Abstract
Background and objective
Cardiac-directed adenylyl cyclase 6 (AC6) expression attenuates left ventricular (LV) hypertrophy and dysfunction in cardiomyopathy, but its effects in the pressure-overloaded heart are unknown.
Methods
Mice with cardiac-directed and regulated expression of AC6 underwent transaortic constriction (TAC) to induce LV pressure overload. Ten days prior to TAC, and for the duration of the 4 week study, cardiac myocyte AC6 expression was activated in one group (AC-On) but not the other (AC-Off). Multiple measures of LV systolic and diastolic function were obtained 4 week after TAC, and LV samples assessed for alterations in Ca2+ signaling.
Results
LV contractility, as reflected in the end-systolic pressure–volume relationship (Emax), was increased (p = 0.01) by activation of AC6 expression. In addition, diastolic function was improved (p < 0.05) and LV dilation was reduced (p < 0.05). LV samples from AC-On mice showed reduced protein expression of sodium/calcium exchanger (NCX1) (p < 0.05), protein phosphatase 1 (PP1) (p < 0.01), and increased phosphorylation of phospholamban (PLN) at Ser16 (p < 0.05). Finally, sarcoplasmic reticulum (SR) Ca2+ content was increased in cardiac myocytes isolated from AC-On mice (p < 0.05).
Conclusions
Activation of cardiac AC6 expression improves function of the pressure-overloaded and failing heart. The predominant mechanism for this favorable adaptation is improved Ca2+ handling, a consequence of increased PLN phosphorylation, reduced NCX1, reduced PP1 expression, and increased SR Ca2+ content.
Keywords: Animal models of human disease, Heart failure, Hypertrophy, Myocardial contraction, Remodeling
1. Introduction
Adenylyl cyclase (AC) is the key effector molecule that regulates cardiac function through generation of cAMP in cardiac myocytes. However, strategies to increase intracellular cAMP to enhance myocardial contractility have generally failed. For example, cardiac-directed expression of β-adrenergic receptors (βAR), the stimulatory GTP-binding protein (Gαs), and protein kinase A (PKA) – similar to inotropic agents – have resulted in left ventricular (LV) chamber dilation, cardiac fibrosis and heart failure [1–3]. Paradoxically, increased expression of AC type 6 (AC6), a predominant AC in cardiac myocytes, has pronounced favorable effects in genetically-induced cardiomyopathy, including increased LV function, increased survival, and prevention of deleterious LV remodeling [4–6]. These experimental data and others have provided the rationale for initiation of a clinical trial of AC6 gene transfer in patients with heart failure [ClinicalTrials.gov NCT00787059].
Heretofore, there have been very few studies of the effects of increased AC6 in the pressure-overloaded LV. There are several reasons to study the effects of AC6 expression in pressure-overload. First, increased expression of AC6 attenuates LV hypertrophy and improves LV function in genetically-induced cardiomyopathy [5,7]. However, the pressure-overloaded LV may be a superior strategy to explore the effects of AC6 expression, vis-à-vis mechanistic insight, because of the weak correlation between clinical and genetic models of heart failure in mice. We achieved pressure overload using transverse aortic constriction [8]. Second, the ability to activate transgene expression when desired provides a means to study transgene effects on the pressure-overloaded heart at selected points in time, an advantage over constitutively expressed transgenes. In the present study, we used a transgenic line that provides cardiac-specific expression of AC6 under tet-regulation [9]. This enabled rapid activation of cardiac transgene AC6 when LV pressure overload was initiated.
Previous reports of the beneficial effects of increased levels of cardiac AC6 in murine cardiomyopathy led us to the hypothesis that activation of AC6 expression would be associated with beneficial effects on the pressure-overloaded LV. An additional goal was to determine mechanisms for improved LV function, if detected. We targeted Ca2+ handling, since disturbances of Ca2+ homeostasis have been shown to be important in the transition from LV hypertrophy to LV failure [10].
2. Materials and methods
2.1. Animals
Animal use and care were in accordance with Institutional and NIH guidelines. Transgenic mice with cardiac-directed and regulated (tet-off) expression of AC6 (congenic C57BL6 background) were generated and previously described in detail [9]. In this line, cardiac transgene AC6 suppression is complete until doxycycline is removed from the water supply whereupon AC6 expression reaches a maximal level 10 days later [9].
A total of 125 mice (91 male, 34 female, 14.8 ± 0.6 weeks old) were used in the present studies: 93 for the primary comparisons and 32 for additional controls (see below). Male–female ratio and mice age were not different between groups. Sixty-three of these 93 survived 4 weeks of pressure overload. Ten days before TAC, doxycycline was stopped in one group (AC-On), but maintained in the other (AC-Off), and this difference vis-à-vis doxycycline was maintained for the 4 week duration of the study. To test the influence of doxycycline per se on LV size and function, 20 transgene-negative sibling mice underwent TAC: 10 received daily doxycycline in their water for 4 weeks, 10 did not. Finally, 12 sham-operated mice were used as an additional control group (no TAC, no doxycycline).
2.2. LV pressure overload
Mice were anesthetized with isoflurane, intubated, and ventilated, and the chest cavity opened in the second intercostal space at the left upper sternal border. A 7-0 suture was placed around the isolated transverse aorta between the right innominate and left common carotid arteries, and was tied firmly against a 27-gauge needle. The needle was then removed and the chest closed. For the sham operation, an identical procedure was performed except that the suture was left untied. Animals were assigned randomly to continue on doxycycline (AC-Off) or have it removed from the water supply (AC-On) 10 days prior to TAC. Data were acquired and analyzed without knowledge of group identity.
