Abstract
A skin mucus lectin exhibiting a homodimeric structure and an S–S bond between subunits of ∼40 kDa was purified from flathead Platycephalus indicus (Scorpaeniformes). This lectin, named FHL (FlatHead Lectin), exhibited mannose-specific activity in a Ca2+-dependent manner. Although FHL showed no homology to any previously reported lectins, it did exhibit ∼20% identity to previously discovered plasma kallikreins and coagulation factor XIs of mammals and Xenopus laevis. These known proteins are serine proteases and play pivotal roles in the kinin-generating system or the blood coagulation pathway. However, alignment analysis revealed that while FHL lacked a serine protease domain, it was homologous to the heavy-chain domain of plasma kallikreins and coagulation factor XI therefore suggesting that FHL is not an enzyme but rather a novel animal lectin. On the basis of this finding, we investigated the lectin activity of human plasma kallikrein and revealed that it could indeed act as a lectin. Other genes homologous to FHL were also found in the genome databases of some fish species, but not in mammals. In contrast, plasma kallikreins and coagulation factor XI have yet to be identified in fish. The present findings suggest that these mammalian enzymes may have originally emerged as a lectin and may have evolved into molecules with protease activity after separation from common ancestors.
Keywords: coagulation factor XI, lectin, plasma kallikrein, skin mucus
Introduction
The family of kallikreins, plasma kallikrein and tissue kallikrein, represent serine proteases defined by their ability to release kinin via the proteolysis of high- and low-molecular-weight kininogen in blood plasma (Werle et al. 1937). The kinin-generating system has many important functions including the modulation of blood pressure, complement activation and the mediation of inflammation (Colman 2004). Of the two kallikreins, tissue kallikrein is composed of only protease sequence, while plasma kallikrein exhibits an N-terminal heavy chain in addition to a C-terminal light chain with protease activity. Much speculation has surrounded the precise function of the heavy chain. Our discovery of a lectin from the skin mucus of flathead, a species of teleost fish, appears to have solved this mystery unexpectedly.
Mature human pre-kallikrein, a precursor of plasma kallikrein, consists of 619 amino acids (Chung et al. 1986), and activation of the pre-kallikrein to kallikrein is induced by cleavage at the amide bond between Arg371 and Ile372 resulting in the production of a heterodimer consisting of an N-terminal heavy chain and a C-terminal light chain containing a Cys364–Cys484 bond (Wuepper and Cochrane 1972). The light chain includes a protease domain with catalytic triad residues. The heavy chain contains four tandem repeats of “apple domains” (Chung et al. 1986), homologs of which are also found in coagulation factor XI (Fujikawa et al. 1986). Human coagulation factor XI, which is a homodimer of 80 kDa subunits linked by a disulfide bond, is also a serine protease that acts in the blood clotting pathway in plasma (Fujikawa et al. 1986). The precise biochemical function of the apple domains, however, has yet to be clarified.
Tissue kallikrein, distributing in a variety of tissues (Richards et al. 1997), is composed of the serine protease domain alone, which is homologous to the light-chain domain of plasma kallikrein. This molecule is found in a variety of vertebrate species including teleosts, while plasma kallikrein and coagulation factor XI have yet to be identified in fish. Moreover, Jiang and Doolitle (2003) reported that no genes homologous to plasma kallikrein and coagulation factor XI have been found in the fugu genome database as yet, suggesting that fish lack plasma kallikrein and coagulation factor XI. The evolutionary relationship between coagulation factor XI, plasma and tissue kallikreins is thus of great interest.
In the process of our comprehensive investigations on lectins, which represent a diverse group of carbohydrate–binding proteins in the skin mucus of many fish species, we discovered a novel lectin, named FHL (FlatHead Lectin), in the flathead Platycephalus indicus (Scorpaeniformes). Interestingly, this lectin exhibited structural similarity to the heavy-chain domain in plasma kallikrein, which has yet to be identified in any teleost species. We propose to define a new family of animal lectins, named “kalliklectins”, into which the various types of lectins should be classified. Our findings prompted us to examine the lectin activity of human plasma kallikrein, and we were successful in providing new evidence of a new functional role for this enzyme. Searches of genome databases suggested that kalliklectin is widely distributed in fish, but not in mammals. Here, we report essential characteristics and the primary structure of our novel lectin and discuss our results in terms of the evolutionary relationships between kallikreins, coagulation factor XI and kalliklectin.
Results
Purification of mannose-binding protein from skin mucus
Mannose-affinity chromatography yielded a single protein peak from skin mucus extract when buffer was changed to phosphate-buffered saline (PBS) (+) containing 50 mM mannose (data not shown). This fraction exhibited hemagglutination activity at a titer of 28. Lectin titer was exclusively reduced by mannose to <21, whereas other tested sugars did not affect the titer (28). Ethylenediaminetetraacetic acid (EDTA) also inhibited hemagglutination activity (<21), indicating that this lectin binds mannose in a Ca2+-dependent manner.
