Abstract
Mouse models of feeding provide a useful tool for elucidating the molecular pathways of energy regulation. The majority of studies in mice have been limited to intake analyses conducted over extended periods of time, which fail to distinguish between a variety of factors that influence nutrient intake. Using licking microstructure analyses we examined both the size and number of licking bursts for water, polycose, sucrose and lecithin in three strains of mice (C57BL/6J, 129Sv/ImJ and C57129F1 hybrids), using pause criteria (250–500, >500 and >1000 ms) that have previously been described in the rat. Burst size and number varied both as a function of tastant concentration and mouse strain; however, these differences were most evident with the >1000 ms pause criterion. Consistent with previous reports, during water consumption C57 mice showed longer mean interlick intervals, a larger number of bursts but reduced burst size relative to the two other strains. F1 mice showed larger burst sizes for polycose, while C57 mice displayed a greater number of bursts for both polycose and sucrose. Both 129 and F1 mice were insensitive to sucrose concentration, whereas C57 mice showed attenuated lecithin intake influenced by a reduction in the size of bursts for this tastant. These results suggest that these strains of mice display differences in the pattern of licking that are most evident with the use of larger pause criteria. These differences in licking behavior might reflect influences of genetic background on pre- and post-ingestive factors controlling intake, the reinforcing properties of each tastant, or native differences in licking style.
Keywords: Ingestive behavior, Mouse genetics, Palatability, Feeding
Introduction
Animal models provide an essential contribution for understanding the basic parameters that regulate components of energy balance. In mice, a variety of genetic models of obesity have proven useful for elucidating the molecular basis of energy regulation, with the hope that such studies will aid in the identification of new drug targets and novel therapeutic strategies (Robinson, Dinulescu, & Cone, 2000). However, the majority of studies concerning energy regulation and food intake in mice have been limited to intake analyses over longer time frames, which fail to distinguish between a variety of variables that are known to influence intake (Davis & Smith, 1992; Sclafani, Cardieri, Tucker, Blusk, & Ackroff, 1993). Here we describe a detailed analysis of licking microstructure, which provides an advantageous strategy by which to gauge the possible contributions of tastant palatability and post-ingestive inhibitory feedback on consumption (Smith, 2001).
Licking in rodents is a highly stereotyped behavior that involves the rhythmic cycling of tongue extensions and retractions thought to be under the control of a group of neurons in medulla oblongata nuclei V, VII and XII, which collectively function as a central pattern generator (Nakamura & Katakura, 1995; Norgren, 1995; Travers & Norgren, 1991). In microstructure analyses the rate of licking is defined by the interlick intervals (ILIs), where the majority of ILIs fall <250 ms and reflect continuous licking bursts (Davis & Smith, 1992). Longer pauses between bursts of licking appear to be relevant for dissecting the various components of meal intake. Davis (1996), Davis and Perez (1993) and Davis and Smith (1992) used two criteria to divide pauses. The pauses between 250 and 500 ms were thought to reflect brief interruptions of licking, such as lateral tongue movements (Grill & Norgren, 1978), whereas pauses >500 ms indicated longer interruptions of licking due to the active engagement of other competing behavior, such as grooming or leaving the food area. The number of licks occurring before the pause intervals defined the size of the licking bursts. This measure was unaffected by sham-feeding preparations and increased as a function of sucrose concentration, indicative of preingestive influences (Davis & Smith, 1992). By contrast, increases in both burst and pause number were seen at 250–500 and >500 ms with sham-feeding preparations. Under normal feeding conditions, the number of bursts in a meal displayed an inverted U-shaped function of concentration with sucrose, reflecting post-ingestive negative feedback (Davis & Smith, 1992; Smith, 2001). Spector, Klumpp, and Kaplan (1998) conducted a systematic assessment to examine pause criteria for the study of licking behaviors in rats, deciding upon a >1000 ms pause criterion, whose burst size and number were associated with pre- and post-ingestive factors, respectively. These results suggest that during the initial stages of sucrose intake, rats rapidly reinstate licking behavior following termination of a licking burst. This pattern leads to increases in both the number of pauses and bursts in the meal. Typically, as the meal progresses the frequency of these events decreases as influences of post-ingestive inhibitory feedback develop.