2.3. Echocardiography
Echocardiography was performed on lightly anesthetized mice as previously described [4].
2.4. In vivo physiology
Mice were anesthetized with sodium pentobarbital (100 mg/kg, ip) and a micromanometer-conductance catheter (SPR-839, Millar Instruments, Houston, TX) was inserted via right carotid artery and advanced into the LV chamber to enable acquisition of LV pressure and volume. Another catheter (SPR-1000) was inserted via left carotid artery, enabling measurement of the pressure difference across the stenosis. After LV pressures were recorded, bilateral vagotomy was performed to minimize confounding effects of reflex activation. Time constant of relaxation (Tau) was calculated by the Weiss method. Inferior vena caval occlusion was performed to reduce LV volume to enable defining the end-systolic pressure–volume relationship (ESPVR). Finally, LV pressure was acquired during intravenous isoproterenol infusion (1 μg/g). Ten sequential beats were averaged for each measurement.
2.5. Western blotting
LV samples were homogenized and underwent Western blotting as described previously [4]. Antibodies to phospholamban (PLN), phospho-PLN (Ser16) and phospho-PLN (Thr17) were obtained from Cell Signaling Technology (Danvers, MA, USA), and antibody to NCX1 from Abcam Inc. (Cambridge, MA, USA). Other antibodies including AC6, cAMP response element binding protein (CREB), phospho-CREB, and PKA catalytic subunit were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.6. Cytoplasmic Ca2+ measurements
Hearts from each group underwent perfusion and cardiac myocytes (CM) were isolated [11] to measure cytoplasmic Ca2+ concentration using Fura-2 fluorescence as we have previously described in detail [12]. To assess SR calcium load, caffeine-induced calcium release was initiated by addition of 10 mM caffeine to Tyrode's solution. Caffeine was applied in close proximity to cells under study to ensure rapid and continuous caffeine availability. Peak Ca2+ concentrations were calculated from the averaged baseline and, in addition, total Ca2+ was assessed by integrating the area under the curve and the time course assessed by measuring the width of the peak at 50% of peak height. Data were acquired and analyzed without knowledge of group identity.
2.7. Statistical analysis
Data are reported as mean ± SE. Between-group comparisons were tested for differences using Student's t-test (2-tailed) or a two-way ANOVA when appropriate. One-way ANOVA was used to compare three groups. The null hypothesis was rejected when p < 0.05. Analyses were performed using GraphPad Prism (GraphPad Software, Inc., San Diego, CA).
3. Results
3.1. Animal model, LV hypertrophy and survival
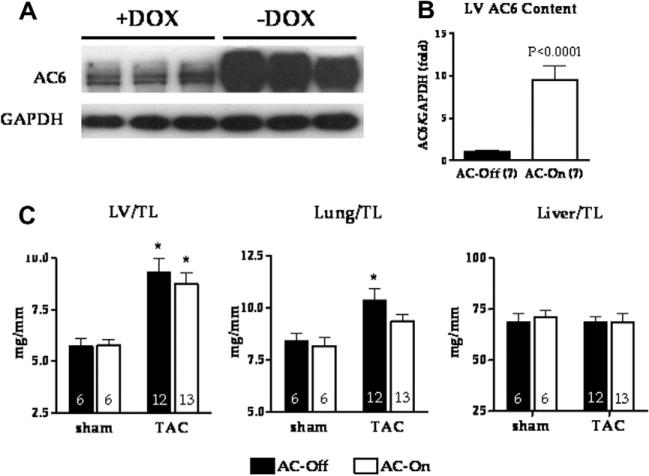
Activation of AC6 expression was associated with a 10-fold increase in LV AC6 protein content (Fig. 1; p < 0.0001), confirming the efficiency of tet-regulated transgene expression. TAC was associated with a similar degree of LV hypertrophy in both groups compared to sham operated animals. LV, lung and liver weight normalized to tibial length did not differ between groups (Fig. 1). The mortality 4 weeks after TAC was 37% (17/46) in AC-Off and 28% (13/47) in AC-On mice (p = 0.40).
Fig. 1.
(A, B) AC6 protein expression in LV homogenates in the presence or absence of doxycycline (DOX). Removing of doxycycline 10 days before TAC (-DOX) was associated with a 10-fold increase in AC6 protein expression 38 days later. (C) LV, lung and liver weight at necropsy normalized to tibial length (TL) (4 weeks after TAC). LV weight was increased 4 weeks after TAC in both groups. Lung weight was increased 4 weeks after TAC in AC-Off mice only. Liver weight was unaffected. No group difference in LV, lung or liver weight was observed. Error bars represent SE. *p < 0.05; two-way ANOVA with Bonferroni post hoc test compared to AC-Off sham.