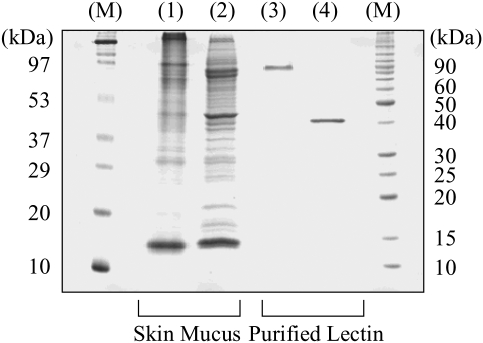
Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS–PAGE) showed that the affinity-purified fraction yielded a single band of ∼40 kDa under reducing conditions but a band of ∼90 kDa under non-reducing conditions (Figure 1). This indicated that the protein was a homodimer with disulfide bonds between subunits whose molecular mass was around 40 kDa. We tentatively named the novel lectin “FHL”.
Fig. 1.
SDS–PAGE analysis of FHL. Skin mucus extract (lanes 1 and 2) and the affinity-purified lectin (lanes 3 and 4) was subject to either non-reducing (lanes 1 and 3) or reducing (lanes 2 and 4) SDS–PAGE. M, molecular markers.
Primary structure of FHL
For structural analysis, purified FHL was at first subjected to Edman degradation. This approach, however, failed to obtain sequence data. This indicated that the N-terminus of the protein was blocked. In order to determine the internal amino acid sequence, FHL was first digested with lysyl endopeptidase, and the resulting fragments separated by reverse-phase high-performance liquid chromatography (HPLC). The amino acid sequences of three fragments were analyzed and the determined sequences were as follows: L1, LFPNTDIPGNDFLNLPAASPEHXQTMXFAHPLXTYVSFD; L2, QANVVSGFFQNAQITQFFN and L3, FSWAIP MIP TVLK.
Based on the amino acid sequence of L2, we synthesized a degenerate primer to use in 3′ RACE (rapid amplification of cDNA end). Subsequently, 5′ RACE was performed with a gene-specific primer designed to the nucleotide sequence obtained by 3′ RACE. The complete cDNA for the protein spanned 1426 bp, including 1134 bp of open reading frame encoding 378 amino acids (Figure 2). The signal peptide was putatively determined to be composed of 20 amino acids releasing a mature protein of 358 amino acids.
Fig. 2.
Nucleotide sequence of cDNA and the deduced amino acid sequence of FHL. Numbers on the right indicate the nucleotide and amino acid position. The dashed lines indicate internal sequences obtained by protein sequencing. Predicted signal sequence is shown in italics, and the polyadenylation signal (AATAAA) is double underlined. Polymorphic mutations at positions 61 (C to A), 77 (T to C), 125 (C to T), 135 (G to C), 137 (T to G), 260 (T to C) and 696 (A to G) are underlined, and resulting amino acid changes (at amino acid positions 17 and 204) are circled.
We found that the nucleotide sequence for FHL exhibited some polymorphic nucleotides. Specifically, of the 13 clones analyzed, 7 clones contained C61, T77, C125, G135, T137, T260 and A696, whereas the remaining 6 clones contained A61, C77, T125, C135, G137, C260 and G696. This variation may result in the production of two types of FHL protein; one with Val17 and Lys204 and the other with Leu17 and Glu204 (Figure 2). These proteins exhibited a high sequence identity with each other (99.5%), suggesting that these were allelic variants or recently duplicated genes. The deduced amino acid sequences included all three of the internal sequences obtained from the fragments digested by lysyl endopeptidase (Figure 2). The calculated molecular mass of the mature protein was ∼41 kDa, which was in good agreement with that estimated by SDS–PAGE analysis (Figure 1, lane 4).
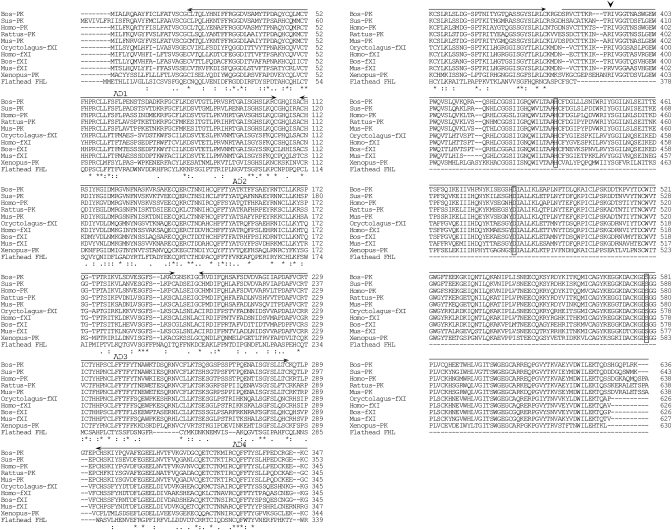
Although no lectins share sequence homology with FHL, we found that plasma kallikreins and coagulation factor XIs in Xenopus laevis and mammals exhibited low e-values (<6 × 1023) in a homology search (Table I). However, we observed a 250-residue deletion at the C-terminal end in the flathead FHL when compared with mammalian and X. laevis kallikreins and coagulation factor XIs (Figure 3). The full-length amino acid sequence of FHL is ∼27.7% identical with the heavy chains of plasma kallikreins and coagulation factor XIs (Table I); heavy chains representing one of the subunits of the activated form.