In the current experiments we evaluated strains of mice that are commonly used in genetic research: C57BL/6J (C57), 129Sv/ImJ (129), and C57129F1 (F1), a hybrid of the previous two. In particular, we were interested in assessing the pattern of licking in these different strains of mice with respect to microstructural variables of burst size and number using pause criteria that had previously been described in the rat (Davis & Smith, 1992; Spector et al., 1998). Previous studies suggest mouse strain differences in lick rates and its microstructure (Boughter, Baird, Bryant, St. John, & Heck, 2007; Glendinning, Feld, Goodman, & Bayor, 2008; Horowitz, Stephan, Smith, & Whitney, 1977). Horowitz et al. (1977) examined licking for water in C57, DBA, and an F1-hybrid strain. C57 mice exhibited the slowest lick rate, F1 an intermediate, and DBA the highest lick rate. More recent examinations with water confirmed slower lick rates and fewer bursts in C57 mice relative to D2, 129 and SWR strains (Boughter et al., 2007; Dotson & Spector, 2005; Glendinning et al., 2005).
Strain differences in licking have also been reported with nutritive tastants. C57 mice showed elevated initial lick rates for sucrose (Glendinning et al., 2008) and increased sucrose and polycose intake (Sclafani, 2006) relative to 129 mice (Glendinning et al., 2005, 2008; Sclafani, 2006). While the taste receptor that mediates glucose polymer taste is unknown, strain differences at low concentrations of sucrose are thought to reflect allelic variations to the T1R3 receptor (Glendinning et al., 2005; Inoue et al., 2007). These results suggest that variations in background genetic strain contribute to factors that influence meal intake. Moreover, targeted gene manipulations of specific neuropeptides (Fintini et al., 2005; Lakaye, Adamantidis, Coumans, & Grisar, 2004) have been shown to influence energy regulation and food intake; however, to date only a few studies have used licking microstructure to assess the role of neuropeptides on feeding behavior (e.g., Baird et al., 2006).
Here we examined lick patterns in C57, F1 and 129 mice during consumption of water and three tastants that differ in their nutritive and caloric properties. In addition to the total intake of each tastant, we examined the size and number of licking bursts. As part of our analysis of licking bursts, we compared three pause criteria (250–500, >500 and >1000 ms) used to define such bursts. In Experiment 1, we examined water consumption to provide an initial assessment of native licking style to a non-nutritive solution in these mice. Next, we assessed the patterns of licking of two commonly studied tastants, the complex polysaccharide polycose and the disaccharide sucrose (Experiments 2 and 3, respectively). Finally, we examined licking microstructure for lecithin (Experiment 4), a principal phospholipid that is used extensively in the food industry as a food additive but has yet to be assessed in animal models.
Methods
Subjects
A total of 66 male mice were used from three strains: C57BL/6J (C57), 129Sv/ImJ (129), and C57129F1 hybrids (F1), obtained from Jackson Laboratory (Bar Harbor, ME). In Experiment 1, eight F1 and 129, and six C57 mice were used, which prior to behavioral testing weighed 33.2 ± 1.5, 31 ± 1.3 and 32.5 ± 1.8 g, respectively. The same mice were also used in Experiment 2. In Experiment 3, seven C57 (23.7 ± 3.5 g) and F1 mice (26.6 ± 0.8 g), and six 129 mice (23.7 ± 1.1 g) from each strain were used. Finally, in Experiment 4, eight mice from each strain were used (weighing 24.3 ± 1.6, 22.6 ± 1.1, and 26.2 ± 1.1 g, respectively). Mice were transferred to the Neurogenetics and Behavior Center at Johns Hopkins University at 6–8 weeks of age and kept on a 12 h light:dark cycle, with lights off at 7 P.M. Following 2 weeks of acclimatization, mice were food-deprived to 85% of their free-feeding weight and were maintained at that weight for the duration of the experiment. Testing took place between the hours of 12 P.M.–5 P.M. Following testing, each mouse was subsequently fed a small food pellet prior to transportation back to the colony room (approximately 5 P.M.). All experiments were conducted under the auspices of the Johns Hopkins University Institutional Animal Care and Use Committee.
