link between miniBax and holin S105, we resorted to an inducible system in which the coupled expression of a holin and an endolysin (R) gene causes bacterial lysis. This system is comprised of the plasmid pS105 or its derivatives and the Δ(SR) E. coli cells. The pS105 plasmid (Fig. 2A) contains the well-characterized λ lysis cassette, consisting of the lysis genes S105, R (endolysin), Rz, and Rz1, downstream from its native λ late promoter, pR′ (Chang et al. 1995; Smith et al. 1998). The Δ(SR) cells harbor a thermally inducible λ prophage, which is lysis-defective due to deletions of both S (S105) and R genes. When carried in the Δ(SR) cells, pS105 directs the expression of the lysis cassette genes upon thermal induction of the prophage, which supplies the late gene activator Q and transactivates the pR′ promoter on the plasmid. As expected, when this system is induced with pS105 or a version in which the S105 gene has been altered to encode a Flag-tagged product (pS105-Flag), abrupt lysis is observed at 30 and 35 min after induction, respectively (Fig. 2B), whereas no lysis is observed for pSam7, which is identical to pS105 except for a nonsense mutation in the S105 gene.
Figure 2.
MiniBax functionally replaces bacteriophage holin (S105), causing endolysin- dependent lysis of Δ(SR) cells. (A) Diagram for the construction of the pS105-based isogenic plasmid replacing S105 with F-miniBax. The DNA segment encoding the first 92 amino acids of S105 in the S105/R lysis cassette within the plasmid pS105 was replaced by the reading frame of F-miniBax. (B) Lysis of Δ(SR) cells by the miniBax/R cassette. The plasmid pSam7 is identical to pS105 except for an Amber mutation in the S105 gene that abolished S105 expression. Upon reaching A550 ∼0.3, the Δ(SR) cells carrying the indicated plasmids were induced by thermal induction for 15 min at 42°C, followed by aeration at 37°C. A550 was measured for each culture at 5-min intervals. The cultures were harvested as described in the Materials and Methods and subjected to SDS-PAGE and Western blot with an α-Flag antibody. (C) Diagram of the lysis cassettes of S105 and F-miniBax with or without functional endolysin (R). (D) Lysis phenotypes of the lysis cassettes listed in C in Δ(SR) cells. Expression of the indicated proteins was detected by Western blot with an α-Flag antibody. (E) Diagram of truncation mutants of miniBax. These miniBax mutants were cloned into the pS105 plasmid by the same strategy as shown in A. (F) Lysis induced by miniBax and its truncation mutants in Δ(SR) cells was measured the same way as in B. The expression of these mutants was detected by Western blot with an α-Flag antibody. (G) Δ(SR) cells carrying either pS-F-miniBax or pS-F-miniBaxH3A were monitored by time-lapse microscopy. Still images of the cells undergoing lysis at different time points are shown. The real-time videos are provided in Supplemental Movies S1 and S2.
By placing the miniBax gene in the same genetic background as S105, the behaviors of these two proteins in bacteria can be directly compared. We therefore generated an isogenic construct based on pS105, replacing S105 with the reading frame of Flag-miniBax (Fig. 2A). Upon thermal induction, this construct showed an abrupt culture-wide lysis, starting at ∼10 min during thermal induction (Fig. 2B). To test the requirement of the endolysin for the observed lysis, we introduced stop codons in the R gene in both pS105-Flag and pS-F-miniBax (Fig. 2C). As shown in Figure 2D, the null mutations of R in both plasmids completely abolished the lysis, indicating that lysis activities of both S105 and miniBax are strictly dependent on the release of the cytoplasmic endolysin R. Furthermore, in the absence of endolysin, both S105 and miniBax caused an abrupt cessation of cell growth, indicative of the triggered formation of lethal membrane lesions (Fig. 2D). Thus, miniBax complements S105 activity.
To further test the specificity of the lysis activity of miniBax, we constructed pS105-based isogenic constructs expressing miniBax mutants that lack each of the five helices (Fig. 2E). Alleles lacking helix 2 (BH3), 5, or 9 exhibited no lysis activity (Fig. 2F). In contrast, mutants in which helix 3 or 4 was replaced by oligo-Ala sequences (miniBaxH3A and miniBaxH4A, respectively) showed lysis kinetics comparable with that of S105, albeit delayed as compared with that of miniBax (Fig. 2F). Time-lapse microscopy was also used to monitor the bacterial lysis by miniBax and miniBaxH3A. In both cases, the lysis event required only a few seconds after onset of morphological change, as observed previously with S105 (Fig. 2G; Supplemental Movies S1, S2; Grundling et al. 2001). These results demonstrate that miniBax can functionally replace S105 in mediating a triggered bacterial lysis, and that helices 2, 5, and 9 are absolutely required for this activity. Furthermore, these lysis phenotypes correlate well with the apoptotic activity of these proteins in Bax−/−/Bak−/− DKO cells (George et al. 2007). It is worth noting that miniBaxH4A showed robust bacterial lysis, although somewhat delayed as compared with miniBax and miniBaxH3A (Fig. 2F), whereas it displayed a reduced apoptosis phenotype in the DKO cells (George et al. 2007). This partial exception may be due to the differential level of expression of this mutant in the two systems.
Next, we examined the membrane localization of miniBax and its truncation mutants by cell fractionation. As shown in Supplemental Figure S1, the majority of miniBax, miniBaxH3A, and especially miniBaxΔH2 are in the membrane fraction, but with a small fraction in the cytosol. While miniBaxH4A displays a decreased level of membrane localization, deletion of helix 5 or 9 shows a predominantly cytosolic localization, suggesting that helices 4, 5, and 9 contribute to the targeting of miniBax in the context of the bacterial cell.