3.2. Echocardiography
No group difference was observed in heart rate (HR) before and 4 week after TAC during echocardiographic assessments (Table 1). Pre-TAC LV function and dimensions showed no group differences. However, LV end-diastolic (p = 0.04) and end-systolic (p = 0.006) dimensions were progressively increased 4 week after TAC in the AC-Off group to a greater degree than were observed in the AC-On group (Table 1). Similarly, the decline in LV fractional shortening (FS) was greater in the AC-Off than in the AC-On group (p = 0.02; Table 1). The FS 4 week after TAC in the AC-On group was 5 percentage units greater than that in the AC-Off group (AC-Off: 24 ± 1%, n = 12; AC-On: 29 ± 1%, n = 13; p < 0.01), a relative increase of 17%. These data indicate that the extent of adverse LV remodeling and LV dysfunction associated with severe pressure overload is reduced by increased LV AC6 expression. To test the effects of doxycycline, we included 2 control groups using transgene-negative littermates; one group received doxycycline from 10 days prior to TAC to 4 weeks after TAC. The other group received no doxycycline. There were no group differences in LV size or function 4 weeks after TAC (Table 2), and respective values were similar to those in the AC-Off group (Table 1).
Table 1.
Echocardiography: effect of AC6 activation on LV size and function during TAC.
| AC-Off (n = 12) |
AC-On (n = 13) |
p | |||||
|---|---|---|---|---|---|---|---|
| Pre-TAC | 4 Week after TAC | Change | Pre-TAC | 4 Week after TAC | Change | ||
| HR (bpm) | 489 ± 18 | 484 ± 14 | –5 ± 23 | 469 ± 17 | 474 ± 22 | 5 ± 27 | 0.85 |
| LVEDD (mm) | 3.86 ± 0.08 | 4.40 ± 0.07 | 0.55 ± 0.08 | 3.77 ± 0.06 | 4.12 ± 0.07 | 0.35 ± 0.04 | 0.04 |
| LVESD (mm) | 2.53 ± 0.07 | 3.36 ± 0.09 | 0.83 ± 0.11 | 2.50 ± 0.06 | 2.93 ± 0.06 | 0.43 ± 0.07 | 0.006 |
| IVS (mm) | 0.67 ± 0.02 | 0.99 ± 0.05 | 0.32 ± 0.04 | 0.66 ± 0.01 | 0.98 ± 0.04 | 0.32 ± 0.03 | 0.97 |
| PW (mm) | 0.66 ± 0.02 | 0.95 ± 0.04 | 0.28 ± 0.03 | 0.63 ± 0.02 | 0.92 ± 0.04 | 0.29 ± 0.04 | 0.85 |
| FS (%) | 35 ± 2 | 24 ± 1 | –11 ± 2 | 34 ± 1 | 29 ± 1 | –5 ± 2 | 0.02 |
Values represent mean ± SE. The p-value was calculated from Student's t-test (unpaired, 2-tailed) comparing changes in each parameter (4 week after TAC data minus pre-TAC data). AC, adenylyl cyclase; LVEDD, LV end-diastolic dimension; LVESD, LV end-systolic dimension; IVS, interventricular septal diastolic thickness; PW, posterior diastolic wall thickness; FS, fractional shortening.
Table 2.
Echocardiography: transgene-negative siblings ± doxycycline.
| –Doxycycline (n = 7) |
+Doxycycline (n = 7) |
p | |||||
|---|---|---|---|---|---|---|---|
| Pre-TAC | 4 Week after TAC | Change | Pre-TAC | 4 Week after TAC | Change | ||
| HR (bpm) | 484 ± 12 | 503 ± 11 | 19 ± 12 | 498 ± 13 | 504 ± 14 | –1 ± 16 | 0.53 |
| LVEDD (mm) | 3.89 ± 0.08 | 4.33 ± 0.10 | 0.44 ± 0.14 | 3.86 ± 0.08 | 4.31 ± 0.06 | 0.42 ± 0.09 | 0.86 |
| LVESD (mm) | 2.56 ± 0.11 | 3.35 ± 0.12 | 0.79 ± 0.19 | 2.54 ± 0.11 | 3.32 ± 0.11 | 0.73 ± 0.11 | 1.0 |
| IVS (mm) | 0.67 ± 0.01 | 0.97 ± 0.04 | 0.30 ± 0.05 | 0.69 ± 0.01 | 0.95 ± 0.03 | 0.26 ± 0.02 | 0.88 |
| PW (mm) | 0.63 ± 0.03 | 0.94 ± 0.03 | 0.31 ± 0.04 | 0.66 ± 0.01 | 0.91 ± 0.02 | 0.27 ± 0.02 | 0.72 |
| FS (%) | 34 ± 2 | 23 ± 2 | –12 ± 2 | 34 ± 2 | 23 ± 2 | –11 ± 2 | 0.92 |
Values represent mean ± SE. The p-value was calculated from Student's t-test (unpaired, 2-tailed) comparing changes in each parameter (4 week after TAC data minus pre- TAC data). AC, adenylyl cyclase; LVEDD, LV end-diastolic dimension; LVESD, LV end-systolic dimension; TVS, interventricular septal diastolic thickness; PW, posterior diastolic wall thickness; FS, fractional shortening.