Apparatus
Behavioral training took place in eight identical chambers, which consisted of clear polycarbonate sides and ceiling, aluminum front and back walls, and floor comprised of parallel, stainless steel rods, all housed in sound-attenuating shells (Med Associates, St. Albans, VT). Chambers were outfitted with a custom-built food cup into which 0.05 ml of liquid reward could be delivered. Food cups were connected to programmable vacuums, which could suction off reward when desired. Infrared photocells installed in the food cup monitored the time spent and the number of entries into the cup. The food cups also contained custom lickometers (Schoenbaum, Garmon, & Setlow, 2001), which used fiber optics to introduce a light beam through the fluid–air interface of a fluid bolus. Licks were detected as disturbances in the amplified light surface at the interface when the fluid was contacted, permitting time-stamping of individual licks. We previously conducted an extensive set of parametric studies to validate the lickometer counts, using a comparison of lick count to slow-motion video of mouse licking behavior that showed accurate lick counting was uncontaminated by licks missed because of fluid bridges sometimes formed with conventional lickometers. This feature, together with the small size of the fiber optics, allows compatibility with a variety of fluid wells, often useful for adapting the experimental apparatus to use with different mouse phenotypes. Furthermore, as with other optical lick detection systems, our device is useful for future studies aimed at examining neural encoding of licking microstructure, unlike more traditional lick sensors that make the animal a part of the electrical circuit (e.g., Davis and Smith, 1992). The time-stamped data were subsequently analyzed for the microstructure of licking of water, polycose (Ross Nutrition, Columbus, OH), sucrose (J.T. Baker, Phillipsburg, NJ) and lecithin (Lewis Labs, Westport, CT), using custom-made programs.
Procedure
Mice received consumption tests for water (Experiment 1), varying concentrations of polycose (2.5%, 5%, 10% and 20%, w/v; Experiment 2), sucrose (2.5%, 5%, 10% and 20%, w/v; Experiment 3) and lecithin (1.25%, 2.5%, 3.75% and 5%, w/v; Experiment 4).
Prior to commencing each consumption test, mice were transferred from the colony to the experimental room. For each experiment, each mouse was assigned an experimental chamber and thereafter tested in that chamber. Mice initially received food cup training, to habituate them to both the experimental context and tastant. In each of the two daily sessions, 60 deliveries of water (Experiment 1) or the second concentration in the series of the particular tastant was provided on a random-time 60 s schedule (i.e., Experiment 2 = 5% polycose; Experiment 3 = 5% sucrose; Experiment 4 = 2.5% lecithin). Next, mice received consumption tests with water (Experiment 1) or each concentration of the tastant (Experiments 2–4) tested on a separate day in a counterbalanced latin square design across strains. We tested mice in this randomized order to minimize the influence of systematic contrast effects. During each test session, in which the photo-beam lickometers were used, the tested tastant was continuously available in the food cup. At the start of the session, 0.05 ml of the tested tastant was available in the food cup, and additional 0.05 ml deliveries occurred every 25 licks as mice consumed the liquid. Prior research showed that under this schedule, tastants were never depleted nor did the food cups overflow. At the end of the session, liquid remaining in the food cup was suctioned off with the vacuum, prior to the running of additional mice.
Dependent variables and data analysis
Total intake (licks during 20-min period) and the pattern of licking rates (in licks/min) over time during the consumption of each tastant were analyzed across strains of mice. In addition, we examined two properties of licking microstructure; the size and number of discontinuous licking bursts, which are thought to reflect different aspects of meal ingestion. To determine an optimal burst definition, we compared three pause criteria, ILIs 250–500 ms, ILIs > 500 ms, and ILIs > 1000 ms. These values were chosen on the basis of prior research with rats (Davis, 1996; Davis & Perez, 1993; Davis & Smith, 1992; Spector et al., 1998). For each pause criterion, a licking burst was defined as two or more consecutive licks, with pauses greater than each criterion determining the licking burst termination. The mean burst size for each pause criterion was calculated by dividing the cumulative number of licks within those bursts by the total number of bursts in the consumption session. Thus, burst number reflected the number of ILIs 250–500 ms, ILIs > 500 ms, and ILIs > 1000 ms.
