Abstract
The effects of genetic background on fear trace conditioning were evaluated in relation to phosphorylated levels of cAMP response element-binding protein (CREB) in the hippocampus using two different inbred strains of mice, C57BL/6 and DBA/2. The male mice received a trace fear conditioning protocol and unpaired control groups were included to assess nonassociative effects on test performance. Both C57BL/6 and DBA/2 mice with paired training displayed higher freezing responses during testing than those with unpaired training, respectively. The C57BL/6 mice with paired training also displayed higher freezing responses to the tone-CS during testing than the DBA/2 mice with paired training. Because much evidence implicates the hippocampus as an important neural substrate for trace fear conditioning, the engagement of the hippocampus was examined after testing by measuring levels of CREB and phosphorylated CREB (pCREB). The results revealed that hippocampal CREB levels in both strains of mice were not significantly altered according to the type of training (unpaired vs. paired). However, the hippocampal pCREB levels were significantly higher in the paired training group than the unpaired control group in C57BL/6 mice, but not in DBA/2 mice. These findings indicate that hippocampal pCREB is closely tied to this form of associative conditioning only in C57BL/6 mice and that different neural substrates may support trace conditioning in C57BL/6 and DBA/2 strains.
Keywords: Trace fear conditioning, cAMP response element-binding, protein (CREB), Phosphorylated CREB (pCREB), Hippocampus Mice
1. Introduction
The transcription factor cyclic AMP response element-binding protein (CREB) is believed to play a critical role in the synaptic processes that underlie learning and memory that are supported by the hippocampus. Indeed, studies have shown that CREB expression is necessary for the hippocampal-dependent form of synaptic plasticity, long-term potentiation (LTP), believed to support long-term memory (Bourtchuladze et al., 1994; Wood et al., 2005), as well as behaviors that rely on the hippocampus. For example, CREB has been found to play a critical role in spatial/reference memory such as that assessed by the water maze (Guzowski and McGaugh, 1997; Wood et al., 2005) as well as in associative tasks that rely on the hippocampus such as contextual fear conditioning (Restivo et al., 2009; Wood et al., 2005).
Recently, we assessed CREB and phosphorylated CREB (pCREB) expression levels in two strains of mice following training in a water maze task (Sung et al., 2008). Specifically, mice were trained to use either a spatial/place strategy or a cued/response strategy. Our results revealed that CREB expression levels were increased in the hippocampus of both strains following spatial training, but not following cued training. However, when we examined pCREB levels in the hippocampus of both strains we found an increase only in the C57BL/6 mice that were given spatial training. These results suggested that these two strains of mice use the cAMP signaling pathway differently in hippocampal learning and memory. Thus, to further characterize this difference, in the present study we examined the CREB and pCREB levels in C57BL/6 and DBA/2 mice following performance in trace fear conditioning, a variant of Pavlovian fear conditioning that is hippocampal-dependent, in addition to the amygdala (Bangasser et al., 2006; Fanselow and Poulos, 2005; Kim et al., 1995).
Pavlovian fear conditioning is a form of associative learning in which an initially neutral stimulus (conditioned stimulus; CS), such as a tone, is paired with the presentation of an unconditioned stimulus (US) with aversive properties such as a footshock. As an expression of associative fear learning, freezing responses are elicited by the presentation of CS alone or by the environment/context cues where the pairing occurred after a number of pairings. There are several variants of fear conditioning in addition to trace, including a delay variant in which the discrete CS and US coterminate and which is dependent upon the amygdala (Fanselow and Poulos, 2005; Maren and Quirk, 2004), and a contextual variant in which the environment serves as the CS and which is dependent upon the hippocampus (Anagnostaras et al., 2001; Maren and Holt, 2000). In trace fear conditioning the CS and US are separated by a stimulus-free trace interval and this variant has been used in numerous studies with genetically modified and inbred mice (Ammassari-Teule et al., 2000; Balogh et al., 2002; Crestani et al., 2002; Huerta et al., 2000; Nguyen et al., 2000a; Nie and Abel, 2001; Owen et al., 1997; Paylor et al., 1994; Stanciu et al., 2001; Voikar et al., 2001). Fear conditioning is widely used because it is an ideal behavioral paradigm for studying associative learning in neurogenetics (Schneider et al., 1999; Sommer et al., 2006). While there are many protocols in the literature that have examined trace fear, for the current study we chose to use the training protocol for trace fear conditioning recently developed by Smith et al. (2007) that incorporated controls for nonassociative effects in mice.
The present experiment examined trace fear conditioning in C57BL/6 and DBA/2 mice, inbred strains commonly used to construct transgenic mouse models, in order to elucidate critical molecular mechanisms for learning and memory function. To date, a number of studies have reported that C57BL/6 and DBA/2 mice can differ in their performances on tasks that are dependent on hippocampal integrity, including a reference memory version of a maze task and context fear conditioning. For example, C57BL/6 mice performed better than DBA/2 mice in locating a stationary hidden platform in the water maze (Paylor et al., 1993). Furthermore, it has also been reported that when given a choice between a spatial/place strategy and a cued/response strategy in either a water maze or a plus maze, C57BL/6 mice preferred a spatial/place strategy whereas DBA/2 mice preferred a cued/response strategy (McDonald and White, 1994; Passino et al., 2002; Sung et al., 2008). Additionally, in the hippocampal-dependent contextual variant of fear conditioning C57BL/6 mice had higher freezing levels in response to the context than DBA/2 mice (Nguyen et al., 2000a; Owen et al., 1997). Finally, the behavioral differences in the performance of hippocampal-dependent tasks between the C57BL/6 and DBA/2 strains are in accordance with reports of differences in hippocampal synaptic plasticity. For instance, LTP persists to a greater extent in C57BL/6 mice than in DBA/2 mice (Matsuyama et al., 1997; Nguyen et al., 2000a,b).
