Abstract
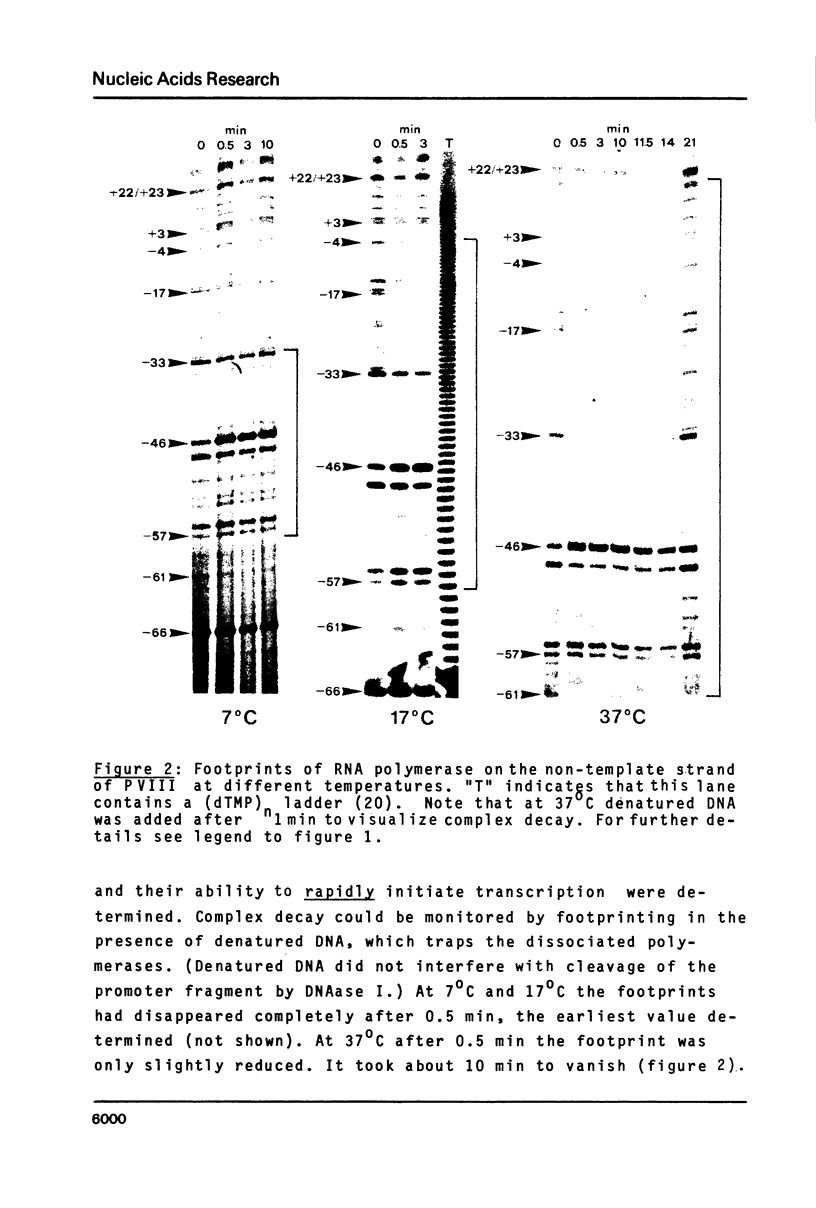
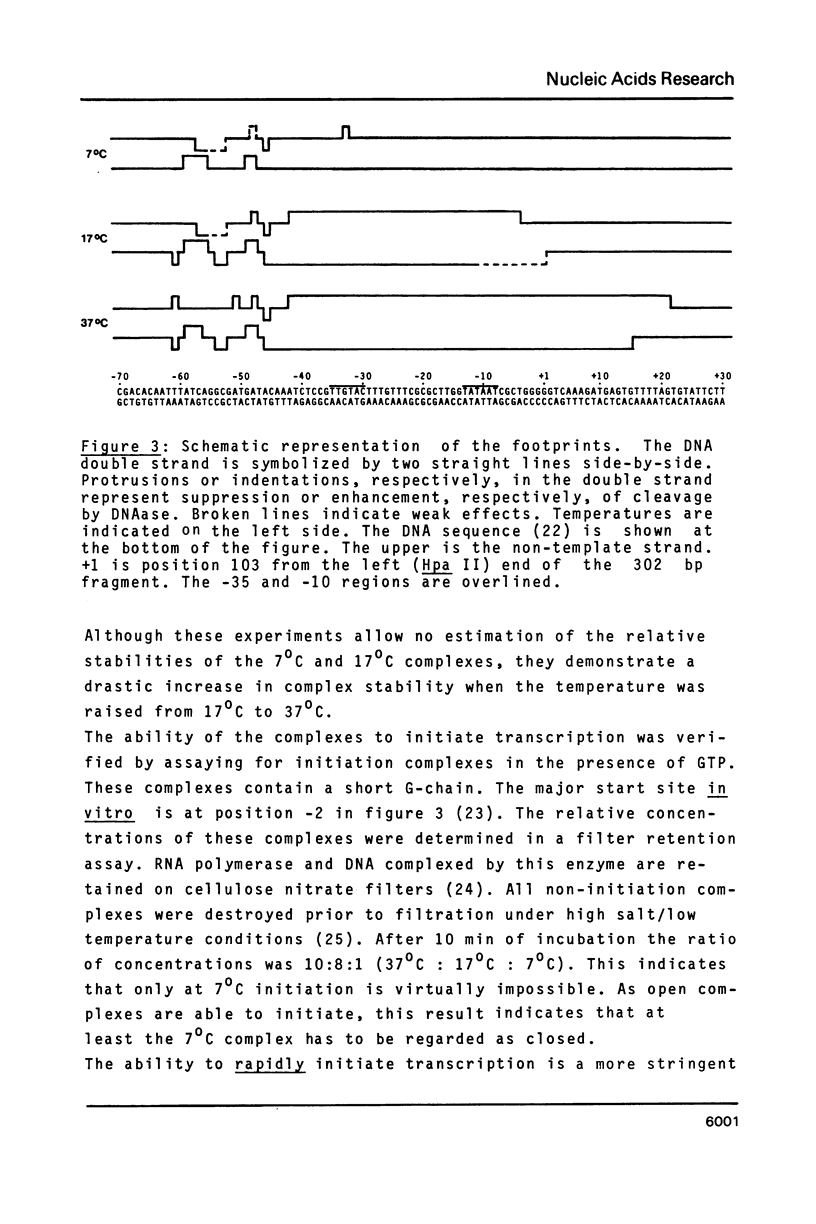
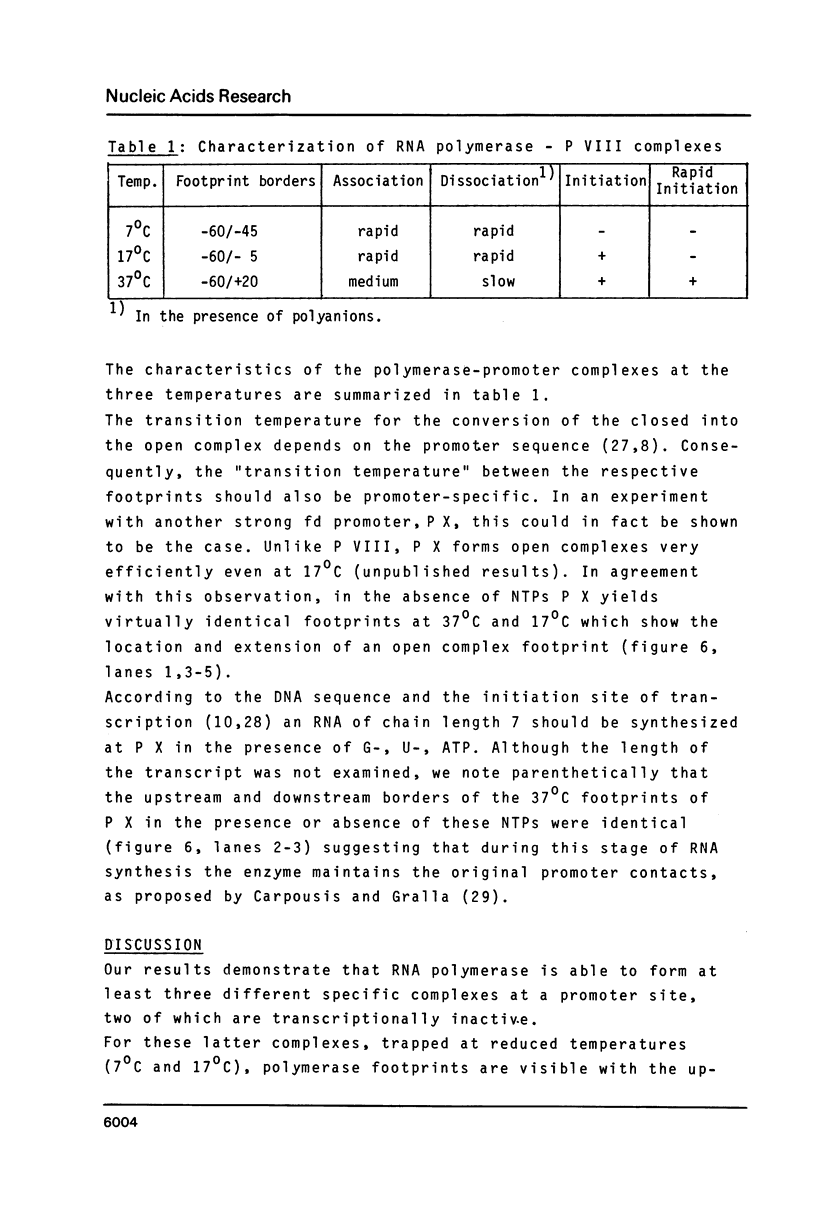
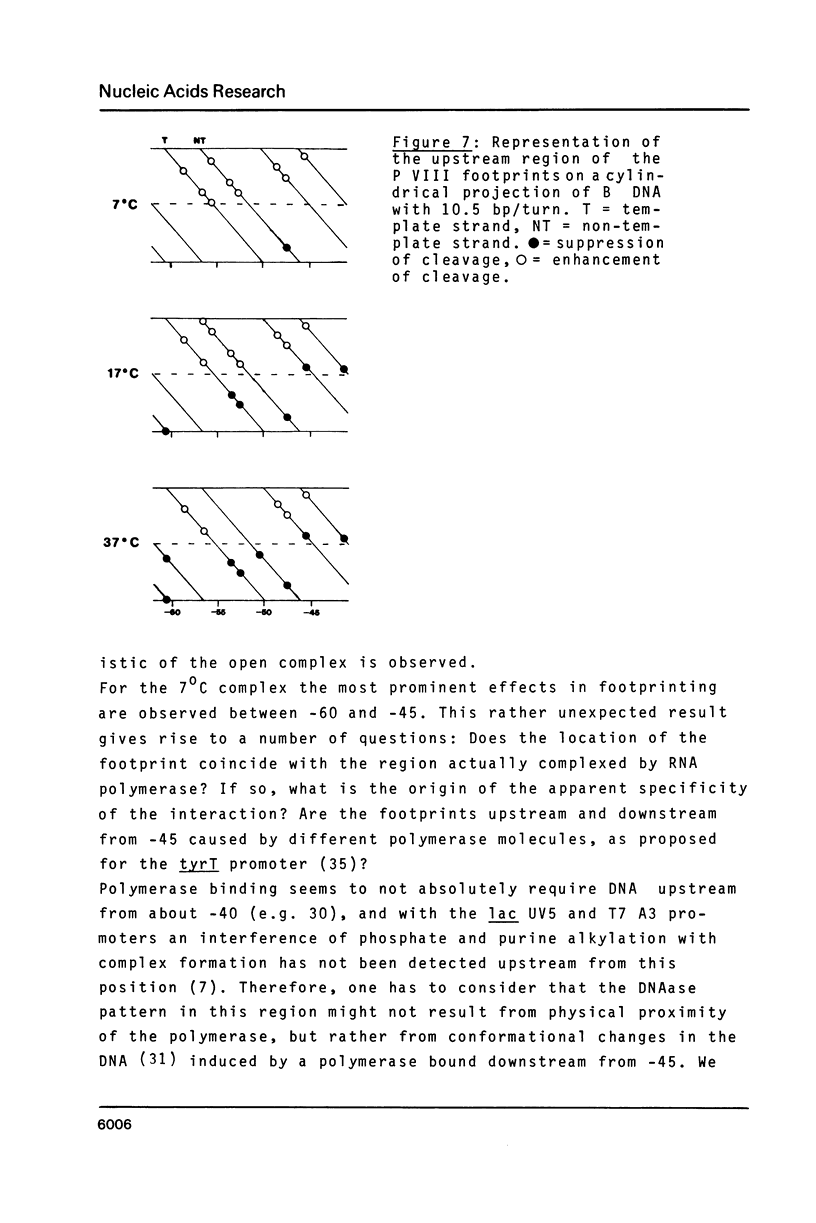
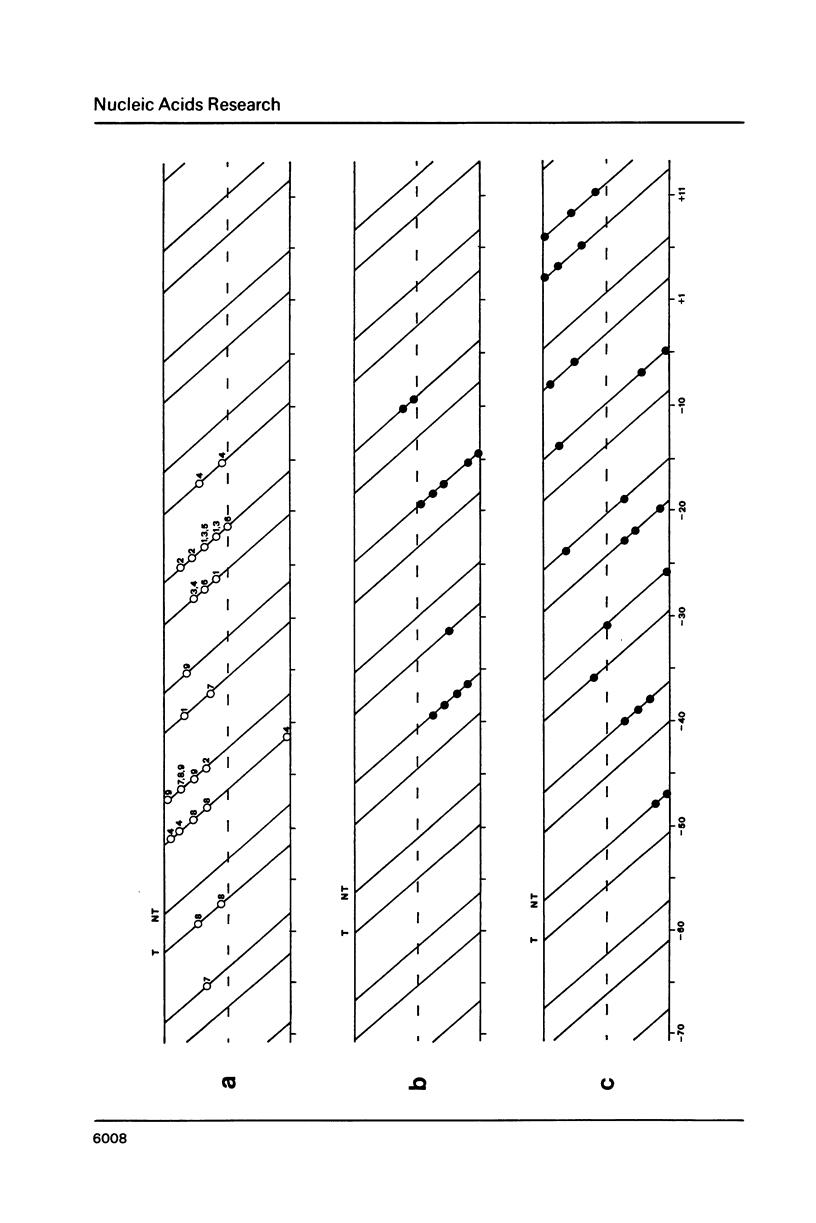
The pathway of E. coli RNA polymerase-promoter complex formation was probed by characterization of low temperature intermediates at the major coat protein promoter of phage fd DMA. Three different complexes could be distinguished. One of them represents the active 'open' complex, the other two have to be regarded as 'closed'. The promoter contacts of the lower temperature complexes were totally comprised within the contacts of the higher temperature complexes. Increase in temperature led to extension of contacts into the downstream direction, while the upstream border of the complexes remained virtually unchanged. Only in the 'open' complex contacts were extended beyond the start site of transcription.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beck E., Sommer R., Auerswald E. A., Kurz C., Zink B., Osterburg G., Schaller H., Sugimoto K., Sugisaki H., Okamoto T. Nucleotide sequence of bacteriophage fd DNA. Nucleic Acids Res. 1978 Dec;5(12):4495–4503. doi: 10.1093/nar/5.12.4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpousis A. J., Gralla J. D. Cycling of ribonucleic acid polymerase to produce oligonucleotides during initiation in vitro at the lac UV5 promoter. Biochemistry. 1980 Jul 8;19(14):3245–3253. doi: 10.1021/bi00555a023. [DOI] [PubMed] [Google Scholar]