In Experiment 1, total licks and mean ILI were analyzed with one-way between-subject ANOVAs across strains (C57, F1, 129), followed by post hoc Tukey's Honestly Significant Difference (HSD) test comparisons, as appropriate. The pattern of water licking was analyzed with two-way mixed ANOVA with between-subject variable of strain and within-subject variable of time bin (1–10 bins), followed by planned individual comparisons designed to evaluate strain differences at each time bin. Licking microstructure was analyzed with one-way between-subject ANOVAs across strains for the size and number of licking bursts for each pause criterion (ILIs 250–500 ms, ILIs > 500 ms, ILIs > 1000 ms), followed by post hoc strain comparisons.
In Experiments 2–4, the total lick data were analyzed with two-way mixed ANOVAs, with a within-subject variable of percent tastant concentration and a between-subject variable of strain, followed by planned individual comparisons designed to evaluate strain differences at each concentration. The pattern of licking for each tastant was analyzed with three-way mixed ANOVAs with strain, time bin and tastant concentration as variables. Strain differences at each time bin were then evaluated using individual comparisons. To assess the effects of tastant concentration on the pattern of licking of each strain, separate two-way within-subject ANOVAs with variables of concentration and time bin were conducted. Additional individual comparisons assessed within-strain differences in the overall lick rate across different tastant concentrations. Finally, licking microstructure (burst size and number) for each pause criterion was assessed with two-way ANOVA with variables of strain and concentration, followed by planned individual comparisons designed to evaluate strain differences at each concentration, and post hoc comparisons used to contrast differences across the three strains. For all statistical analyses the level of significance adopted for ANOVAs and all planned and post hoc comparisons was p < 0.05.
Results
Experiment 1: water consumption
Total consumption and ILI distributions for C57, F1 and 129 mice
When allowed free access to water, all mice showed comparable intake as evidenced by total licks during the consumption session (Fig. 1; right panel). ANOVA revealed no strain differences (F(2,19) = 0.23, p = 0.79). The interlick intervals (ILIs) for continuous licking of water were also quantified to compare previously established licking topographies (Boughter et al., 2007; Dotson & Spector, 2005) with those seen using the current apparatus. The ILI was defined as the time between the onset of one lick and the onset of a succeeding lick. To assess mean ILI for each strain, we only included licks that occurred <250 ms, as they are thought to reflect continuous licking (Davis & Smith, 1992). Over 90% of licks occurred at this interval. The average ILI differed across groups, with C57 mice displaying the longest ILI (129 ± 2.75 ms) compared with both F1 (108 ± 9.25 ms) and 129 mice (105 ± 8.13 ms). ANOVA conducted on the mean ILIs revealed a main effect of strain (F(2,19) = 7.71, p < 0.01), with C57 mice differing from both 129 (p = 0.003) and F1 mice (p = 0.01); the ILIs were similar for 129 and F1 mice (p = 0.72).
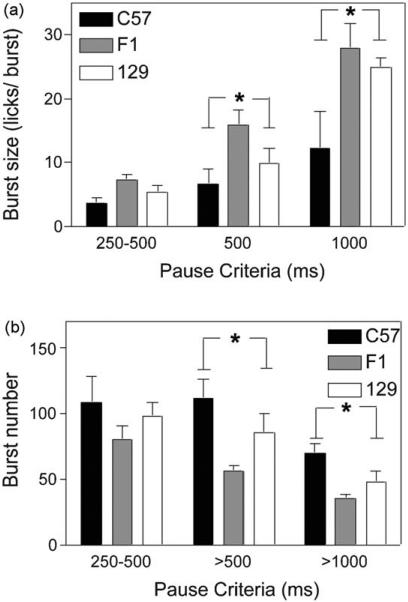
Fig. 1.