As illustrated above, many studies have examined the difference between C57BL/6 and DBA/2 mice on many hippocampal-dependent tasks; however, none have examined the two strains in trace fear conditioning. Therefore, in the current study the involvement of the hippocampus was investigated in both C57BL/6 and DBA/2 mice by measuring the CREB and pCREB levels following training in the trace fear conditioning protocol.
2. Results
2.1. Behavioral data
The experimental protocol and parameters employed in the present study were developed to minimize nonassociative effects in Pavlovian fear conditioning in mice (Smith et al., 2007). We employed a slightly modified version of this protocol that involved three days of behavioral experimentation. A total of 48 mice (24 from each strain) were assigned to either a paired or an unpaired group. On day 1, the mice from the unpaired control group received six tone-alone (80 dB, 20-s duration) presentations while the mice of paired groups were exposed to the experimental environment in the corresponding period. On day 2, the paired group received six trials in the training session, each consisting of a tone-CS (80 dB, 20-s duration) and a shock US (0.5 mA, 2-s duration) with those stimuli separated by a trace interval of 18 s. The unpaired groups received six US-alone presentations at the point that US was given to the paired groups. Assessment of trace conditioning, as indexed by freezing in the two strains, was carried out on day 3 in a novel context when the CS alone was presented. A schematic of the protocol is depicted in Fig. 1.
Fig. 1.
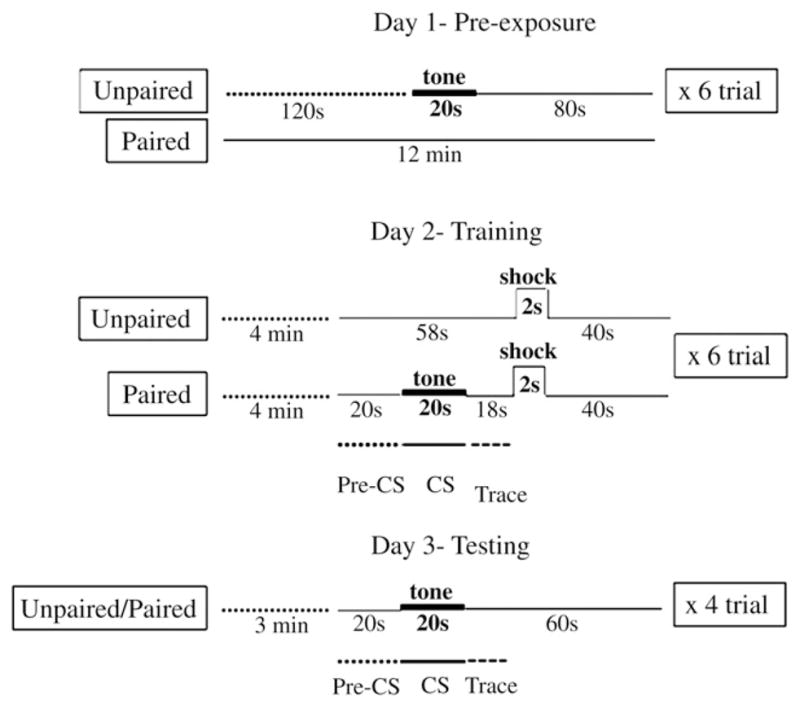
Schematic representation of the protocol for trace fear conditioning. The third inset in day 2 (Training) and day 3 (Testing) shows the time interval used for the analysis of freezing levels. The testing in day 3 was conducted in a novel test chamber. Note that the times represented by the dashed lines are not repeated.
The freezing rates across six trials during day 2 were analyzed in a two-trial block to confirm the increasing freezing rates in the course of training. To characterize changes of freezing levels throughout the entire trial, the trial period was divided into three epochs: 1) (Pre-CS) period prior to tone-CS presentation (20-s interval); 2) (CS) period of tone-CS presentation (40-s interval); 3) (Trace) encompassing the empty trace interval until the shock occurred during training (60-s interval). The freezing rates from the unpaired group were analyzed with the corresponding periods of the paired group based on the US presentation point. Table 1 shows the mean percentage of freezing exhibited by training and strain. No mice in any groups showed freezing behaviors during Pre-CS in the first trial. Statistical analysis using a mixed four-way factorial ANOVA revealed a significant main effect of Strain (C57BL/6 vs. DBA/2) (F(1,44) =19.33, p <0.001), Group (paired vs. unpaired) (F(1,44) =6.04, p=0.02), Trial Epoch (F(2,88) =123.67, p<0.001), and Trial Block (F(2,88) =13.13, p <0.001) as well as a significant interaction between Trial Epoch × Strain (F(2,88) = 19.77, p<0.001), Trial Block × Strain (F(2,88) =3.31, p =0.041), Trial Epoch × Group (F(2,88) =23.14, p <0.001), the Trial Block × Trial Epoch (F(4,176) =8.11, p <0.001), and Trial Block × Trial Epoch × Group (F(4,176) =9.37, p <0.001). There were no other two-way or three-way interaction effects. Most importantly, no such interactions involving the Group × Strain were found. Statistical analysis also revealed that both C57BL/6 mice and DBA/2 mice showed evidence of associative learning as indicated by a greater response when the paired groups were compared to the unpaired groups in each corresponding strain. However, in both paired and unpaired groups, the overall freezing levels of DBA/2 mice were lower than those of C57BL/6 mice. Additionally, the overall level of freezing in both strains of mice increased over the course of training independent of any specific epoch.
Table 1.