- Chakhmakhcheva O. G., Efimov V. A., Ovchinnikov YuA Chemical-enzymatic synthesis of biologically active DNA fragments. Nucleic Acids Symp Ser. 1980;(7):345–363. [PubMed] [Google Scholar]
- Chamberlin M. J. The selectivity of transcription. Annu Rev Biochem. 1974;43(0):721–775. doi: 10.1146/annurev.bi.43.070174.003445. [DOI] [PubMed] [Google Scholar]
- Chenchick A., Beabealashvilli R., Mirzabekov A. Topography of interaction of Escherichia coli RNA polymerase subunits with lac UV5 promoter. FEBS Lett. 1981 Jun 1;128(1):46–50. doi: 10.1016/0014-5793(81)81076-9. [DOI] [PubMed] [Google Scholar]
- Dausse J. P., Sentenac A., Fromageot P. Interaction of RNA polymerase from Escherichia coli with DNA. Effect of temperature and ionic strength on selection of T7 DNA early promoters. Eur J Biochem. 1976 Jun 1;65(2):387–393. doi: 10.1111/j.1432-1033.1976.tb10352.x. [DOI] [PubMed] [Google Scholar]
- Frank R., Köster H. DNA chain length markers and the influence of base composition on electrophoretic mobility of oligodeoxyribonucleotides in polyacrylamide-gels. Nucleic Acids Res. 1979;6(6):2069–2087. doi: 10.1093/nar/6.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawley D. K., McClure W. R. Compilation and analysis of Escherichia coli promoter DNA sequences. Nucleic Acids Res. 1983 Apr 25;11(8):2237–2255. doi: 10.1093/nar/11.8.2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyden B., Nüsslein C., Schaller H. Initiation of transcription within an RNA-polymerase binding site. Eur J Biochem. 1975 Jun 16;55(1):147–155. doi: 10.1111/j.1432-1033.1975.tb02147.x. [DOI] [PubMed] [Google Scholar]