Experiment 1: Total intake and pattern of licking for water. Total licks (right panel) and pattern of licking in licks/min (left panel) for C57 (closed shapes), F1 (grey shapes) and 129 mice (open shapes). Approximately 400 total licks reflected 0.8 ml delivery of water.
Pattern of licking
The pattern of licking across time bins differed as a function of strain (Fig. 1; left panel). While all mice showed avid lick rates at the start of the consumption session, lick rates were lowest in C57 mice and highest in the F1 group. ANOVA with factors of strain time bin revealed no effect of strain (F(2,19) = 0.06, p = 0.93), a main effect of time bin (F(9,18) = 45.18, p < 0.00001) and a significant interaction between the variables (F(18,171) = 2.72, p < 0.001). Planned comparisons conducted at each time bin revealed for C57 mice reduced lick rates at bin 1 and elevated lick rates at bin 4 compared to all other mice (Fs > 5.25, ps < 0.03). No other strain differences were revealed (largest F-value; bin 6 C57 vs. F1, F(1,19) = 3.57, p = 0.07).
Licking microstructure
Burst size
Strain differences in burst size depended on the pause criteria used for analysis (Fig. 2a). For the smallest pause criterion, there were no differences in the size of bursts among the strains (F(2,19) = 2.74, p = 0.08). However, when the larger pause criterion of >500 ms was used significant strain differences were revealed (F(2,19) = 3.42, p = 0.05) with significantly smaller bursts in C57 than in F1 mice (p < 0.05). Similarly, the use of the >1000 ms pause criterion also revealed a main effect of strain (F(2,19) = 3.89, p < 0.05), with C57 showing significantly smaller busts than either 129 (p = 0.03) or F1 mice (p = 0.01).
Fig. 2.
Experiment 1: Water consumption: licking microstructure. Size (a) and number (b) of licking bursts for 250–500, >500, and >1000 ms pause criteria in C57 (closed bars), F1 (grey bars) and 129 mice (open bars). Asterisk indicates main effect of strain at selected pause criterion.
Burst number
The number of bursts initiated also differed both as a function of strain and pause criteria (Fig. 2b). With the 250–500 ms pause criterion there were no significant effects of strain (F(2,19) = 0.88, p = 0.43). However, using the >500 ms pause criterion, significant strain differences were noted (F(2,19) = 4.72, p = 0.02) with more bursts in C57 than in F1 mice (p = 0.01). With the >1000 ms pause criterion, strain differences were also found (F(2,19) = 6.63, p < 0.01), with more bursts in C57 mice than in each of the other strains (ps < 0.05).
Summary
Although overall water intake was comparable across the strains of mice, analysis of initial lick rates and lick microstructure revealed substantial differences in the licking profile among the strains. C57 mice showed lower initial lick rates and longer ILIs under continuous licking, as well as more bursts, but smaller burst sizes, than the other mice.
Experiment 2: polycose consumption
At each concentration tested, all strains of mice showed similar total licks for polycose (Fig. 3; right panels). Two-way strain × concentration ANOVA revealed no effect of strain (F(2,19) = 0.19, p = 0.82), a main effect of concentration (F(3,57) = 10.91, p < 0.00001), but no interaction between the variables (F(6,57) = 1.16, p = 0.33). Additionally, there were no strain differences in intake at any concentration (largest F-value; 2.5% concentration; F(2,19) = 0.91, p = 0.41).
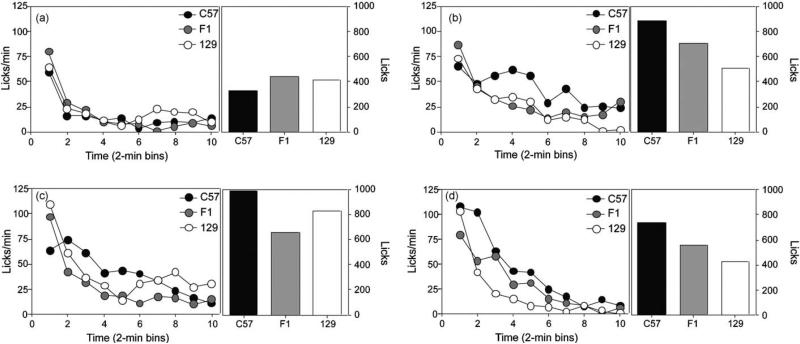
Fig. 3.