Mean (± SEM) freezing rates (training) during the three intervals of the trial epoch for paired and unpaired groups of mice during day 2. Unit: %.
| Group | Strain | Block | Pre-CS | CS | Trace |
|---|---|---|---|---|---|
| Paired | C57BL/6 | 1 | 1.67±0.71 | 7.50±2.09 | 11.25±2.23 |
| 2 | 37.50±5.79 | 35.42±4.54 | 54.58±5.95 | ||
| 3 | 70.83±3.63 | 38.75±4.23 | 71.67±3.66 | ||
| DBA/2 | 1 | 8.75±4.40 | 3.75±2.05 | 7.50±3.92 | |
| 2 | 25.00±8.66 | 12.08±3.82 | 24.58±9.08 | ||
| 3 | 37.92±8.91 | 13.75±3.80 | 44.58±8.80 | ||
| Unpaired | C57BL/6 | 1 | 0.00±0.00 | 0.83±0.56 | 0.00±0.00 |
| 2 | 21.67±5.05 | 29.17±6.15 | 29.17±3.53 | ||
| 3 | 52.08±7.27 | 54.58±7.16 | 50.42±5.95 | ||
| DBA/2 | 1 | 5.00±2.68 | 1.67±1.28 | 0.42±0.42 | |
| 2 | 16.25±4.93 | 16.25±6.28 | 10.42±4.67 | ||
| 3 | 25.00±6.37 | 22.92±4.94 | 19.17±6.45 |
During day 2, a paired group received six CS-US trials, while an unpaired group received US only (received CS day 1). Freezing rates across six trials were presented in a two-trial block. Trial periods: (Pre-CS) period prior to tone-CS presentation (20-s interval); (CS) period of tone-CS presentation (40-s interval); (Trace) encompasses the empty trace interval until the shock occurred during training (60-s interval). Freezing rates in the unpaired group were analyzed in the corresponding periods of the paired group on the basis of the US presentation point. See the results for statistical significance.
Test of trace conditioning, as indexed by freezing to the tone-CS in the two strains, was carried out on day 3 in a novel context and is presented in Table 2. Table 2 shows the mean freezing rates during the three intervals (epoch) of the trial, averaged across four tone-CS trials for paired and unpaired groups. As shown in Table 2, both C57BL/6 and DBA/2 showed evidence of associative learning as indicated by the greater freezing responses in the paired groups relative to the unpaired groups for each strain. However, DBA/2 mice in the unpaired group showed more moderate freezing levels during the entire epoch when compared to C57BL/6. These findings were confirmed by a mixed three-way factorial ANOVA, which revealed significant main effects of Strain (C57BL/6 vs. DBA/2: F(1,44) =58.61, p<0.001), Group (paired vs. unpaired: F(1,44) = 12.21, p=0.001), and Trial Epoch (F(2,88) =63.59, p<0.001), as well as a significant interaction between Trial Epoch × Strain (F(2,88) =7.12, p =0.001) and Trial Epoch × Group (F(2,88) =6.59, p=0.002). There were no other two-way or three-way interaction effects. Most importantly, no such interactions were found involving the Group × Strain. The post hoc test was conducted on interaction between the Trial Epoch × Strain and the Trial Epoch. The freezing rate during the trace epoch in C57BL/6 was significantly higher than during the other epochs in C57BL/6 and all epochs in DBA/2. Similarly, the freezing rate during the trace epoch in paired group was significantly higher than the other epochs in the paired and all epochs in the unpaired group. Moreover, the freezing rate was measured on day 3 (testing) 3 min before presentation of the first CS in novel context. The freezing rate of C57BL/6 (unpaired: 19.54±4.01; paired: 22.13±5.78) was significantly higher than that of DBA/2 (unpaired: 2.68±0.93; paired: 2.96±2.46), regardless of training type (unpaired vs. paired).
Table 2.
Mean (± SEM) freezing rates during the three intervals of the trial epoch, averaged across four tone-CS trials for paired and unpaired groups of mice during day 3 Unit: %.
| Group | Strain | Pre-CS | CS | Trace |
|---|---|---|---|---|
| Paired | C57BL/6 | 37.29±8.66 | 43.54±4.43 | 84.17±3.67 |
| DBA/2 | 15.63±5.40 | 11.67±5.69 | 36.25±6.68 | |
| Unpaired | C57BL/6 | 34.17±6.54 | 26.46±3.35 | 54.17±4.13 |
| DBA/2 | 5.83±2.37 | 5.21±1.55 | 16.67±4.07 |
During day 3, both the paired group and the unpaired group received four tone-CS trials. Trial periods: (Pre-CS) period prior to tone-CS presentation (20-s interval); (CS) period of tone-CS presentation (40-s interval); (Trace) encompasses the empty trace interval until the shock occurred during training (60-s interval).
2.2. CREB and pCREB levels after testing
All mice were used to evaluate the hippocampal CREB and pCREB levels 30 min following testing on day 3. Fig. 2 shows the representative immunoblots of the hippocampal CREB and pCREB. The relationship between the training conditions and levels of CREB and pCREB was analyzed using two-factor ANOVAs with CREB or pCREB levels as dependent variables. The independent variables were Training Group (paired vs. unpaired) and Strain (C57BL/6 vs. DBA/2).
Fig. 2.