- Hofer B., Köster H. On the influence of thymidine analogues on the activity of phage fd promoters in vitro. Nucleic Acids Res. 1980 Dec 20;8(24):6143–6162. doi: 10.1093/nar/8.24.6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofer B., Ruhe G., Koch A., Köster H. Primary and secondary structure specificity of the cleavage of 'single-stranded' DNA by endonuclease Hinf I. Nucleic Acids Res. 1982 May 11;10(9):2763–2773. doi: 10.1093/nar/10.9.2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones O. W., Berg P. Studies on the binding of RNA polymerase to polynucleotides. J Mol Biol. 1966 Dec 28;22(2):199–209. doi: 10.1016/0022-2836(66)90126-4. [DOI] [PubMed] [Google Scholar]
- Kanazawa H., Mabuchi K., Futai M. Nucleotide sequence of the promoter region of the gene cluster for proton-translocating ATPase from Escherichia coli and identification of the active promotor. Biochem Biophys Res Commun. 1982 Jul 30;107(2):568–575. doi: 10.1016/0006-291x(82)91529-7. [DOI] [PubMed] [Google Scholar]
- Kirkegaard K., Buc H., Spassky A., Wang J. C. Mapping of single-stranded regions in duplex DNA at the sequence level: single-strand-specific cytosine methylation in RNA polymerase-promoter complexes. Proc Natl Acad Sci U S A. 1983 May;80(9):2544–2548. doi: 10.1073/pnas.80.9.2544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppe K., Ohtsuka E., Kleppe R., Molineux I., Khorana H. G. Studies on polynucleotides. XCVI. Repair replications of short synthetic DNA's as catalyzed by DNA polymerases. J Mol Biol. 1971 Mar 14;56(2):341–361. doi: 10.1016/0022-2836(71)90469-4. [DOI] [PubMed] [Google Scholar]