Bacterial lysis activity of miniBax is dependent on homo-oligomerization
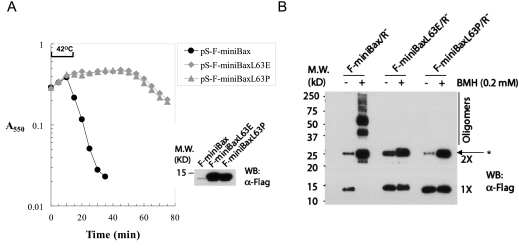
Homo-oligomerization has been shown to be critical for the apoptotic activity of Bax/Bak (Antonsson et al. 2001; George et al. 2007; Dewson et al. 2008) and for S105 (Grundling et al. 2000b). In addition, the BH3 domain (helix 2) has been shown to be critical for homo-oligomerization of Bax/Bak (George et al. 2007; Dewson et al. 2008). We therefore examined the role of the BH3 domain in the bacterial lysis activity of miniBax. As shown in Figure 3A, unlike miniBax, the mutants that contain either a L63P or L63E missense mutation in the BH3 domain were lysis-defective, despite unperturbed membrane targeting (Supplemental Fig. S2). The homo-oligomerization of these proteins was examined by BMH (bis-maleimidohexane)-mediated cross-linking. To avoid the potential nonspecific effects on homo-oligomerization by bacterial lysis, the BMH assay was carried out in an R− background. Both BH3 mutants lost the triggering phenotype (Supplemental Fig. S2A) and were detected predominantly in the membrane fractions (Supplemental Fig. S2B). Not surprisingly, miniBax displayed a robust homo-oligomerization, whereas little or no homo-oligomerization was detected for the two BH3 mutants (Fig. 3B). These results indicate that homo-oligomerization is essential for the bacterial lysis activity of miniBax, similar to its role in full-length Bax-mediated mitochondrial damage and apoptosis.
Figure 3.
Dependence of the bacterial lysis activity of miniBax on homo-oligomerization. (A) Lysis phenotypes of miniBax and its two BH3 mutants, miniBaxL63E and miniBaxL63P, in Δ(SR) cells. The expression of these proteins was detected by Western blot as carried out in Figure 2B. (B) Homo-oligomerization of miniBax and its mutants. BMH cross-linking was carried out as described in the Materials and Methods, followed by SDS-PAGE and Western blot analysis with an α-Flag antibody. The asterisk (*) stands for nonspecific protein.
Active mutants of Bax and Bak, but not any other Bcl-2 family member, are competent for bacterial lysis
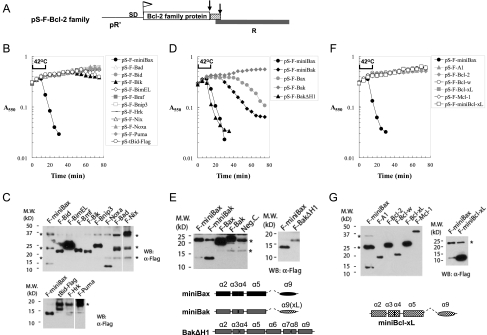
In view of the sequence and structural homology among the Bcl-2 family members, we generated isogenic constructs based on pS105, replacing S105 gene with the reading frame of each Bcl-2 family protein (Fig. 4A), and tested them for bacterial lysis activity in the Δ(SR) cells. Of the 10 BH3-only proteins, including truncated Bid (tBid), the active form of Bid, none showed lysis activity (Fig. 4B,C). We next tested the Bax/Bak proteins, which are considered to act as gateway proteins for mitochondria-dependent apoptosis (Wei et al. 2001). Due to the close sequence and structural homology between Bax and Bak, we also generated the corresponding “miniBak,” which contains helices 2–5 of Bak fused to the helix 9 of Bcl-xL, a well-characterized mitochondrial targeting sequence (Fig. 4E; Kaufmann et al. 2003). In addition, a Bak mutant that lacks only helix 1 (BakΔH1), which showed constitutive apoptotic activity in Bax/Bak DKO cells, was generated in the pS105 replacement system. While full-length Bax displayed a much delayed lysis phenotype as compared with that of miniBax, full-length Bak failed to show any lysis activity. In contrast, miniBak, especially BakΔH1, showed a potent lysis activity (Fig. 4D,E). Lastly, when the anti-apoptotic Bcl-2 proteins were tested, none showed any lysis activity (Fig. 4F,G). However, since Bcl-xL and Bax share similar 9-α helix structures, we asked whether an arrangement of the Bcl-xL helices similar to that of miniBax would result in an “active” Bcl-xL mutant. We therefore generated “miniBcl-xL,” which contains helices 2–5, and 9 of Bcl-xL in the chimeric lysis cassette (Fig. 4G). Despite a higher level of expression than that of miniBax, miniBcl-xL did not cause lysis in Δ(SR) cells (Fig. 4F,G). Together, these results strongly suggest that, among the Bcl-2 family members, the holin-like bacterial lysis activity is specific for the active Bax/Bak proteins.
Figure 4.
Lysis of Δ(SR) cells by active mutants of Bax or Bak, but not by any other Bcl-2 family protein. (A) Diagram for the replacement of the first 92 amino acids of S105 by Bcl-2 family proteins in the S105/R lysis cassette in pS105. (B) Lysis curves for the BH3-only proteins were measured as in Figure 2B. (C) Expression of the indicated BH3-only proteins in Δ(SR) cells. Protein samples from cells expressing F-Bid, F-BimEL, F-Bmf, and F-Bik were diluted 1:10. Protein samples from F-Bnip3 and F-Noxa were diluted 1:5. The asterisk (*) stands for nonspecific protein. (D) Lysis phenotypes of the Bax/Bak and their mutants in Δ(SR) cells. (E) Expression of Bax/Bak and their mutants. Due to low expression, the protein sample from F-BakΔH1-expressing cells was concentrated fourfold. The asterisk (*) stands for nonspecific protein. Diagrams of miniBax, miniBak, and BakΔH1 are shown at the bottom. (F) Lysis phenotypes of anti-apoptotic Bcl-2 family proteins. (G) Expression of the indicated anti-apoptotic Bcl-2 family proteins. Protein samples from cells expressing these proteins were diluted 1:20 as compared with F-miniBax. A diagram of a truncation mutant of Bcl-xL, “miniBcl-xL,” is shown at the bottom.