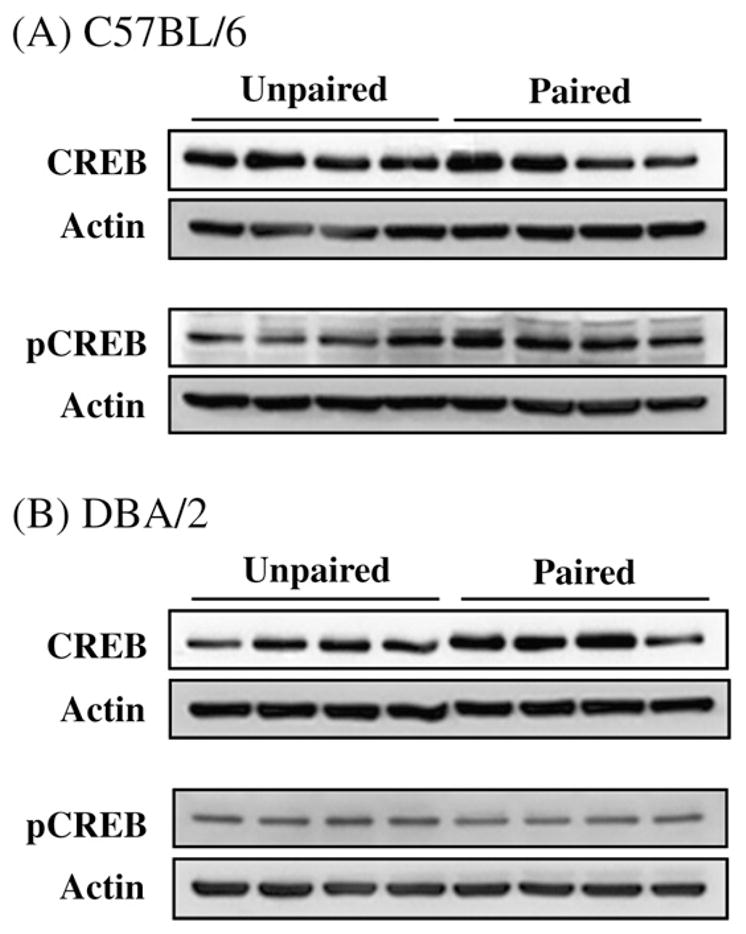
Representative immunoblots of hippocampal CREB and pCREB from C57BL/6 (A) and DBA/2 (B) mice 30 min after the last tone-CS presentation on day 3.
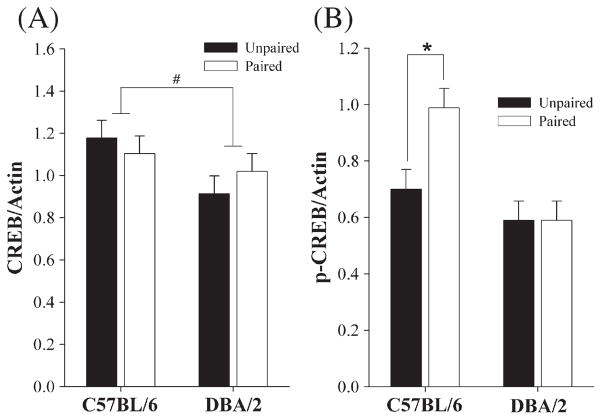
Hippocampal CREB levels in the paired group were not different from those in the unpaired group in both strains, but the overall hippocampal CREB levels of the C57BL/6 mice were significantly higher than those of the DBA/2 mice (F(1,44) =4.33, p =0.04; Fig. 3A). On the contrary, the hippocampal pCREB levels were significantly influenced by Training Group (F(1,44) =4.37, p =0.04) but in a strain-dependent manner (Fig. 3B). Both a significant effect of Strain (F(1,44) =13.72, p=0.001) and a significant Training Group × Strain interaction (F(1,44) =4.35, p =0.04) were evidently significant in the pCREB analysis. Subsequent post hoc comparisons revealed that levels of hippocampal pCREB in C57BL/6 mice with paired training were significantly higher in comparison to those of the unpaired group of mice in the same strain, and to those of the DBA/2 mice regardless of the training group (p<0.05, Fig. 3B).
Fig. 3.
Quantification of hippocampal CREB and pCREB levels (mean±S.E.M) from C57BL/6 and DBA/2 mice 30 min after the last tone-CS presentation on day 3. Data are expressed as the ratio of CREB/actin (A) and pCREB/actin (B) in the hippocampus. The overall hippocampal CREB levels of the C57BL/6 mice were statistically higher than those of the DBA/2 mice (#, A). (*) indicates significantly greater hippocampal pCREB following tone-CS presentationin comparison with the unpaired training in C57BL/6 mouse strains (B).
3. Discussion
Previously we reported that the levels of CREB were increased in C57BL/6 and DBA/2 mice following spatial/place training in the water maze compared to cued/response training, but that pCREB was increased only in the C57BL/6 mice that received place/spatial training (Sung et al., 2008). Those findings suggested a difference between the two strains that may contribute to behavioral outcomes in hippocampal-dependent learning. Thus, the aim of the current study was to further characterize the levels of CREB and pCREB in these two strains of mice following training in another hippocampal-dependent task. Our results show that 1) overall, C57BL/6 mice greater freezing to the tone-CS than the DBA/2 mice, 2) significant associative learning, however, was evident in both strains as a comparison of paired and unpaired performance, 3) following trace fear testing hippocampal CREB levels were greater in C57BL/6 mice than DBA/2 mice, and 4) hippocampal pCREB levels were higher only in the paired group of C57BL/6 mice. These results suggest that in C57BL/6 mice pCREB levels serve as an index of hippocampal involvement in associative learning in a trace paradigm. However, DBA/2 mice exhibit significant trace learning without a corresponding increase in hippocampal pCREB levels.