- Lomonossoff G. P., Butler P. J., Klug A. Sequence-dependent variation in the conformation of DNA. J Mol Biol. 1981 Jul 15;149(4):745–760. doi: 10.1016/0022-2836(81)90356-9. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melnikova A. F., Beabealashvilli R., Mirzabekov A. D. A study of unwinding of DNA and shielding of the DNA grooves by RNA polymerase by using methylation with dimethylsulphate. Eur J Biochem. 1978 Mar;84(1):301–309. doi: 10.1111/j.1432-1033.1978.tb12169.x. [DOI] [PubMed] [Google Scholar]
- Pribnow D. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci U S A. 1975 Mar;72(3):784–788. doi: 10.1073/pnas.72.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptashne M., Backman K., Humayun M. Z., Jeffrey A., Maurer R., Meyer B., Sauer R. T. Autoregulation and function of a repressor in bacteriophage lambda. Science. 1976 Oct 8;194(4261):156–161. doi: 10.1126/science.959843. [DOI] [PubMed] [Google Scholar]
- Russell D. R., Bennett G. N. Characterization of the beta-lactamase promoter of pBR322. Nucleic Acids Res. 1981 Jun 11;9(11):2517–2533. doi: 10.1093/nar/9.11.2517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saucier J. M., Wang J. C. Angular alteration of the DNA helix by E. coli RNA polymerase. Nat New Biol. 1972 Oct 11;239(93):167–170. doi: 10.1038/newbio239167a0. [DOI] [PubMed] [Google Scholar]