3.3. LV systolic and diastolic function
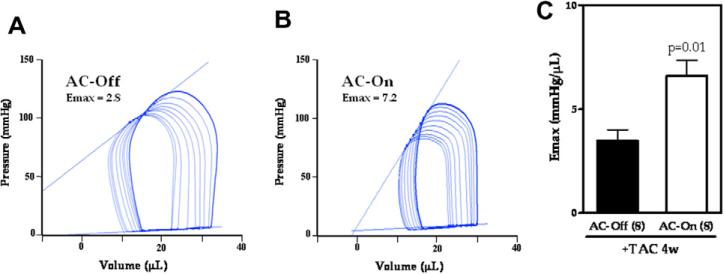
To assess the effect of AC6 transgene expression on contractile function of the pressure-overloaded heart, we measured LV pressure and volume 4 week after TAC. There were no group differences in basal measures of heart rate (AC-Off: 422 ± 31 bpm, n = 8; AC-On: 430 ± 28 bpm, n = 8; p = 0.86), pressure gradient across the TAC (AC-Off: 51 ± 9 mm Hg, n = 8; AC-On: 47 ± 9 mm Hg, n = 8, p = 0.82), and LVSP (AC-Off: 101 ± 12 mm Hg, n = 8; AC-On: 100 ± 13 mm Hg, n = 8; p = 0.97). LV max dP/dt and min dP/dt, LVEDP, CO, and Tau did not differ between groups at baseline, but were improved in the AC-On group during bAR stimulation (isoproterenol, 1 μg/g, iv) (Table 3). For example, AC-On animals showed increased isoproterenol-stimulated peak LV + dP/dt (p < 0.05; Table 3). In addition, improvements in peak negative LV dP/dt (p < 0.05) and Tau (p < 0.05) were observed, indicating improved LV diastolic function (Table 3). Emax, derived from the ESPVR, a relatively load-independent measure of contractile function, was increased 1.9-fold (AC-Off: 3.5 ± 0.5 mm Hg/μL, n = 8; AC-On: 6.6 ± 0.7 mm Hg/μL, n = 8; p = 0.01) by activation of AC6 expression (Fig. 2).
Table 3.
Hemodynamic assessment.
| AC-Off (n = 8) |
AC-On (n = 8) |
p | |||||
|---|---|---|---|---|---|---|---|
| Baseline | +ISO | Change | Baseline | +ISO | Change | ||
| HR (bpm) | 422 ± 31 | 578 ± 34 | 155 ± 16 | 430 ± 28 | 602 ± 17 | 172 ± 27 | 0.59 |
| LVSP (mm Hg) | 101 ± 12 | 128 ± 15 | 26 ± 8 | 100 ± 13 | 143 ± 20 | 43 ± 16 | 0.37 |
| Max dP/dt (mm Hg/s) | 5122 ± 785 | 9579 ± 1039 | 4457 ± 813 | 5129 ± 632 | 12,591 ± 644 | 7462 ± 887 | 0.026 |
| Min dP/dt (mm Hg/s) | –4274 ± 697 | –5497 ± 786 | –1223 ± 725 | –4552 ± 725 | –9049 ± 1041 | –4497 ± 937 | 0.015 |
| LVEDP (mm Hg) | 9.4 ± 1.2 | 7.9 ± 1.1 | –1.5 ± 0.3 | 8.6 ± 1.1 | 5.9 ± 0.8 | –2.8 ± 0.4 | 0.023 |
| CO (mL/min) | 6.6 ± 0.7 | 9.5 ± 1.1 | 2.8 ± 0.6 | 6.1 ± 0.8 | 11.7 ± 10.9 | 5.5 ± 1.0 | 0.042 |
| Tau (ms) | 6.5 ± 0.5 | 5.5 ± 0.4 | –1.0 ± 0.3 | 7.1 ± 0.4 | 5.0 ± 0.2 | –2.1 ± 0.3 | 0.019 |
Values represent mean ± SE. The p-value was calculated Student's t-test (unpaired, 2-tailed) comparing changes in each parameter (+ISO data minus baseline data). AC, adenylyl cyclase; HR, heart rate; LVSP, LV systolic pressure; LVEDP, LV end-diastolic pressure; CO, cardiac output; ISO, isoproterenol, 1 μg/g, iv.
Fig. 2.
(A, B) Representative pressure–volume curves for an animal in each group (4 weeks after TAC). Emax was obtained from the slope of the end-systolic pressure–volume points on successive loops during inferior vena cava occlusion. (C) AC-On animals showed increased Emax values, indicative of increased LV contractility. Error bars represent SE.
3.4. Caffeine-induced Ca2+ release
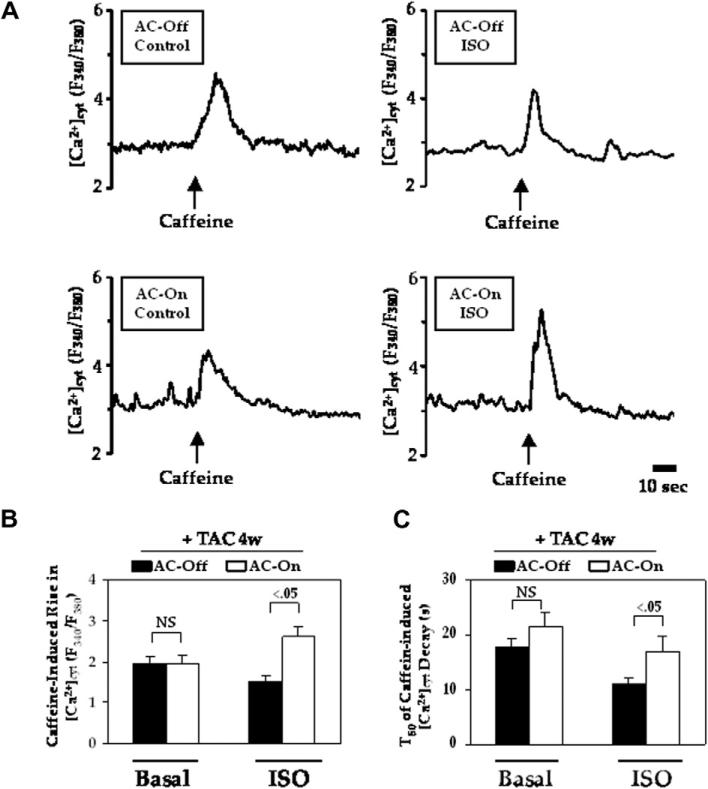
Fig. 3A shows representative cytoplasmic Ca2+ concentrations in cardiac myocytes isolated from the two groups of animals 4 week after TAC. No group difference was seen before stimulation, but after isoproterenol stimulation, cardiac myocytes from AC-On mice showed increased caffeine-induced Ca2+ release (Fig. 3B). T50, the time required for 50% reduction of the peak caffeine-induced intracellular Ca2+ level, was increased during isoproterenol stimulation in cardiac myocytes from AC-On mice (Fig. 3C).