3.1. Significant differences of conditioned responses to the tone-CS between C57BL/6 mice and DBA/2 mice in the trace fear conditioning
Overall, we found evident differences between the strains and training groups during trace interval on day 3 such that the C57BL/6 mice exhibited greater freezing than the DBA/2 mice. Without the inclusion of unpaired control mice, the data for the paired groups might have suggested better learning in the C57BL/6 strain. However, both strains acquired significant conditioning by comparison of paired and unpaired groups within each strain and there was no significant Group × Strain interaction. The considerable levels of freezing in the unpaired C57BL/6 mice would appear inconsistent with the finding of Smith et al. (2007) who reported much lower levels of freezing in unpaired C57BL/6 mice in a similar trace conditioning protocol. Our protocol was based on that developed by Smith et al. (2007) in which three strains (C57BL/6, 129, and a hybrid strain (F1) of the two) were trained and tested, and which included unpaired controls for each strain to serve as a baseline for nonassociative effects. It should be noted, however, that the protocol in the current study was modified slightly to include an 80 dB tone-CS rather than a 70 dB tone-CS (used by Smith et al., 2007), and that this difference may account for the somewhat higher levels of freezing during tone-CS testing in the unpaired group of C57BL/6 mice in the current study (33%) relative to those reported by Smith et al. (2007) (15%). Nonetheless, substantial associative learning was still evident in that strain by comparison of the paired and unpaired groups in the current study.
The low levels of freezing we observed in the DBA/2 mice agree with other reports. Although several studies have compared the performances of various strains of mice on fear conditioning including C57BL/6 and DBA/2 mice (Balogh et al., 2002; Balogh and Wehner, 2003; Paylor et al., 1994), few have examined trace fear conditioning. To date only one study has compared C57BL/6 and DBA/2 strains using trace fear conditioning. Holmes et al. (2002) trained males and females of three strains (C57BL/6, DBA/2, and 129S) and reported poor performance of the DBA/2 (<20%) mice compared to performance of the C57BL/6 (>50%) mice. Our behavioral results, indicating relatively low levels of freezing in tests, are consistent with the findings of Holmes and colleagues. Most importantly, however, the current study expanded on that of Holmes et al. (2002) because we also included unpaired controls. To our knowledge, our study is the first study of strain differences comparing DBA/2 and C57BL/6 mice in trace fear conditioning using an appropriate unpaired control group. The behavioral results clearly demonstrate significant learning in each strain.
The major reason for the poor conditioning of DBA/2 in trace fear conditioning when compared to C57BL/6 might be dysfunction of the hippocampus of DBA/2 (Paylor et al., 1993). However, the poor conditioning of DBA/2 might have been due to other strain differences. For example, the encoding function of the CS tone might have been weakened due to poor hearing in DBA/2 mice (Turner et al., 2005). In addition, the reversal learning in water maze task, which require prefrontal cortex is performed poorly by DBA/2 when compared to C57BL/6; therefore, execution of working memory might be limited in DBA/2 (Voikar et al., 2005). Moreover, these two strains also differ in their emotional characteristics, with DBA/2 mice being more exploratory and less anxious in the open field and the elevated plus maze than C57BL/6 mice (Trullas and Skolnick, 1993). In particular, the freezing levels of C57BL/6 and DBA/2 mice in trace fear conditioning might reflect strain differences in emotionality.
Podhorna and Brown (2002) conducted a series of tests including complex learning tasks and emotional tasks to determine if stain differences in emotionality could account for strain differences in learning and memory performance. They reported that the anxiety levels were significantly correlated with all measures of learning and memory in the object recognition task, passive avoidance task, and water maze task, but, through a sophisticated statistical analysis such as analysis of covariance, they concluded that strain differences in activity and anxiety did not account for stain differences in learning and memory by C57BL/6 and DBA/2 (Podhorna and Brown, 2002). Nevertheless, it is still valuable to consider possible effects of anxiety levels in mice on the performances of trace fear conditioning.
3.2. Hippocampal pCREB levels in C57BL/6 mice with paired training were significantly greater than the C57BL/6 mice with an unpaired control and DBA/2 mice with either paired or unpaired control
In the present study, hippocampal CREB and pCREB levels in C57BL/6 and DBA/2 mice were assessed 30 min after the completion of a trace tone-CS test session in a novel context chamber. Our results show that compared to the unpaired control group, hippocampal CREB levels of the paired group of mice did not change in either C57BL/6 or DBA/2 mice as a function of training condition, however, the C57BL/6 mice (paired and unpaired) had a higher overall level of expression relative to DBA/2 mice (paired and unpaired). In our report using the water maze task, C57BL/6 and DBA/2 mice given hippocampal-dependent spatial learning in the water maze had similarly increased levels of hippocampal CREB compared to mice given cued/response training (Sung et al., 2008). However, because the unpaired condition in trace fear conditioning as a comparison control is not comparable to the cued/response training in the water maze task, these discrepancies between studies may be accounted for by differences in comparison controls in the two hippocampal-dependent tasks.
In contrast to CREB levels of pCREB (activated CREB) were only significantly higher in the paired group of C57BL/6 mice compared to the unpaired C57BL/6 and both groups of DBA/2 mice. These results are consistent with our previous findings of increased pCREB expression levels after hippocampal-dependent learning in C57BL/6 mice but not in DBA/2 (Sung et al., 2008). Taken together, these finding strongly suggest that C57BL/6 mice use the cAMP signaling pathway in hippocampal-dependent learning and memory, while the DBA/2 mice do not. At the same time, our results show that the paired DBA/2 mice showed higher freezing responses to trace-CS conditioning than the corresponding unpaired group reflecting learning in that strain. Thus, it is possible that this conditioning in the DBA/2 mice reflects the engagement of other neural structures aside from the hippocampus, or that other signaling pathways independent of the cAMP signaling pathway in the hippocampus might be used. For example, recent research has provided evidence of the involvement of prefrontal cortex in trace conditioning paradigms (Quirk et al., 2006). Thus some compensatory process could subserve trace learning in the DBA/2 strain.
3.3. Summary and conclusions
In summary, we have shown that both C57BL/6 and DBA/2 mice with paired training displayed higher freezing responses to the tone-CS during testing than those with corresponding unpaired training. We also found that hippocampal pCREB levels were significantly higher in the paired training group than in the unpaired control group in C57BL/6 mice, not in DBA/2 mice.