Table I.
Plasma kallikreins and coagulation factor XIs exhibit lower e-values in homology searches
| Gene | Species | % identity (FL vs FHL)* | % identity (HC vs FHL)** | e-value | Accession no. |
|---|---|---|---|---|---|
| Plasma kallikrein | Xenopus laevis | 18.7 | 27.7 | 3e-36 | BC077417 |
| Coagulation factor XI | Mus musculus | 18.4 | 25.3 | 1e-34 | BC019485 |
| Plasma kallikrein | Bos taurus | 16.8 | 26.8 | 2e-34 | BC105498 |
| Plasma kallikrein | Rattus norvegicus | 16.1 | 27.1 | 3e-34 | BC089815 |
| Plasma kallikrein | Rattus norvegicus | 17.2 | 27.1 | 3e-34 | M58590 |
| Plasma kallikrein | Rattus norvegicus | 16.9 | 26.9 | 3e-34 | M30282 |
| Plasma kallikrein | Sus scrofa | 16.0 | 25.8 | 5e-34 | AB022425 |
| Coagulation factor XI | Oryctolagus cuniculus | 19.9 | 26.4 | 8e-34 | AF395821 |
| Coagulation factor XI | Mus musculus | 15.9 | 26.0 | 2e-33 | AF356627 |
| Plasma kallikrein | Mus musculus | 16.1 | 26.6 | 7e-33 | BC026555 |
| Plasma kallikrein | Mus musculus | 16.2 | 26.6 | 7e-33 | M58588 |
| Plasma kallikrein | Homo sapiens | 15.8 | 25.9 | 1e-32 | BC117351 |
| Plasma kallikrein | Homo sapiens | 15.8 | 25.9 | 1e-32 | BC117349 |
| Plasma kallikrein | Homo sapiens | 15.8 | 25.9 | 1e-32 | M13143 |
| Coagulation factor XI | Bos taurus | 16.3 | 25.9 | 5e-31 | AB196307 |
| Coagulation factor XI | Homo sapiens | 15.8 | 25.1 | 2e-29 | BC122863 |
| Coagulation factor XI | Homo sapiens | 15.8 | 25.1 | 2e-29 | BC119014 |
| Coagulation factor XI | Homo sapiens | 15.8 | 25.1 | 2e-29 | M13142 |
| Coagulation factor XI | Homo sapiens | 17.6 | 21.1 | 6e-23 | AF045649 |
*FL vs FHL; percent identity of full-length protein to FHL.
**HC vs FHL; percent identity of heavy chain to FHL.
Fig. 3.
Comparison of the amino acid sequence of FHL with the plasma kallikreins and coagulation factor XIs of mammals and X. laevis. Numbers on the right indicate the amino acid position. Identical (*) and similar (: or .) residues identified by Clustal W are indicated. The cleavage site for enzyme activation in plasma kallikreins is indicated by arrowhead, and the catalytic triad residues (His, Asp and Ser) are boxed. Four apple domains (corresponding to human plasma kallikrein residues 21–104, 111–194, 201–284 and 292–375) are shown by arrows, and the upper line represents the serine protease domain. Abbreviations and accession numbers are as follows: Homo-PK, human H. sapiens plasma kallikrein (BC117351); Mus-PK, mouse M. musculus plasma kallikrein (BC026555); Rattus-PK, rat Rattus norvegicus plasma kallikrein (BC089815); Bos-PK, bovine Bos taurus plasma kallikrein (BC105498); Sus-PK, pig Sus scrofa plasma kallikrein (AB022425); Xenopus-PK, X. laevis plasma kallikrein (BC077417); Homo-fXI, H. sapiens factor XI (BC122863); Mus-fXI, M. musculus factor XI (BC019485); Bos-fXI, Bos taurus factor XI (AB196307) and Oryctolagus-fXI, rabbit Oryctolagus cuniculus factor XI (AF395821).
Gene expression of FHL in various tissues
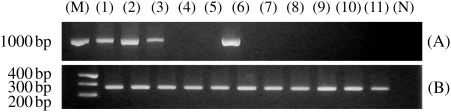
Reverse transcription (RT)–polymerase chain reaction (PCR) analysis, with two specific primers (−3 and −4), yielded positive bands for FHL of predicted size (∼1000 bp) in samples from the skin, gill, esophagus and liver whereas the stomach, intestine, kidney, heart, spleen, ovary and muscle were negative (Figure 4).
Fig. 4.