Fig. 3.
Isoproterenol stimulated caffeine-induced Ca2+ release in isolated cardiac myocytes 4 weeks after TAC. (A) Representative [Ca2+]cyt traces in isoproterenol-stimulated myocytes, averaged from 5 to 8 individual cells. (B) Caffeine-induced rise in [Ca2+]cyt, indicating Ca2+ release from SR. (C) The time required for a 50% reduction of the peak caffeine-induced intracellular Ca2+ level (T50) was longer in AC-On when stimulated with isoproterenol. Error bars denote SE. Between-group comparisons were made using two-way ANOVA with Bonferroni post hoc test.
3.5. Phospholamban (PLN) phosphorylation and NCX1 and PP1 expression
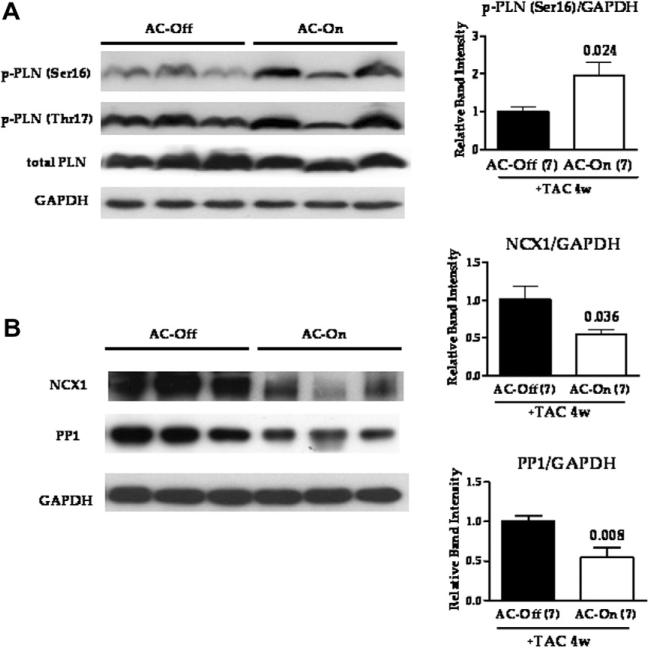
Increased LV expression of AC6 was associated with increased PLN phosphorylation at Ser16 (p = 0.024, Fig. 4). PLN phosphorylation at Thr17 and PLN expression were unchanged (Fig. 4). LV NCX1 and PP1 expression were reduced in AC-On mice (p = 0.036 and 0.008, respectively, Fig. 4). LV expression of β1AR, β2AR, Gs, Gq, Gi, PP2A, SERCA2a, calsequestrin and RyR levels showed no group differences. Expression of PKA catalytic subunit, CREB, and phospho-CREB showed no group differences.
Fig. 4.
LV protein expression 4 weeks after TAC. (A) Phospholamban (PLN) protein expression and phosphorylation at Ser16 or Thr17. Activation of AC6 expression increased p-PLN (Ser16), but not p-PLN (Thr17) or total PLN expression. (B) Sodium/calcium exchanger (NCX1) and type 1 protein phosphatase (PP1) expression. LV expression of NCX1 and PP1 were reduced by the activation of AC6 expression. In all graphs, bars represent mean values and error bars denote SE. Numbers above bars represent p-values from Student's t-test (2-tailed).
4. Discussion
We found that AC6 expression reduces LV dilation and dysfunction in pressure-overload. Activation of AC6 expression was associated with improvements in both LV systolic and diastolic function. Cardiac myocytes from AC-On mice showed increased caffeine-induced SR Ca2+ release associated with increased PLN phosphorylation and reduced expression of NCX1 and PP1 – alterations that would be anticipated to increase LV function.
Increased caffeine-induced SR Ca2+ release was observed in cardiac myocytes from AC-On mice when stimulated with isoproterenol, uncovering potential mechanisms for improved calcium handling during stress that were not evident in the basal state. Furthermore, PLN phosphorylation at Ser16 was increased in LV samples from AC-On mice. These changes provide a plausible mechanism for the beneficial effects conferred by AC6 expression on LV systolic and diastolic function.