Many studies have used transgenic and knockout mice to uncover key molecular mechanisms of learning and memory (Picciotto and Wickman, 1998; Wehner et al., 2001). The current data, consistent with earlier findings, indicate that the C57BL/6 mouse strain may be the superior background strain for the genetic analysis of molecular mechanisms underlying learning and memory in the hippocampal system. Because DBA/2 mice present a behavioral profile that exhibits learning coupled with a deficiency in a major plasticity pathway in the hippocampus, this strain may provide a particularly suitable model for experimental analysis with the goal of enhancing hippocampal-dependent learning and memory. Those mice may also be informative as to alternative pathways/circuits that can contribute to associative learning processes.
4. Experimental procedures
4.1. Subjects
Twenty-four male C57BL/6 and twenty-four male DBA/2 mice (SPF) obtained from Charles River Co. (Gapeung, South Korea) were 3 months at the beginning of the experiments. Mice were housed in groups of 4 to a cage, in a temperature and humidity-controlled room, with a 12 h light/dark cycle (lights on, 07:00–19:00 h). Food and water were available ad libitum. All testing was conducted during the light cycle. Experiments were conducted in compliance with the Konkuk University’s Council Directive for the use and care of laboratory animals.
4.2. Apparatus
The trace fear conditioning task was carried out in square (17.78 cm W×17.78 cm D×30.48 cm H, Coulbourn) and octagonal (rad 21.59 cm, 30.48 cm H, Coulbourn) chambers. During training each chamber was equipped with a grid floor through which a footshock could be delivered. During testing, the grid floors of octagonal chambers were replaced with a solid, black-colored wooden floor coated with a clear sealant. The octagonal chambers were altered such that the Plexiglas walls were interspersed with opaque black and white tiles to form a checkered pattern along four of the eight angles. Furthermore, the octagonal chambers used as the testing box were scented with pine smell. The walls of the chambers were made of clear Plexiglas. All of the chambers were mounted within specially designed sound-attenuating shells constructed of polypropylene and PVC. Each shell was equipped with an exhaust fan (which also served as a background noise generator (68 dB), a speaker mounted on the back wall through which a tone could be delivered, and a red ambient (8 W) overhead house light. The tone (80 dB) and shock were created via a peripheral Coulbourn programmable tone generator (model #A69-20) and Coulbourn programmable precision regulated animal shocker (model #H13-16), and all stimuli onset and duration were controlled by a PC interfaced with Coulbourn Graphic State software. All chambers were cleaned with 70% ethanol before and after each use.
4.3. Procedure
Procedures were same as those described by Smith et al. (2007), with the exception that the tone-CS in the current study was increased to 80 dB. Procedures for the protocol involved three phases: (1) pre-exposure, which allowed the mice to acclimate and become familiar with the training chamber. (2) training, during which the mice were presented with the CS and or US stimuli, and (3) testing, in which mice were observed for freezing in response to the tone and trace-CS. Each phase occurred at 24-h intervals.
4.3.1. Pre-exposure
On day 1, all mice were individually placed into a training chamber for 12 min. During pre-exposure (day 1) the acclimation period was 2 min before tone presentation began for the mice assigned to the unpaired groups. For those mice, presentation of the 20-s tone cue was followed by an 80-s interval, and was repeated a total of six times. For the mice assigned to the paired groups, the acclimation period was 12 min, corresponding to the duration of the pre-exposure session for the unpaired groups (see Fig. 1).
4.3.2. Training
On the training day (day 2) mice were placed into the same training chamber as on day 1, and allowed to acclimate for 4 min, six conditioning trials involving a 80-dB, 2000 Hz, tone-CS and a 2-s, 0.5 mA shock US were run in trial epochs that lasted 100 s (including the intertrial interval). For the paired groups of mice, each trial consisted of a 20-s “baseline” interval, a 20-s tone presentation, an 18-s trace interval, a 2-s shock, and a 40-s postshock interval. Trials were the same for the unpaired groups of mice except that the tone was omitted (i.e., a 58-s interval, a 2-s shock, and 40-s postshock interval) (see Fig. 1).
4.3.3. Testing
On the testing day (day 3), mice were placed in a novel octagonal shaped and scented test chamber and allowed to move about freely for 3 min. All mice received four 100-s testing trials. For all mice, each trial began with a 20-s interval, followed a 20-s 80 dB tone presentation and then was by a 60-s interval.
4.3.4. Scoring
All scoring was done at the conclusion of testing from video by a trained observer blind to experimental conditions. During this time the amount of freezing was observed in 1-s increments throughout each context test exposure and each 60-s trial epoch (20-s baseline, 20-s tone, 20-s trace). Scoring was conducted throughout the entire trial epoch and intertrial interval. To assess the objectivity, the data from several randomly selected sessions in each phase of the experiment were scored by Y.K.H and J.-C.S. The two observers agreed on 87% of over 9000 observations.
4.4. CREB and pCREB measurements
Thirty minutes after the last testing trial on the third day, all mice were sacrificed. The hippocampi and prefrontal cortex were then rapidly dissected and frozen at −80 °C until further processing.
4.5. Western blot analysis
Proteins for the analysis of CREB and pCREB were extracted in the following manner. Individual tissue samples were weighed and then homogenized in 5 vol of ice-cold buffer containing 20 mM Tris, pH 7.5, 5% glycerol, 1.5 mM EDTA, 40 mM KCl, 0.5 mM dithiothreitol, and protease inhibitors (No. 539131, Calbiochem). Homogenates were centrifuged at 20,800×g for 30 min at 4 °C. The supernatant was removed from each sample, and an aliquot was taken for determination of total protein concentration using Bradford Reagent. The proteins were then separated by SDS-polyacrylamide gel electrophoresis and transferred to a PVDF membrane. The membrane was incubated with a primary antibody (Ab) against CREB (1:1000, Cell Signaling) and pCREB, phosphorylated on serine-133 (1:1000, Upstate). Following primary incubation, blots were incubated with the HRP-conjugated secondary Ab (1:2500, Amersham Biosciences). Blots were visualized using an ECL system, and developed using Hyper-film (Amersham). The relative levels of CREB and pCREB were determined by densitometry and normalization to β-actin (1:5000, Sigma), an invariant cytoskeletal protein.