Generation of chimeric phages by replacing S105 with mutants of Bax/Bak in the λ genome
A more stringent test for the functional homology between miniBax and S105 would be to examine the generation of recombinant phages that contain miniBax in the place of S105 in the λ genome. A convenient way to assess this is to quantify the production of plaque-forming units (PFUs) from the inductions described above, relying on homologous recombination in the induced cells to generate chimeric phages (Zheng et al. 2008). To our surprise, while Δ(SR) cells harboring the pS105 cassette produced large numbers of plaques, cells harboring the pS-F-miniBax plasmid failed to produce any plaques, despite complete lysis following thermal induction (Fig. 5B,C). Considering the observation that miniBax causes lysis consistently earlier than S105 (Fig. 2), we reasoned that the early lysis induced by miniBax may not allow enough time for the packaging of the chimeric phage particles. To test this possibility, it is necessary to generate mutants of Bax or Bak that exhibit delayed onset of lysis, preferably similar to the lysis timing of S105. Three mutants in our collection—miniBaxH3A, BaxΔH6 (George et al. 2007, 2010), and miniBak (Fig. 5A)—were compared with miniBax in the lysis assay. Following thermal induction, these alleles exhibited retarded onset of lysis as compared with miniBax (Fig. 5B). Strikingly, with all three alleles, the ability to support the generation of plaque-forming recombinants was similar to that of S105 (Fig. 5C; Supplemental Table S1). Furthermore, these chimeric phages were able to generate lysogens expressing the relevant proteins (Supplemental Fig. S3). For unclear reasons, however, the plaques from these alleles were consistently smaller in size than those from S105 (Fig. 5C). Together, these results indicate that Bax/Bak mutants with lysis times comparable with S105 can replace the latter in vivo for the generation of viable phages.
Figure 5.
Replacement of the holin gene by functional Bax/Bak mutants generates viable chimeric λ phages. (A) Diagrams of Bax and Bak mutants. These mutants were cloned into the pS105 plasmid as shown in Figure 2A to replace S105. (B) Lysis phenotypes of the indicated Bax/Bak mutants. Expression of the indicated Bax and Bak mutants in Δ(SR) cells was detected by Western blot with an α-Flag antibody as carried out in Figure 2B. (C) Plaque formation by Bax/Bak mutants. At 75 min, the cultures were centrifuged at 22,000g for 10 min, and the supernatant was assayed for plaque formation using the MC4100ΔtonA as indicator cells as described in the Materials and Methods.
Sizing the holes produced by active Bax mutants
Our results indicate that active Bax, like S105, can form membrane lesions that allow the release of a functional endolysin (Figs. 2–5). We next set out to address the size of these lesions. The earliest estimates of the S105 lesion size were obtained by characterizing the lytic function of recombinant phages in which the R endolysin gene (158 amino acids) was fused in-frame to the full-length lacZ gene (1024 amino acids) (Wang et al. 2003). The chimeric R-LacZ product was shown to have normal β-galactosidase activity and formed a stable tetramer of >500 kDa (Wang et al. 2003; Matthews 2005). Despite the gross increase in the size of the endolysin, S105 retained the ability to allow the release of R-LacZ into the periplasm, as judged by the full plaque-forming ability and lytic function of the recombinant phage (Wang et al. 2003). The analogous F-miniBax/R-LacZ cassette was constructed based on pS105/R-LacZ and tested in Δ(SR) cells for lytic activity (Fig. 6A). Unlike S105/R-LacZ, lysis by F-miniBax/R-LacZ or F-miniBaxH3A/R-LacZ is markedly impaired (Fig. 6B). Since all three constructs showed comparable levels of R-LacZ expression (Supplemental Fig. S4), these results suggest that most of the holes induced by miniBax or miniBaxH3A were not large enough to allow the passage of the 500-kDa R-LacZ complex (Fig. 6B).
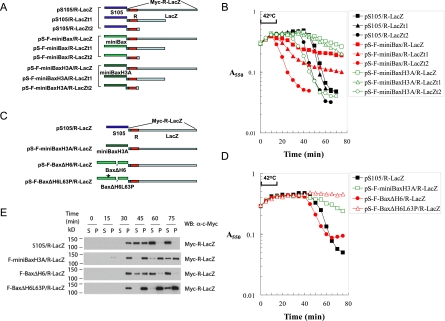
Figure 6.
Sizing the membrane holes induced by active Bax mutants through the R-LacZ fusion proteins. (A) Diagram of the various lysis cassettes with LacZ or its truncation mutants fused to the reading frame of R. The R-LacZ fusion protein has a Myc tag on the N terminus. R-LacZt1 is the fusion between R and the first 497 amino acids of LacZ. R-LacZt2 is the fusion between R and the first 8 amino acids of LacZ. (B) Lysis curves of the different lysis cassettes listed in A in Δ(SR) cells. (C) Diagram of the lysis cassettes expressing Bax mutants in conjunction with R-LacZ. (D) Lysis curves of the indicated Bax mutants with R-LacZ cassettes in Δ(SR) cells. (E) Δ(SR) cells carrying the indicated plasmids were thermally induced. At the indicated time points, 1-mL samples of the culture were collected and pelleted by centrifugation at 22,000g at 4°C. The supernatant (S) and the pellet (P), which was resuspended in 1 mL of EBC buffer, were loaded onto SDS-PAGE followed by Western blot analysis with an α-Myc antibody.
The above-mentioned possibility would predict that the lysis defect of miniBax/R-LacZ or miniBaxH3A/R-LacZ may be overcome by reducing the size of R-LacZ. We therefore constructed F-miniBax/R-LacZt1 and F-miniBax/R-LacZt2 cassettes, which express R-LacZ fusion proteins with 497 amino acids and 8 amino acids, respectively, from the N terminus of β-galactosidase following the reading frame of R (Fig. 6A). Indeed, while miniBax/R-LacZt1 shows a weak but somewhat improved lysis as compared with that of miniBax/R-LacZ, miniBax/R-LacZt2 regains full lytic activity (Fig. 6B). These results indicate that the induced miniBax hole is not much in excess of the size required for the passage of the monomeric R endolysin (16 kDa) and nonpermissive even for an R endolysin with an ∼50-kDa addition (R-LacZt1). Similar results were obtained with miniBaxH3A in conjunction with different R-LacZ derivatives (Fig. 6B). These results strongly suggest that the majority of the membrane holes generated by miniBax and miniBaxH3A are significantly smaller than those generated by S105.