PLN is a key regulator of SERCA2 activity and cardiac contractility [13]. SERCA2 is under the inhibitory control of PLN in its dephosphorylated form. PLN phosphorylation relieves this inhibition and thereby increases SR Ca2+ uptake [14]. PLN deletion, which increases sequestration of Ca2+, increases cardiac function [15]. Reduction in PLN phosphorylation (Ser16) and SERCA2a activity are seen in failing myocardium [16]. In the present study, we found that the expression of other Ca2+-related proteins such as SERCA2a, total PLN, and calsequestrin showed no group differences. In a previous study from our group, we found reduced PLN expression in cultured neonatal rat cardiac myocytes following adenovirus-mediated AC6 gene transfer [17]. In contrast, in the present study, we used LV homogenates from transgenic mice with pressure overload. Reasons for differences are likely related to differences in species (mouse vs rat), age (neonatal vs adult), preparation (homogenates vs intact cells), or, even more important, the presence of pressure overload. A plausible explanation for the beneficial effects of AC6 in the present study is increased SERCA2 activity and Ca2+ uptake through increased PLN phosphorylation.
The time decay of [Ca2+]cyt with isoproterenol was increased in AC-On mice compared with AC-Off mice. The major mechanisms for removing cytosolic calcium after caffeine-induced SR Ca2+ release are uptake of calcium into the SR by SERCA2, which would increase with increases in PLN phosphorylation and Na+–Ca2+ exchanger (NCX) activity. The prolonged T50 most likely reflects decreased NCX activity. In the failing heart, expression and activity of NCX are increased relative to SERCA activity, causing enhanced Ca2+ efflux and decreased cardiac systolic and diastolic function [18,19]. In isolated cardiac myocytes, increased NCX expression reduces fractional shortening and caffeine-induced contraction, indicating reduced SR Ca2+ load [20]. In addition, pharmacological inhibition of NCX increases Ca2+ transients and contractile function in normal and failing cardiac myocytes [21,22]. These data indicate that NCX inhibition may have beneficial effects on the failing heart and NCX levels may influence SR Ca2+ release and cardiac contractility. The improvements in LV function observed by activation of AC6 expression were associated with decreased NCX activity, and improved Ca2+ handling.
Many factors contribute to change in LV contractile function. In the present study caffeine-induced SR Ca2+ release was chosen as an integrative measure to test the functional significance of several alterations that would affect SR calcium content and the removal of cytosolic calcium. This provided a functional correlate that reflected changes with in vivo measurements of function. Our group has previously shown that such studies are tightly correlated with Ca2+ uptake capacity measured in SR activity assays in LV homogenates [12].
AC6 expression also was associated with reduced PP1 expression. PP1 is a pivotal negative regulator of cardiac contraction, which dephosphorylates PLN and other proteins [23]. Increased LV PP1 expression and activity are seen in failed human hearts and animal models of heart failure [24,25]. In addition, increased LV PP1 levels results in depressed cardiac function and dilated cardiomyopathy [26]. In contrast, inhibition of LV PP1 activity by inhibitor-2 gene transfer increased LV contractility and Ca2+ handling, which was associated with increased PLN phosphorylation [27]. Therefore, decreased LV PP1 expression and activity, as occurred after AC6 expression in the current study, provides a plausible mechanism for increased PLN phosphorylation, improved Ca2+ handling, and increased LV contractile function.
Previous studies of AC6 expression in cultured neonatal rat cardiac myocytes showed abundant transgene AC6 in the nuclear envelope, suggesting the possibility of AC6-regulation of nuclear transcriptional activity. A markedly increased expression of the nuclear transcriptional factor ATF3 was found to be associated with AC6 gene transfer. Further studies demonstrated that ATF3 inhibits PLN promoter activity and thereby reduces PLN expression [17]. In the present study, expression of CREB (cAMP response binding protein) transcription factor and its phosphorylation showed no group differences, indicating that CREB may not be involved. However, ATF3 expression was too low to detect group differences. Whether AC6 influences transcription of PP1 and NCX1 is unknown, but is a focus of our laboratory.
Previously we reported that increased cardiac AC6 expression increased cardiac function, and reduced LV dilation and hypertrophy in Gq-associated cardiomyopathy [5,7]. In the present study, AC6 activation during LV pressure overload favorably modified LV function without inhibiting hypertrophic growth. However, external mechanical stress per se, such as occurs in pressure overload, remains constant even after gene activation unlike the case with the heart failure model. It is worth noting that cardiac activation of AC6 in CHF mice induced by MI also resulted in increased LV function without any change in LV/body weight and LV wall thickness [4].
In this study, we removed doxycyline 10 days prior to TAC to activate cardiac AC6 expression maximally by the time of TAC. This timeline provided continuously increased AC6 expression during pressure overload, and enabled us to explore its effect on LV hypertrophic response, remodeling and change in function. In clinical settings, however, AC6 gene transfer would be applied when LV hypertrophy was already well-developed. Additional studies in which activation of AC6 expression is initiated in a late stage after TAC will be required to address this important issue.
There is a single paper in the literature regarding cardiac AC6 expression and pressure overload, which reported reduced, but still normal LVEF (AC6: 58 ± 1%; control: 65 ± 1%) 4 week after TAC in FVN mice with cardiac-directed constitutive expression of AC6 [28]. In contrast, we found that transgene negative C57B6 mice showed abnormal LVEF 4 week after TAC (48 ± 1%), and that activation of cardiac AC6 expression at the time of TAC placement resulted in increased LVEF, fractional shortening and LV function 4 week after TAC. The mice used in their study [28] had increased cardiac AC6 expression from birth, while ours had AC6 activated only just before TAC placement, suggesting that timing of trans-gene expression is an important determinant of the physiological response. In addition, strain differences (FVN vs C57B6) may also be important.