Gene expression of FHL in various tissues. Identical amounts of total RNA from the skin (1), gill (2), esophagus (3), stomach (4), intestine (5), liver (6), kidney (7), heart (8), spleen (9), ovary (10) and muscle (11) were isolated and reverse-transcribed to produce cDNA. PCR amplification was performed with specific primer-3 and -4 to amplify a partial sequence of FHL (A). Corresponding β-actin bands for these samples are shown in (B). M, marker; N, negative control.
Homologous genes in other species
We performed a BLASTP search with the full-length FHL protein against the genome sequence databases of four fish, and two mammals to investigate whether homologous genes were present in other animals. In the fugu genome database, a predicted protein (GENSCANSLICE00000025120), which was found on scaffold_755 at locations 1915–4673, showed the lowest e-value (1.4 × 1089), and 39.1% identity to FHL. Tetraodon and medaka genome databases also gave us two FHL-like transcripts, GIDT00014929001 (5.5 × 10110, 41.3% identity) and GENSCAN00000012567 (1.4 × 1093, 41.5% identity). It is notable that these genes coding FHL-like proteins possess stop codons corresponding to that of the FHL gene such that the predicted proteins also lack a serine protease domain, just like FHL. On the other hand, we could not verify the presence of an FHL-like sequence in the zebrafish genome database, because e-values of predicted proteins were not so low (>3.8 × 109). In the mouse genome database, there were two hit genes (ENSMUSG00000031645 and GENSCAN00000391967) with e-values of <1 × 1010, but the former encoded mouse coagulation factor XI and the latter appeared not to be FHL-like because of an excessively large number of coding amino acid residues (1948 residues). We found four FHL-like sequences (KLKB1_HUMAN, NM_000892.3, FA11_HUMAN and P03951-2) in the human genome database; KLKB1_HUMAN and NM_000892.3 were plasma kallikreins, whereas FA11_HUMAN and P03951-2 were coagulation factor XIs.
Human plasma kallikrein can act as a lectin
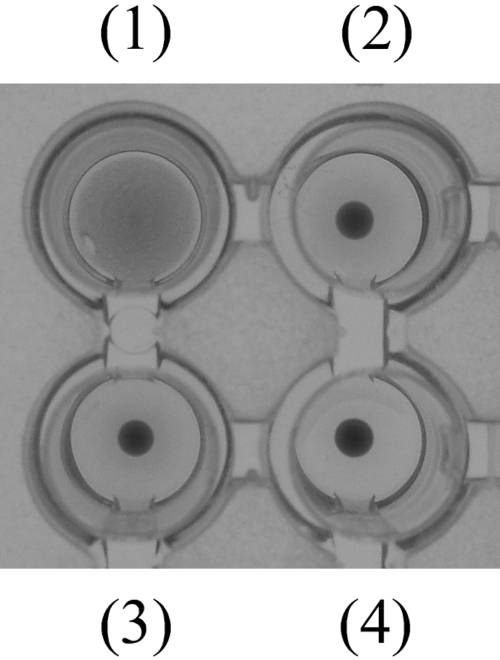
Significant similarity between plasma kallikrein and FHL prompted us to investigate the possibility that human plasma kallikrein might act as a lectin. Human plasma kallikrein exhibited hemagglutination activity (Figure 5). Fetuin having multiple types of sugars inhibited the agglutination (Figure 5), indicating that the hemagglutination was caused by the sugar-binding ability in human plasma kallikrein. However, the hemagglutination was not inhibited by mannose and lactose (data not shown).
Fig. 5.
Hemagglutination activity of human plasma kallikrein. Human plasma kallikrein was incubated with PBS (+) only (1) or the same buffer including 1 mg/mL of fetuin (2) at room temperature for 1 h. Then, 2% rabbit red blood cell suspension was added. PBS (+) only (3) and fetuin-containing PBS (+) (4) were also used for negative controls.
Discussion
This study provides two important advances. One is the discovery of a novel lectin from the mucus of fish skin. As already reported, various types of lectins, many of which are quite unique, have been identified from the body surface of fish (Muramoto and Kamiya 1992; Muramoto et al. 1999; Tasumi et al. 2002, 2004; Tsutsui et al. 2003, 2007, 2009; Okamoto et al. 2005). In the present study, we describe FHL, a novel molecule which provides additional knowledge concerning the diversity of lectins from the mucus of fish skin. The other advance is the discovery of a new functional role of mammalian plasma kallikrein, namely lectin activity. Its potential impact is highly significant since lectins relate to the recognition of pathogen-associated molecular patterns and our discovery may lead to further advancement of our present understanding of mammalian plasma kallikreins in self-defense mechanisms.