4.6. Data analysis
Percent freezing during training was analyzed using repeated measures with a mixed three or four factorial analysis of variance (Strain (C57BL/6 vs. DBA/2) × Training Group (paired vs. unpaired) × Trial Epoch (Pre-CS, CS, trace) × Block (two-trial block)) to evaluate acquisition in trace fear training. For analysis of performance during the testing, freezing percents were analyzed using a repeated measures three factor analysis of variance (Strain (C57BL/6 vs. DBA/2) × Training Group (paired vs. unpaired) × Trial Epoch (Pre-CS, CS, Trace). Two-factor ANOVA was conducted with levels of CREB and pCREB as dependent variables. Independent variables were Training Group (paired vs. unpaired) and Strain (C57BL/6 vs. DBA/2), followed by Bonferroni’s post hoc comparisons, if necessary. A p-value less than 0.05 was considered significant.
Acknowledgments
This work was supported by research grant from Institute of Biomedical Science and Technology, Konkuk University (2007) to Seol-Heui Han and the Korea Research Foundation Grant funded by the Korean Government (MOEHRD), Basic Research Promotion Fund (KRF-2009-0075581) to Jung-Soo Han, and NCRR grant 1PO40 RR017688 to Michela Gallagher.
References
- Ammassari-Teule M, Passino E, Restivo L, de Marsanich B. Fear conditioning in C57/BL/6 and DBA/2 mice: variability in nucleus accumbens function according to the strain predisposition to show contextual- or cue-based responding. Eur J Neurosci. 2000;12:4467–4474. [PubMed] [Google Scholar]
- Anagnostaras SG, Gale GD, Fanselow MS. Hippocampus and contextual fear conditioning: recent controversies and advances. Hippocampus. 2001;11:8–17. doi: 10.1002/1098-1063(2001)11:1<8::AID-HIPO1015>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Balogh SA, Wehner JM. Inbred mouse strain differences in the establishment of long-term fear memory. Behav Brain Res. 2003;140:97–106. doi: 10.1016/s0166-4328(02)00279-6. [DOI] [PubMed] [Google Scholar]
- Balogh SA, Radcliffe RA, Logue SF, Wehner JM. Contextual and cued fear conditioning in C57BL/6J and DBA/2J mice: context discrimination and the effects of retention interval. Behav Neurosci. 2002;116:947–957. doi: 10.1037//0735-7044.116.6.947. [DOI] [PubMed] [Google Scholar]
- Bangasser DA, Waxler DE, Santollo J, Shors TJ. Trace conditioning and the hippocampus: the importance of contiguity. J Neurosci. 2006;26:8702–8706. doi: 10.1523/JNEUROSCI.1742-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- Crestani F, Keist R, Fritschy JM, Benke D, Vogt K, Prut L, Bluthmann H, Mohler H, Rudolph U. Trace fear conditioning involves hippocampal alpha5 GABA(A) receptors. Proc Natl Acad Sci USA. 2002;99:8980–8985. doi: 10.1073/pnas.142288699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanselow MS, Poulos AM. The neuroscience of mammalian associative learning. Annu Rev Psychol. 2005;56:207–234. doi: 10.1146/annurev.psych.56.091103.070213. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci USA. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Wrenn CC, Harris AP, Thayer KE, Crawley JN. Behavioral profiles of inbred strains on novel olfactory, spatial and emotional tests for reference memory in mice. Genes Brain Behav. 2002;1:55–69. doi: 10.1046/j.1601-1848.2001.00005.x. [DOI] [PubMed] [Google Scholar]
- Huerta PT, Sun LD, Wilson MA, Tonegawa S. Formation of temporal memory requires NMDA receptors within CA1 pyramidal neurons. Neuron. 2000;25:473–480. doi: 10.1016/s0896-6273(00)80909-5. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Maren S, Holt W. The hippocampus and contextual memory retrieval in Pavlovian conditioning. Behav Brain Res. 2000;110:97–108. doi: 10.1016/s0166-4328(99)00188-6. [DOI] [PubMed] [Google Scholar]
- Maren S, Quirk GJ. Neuronal signalling of fear memory. Nat Rev Neurosci. 2004;5:844–852. doi: 10.1038/nrn1535. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Namgung U, Routtenberg A. Long-term potentiation persistence greater in C57BL/6 than DBA/2 mice: predicted on basis of protein kinase C levels and learning performance. Brain Res. 1997;763:127–130. doi: 10.1016/s0006-8993(97)00444-7. [DOI] [PubMed] [Google Scholar]
- McDonald RJ, White NM. Parallel information processing in the water maze: evidence for independent memory systems involving dorsal striatum and hippocampus. Behav Neural Biol. 1994;61:260–270. doi: 10.1016/s0163-1047(05)80009-3. [DOI] [PubMed] [Google Scholar]
- Nguyen PV, Abel T, Kandel ER, Bourtchouladze R. Strain-dependent differences in LTP and hippocampus-dependent memory in inbred mice. Learn Mem. 2000a;7:170–179. doi: 10.1101/lm.7.3.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Duffy SN, Young JZ. Differential maintenance and frequency-dependent tuning of LTP at hippocampal synapses of specific strains of inbred mice. J Neurophysiol. 2000b;84:2484–2493. doi: 10.1152/jn.2000.84.5.2484. [DOI] [PubMed] [Google Scholar]