5. Conclusions
Activation of cardiac AC6 expression improves function of the pressure-overloaded and failing heart. The predominant mechanism for this favorable adaptation is improved Ca2+ handling, a consequence of increased PLN phosphorylation, reduced NCX1, reduced PP1 expression, and increased SR Ca2+ content.
Acknowledgments
This work was supported by NIH Grants 5P01HL066941, HL081741, and HL088426 (H.K.H.), Merit Review Awards from the Department of Veteran's Affairs (H.K.H.), American Heart Association Western States Affiliate Grant-in-Aid 0765064Y (M.H.G.) and Postdoctoral Fellowship 09POST2250887 (Y.S.). We thank Diane Huang and Matthew Spellman for technical assistance and Dr. Glenn I. Fishman for providing the tTA mice.
References
- 1.Engelhardt S, Hein L, Wiesmann F, Lohse MJ. Progressive hypertrophy and heart failure in beta1-adrenergic receptor transgenic mice. Proc. Natl. Acad. Sci. USA. 1999;96:7059–7064. doi: 10.1073/pnas.96.12.7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gaudin C, Ishikawa Y, Wight DC, Mahdavi V, Nadal-Ginard B, Wagner TE, Vatner DE, Homcy CJ. Overexpression of Gs alpha protein in the hearts of transgenic mice. J. Clin. Invest. 1995;95:1676–1683. doi: 10.1172/JCI117843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Antos CL, Frey N, Marx SO, Reiken S, Gaburjakova M, Richardson JA, Marks AR, Olson EN. Dilated cardiomyopathy and sudden death resulting from constitutive activation of protein kinase a. Circ. Res. 2001;89:997–1004. doi: 10.1161/hh2301.100003. [DOI] [PubMed] [Google Scholar]
- 4.Lai NC, Tang T, Gao MH, Saito M, Takahashi T, Roth DM, Hammond HK. Activation of cardiac adenylyl cyclase expression increases function of the failing ischemic heart in mice. J. Am. Coll. Cardiol. 2008;51:1490–1497. doi: 10.1016/j.jacc.2008.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roth DM, Bayat H, Drumm JD, Gao MH, Swaney JS, Ander A, Hammond HK. Adenylyl cyclase increases survival in cardiomyopathy. Circulation. 2002;105:1989–1994. doi: 10.1161/01.cir.0000014968.54967.d3. [DOI] [PubMed] [Google Scholar]
- 6.Tang T, Gao MH, Roth DM, Guo T, Hammond HK. Adenylyl cyclase type VI corrects cardiac sarcoplasmic reticulum calcium uptake defects in cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2004;287:H1906–H1912. doi: 10.1152/ajpheart.00356.2004. [DOI] [PubMed] [Google Scholar]
- 7.Roth DM, Gao MH, Lai NC, Drumm J, Dalton N, Zhou JY, Zhu J, Entrikin D, Hammond HK. Cardiac-directed adenylyl cyclase expression improves heart function in murine cardiomyopathy. Circulation. 1999;99:3099–3102. doi: 10.1161/01.cir.99.24.3099. [DOI] [PubMed] [Google Scholar]
- 8.Rockman HA, Ross RS, Harris AN, Knowlton KU, Steinhelper ME, Field LJ, Ross J, Jr., Chien KR. Segregation of atrial-specific and inducible expression of an atrial natriuretic factor transgene in an in vivo murine model of cardiac hypertrophy. Proc. Natl. Acad. Sci. USA. 1991;88:8277–8281. doi: 10.1073/pnas.88.18.8277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gao MH, Bayat H, Roth DM, Yao Zhou J, Drumm J, Burhan J, Hammond HK. Controlled expression of cardiac-directed adenylylcyclase type VI provides increased contractile function. Cardiovasc. Res. 2002;56:197–204. doi: 10.1016/s0008-6363(02)00539-4. [DOI] [PubMed] [Google Scholar]
- 10.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu. Rev. Physiol. 2008;70:23–49. doi: 10.1146/annurev.physiol.70.113006.100455. [DOI] [PubMed] [Google Scholar]
- 11.Wolska BM, Solaro RJ. Method for isolation of adult mouse cardiac myocytes for studies of contraction and microfluorimetry. Am. J. Physiol. 1996;271:H1250–H1255. doi: 10.1152/ajpheart.1996.271.3.H1250. [DOI] [PubMed] [Google Scholar]
- 12.Tang T, Gao MH, Lai NC, Firth AL, Takahashi T, Guo T, Yuan JX, Roth DM, Hammond HK. Adenylyl cyclase type 6 deletion decreases left ventricular function via impaired calcium handling. Circulation. 2008;117:61–69. doi: 10.1161/CIRCULATIONAHA.107.730069. [DOI] [PubMed] [Google Scholar]
- 13.Hasenfuss G, Meyer M, Schillinger W, Preuss M, Pieske B, Just H. Calcium handling proteins in the failing human heart. Basic Res. Cardiol. 1997;92(Suppl. 1):87–93. doi: 10.1007/BF00794072. [DOI] [PubMed] [Google Scholar]
- 14.Luo W, Chu G, Sato Y, Zhou Z, Kadambi VJ, Kranias EG. Transgenic approaches to define the functional role of dual site phospholamban phosphorylation. J. Biol. Chem. 1998;273:4734–4739. doi: 10.1074/jbc.273.8.4734. [DOI] [PubMed] [Google Scholar]