This paper reports the purification, characterization and sequencing of a unique protein from flathead skin with similar structure to plasma kallikrein and its homolog, coagulation factor XI. However, it was notable that the flathead protein completely lacked a serine protease domain including a cleavage site and catalytic triad residues (Figure 3). In addition, this protein has binding activity to mannose in a Ca2+-dependent manner. Since lectins are defined as carbohydrate–binding proteins that are neither antibodies against sugars nor enzymes whose substrates are saccharides (Sharon and Lis 1972), the flathead protein should be classified as a novel lectin but not an enzyme. Genome database searches revealed that FHL-like genes are present in medaka, Tetraodon and fugu. Additionally, we have already obtained an FHL-like sequence, which also lacks a serine protease domain, from a cDNA library derived from the skin of goosefish Lophiomus setigerus (Lophiiformes; accession number AB291615). These findings suggest that FHL-like proteins are widely distributed in fish species. Here, we propose to define a new family of animal lectins, named “kalliklectins”, into which FHL-like lectins should be classified in future. Furthermore, we revealed that human plasma kallikrein agglutinated rabbit red blood cells (Figure 5). This finding suggested that mammalian plasma kallikreins and coagulation factor XIs have carbohydrate-binding activities and act as lectins, in addition to their protease activities, in blood plasma.
Fetuin inhibited hemagglutination activity of human plasma kallikrein (Figure 5), but mannose and lactose did not. Substitution of amino acid residues in the carbohydrate recognition domain often results in alteration of specific sugar; for example, alteration of the Glu-Pro-Asn sequence to the Gln-Pro-Asp in a C-type lectin induces the change of recognized sugar from mannose to lactose (Drickamer 1992). Our data suggest that mammalian plasma kallikreins bind to other carbohydrate than mannose because of the substitution of an amino acid sequence.
RT–PCR clearly demonstrated that flathead FHL was widely produced in the skin, gill, esophagus and liver (Figure 4). This was in contrast to the case of mammalian plasma kallikrein and coagulation factor XI, which are predominantly synthesized in the liver as pre-kallikrein (Ciechanowicz et al. 1993). The expression of FHL in the liver may imply that FHL produced in the liver is secreted into the blood plasma in a similar manner as mammalian plasma kallikrein and coagulation factor XI.
Another type of kallikrein, called “tissue kallikrein”, is found in various tissues of many vertebrate species including teleosts (Richards et al. 1997). This type of kallikrein has been detected in the skin mucus of salmon Salmo salar, where it is believed to function as a self-defense factor since levels elevate following challenge with several bacterial pathogens (Haussmann et al. 2006). However, this molecule is clearly different from FHL. Tissue kallikrein contains a serine protease domain but lacks lectin domains. On the other hand, FHL, which lacks a protease domain, does not function in the kinin-generating system or the blood coagulation pathway. The skin of fish is constantly exposed to water-born pathogens. Thus, the fact that FHL exhibits binding activity to mannose may act as a pattern recognition protein in the self-defense system operating on the skin surface of flathead in a similar manner as other skin mucus lectins with agglutination activities against some bacteria, as well as opsonic activities (Kamiya and Shimizu 1980; Kamiya et al. 1988; Tasumi et al. 2002, 2004; Nakamura et al. 2006; Tsutsui et al. 2006, 2007). Functional studies are required to investigate why the flathead secrets a plasma kallikrein-like lectin into the skin mucus.
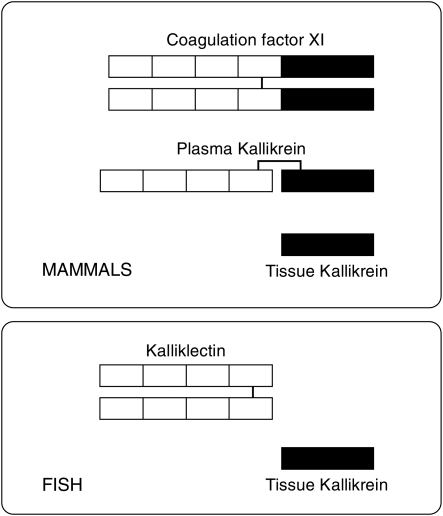
To our knowledge, plasma kallikrein and coagulation factor XI genes have yet to be identified in fish, although kallikrein-like sequences have been obtained from brook trout Salvelinus fontinalis (Hajnik et al. 1998) and medaka (accession no. AB242321). Jiang and Doolitle (2003) also reported that they could not identify genes homologous to plasma kallikrein or coagulation factor XI in the fugu genome database. These facts suggest that fish lack plasma kallikrein and coagulation factor XI. In contrast, we could not identify the kalliklectin-like sequences in either the human or the mouse genome databases, although these sequences were found in some fish genome databases. This finding may mean that mammals do not have this type of lectin. We also clearly demonstrated that human plasma kallikrein exhibits lectin activity (Figure 5). Because one human plasma kallikrein molecule contains a single heavy chain, our results may suggest that the heavy chain contains multiple carbohydrate recognition domains; four tandem-repeated apple domains are likely to possess the domains. The lectin activity of human plasma kallikrein may cause substrate specificity, since kininogen, the substrate of plasma kallikrein, is a glycoprotein. Structural relationships and the possible distribution of these molecules in fish and mammals are shown in Figure 6. Based on these findings, we propose the hypothesis that kalliklectin and tissue kallikrein in fish are ancient forms of mammalian plasma kallikreins and coagulation factor XI. Detailed genomic studies on a wide variety of different species from invertebrates to mammals are expected to reveal evolutionary relationships among kalliklectin, coagulation factor XI, tissue kallikrein and plasma kallikrein.