In theory, an alternative strategy to rescue the lysis defect of miniBax/R-LacZ or miniBaxH3A/R-LacZ is to increase the size of the hole. Considering the extensive deletion of helices from Bax that resulted in miniBax, we wanted to test the possibility that Bax mutants with fewer deletions might support the formation of larger holes. We therefore replaced S105 in pS105/R-LacZ with another active Bax mutant, BaxΔH6 (Fig. 5A), which contains three additional helices as compared with miniBax (Fig. 6C). Unlike miniBaxH3A, BaxΔH6 was proficient in lysis under the R-LacZ background, whereas its BH3 mutant, BaxΔH6L63P, failed to cause lysis in the same setting (Fig. 6D). This is in contrast to the observation under the R background, in which miniBaxH3A showed an even earlier lysis than BaxΔH6 (Fig. 5B). In addition, we also monitored the release of the soluble R-LacZ fusion protein into the culture medium during induced lysis. As shown in Figure 6E, similar to S105, BaxΔH6 was able to cause the release of R-LacZ following thermal induction. In contrast, miniBaxH3A and BaxΔH6L63P were both unable to efficiently release R-LacZ following thermal induction. Consistently, under the R-LacZ background, the lysis defect was also observed for another miniBax-derived mutant, miniBaxY115AF116A, but not for full-length Bax (Supplemental Fig. S5). Taken together, these results suggest that the minimum homo-oligomerization domain (helices 2–5 in miniBax or miniBaxH3A) (George et al. 2007) predominantly produces membrane holes with sizes only comparable with the endolysin (R), whereas additional helices (helices 1, 7, and 8 in BaxΔH6) facilitate the formation of much bigger holes, which allow the release of proteins as large as the 500-kDa LacZ tetramer (R-LacZ).
Regulation of the lysis activity of Bax parallels the control of MOMP
As the apoptotic activity of Bax is regulated by other Bcl-2 family proteins, we set out to test whether the bacterial lysis activity of Bax is similarly regulated. The phages carrying Bax or its derivatives allowed for the generation of lysogens with prophages directing the expression of the corresponding proteins, albeit at a lower level. Expression plasmids can then be introduced into these lysogens for the coexpression of Bax or its derivatives with other proteins. This lysogen-based coexpression system was first used to test the effect of Bcl-xL on the lysis activity of active Bax mutants. MiniBaxH3A lysogens were generated through the corresponding recombinant phages (Fig. 5C). Subsequently, a plasmid expressing Bcl-xL was introduced into these cells, in which both miniBaxH3A and Bcl-xL were expressed following thermal induction. As shown in Figure 7A, expression of Bcl-xL inhibited the lysis activity of miniBaxH3A. In contrast, the expression of GFP or a mutant Bcl-xL, Bcl-xLmt8, which was shown to be defective in anti-apoptotic function (Cheng et al. 1996), had little effect. Similarly, Bcl-xL showed a strong inhibitory effect on the lysis mediated by BaxΔH6 (Fig. 7B). The inhibitory effect of Bcl-xL on lysis is likely through an inhibition on the homo-oligomerization of the effectors. Indeed, using the BMH cross-linking assay, we found that the homo-oligomerization of miniBaxH3A was strongly inhibited by Bcl-xL, but not by GFP or Bcl-xLmt8 (Supplemental Fig. S6). These results suggest that the anti-apoptotic Bcl-2 family proteins inhibit the activities of Bax/Bak in bacteria.
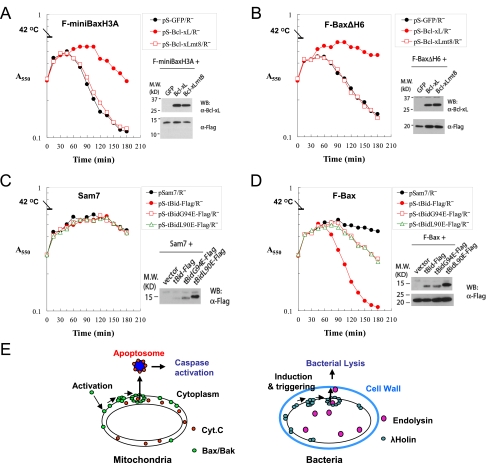
Figure 7.
Regulation of Bax-mediated bacterial lysis by Bcl-xL and tBid. (A) Suppression of miniBaxH3A-mediated bacterial lysis by Bcl-xL. Lysis curves of F-miniBaxH3A lysogens carrying the indicated plasmids were measured at a 15-min interval. The expression of Bcl-xL and Bcl-xLmt8 at 15 min was detected using an α-Bcl-xL antibody, and the expression of miniBaxH3A was detected using an α-Flag antibody. (B) Suppression of BaxΔH6-mediated bacterial lysis by Bcl-xL. (C) Lysis phenotypes of sam7 lysogen carrying the indicated plasmids. The expression of tBid, its mutants, and Bax at 60 min was detected by Western blot with an α-Flag antibody. (D) Lysis phenotypes of F-Bax lysogen carrying indicated plasmids. (E) A diagram depicting the functional homology between MOMP and hole formation in bacterial membrane.
Next, to test the potential regulation of the lysis activity of Bax by the BH3-only proteins, we introduced an expression plasmid for tBid or its BH3 domain mutants into cells that contain a prophage expressing either a nonsense mutant of holin S105 (Sam7 lysogen) or the wild-type Bax (Bax lysogen). As expected, in the absence of Bax (the Sam7 lysogen), tBid or its BH3 mutants, tBidL90E and tBidG94E, showed no lysis activity (Fig. 7D). When introduced into the Bax-expressing cells, tBid greatly stimulated the lysis activity of Bax, whereas tBidL90E or tBidG94E displayed only minor effects, suggesting that tBid stimulates the Bax-mediated lysis through a BH3-dependent mechanism (Fig. 7E). Together, these results suggest that the lysis activity of Bax is regulated through mechanisms similar to those regulating it during MOMP.