- 15.Luo W, Grupp IL, Harrer J, Ponniah S, Grupp G, Duffy JJ, Doetschman T, Kranias EG. Targeted ablation of the phospholamban gene is associated with markedly enhanced myocardial contractility and loss of beta-agonist stimulation. Circ. Res. 1994;75:401–409. doi: 10.1161/01.res.75.3.401. [DOI] [PubMed] [Google Scholar]
- 16.Schwinger RH, Munch G, Bolck B, Karczewski P, Krause EG, Erdmann E. Reduced Ca(2+)-sensitivity of SERCA 2a in failing human myocardium due to reduced serin-16 phospholamban phosphorylation. J. Mol. Cell. Cardiol. 1999;31:479–491. doi: 10.1006/jmcc.1998.0897. [DOI] [PubMed] [Google Scholar]
- 17.Gao MH, Tang T, Guo T, Sun SQ, Feramisco JR, Hammond HK. Adenylyl cyclase type VI gene transfer reduces phospholamban expression in cardiac myocytes via activating transcription factor 3. J. Biol. Chem. 2004;279:38797–38802. doi: 10.1074/jbc.M405701200. [DOI] [PubMed] [Google Scholar]
- 18.Hasenfuss G, Schillinger W, Lehnart SE, Preuss M, Pieske B, Maier LS, Prestle J, Minami K, Just H. Relationship between Na+–Ca2+exchanger protein levels and diastolic function of failing human myocardium. Circulation. 1999;99:641–648. doi: 10.1161/01.cir.99.5.641. [DOI] [PubMed] [Google Scholar]
- 19.Studer R, Reinecke H, Vetter R, Holtz J, Drexler H. Expression and function of the cardiac Na+/Ca2+ exchanger in postnatal development of the rat, in experimental-induced cardiac hypertrophy and in the failing human heart. Basic Res. Cardiol. 1997;92(Suppl. 1):53–58. doi: 10.1007/BF00794068. [DOI] [PubMed] [Google Scholar]
- 20.Schillinger W, Janssen PM, Emami S, Henderson SA, Ross RS, Teucher N, Zeitz O, Philipson KD, Prestle J, Hasenfuss G. Impaired contractile performance of cultured rabbit ventricular myocytes after adenoviral gene transfer of Na(+)–Ca(2+) exchanger. Circ. Res. 2000;87:581–587. doi: 10.1161/01.res.87.7.581. [DOI] [PubMed] [Google Scholar]
- 21.Hobai IA, Maack C, O'Rourke B. Partial inhibition of sodium/calcium exchange restores cellular calcium handling in canine heart failure. Circ. Res. 2004;95:292–299. doi: 10.1161/01.RES.0000136817.28691.2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liron B, Reuben H, Beni S, Chagit S, Khananshvili D. Purified endogenous inhibitor of the Na/Ca exchanger can enhance the cardiomyocytes contractility and calcium transients. Biochem. Biophys. Res. Commun. 2006;346:1100–1107. doi: 10.1016/j.bbrc.2006.06.020. [DOI] [PubMed] [Google Scholar]
- 23.MacDougall LK, Jones LR, Cohen P. Identification of the major protein phosphatases in mammalian cardiac muscle which dephosphorylate phospholamban. Eur. J. Biochem. 1991;196:725–734. doi: 10.1111/j.1432-1033.1991.tb15871.x. [DOI] [PubMed] [Google Scholar]
- 24.Neumann J, Eschenhagen T, Jones LR, Linck B, Schmitz W, Scholz H, Zimmermann N. Increased expression of cardiac phosphatases in patients with end-stage heart failure. J. Mol. Cell. Cardiol. 1997;29:265–272. doi: 10.1006/jmcc.1996.0271. [DOI] [PubMed] [Google Scholar]
- 25.Boknik P, Fockenbrock M, Herzig S, Knapp J, Linck B, Luss H, Muller FU, Muller T, Schmitz W, Schroder F, Neumann J. Protein phosphatase activity is increased in a rat model of long-term beta-adrenergic stimulation. Naunyn Schmiedebergs Arch. Pharmacol. 2000;362:222–231. doi: 10.1007/s002100000283. [DOI] [PubMed] [Google Scholar]
- 26.Carr AN, Schmidt AG, Suzuki Y, del Monte F, Sato Y, Lanner C, Breeden K, Jing SL, Allen PB, Greengard P, Yatani A, Hoit BD, Grupp IL, Hajjar RJ, DePaoli-Roach AA, Kranias EG. Type 1 phosphatase, a negative regulator of cardiac function. Mol. Cell. Biol. 2002;22:4124–4135. doi: 10.1128/MCB.22.12.4124-4135.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada M, Ikeda Y, Yano M, Yoshimura K, Nishino S, Aoyama H, Wang L, Aoki H, Matsuzaki M. Inhibition of protein phosphatase 1 by inhibitor-2 gene delivery ameliorates heart failure progression in genetic cardiomyopathy. FASEB J. 2006;20:1197–1199. doi: 10.1096/fj.05-5299fje. [DOI] [PubMed] [Google Scholar]
- 28.Guellich A A, Gao S, Hong C, Yan L, Wagner TE, Dhar SK, Ghaleh B, Hittinger L, Iwatsubo K, Ishikawa Y, Vatner SF, Vatner DE. Effects of cardiac overexpression of type 6 adenylyl cyclase affects on the response to chronic pressure overload. Am. J. Physiol. 2010;299:H707–H712. doi: 10.1152/ajpheart.00148.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]