- Schaller H., Gray C., Herrmann K. Nucleotide sequence of an RNA polymerase binding site from the DNA of bacteriophage fd. Proc Natl Acad Sci U S A. 1975 Feb;72(2):737–741. doi: 10.1073/pnas.72.2.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A., Galas D. J. The interaction of RNA polymerase and lac repressor with the lac control region. Nucleic Acids Res. 1979 Jan;6(1):111–137. doi: 10.1093/nar/6.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebenlist U. RNA polymerase unwinds an 11-base pair segment of a phage T7 promoter. Nature. 1979 Jun 14;279(5714):651–652. doi: 10.1038/279651a0. [DOI] [PubMed] [Google Scholar]
- Siebenlist U., Simpson R. B., Gilbert W. E. coli RNA polymerase interacts homologously with two different promoters. Cell. 1980 Jun;20(2):269–281. doi: 10.1016/0092-8674(80)90613-3. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Okamoto T., Sugisaki H., Takanami M. The nucleotide sequence of an RNA polymerase binding site on bacteriophage fd DNA. Nature. 1975 Feb 6;253(5491):410–414. doi: 10.1038/253410a0. [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Sugisaki H., Okamoto T., Takanami M. Studies on bacteriophage fd DNA. IV. The sequence of messenger RNA for the major coat protein gene. J Mol Biol. 1977 Apr 25;111(4):487–507. doi: 10.1016/s0022-2836(77)80065-x. [DOI] [PubMed] [Google Scholar]
- Taniguchi T., de Crombrugghe B. Interactions of RNA polymerase and the cyclic AMP receptor protein on DNA of the E. coli galactose operon. Nucleic Acids Res. 1983 Aug 11;11(15):5165–5180. doi: 10.1093/nar/11.15.5165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travers A. A., Lamond A. I., Mace H. A., Berman M. L. RNA polymerase interactions with the upstream region of the E. coli tyrT promoter. Cell. 1983 Nov;35(1):265–273. doi: 10.1016/0092-8674(83)90229-5. [DOI] [PubMed] [Google Scholar]