Fig. 6.
Structural relationship among plasma kallikrein, tissue kallikrein, coagulation factor XI and kalliklectin and their possible distribution. Black and white boxes represent the serine protease domain and the apple domain, respectively. Bar indicates S–S bond.
In conclusion, we propose the existence of a novel family of animal lectins, “kalliklectins”, homologous to the heavy-chain domain of mammalian plasma kallikreins and coagulation factor XI. Genes homologous to FHL were also found in the genome databases of some fish species, but not in mammals. We also discovered that human plasma kallikrein can function as a saccharide-binding protein. These results suggest that the origin of mammalian plasma kallikreins and coagulation factor XIs may be a protein similar to fish kalliklectin, and these molecules have acquired enzymic activities after separation from fish linage.
Materials and methods
Purification of mannose-binding protein
Skin mucus from flathead was homogenized with 2 volumes of PBS containing 0.9 mM CaCl2 and 0.33 mM MgCl2 (PBS (+)). The homogenate was then centrifuged and the supernatant loaded onto a column packed with mannose-agarose saline suspension (Sigma). The column was then washed with PBS (+), and the bound protein eluted with PBS (+) containing 50 mM mannose. We then performed a hemagglutination assay and an inhibition assay with sugars and EDTA using the eluted fraction, as described previously (Muramoto and Kamiya 1992).
Protein sequencing
Protein was purified by affinity chromatography and subjected to SDS–PAGE under reducing conditions. Protein bands were electrically transferred from the gel onto a polyvinylidene difluoride membrane (ATTO). After CBB staining, the N-terminal amino acid sequence of the protein was analyzed using a 476A Protein sequencer (Perkin Elmer Applied Biosystems). Enzymatic digestion was also carried out as described (Muramoto and Kamiya 1992) to determine the internal amino acid sequences of the protein. The digest was separated by HPLC (Hitachi) on a TSKgel ODS-120T reversed-phase column (Tosoh). Separated fragments were also subjected to protein sequencing.
cDNA cloning
Total RNA was extracted from the skin of flathead using RNA extraction solution (ISOGEN, Nippon gene). First-strand cDNA was synthesized from 1 µg of the total RNA with the SMART™ RACE cDNA amplification kit (Clontech) for 5′ and 3′ RACE. Each PCR described hereafter was carried out using an i-Cycler (Bio-Rad) in a total volume of 20 µL with Taq-DNA polymerase (Takara) and 500 nM of each forward and reverse primers.
At first, we performed 3′ RACE with a degenerate (primer-1; 5′-CARATHACICARTTYTTYAA-3′), corresponding to the amino acid QITQFFN in fragment L2, and Nested Universal Primer-A (NUP-A; Clontech) for 30 cycles at 94°C for 30 s, 50°C for 30 s and 72°C for 40 s. For 5′ RACE, a gene-specific primer (primer-2; 5′-ATTTCCTGGGATG TCAGTGTTGGG-3′) was synthesized on the basis of the nucleotide sequence obtained by 3′ RACE. This was then used in PCR with NUP-A. Thirty cycles of PCR amplification were carried out consisting of incubations at 94°C for 30 s, 58°C for 30 s and 72°C for 1 min.
The nucleotide sequence obtained by 3′ and 5′ RACE was confirmed by PCR amplification with two specific primers: primer-3 (5′-GGTCAAGAATGTAATCAACAGC-3′) and primer-4 (5′-TAACTTTGGGAGGAGCAGGCA-3′). Denaturation was performed at 94°C for 3 min, followed by 30 cycles at 94°C for 30 s, 58°C for 30 s and 72°C for 1 min.
Each of the PCR products generated was subcloned into the pCR®2.1-TOPO vector (Invitrogen). Plasmid DNA was then purified and sequenced with a 310 Genetic Analyzer (ABI).
Computer analyses
Homolog searches were performed with the BLASTX program (http://www.ddbj.nig.ac.jp/search/blast-j.html). Alignment of the full-length lectin amino acid sequences was carried out using Clustal W. The signal peptide in the deduced amino acid sequence was predicted by SignalP (version 3.0; http://www.cbs.dtu.dk/services/SignalP/).
Gene expression analysis by RT–PCR
The isolation of total RNA and the synthesis of cDNA from the skin, gill, esophagus, stomach, intestine, liver, heart, kidney, spleen, ovary and muscle were carried out as described earlier. For RT–PCR analysis, FHL-specific primers-3 and -4 were used with a protocol of 30 cycles at 94°C for 30 s, 58°C for 30 s and 72°C for 1 min, giving a product size of 999 bp. cDNA encoding for β-actin was amplified as a positive control. The PCR products were visualized by electrophoresis on a 1.8% agarose gel and staining with ethidium bromide.