Discussion
Here we present evidence that constitutively active Bax/Bak proteins competent for mitochondrial damage are capable of serving as holins in phage-mediated bacterial cell lysis. These results suggest a mechanistic link between MOMP and hole formation in the bacterial membrane and provide new perspectives on the size control of the Bax/Bak-induced pore.
A close correlation between the apoptotic activity of Bax/Bak in the DKO cells and their bacterial lysis activity in bacteria
Activated Bax and Bak function as essential effectors in MOMP and apoptosis by causing damage on the mitochondrial outer membrane (Wei et al. 2001; Youle and Strasser 2008; Tait and Green 2010). In this study, we demonstrate that the expression of active mutants of Bax/Bak potently causes membrane lesions and the lysis of bacteria. Of the entire collection of Bcl-2 family members, only active mutants of Bax/Bak possess this novel activity. In addition, miniBax and miniBak displayed significantly more potent lysis activities as compared with their respective full-length proteins (Fig. 4), consistent with an activation of Bax or Bak through structural changes (George et al. 2007). Furthermore, the apoptotic activity of Bax in Bax−/−/Bak−/− DKO cells and the bacterial lysis activity seem to be mediated essentially by the same structural elements (Fig. 2; George et al. 2007). Of note, similar to the regulation of the apoptotic activity of Bax in the mammalian cells, the bacterial lysis activities of active Bax mutants were found to be inhibited by Bcl-xL, and on the other hand, the lysis activity of Bax is stimulated by tBid in a BH3-dependent manner (Fig. 7). These results strongly suggest that the apoptotic activity (MOMP-inducing activity) of Bax/Bak is functionally equivalent to the bacterial lysis activity. Thus, we speculate that the bacterial system may be suitable for studying the regulation of Bax and Bak by Bcl-2 or even non-Bcl-2 family proteins.
Active Bax and Bak as functional holins, mechanistically linking MOMP and bacterial lysis
The holins constitute a large functional group of small membrane proteins with diverse amino acid sequences (Wang et al. 2000). To date, 105 known or putative holins within >30 unrelated ortholog groups have been identified in bacteriophages. Most holins are <150 amino acids in length, with two or three α-helical and hydrophobic transmembrane (TM) domains followed by a short C-terminal cytoplasmic domain enriched in basic residues. Importantly, holins share the ability to permeabilize the bacterial cell membrane. Several salient features are shared by Bax/Bak, especially active Bax/Bak (i.e., miniBax/Bak) and typical holins. These include the following: (1) MiniBax contains at least two bona fide hydrophobic TM domains—helix 5 and helix 9, both of which have been shown to insert into the OMM (Garcia-Saez et al. 2004; Annis et al. 2005)—and a short stretch of basic residues at the C terminus (Supplemental Fig. S7). (2) Both miniBax/Bak and the canonical holin S105 are primarily composed of α helices (Smith et al. 1998; Grundling et al. 2000a). (3) Bax/Bak and S105 both exist as monomer/dimers before triggering to a pore-forming state, but require the formation of large homo-oligomers in the membrane for function. (4) Both active Bax/Bak and S105 inflict irreparable damages to the membrane in a lethal process. (5) Both form large oligomeric membrane pores that allow the escape of fully folded functional proteins, which are effectors for downstream lethal events. (6) Both proteins were shown to permeabilize liposomes in a reconstituted in vitro system (Smith et al. 1998; Saito et al. 2000; Kuwana et al. 2002). (7) Both are inhibited by closely related endogenous proteins: Bcl-xL and anti-holin, respectively (Wang et al. 2000; Rice and Bayles 2008). It is worth noting, however, that Bax/Bak showed at least one difference from a typical holin. The holins are sensitive to the energization state of the bacterial membrane, in that treatment with energy poisons or uncouplers can trigger holin-mediated hole formation prematurely (Wang et al. 2000), whereas active Bax/Bak did not exhibit such a feature (data not shown). The sensitivity to the membrane potential is thought to be critical for the precise control of lysis timing in holins (Wang et al. 2000). This appears to be absent in Bax/Bak, which may partially account for the smaller plaque size of the chimeric λ Bax/Bak derivatives (Fig. 5). Nonetheless, the common features and the functional replacement of holin by active Bax/Bak in triggered bacterial lysis and phage production strongly indicate that active Bax/Bak are functional holins. Of note, when expressed in mammalian cells, S105 has been reported to cause a caspase-independent and nonapoptotic cell death in eukaryotic cells (Agu et al. 2007). It will be interesting to test whether S105 can functionally replace Bax/Bak in the mitochondrial pathway of apoptosis when it is properly targeted to the mitochondria.
In the mitochondria-dependent pathway of apoptosis, activated Bax/Bak causes the formation of nonspecific OMM lesions of sufficient size to allow the nonspecific egress of fully folded native proteins such as cytochrome c and SMAC to initiate the caspase cascade. Similarly, holins initiate the lysis pathway by formation of “holes,” nonspecific lesions, in the cytoplasmic membrane, allowing fully folded, active muralytic enzymes to escape and attack the peptidoglycan cell wall (Fig. 7F). The functional replacement of the effector for bacterial lysis (holin) by the effector (active Bax/Bak) for MOMP suggests a mechanistic link between these two operationally similar processes.
The size control and composition of the pore
Although the observation of cytochrome c release from mitochondria during apoptosis has been reported more than a decade ago (Liu et al. 1996; Kluck et al. 1997; Yang et al. 1997), efforts to define the physical nature of the OMM pore have not been fruitful (Tait and Green 2010). The characterization of bacterial membrane lesions caused by holins, however, has been more advanced. It has been demonstrated that S105 allows the escape of a tetrameric R-LacZ chimeric protein with a molecular weight of ∼500 kDa (Wang et al. 2003). Using the same endolysin-LacZ chimera strategy, the miniBax derivatives were found to exhibit relatively slow and incomplete lysis, suggesting that either the pores were relatively small and restricted the egress of the chimeric endolysin or the pore sizes were heterogeneous, with only a subset of sufficient size. However, a full-length Bax lacking only the helix 6 (BaxΔH6) was proficient at lysis with R-LacZ (Fig. 6). This suggests that the domains deleted in our miniBax construct, although not essential for holin-like function in bacteria or OMM permeabilization in mitochondria, serve a modulating role in controlling the size of the hole. In support of this notion, full-length Bax was previously found to be much more efficient at making high-order oligomers in the OMM than the miniBax constructs (George et al. 2007). The simplest idea is that these modulating domains influence the degree of homo-oligomerization that occurs before concerted hole formation. Alternatively, these helices may affect the architecture and shape of the hole. Of importance, the novel notion that helices in Bax play a role in modulating the size of the pore provides strong support for the hypothesis that the membrane pores are proteinaceous in nature and are composed of Bax/Bak homo-oligomers (Tait and Green 2010).