- Nie T, Abel T. Fear conditioning in inbred mouse strains: an analysis of the time course of memory. Behav Neurosci. 2001;115:951–956. doi: 10.1037//0735-7044.115.4.951. [DOI] [PubMed] [Google Scholar]
- Owen EH, Logue SF, Rasmussen DL, Wehner JM. Assessment of learning by the Morris water task and fear conditioning in inbred mouse strains and F1 hybrids: implications of genetic background for single gene mutations and quantitative trait loci analyses. Neuroscience. 1997;80:1087–1099. doi: 10.1016/s0306-4522(97)00165-6. [DOI] [PubMed] [Google Scholar]
- Passino E, Middei S, Restivo L, Bertaina-Anglade V, Ammassari-Teule M. Genetic approach to variability of memory systems: analysis of place vs. response learning and fos-related expression in hippocampal and striatal areas of C57BL/6 and DBA/2 mice. Hippocampus. 2002;12:63–75. doi: 10.1002/hipo.10007. [DOI] [PubMed] [Google Scholar]
- Paylor R, Baskall L, Wehner JM. Behavioral dissociations between C57BL/6 and DBA/2 mice on learning and memory tasks: a hippocampal-dysfunction hypothesis. Psychobiology. 1993;21:11–26. [Google Scholar]
- Paylor R, Tracy R, Wehner J, Rudy JW. DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci. 1994;108:810–817. doi: 10.1037//0735-7044.108.4.810. [DOI] [PubMed] [Google Scholar]
- Picciotto MR, Wickman K. Using knockout and transgenic mice to study neurophysiology and behavior. Physiol Rev. 1998;78:1131–1163. doi: 10.1152/physrev.1998.78.4.1131. [DOI] [PubMed] [Google Scholar]
- Podhorna J, Brown RE. Strain differences in activity and emotionality do not account for differences in learning and memory performance between C57BL/6 and DBA/2 mice. Genes Brain Behav. 2002;1:96–110. doi: 10.1034/j.1601-183x.2002.10205.x. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Restivo L, Tafi E, Ammassari-Teule M, Marie H. Viral-mediated expression of a constitutively active form of CREB in hippocampal neurons increases memory. Hippocampus. 2009;19:228–234. doi: 10.1002/hipo.20527. [DOI] [PubMed] [Google Scholar]
- Schneider F, Weiss U, Kessler C, Muller-Gartner HW, Posse S, Salloum JB, Grodd W, Himmelmann F, Gaebel W, Birbaumer N. Subcortical correlates of differential classical conditioning of aversive emotional reactions in social phobia. Biol Psychiatry. 1999;45:863–871. doi: 10.1016/s0006-3223(98)00269-8. [DOI] [PubMed] [Google Scholar]
- Smith DR, Gallagher M, Stanton ME. Genetic background differences and nonassociative effects in mouse trace fear conditioning. Learn Mem. 2007;14:597–605. doi: 10.1101/lm.614807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer M, Hajak G, Dohnel K, Schwerdtner J, Meinhardt J, Muller JL. Integration of emotion and cognition in patients with psychopathy. Prog Brain Res. 2006;156:457–466. doi: 10.1016/S0079-6123(06)56025-X. [DOI] [PubMed] [Google Scholar]
- Stanciu M, Radulovic J, Spiess J. Phosphorylated cAMP response element binding protein in the mouse brain after fear conditioning: relationship to Fos production. Brain Res Mol Brain Res. 2001;94:15–24. doi: 10.1016/s0169-328x(01)00174-7. [DOI] [PubMed] [Google Scholar]
- Sung JY, Goo JS, Lee DE, Jin DQ, Bizon JL, Gallagher M, Han JS. Learning strategy selection in the water maze and hippocampal CREB phosphorylation differ in two inbred strains of mice. Learn Mem. 2008;15:183–188. doi: 10.1101/lm.783108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trullas R, Skolnick P. Differences in fear motivated behaviors among inbred mouse strains. Psychopharmacology (Berl) 1993;111:323–331. doi: 10.1007/BF02244948. [DOI] [PubMed] [Google Scholar]
- Turner JG, Parrish JL, Hughes LF, Toth LA, Caspary DM. Hearing in laboratory animals: strain differences and nonauditory effects of noise. Comp Med. 2005;55:12–23. [PMC free article] [PubMed] [Google Scholar]
- Voikar V, Koks S, Vasar E, Rauvala H. Strain and gender differences in the behavior of mouse lines commonly used in transgenic studies. Physiol Behav. 2001;72:271–281. doi: 10.1016/s0031-9384(00)00405-4. [DOI] [PubMed] [Google Scholar]
- Voikar V, Polus A, Vasar E, Rauvala H. Long-term individual housing in C57BL/6J and DBA/2 mice: assessment of behavioral consequences. Genes Brain Behav. 2005;4:240–252. doi: 10.1111/j.1601-183X.2004.00106.x. [DOI] [PubMed] [Google Scholar]
- Wehner JM, Radcliffe RA, Bowers BJ. Quantitative genetics and mouse behavior. Annu Rev Neurosci. 2001;24:845–867. doi: 10.1146/annurev.neuro.24.1.845. [DOI] [PubMed] [Google Scholar]
- Wood MA, Kaplan MP, Park A, Blanchard EJ, Oliveira AM, Lombardi TL, Abel T. Transgenic mice expressing a truncated form of CREB-binding protein (CBP) exhibit deficits in hippocampal synaptic plasticity and memory storage. Learn Mem. 2005;12:111–119. doi: 10.1101/lm.86605. [DOI] [PMC free article] [PubMed] [Google Scholar]