Genome database research
Genome databases of fugu (version 4), Tetraodon (version 7), medaka (version 1) and zebrafish Danio rerio (version 6) were screened with the full-length amino acid sequence of FHL as a probe using the BLASTP platform in Ensemble Genome Browser (Sanger Institute, http://www.ensembl.org/). This was carried out in order to investigate whether genes coding for homologous proteins are present in other fish. We also performed BLASTP searches against the mouse Mus musculus and human Homo sapiens genome databases (Ensemble Genome Browser) to survey the flathead-like sequence in mammals. Predicted amino acid sequences whose e-values were <1 × 1010 were considered to have significant similarity and were subsequently aligned with the amino acid sequence of FHL using Clustal W to analyze identity.
Hemagglutination test for human plasma kallikrein
Lyophilized human plasma kallikrein (Acris) was dissolved in 20 mM Tris–HCl (pH 7.8) containing 100 mM NaCl, 20 mM CaCl2, 20 mM MgCl2 and 0.2% protease inhibitor cocktail (Sigma) at a final concentration of 500 µg/mL. To test the hemagglutination activity of human plasma kallikrein, 12.5 µL of the solution was incubated with either PBS (+) or the same buffer containing fetuin (1 mg/mL, from fetal calf serum, Sigma), 200 mM mannose and 200 mM lactose at room temperature for 1 h. Then, 25 µL of 2% rabbit erythrocyte suspension was added and hemagglutination activity assessed after 1 h.
Funding
This study was partly supported by Grant-in-aid for Creative Basic Research 12NP0201 from the Ministry of Education, Science, Sports and Culture of Japan.
Conflict of interest
None declared.
Abbreviations
EDTA, ethylenediaminetetraacetic acid; FHL, FlatHead Lectin; HPLC, high-performance liquid chromatography; NUP-A, Nested Universal Primer-A; PBS, phosphate-buffered saline; RACE, rapid amplification of cDNA ends; RT–PCR, reverse transcription–polymerase chain reaction; SDS–PAGE, sodium dodecyl sulfate–polyacrylamide gel electrophoresis.
Acknowledgment
We are grateful to Dr. Misako Nakaya, The University of Tokyo, for her kind assistance in the protein sequencing.
References
- Chung DW, Fujikawa K, McMullen BA, Davie EW. Human plasma prekallikrein, a zymogen to a serine protease that contains four tandem repeats. Biochemistry. 1986;25:2410–2417. doi: 10.1021/bi00357a017. doi:10.1021/bi00357a017. [DOI] [PubMed] [Google Scholar]
- Ciechanowicz A, Bader M, Wagner J, Ganten D. Extrahepatic transcription of plasma prekallikrein gene in human and rat tissues. Biochem Biophys Res Commun. 1993;197:1370–1376. doi: 10.1006/bbrc.1993.2628. doi:10.1006/bbrc.1993.2628. [DOI] [PubMed] [Google Scholar]
- Colman RW. Plasma prekallikrein and kallikrein. In: Barret AJ, Rawlings ND, Woessner JF, editors. The Handbook of Proteolytic Enzymes. 2nd ed. Oxford (UK): Elsevier Science; 2004. pp. 1644–1651. [Google Scholar]
- Drickamer T. Engineering galactose-binding activity into-a C-type mannose-binding protein. Nature. 1992;360:183–186. doi: 10.1038/360183a0. doi:10.1038/360183a0. [DOI] [PubMed] [Google Scholar]
- Fujikawa K, Chung DW, Hendrickson LE, Davie EW. Amino acid sequence of human factor XI, a blood coagulation factor with four tandem repeats that are highly homologous with plasma prekallikrein. Biochemistry. 1986;25:2417–2424. doi: 10.1021/bi00357a018. doi:10.1021/bi00357a018. [DOI] [PubMed] [Google Scholar]
- Hajnik CA, Goetz FW, Hsu SY, Sokal N. Characterization of a ribonucleic acid transcript from the brook trout (Salvelinus fontinalis) ovary with structural similarities to mammalian adipsin/complement factor D and tissue kallikrein, and the effects of kallikrein-like serine proteases on follicle contraction. Biol Reprod. 1998;58:887–897. doi: 10.1095/biolreprod58.4.887. doi:10.1095/biolreprod58.4.887. [DOI] [PubMed] [Google Scholar]