Using electron microscopy and recombinant proteins purified in nonionic detergent, Savva (et al. 2008) found that functional S105 formed multimeric ring structures, which likely reflect a minimum energy arrangement in the membrane. In addition, recent cryo-electron microscopy and tomography studies revealed that the lesions caused by holin S105 of phage λ could be visualized as interruptions in the bilayer spanning very large areas of the cytoplasmic membrane, averaging >350 nm in diameter (Dewey et al. 2010). Assuming these lesions correspond to the holes, this latter observation fully accounted for the ability of holins to allow the rapid, nonspecific escape of proteins not only of endolysins, of which the λ R endolysin, a soluble, 15-kDa monomeric protein is typical, but also of much larger proteins, including the ∼500-kDa R-LacZ chimeras (Dewey et al. 2010). However, the lesions that are formed by the Bax proteins in the bacterial cytoplasmic membrane do not appear to be of the near micron-scale holes like those formed by the S105 holin, since they could not be observed using cryo-electron microscopy at a 50-nm resolution (data not shown). Higher-resolution cryo-electron microscopy and tomography studies should help uncover the physical nature of active Bax/Bak and the membrane lesions.
Overall, results presented in this study establish a functional homology between active Bax/Bak and bacteriophage holin. Considering the similarities and evolutionary relationships between bacteria and mitochondria, our results also suggest that the phage/bacteria system is potentially a powerful model system to study the mechanisms and regulation of MOMP.
Materials and methods
Reagents and antibodies
The following chemicals were used in this study. These include ampicillin (Fisher Scientific), kanamycin (Fisher Scientific), IPTG (Acros Organics), maltose (Fisher Scientific), and BMH (Pierce Biotechnology). The antibodies used are anti-Flag antibody (Sigma), α-Myc antibody (Santa Cruz Biotechnologies, Inc.), and α-Bcl-xL antibody (Cell signaling Technology, Inc.).
Bacterial strains and culture growth
Bacterial strains used in this study include BL21(DE3)pLysS (Novagen), MC4100[λΔ(SR)] (Smith et al. 1998; Grundling et al. 2001), and MC4100ΔtonA (Zheng et al. 2008) . For convenience, MC4100[λΔ(SR)] cells were referred to as Δ(SR) cells in this study. Bacterial cultures were grown in LB medium supplemented with 50 μg/mL kanamycin or 100 μg/mL ampicillin as appropriate. Lysis curves were obtained by measuring A550 after IPTG or thermal induction. Since the plasmids containing cDNA of Bax were easily mutated after overgrowth of the host (Asoh et al. 1998), all of the cultures used for the lysis study avoided the stationary state. Briefly, cells from a single colony were inoculated into a 5-mL culture, grown until A550 reached ∼1.0, and kept at 4°C overnight. The culture was diluted into a 25-mL volume of medium to achieve a starting A550 of ∼0.04. BL21(DE3)pLysS cells carrying pET24b+-derived plasmids were grown at 37°C and induced by IPTG (0.3 mM) at A550 ∼0.3. For lysis curves of Δ(SR) cells carrying pS105-derived plasmids, cultures were grown at 30°C until A550 ∼0.3, thermally induced by aeration for 15 min at 42°C, and aerated at 37°C thereafter.
Plasmid construction
The parental plasmids used in this study are pET24b(+) (Novagen), pS105 (Smith et al. 1998), or pS105/R-LacZ, which was called pS105mycRфlacZ in an earlier study (Wang et al. 2003). The pS105 plasmid is a medium-copy-number vector with the entire λ SRRzRz1 lysis cassette placed downstream from S gene's native pR′ promoter. This plasmid and its derivatives were carried in a lysogenic host, Δ(SR) cells, and expression of genes in the lysis cassette was induced by thermal induction from the prophage, which supplied the late gene activator Q to transactivate the pR′ promoter on the plasmid. The pS105/R-LacZ plasmid was identical to pS105, except that the R gene was replaced by the cDNA encoding a c-Myc-tagged R fused to the LacZ reading frame (Wang et al. 2003). Newly constructed plasmids were created by site-directed mutagenesis using the QuickChange kit from Stratagene or by ligation and were verified by DNA sequencing. Detailed descriptions of these constructs are provided in the Supplemental Material.
Bacterial extracts for SDS-PAGE and Western blot
Unless otherwise indicated, the cells in 1 mL of culture were collected by centrifugation following thermal induction by centrifugation at 22,000g for 10 min at 4°C 1 h after induction by IPTG or by thermal shift. The cell pellets were lysed in EBC buffer (0.5% NP-40, 120 mM Tris-HCl at pH 7.5, 120 mM NaCl, 1 mM EDTA) for 1 h at 4°C with gentle rotation. The extracts were cleared by centrifugation at 22,000g for 20 min. The supernatant was mixed with 4× SDS sample buffer and subjected to SDS-PAGE. For Figure 2D, the EBC lysates were directly mixed with 4× SDS protein sample buffer and subjected to SDS-PAGE without removing the pellet.
Time-lapse microscopy
Cultures of Δ(SR) cells transformed with pS-F-miniBax or pS-F-miniBaxH3A were grown to A550 ∼0.3 at 30°C, induced for 15 min at 42°C, and then grown at 37°C, as described above, except for the pS-F-miniBax cultures, which were induced for only 5 min at 42°C. Ten-microliter aliquots of the culture were collected ∼5 min before the onset of lysis (as judged from lysis curves) and placed on preheated microscope slides (37°C). Cells were visualized at 400× magnification by phase-contrast illumination on a Zeiss Axio Observer inverted microscope and recorded at 4 frames per second until the end of the experiment. Videos were processed using the AxioVision software from Zeiss.