- Walter G., Zillig W., Palm P., Fuchs E. Initiation of DNA-dependent RNA synthesis and the effect of heparin on RNA polymerase. Eur J Biochem. 1967 Dec;3(2):194–201. doi: 10.1111/j.1432-1033.1967.tb19515.x. [DOI] [PubMed] [Google Scholar]
- Wang J. C., Jacobsen J. H., Saucier J. M. Physiochemical studies on interactions between DNA and RNA polymerase. Unwinding of the DNA helix by Escherichia coli RNA polymerase. Nucleic Acids Res. 1977;4(5):1225–1241. doi: 10.1093/nar/4.5.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Chamberlin M. J. Electron microscope studies of transient complexes formed between Escherichia coli RNA polymerase holoenzyme and T7 DNA. Proc Natl Acad Sci U S A. 1977 Sep;74(9):3740–3744. doi: 10.1073/pnas.74.9.3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishart W. L., Machida C., Ohtsubo H., Ohtsubo E. Escherichia coli RNA polymerase binding sites and transcription initiation sites in the transposon Tn3. Gene. 1983 Sep;24(1):99–113. doi: 10.1016/0378-1119(83)90135-x. [DOI] [PubMed] [Google Scholar]
- deHaseth P. L., Goldman R. A., Cech C. L., Caruthers M. H. Chemical synthesis and biochemical reactivity of bacteriophage lambda PR promoter. Nucleic Acids Res. 1983 Feb 11;11(3):773–787. doi: 10.1093/nar/11.3.773. [DOI] [PMC free article] [PubMed] [Google Scholar]