- Haussmann D, Vidal R, Figueroa J. Tissue expression of glandular kallikrein and its response to 17ß-estradiol in the acclimatized carp. Zoolog Sci. 2006;23:507–516. doi: 10.2108/zsj.23.507. doi:10.2108/zsj.23.507. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Doolitle RF. The evolution of vertebrate blood coagulation as viewed from a comparison of puffer fish and sea squirt genomes. Proc Natl Acad Sci USA. 2003;100:7527–7532. doi: 10.1073/pnas.0932632100. doi:10.1073/pnas.0932632100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya H, Muramoto K, Goto R. Purification and properties of agglutinins from conger eel, Conger myriaster. Dev Comp Immunol. 1988;12:309–318. doi: 10.1016/0145-305x(88)90007-9. doi:10.1016/0145-305X(88)90007-9. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Shimizu Y. Marine biopolymers with cell specificity II. Purification and characterization of agglutinins from mucous of windowpane flounder Lophopsetta maculata. Biochim Biophys Acta. 1980;622:171–178. doi: 10.1016/0005-2795(80)90028-8. [DOI] [PubMed] [Google Scholar]
- Muramoto K, Kagawa D, Sato T, Ogawa T, Nishida Y, Kamiya H. Functional and structural characterization of multiple galectins from the skin mucus of conger eel, Conger myriaster. Comp Biochem Physiol. 1999;123B:33–45. doi: 10.1016/s0305-0491(99)00037-1. [DOI] [PubMed] [Google Scholar]
- Muramoto K, Kamiya H. The amino-acid sequence of a lectin from conger eel, Conger myriaster, skin mucus. Biochim Biophys Acta. 1992;1116:129–136. doi: 10.1016/0304-4165(92)90109-8. [DOI] [PubMed] [Google Scholar]
- Nakamura O, Matsuoka H, Ogawa T, Muramoto K, Kamiya H, Watanabe T. Opsonic effect of congerin, a mucosal galectin of the Japanese conger, Conger myriaster (Brevoort) Fish Shellfish Immunol. 2006;20:433–435. doi: 10.1016/j.fsi.2005.06.004. doi:10.1016/j.fsi.2005.06.004. [DOI] [PubMed] [Google Scholar]
- Okamoto M, Tsutsui S, Tasumi S, Suetake S, Kikuchi K, Suzuki Y. Tandem repeat L-rhamnose-binding lectin from the skin mucus of ponyfish, Leiognathus nuchalis. Biochem Biophys Res Commun. 2005;333:463–469. doi: 10.1016/j.bbrc.2005.05.118. doi:10.1016/j.bbrc.2005.05.118. [DOI] [PubMed] [Google Scholar]
- Richards GP, Chao L, Chao J. Distribution of tissue kallikreins in lower vertebrates: Potential physiological roles for fish kallikreins. Comp Biochem Physiol. 1997;118C:49–58. doi: 10.1016/s0742-8413(97)00031-5. [DOI] [PubMed] [Google Scholar]
- Sharon N, Lis H. Lectins: Cell-agglutinating and sugar-specific proteins. Science. 1972;177:949–959. doi: 10.1126/science.177.4053.949. doi:10.1126/science.177.4053.949. [DOI] [PubMed] [Google Scholar]
- Tasumi S, Ohira T, Kawazoe I, Suetake H, Suzuki Y, Aida K. Primary structure and characteristics of a lectin from skin mucus of the Japanese eel Anguilla japonica. J Biol Chem. 2002;277:27305–27311. doi: 10.1074/jbc.M202648200. doi:10.1074/jbc.M202648200. [DOI] [PubMed] [Google Scholar]
- Tasumi S, Yang WJ, Usami T, Tsutsui S, Ohira T, Kawazoe I, Wilder MN, Aida K, Suzuki Y. Characteristics and primary structure of a galectin in the skin mucus of the Japanese eel, Anguilla japonica. Dev Comp Immunol. 2004;28:325–335. doi: 10.1016/j.dci.2003.08.006. doi:10.1016/j.dci.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Tsutsui S, Iwamoto K, Nakamura O, Watanabe T. Yeast-binding C-type lectin with opsonic activity from conger eel (Conger myriaster) skin mucus. Mol Immunol. 2007;44:691–702. doi: 10.1016/j.molimm.2006.04.023. doi:10.1016/j.molimm.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Tsutsui S, Nishikawa H, Mano N, Hirose H, Tasumi S, Suetake H, Suzuki Y. Possible role of a skin mucus lectin from fugu Takifugu rubripes in excluding marine bacteria from the body surface. Fish Sci. 2006;72:455–457. doi:10.1111/j.1444-2906.2006.01172.x. [Google Scholar]
- Tsutsui S, Tasumi S, Suetake H, Suzuki Y. Lectins homologous to those of monocotyledonous plants in the skin mucus and intestine of pufferfish, Fugu rubripes. J Biol Chem. 2003;278:20882–20889. doi: 10.1074/jbc.M301038200. doi:10.1074/jbc.M301038200. [DOI] [PubMed] [Google Scholar]
- Tsutsui S, Yamaguchi M, Hirasawa A, Nakamura O, Watanabe T. Common skate (Raja kenojei) secretes pentraxin into the cutaneous secretion: The first skin mucus lectin in cartilaginous fish. J Biochem. 2009;146:295–306. doi: 10.1093/jb/mvp069. doi:10.1093/jb/mvp069. [DOI] [PubMed] [Google Scholar]
- Werle E, Götze W, Keppler A. Über die wirkung des kallikreins auf den isolierten darm und über eine neue darmkontrahierende substanz. Biochem Z. 1937;289:217–233. [Google Scholar]
- Wuepper KD, Cochrane CG. Plasma prekallikrein: Isolation, characterization, and mechanism of activation. J Exp Med. 1972;135:1–20. doi: 10.1084/jem.135.1.1. doi:10.1084/jem.135.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]