R-LacZ release assay
Cultures of Δ(SR) cells carrying the indicated plasmids in Figure 6D were thermally induced as described before. Samples from 1 mL of culture each were collected at the indicated time points and precipitated by centrifugation at 22,000g for 10 min at 4°C. The pellet was then lysed in 1 mL of EBC buffer for 1 h with gentle rotation at 4°C. Equal volumes of the supernatant and lysate of the pellet were mixed with 4× SDS sample buffer and subjected to SDS-PAGE. R-LacZ was detected by an α-c-myc antibody.
Plaque-forming assay
Recombinant phages were isolated from lysates of thermally induced Δ(SR) cells carrying pS105-derived plasmids as described earlier (Zheng et al. 2008). Briefly, the lysates were collected at 75 min by centrifugation at 22,000g for 10 min at 4°C. The supernatant was diluted in 300 μL of SM buffer (100 mM NaCl, 10 mM MgSO4, 50 mM at pH 7.5 Tris-Cl) plus gelatin (0.01% w/v) and mixed with another 300 μL of a culture of MC4100ΔtonA (A550 ∼0.6, freshly grown in LB supplemented with 10 mM MgSO4, 0.2% maltose). As a negative control, SM buffer plus gelatin without lysate was also mixed with MC4100ΔtonA cells. After 25 min at room temperature, the mixtures were added to 10 mL of LB-agar (LB with 0.5% agar, 10 mM MgSO4) on a 10-cm plate and incubated for 12–16 h at 37°C.
Generation of lysogens by recombinant λ phage and induction
Individual plaques of recombinant λ phage were picked by pipette and washed out into the tube by 500 μL SM buffer. Bacteriophage particles were allowed to diffuse into the SM buffer from the agar by gentle shaking for 2–3 h at room temperature. One-hundred microliters of the bacteriophage suspension was diluted in SM buffer with gelatin and subjected to plaque-forming assay. The whole plate of bacteriophage plaques were eluted with 5 mL of SM buffer. Phages, which were purified by replating and picked again three times, were then used to lysogenize MC4100ΔtonA cells. Lysogens were selected by plating infected cells on plain LB plates at 30°C and were screened by PCR for the presence of prophage. Lysogens carrying indicated plasmids were also grown and thermally induced as described earlier for Δ(SR) cells. A550 of each culture was measured at 15-min intervals.
BMH cross-linking
Expression from the plasmids pS-F-miniBax/R−, pS-F-miniBaxΔH2/R−, and pS-F-miniBaxL63P/R− was induced in Δ(SR) cells at A550 ∼0.3. At 45 min, cultures from 50 mL were passed through EmulsiFlex-C5 twice at the pressure of 16,000 psi. The membranes were collected by ultracentrifugation at 100,000g for 60 min at 4°C and resuspended in 250 μL of PBS. For the cross-linking reaction, the protein amount used of each reaction was adjusted by normalizing cell amount and assessed as total A550 units collected. Typically, a final reaction mixture of 50 μL with or without BMH (0.2 mM) was incubated for 1 h at room temperature with gentle shaking. The reaction was stopped by the addition of DTT (50 mM) and incubation for another 15 min at room temperature. The membranes were collected again by ultracentrifugation as described above and solubilized by EBC buffer for 1 h at 4°C with gentle rotation. The insoluble material was removed by centrifugation at 22,000g for 20 min at 4°C. The soluble fraction was mixed with 4× SDS sample buffer, subjected to SDS-PAGE, and analyzed by Western blotting with an anti-Flag antibody.
Acknowledgments
The cDNAs for Puma and Hrk were kind gifts from Drs. Karen Vousden and Gabriel Nunez, respectively. We thank Drs. Robert Lewis, Michael Brattain, Angie Rizzino, and Michel Ouellette for helpful discussions. This study was supported by GM76237, GM76237S1, and 11GRNT7850008 (AHA), and a pilot grant from the Nebraska Center for Cell Signaling (5P20-RR018759) to X.L.; R01AI038901 and P01AI83211 to K.W.B.; and GM27099 to R.F.Y.
Footnotes
Supplemental material is available for this article.
Article published online ahead of print. Article and publication date are online at http://www.genesdev.org/cgi/doi/10.1101/gad.171645.111.
References
- Agu CA, Klein R, Lengler J, Schilcher F, Gregor W, Peterbauer T, Blasi U, Salmons B, Gunzburg WH, Hohenadl C 2007. Bacteriophage-encoded toxins: the λ-holin protein causes caspase-independent non-apoptotic cell death of eukaryotic cells. Cell Microbiol 9: 1753–1765 [DOI] [PubMed] [Google Scholar]
- Altman E, Altman RK, Garrett JM, Grimaila RJ, Young R 1983. S gene product: identification and membrane localization of a lysis control protein. J Bacteriol 155: 1130–1137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, Andrews DW 2005. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J 24: 2096–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antignani A, Youle RJ 2006. How do Bax and Bak lead to permeabilization of the outer mitochondrial membrane? Curr Opin Cell Biol 18: 685–689 [DOI] [PubMed] [Google Scholar]
- Antonsson B, Montessuit S, Sanchez B, Martinou JC 2001. Bax is present as a high molecular weight oligomer/complex in the mitochondrial membrane of apoptotic cells. J Biol Chem 276: 11615–11623 [DOI] [PubMed] [Google Scholar]
- Asoh S, Nishimaki K, Nanbu-Wakao R, Ohta S 1998. A trace amount of the human pro-apoptotic factor Bax induces bacterial death accompanied by damage of DNA. J Biol Chem 273: 11384–11391 [DOI] [PubMed] [Google Scholar]
- Bayles KW 2007. The biological role of death and lysis in biofilm development. Nat Rev Microbiol 5: 721–726 [DOI] [PubMed] [Google Scholar]
- Bienkowska-Szewczyk K, Lipinska B, Taylor A 1981. The R gene product of bacteriophage λ is the murein transglycosylase. Mol Gen Genet 184: 111–114 [DOI] [PubMed] [Google Scholar]
- Chang CY, Nam K, Young R 1995. S gene expression and the timing of lysis by bacteriophage λ. J Bacteriol 177: 3283–3294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Levine B, Boise LH, Thompson CB, Hardwick JM 1996. Bax-independent inhibition of apoptosis by Bcl-XL. Nature 379: 554–556 [DOI] [PubMed] [Google Scholar]
- Chipuk JE, Green DR 2008. How do BCL-2 proteins induce mitochondrial outer membrane permeabilization? Trends Cell Biol 18: 157–164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danial NN, Korsmeyer SJ 2004. Cell death: critical control points. Cell 116: 205–219 [DOI] [PubMed] [Google Scholar]
- Dewey JS, Savva CG, White RL, Vitha S, Holzenburg A, Young R 2010. Micron-scale holes terminate the phage infection cycle. Proc Natl Acad Sci 107: 2219–2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, Kluck RM 2008. To trigger apoptosis, Bak exposes its BH3 domain and homodimerizes via BH3:groove interactions. Mol Cell 30: 369–380 [DOI] [PubMed] [Google Scholar]
- Dyall SD, Brown MT, Johnson PJ 2004. Ancient invasions: from endosymbionts to organelles. Science 304: 253–257 [DOI] [PubMed] [Google Scholar]
- Galonek HL, Hardwick JM 2006. Upgrading the BCL-2 network. Nat Cell Biol 8: 1317–1319 [DOI] [PubMed] [Google Scholar]
- Garcia-Saez AJ, Mingarro I, Perez-Paya E, Salgado J 2004. Membrane-insertion fragments of Bcl-xL, Bax, and Bid. Biochemistry 43: 10930–10943 [DOI] [PubMed] [Google Scholar]
- George NM, Evans JJ, Luo X 2007. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev 21: 1937–1948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George NM, Targy N, Evans JJ, Zhang L, Luo X 2010. Bax contains two functional mitochondrial targeting sequences and translocates to mitochondria in a conformational change- and homo-oligomerization-driven process. J Biol Chem 285: 1384–1392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundling A, Blasi U, Young R 2000a. Biochemical and genetic evidence for three transmembrane domains in the class I holin, λ S. J Biol Chem 275: 769–776 [DOI] [PubMed] [Google Scholar]
- Grundling A, Blasi U, Young R 2000b. Genetic and biochemical analysis of dimer and oligomer interactions of the λ S holin. J Bacteriol 182: 6082–6090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundling A, Manson MD, Young R 2001. Holins kill without warning. Proc Natl Acad Sci 98: 9348–9352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang X, Wang X 2004. Cytochrome C-mediated apoptosis. Annu Rev Biochem 73: 87–106 [DOI] [PubMed] [Google Scholar]
- Kaufmann T, Schlipf S, Sanz J, Neubert K, Stein R, Borner C 2003. Characterization of the signal that directs Bcl-x(L), but not Bcl-2, to the mitochondrial outer membrane. J Cell Biol 160: 53–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH 2006. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol 8: 1348–1358 [DOI] [PubMed] [Google Scholar]
- Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH 2009. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell 36: 487–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD 1997. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science 275: 1132–1136 [DOI] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, Green DR, Newmeyer DD 2002. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell 111: 331–342 [DOI] [PubMed] [Google Scholar]
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X 1996. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell 86: 147–157 [DOI] [PubMed] [Google Scholar]
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, Andrews DW 2008. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell 135: 1074–1084 [DOI] [PubMed] [Google Scholar]
- Matthews BW 2005. The structure of E. coli β-galactosidase. C R Biol 328: 549–556 [DOI] [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Hsu YT, Youle RJ 1999. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J 18: 2330–2341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer DD, Ferguson-Miller S 2003. Mitochondria: releasing power for life and unleashing the machineries of death. Cell 112: 481–490 [DOI] [PubMed] [Google Scholar]
- Rice KC, Bayles KW 2008. Molecular control of bacterial death and lysis. Microbiol Mol Biol Rev 72: 85–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Korsmeyer SJ, Schlesinger PH 2000. BAX-dependent transport of cytochrome c reconstituted in pure liposomes. Nat Cell Biol 2: 553–555 [DOI] [PubMed] [Google Scholar]
- Savva CG, Dewey JS, Deaton J, White RL, Struck DK, Holzenburg A, Young R 2008. The holin of bacteriophage λ forms rings with large diameter. Mol Microbiol 69: 784–793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DL, Struck DK, Scholtz JM, Young R 1998. Purification and biochemical characterization of the λ holin. J Bacteriol 180: 2531–2540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tait SW, Green DR 2010. Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11: 621–632 [DOI] [PubMed] [Google Scholar]
- Wang IN, Smith DL, Young R 2000. Holins: the protein clocks of bacteriophage infections. Annu Rev Microbiol 54: 799–825 [DOI] [PubMed] [Google Scholar]
- Wang IN, Deaton J, Young R 2003. Sizing the holin lesion with an endolysin-β-galactosidase fusion. J Bacteriol 185: 779–787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ 2001. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science 292: 727–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, Ierino H, Lee EF, Fairlie WD, Bouillet P, et al. 2007. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak. Science 315: 856–859 [DOI] [PubMed] [Google Scholar]
- Yang J, Liu X, Bhalla K, Kim CN, Ibrado AM, Cai J, Peng TI, Jones DP, Wang X 1997. Prevention of apoptosis by Bcl-2: release of cytochrome c from mitochondria blocked. Science 275: 1129–1132 [DOI] [PubMed] [Google Scholar]
- Youle RJ, Strasser A 2008. The BCL-2 protein family: opposing activities that mediate cell death. Nat Rev Mol Cell Biol 9: 47–59 [DOI] [PubMed] [Google Scholar]
- Zheng Y, Struck DK, Dankenbring CA, Young R 2008. Evolutionary dominance of holin lysis systems derives from superior genetic malleability. Microbiology 154: 1710–1718 [DOI] [PMC free article] [PubMed] [Google Scholar]