Experiment 2: Total intake and pattern of licking for polycose. Total licks (right panel) and pattern of licking (left panel) for C57 (closed shapes), F1 (grey shapes) and 129 mice (open shapes) during (a) 2.5%, (b) 5%, (c) 10% and (d) 20% polycose consumption sessions.
Pattern of licking
While overall intake was similar, there were substantial differences in the initial lick rates (Fig. 3; left panels). F1 mice showed higher initial lick rates than the 129 and C57 mice, which displayed similar initial lick rates, but C57 mice maintained higher lick rates during later stages of consumption. Three-way ANOVA with variables of strain, polycose concentration (2.5%, 5%, 10% and 20%), and time bins (1–10) revealed no main effect of strain (F(2,19) = 1.05, p = 0.36), but main effects of concentration (F(3,57) = 8.94, p < 0.0001) and time bin (F(9,171) = 26.08, p < 0.00001) and a significant strain × time bin interaction (F(18,171) = 3.19, p < 0.00001). No other significant interactions with strain were noted (largest F-value; three-way interaction, F(54,513) = 1.26, p = 0.10). Planned comparisons conducted on the strain × time bin interaction revealed significantly greater licking by F1 than C57 mice during bins 1–3 (smallest F-value; bin 1, F(1,19) = 5.27, p < 0.05). Further differences were noted in the later stages of the consumption session, with C57 mice showing more licking than F1 mice at bins 8–10 (smallest F-value, bin 9, F(1,19) = 4.36, p = 0.05). No differences were noted between F1 and 129 mice during these time points (Fs < 1, ps > 0.33).
In C57 mice, separate ANOVAs for each strain with factors of concentration × time bin revealed main effects of concentration (F(3,15) = 5.49, p < 0.01) and time bin (F(9,45) = 1.96, p = 0.05), but no interaction between these factors (F(27,135) = 1.3, p = 0.16). Differences in overall lick rate were noted between the first two concentrations compared to the last two concentrations (smallest F-value; 2.5% vs. 20%, F(1,5) = 6.50, p = 0.05). A similar analysis for F1 mice revealed main effects of concentration (F(3,21) = 7.45, p = 0.001) and time bin (F(9,63) = 16.13, p = 0.0001) but no concentration × time bin interaction (F(27,189) = 1.32, p = 0.14). In these mice, the 2.5% polycose differed significantly from all other concentrations (smallest F-value; 2.5% vs. 10%, F(1,7) = 9.14, p = 0.01). Finally, in 129 mice there was a main effect of time bin (F(9,63) = 16.12, p < 0.0001), with differences in the pattern of licking between 2.5% and 20% polycose (F(1,7) = 6.48, p = 0.04).
Licking microstructure
Burst size
For the 250–500 ms criterion C57 mice generally showed the smallest burst size relative to the two other strains (Fig. 4a). Two-way ANOVA with factors of strain × concentration revealed no main effects or interactions (largest F-value; strain, F(2,19) = 3.07, p = 0.06); however, planned strain comparisons at each concentration revealed a significant strain effect at the 10% concentration (F(2,19) = 4.73, p = 0.02) with C57 differing from both 129 and F1 strains (ps < 0.05). Analysis of licking bursts for the >500 ms pause criterion (Fig. 4b) revealed a main effect of strain (F(2,19) = 3.29, p = 0.05), but no effect of concentration or strain concentration interaction (largest F-value; concentration, F(3,57) = 1.39, p = 0.25). Planned comparisons revealed strain differences at the 10% concentration (F(2,19) = 5.13, p = 0.01), with C57 differing from 129 and F1 strains (ps < 0.05). Similarly, for the >1000 ms criterion (Fig. 4c) strain differences were noted (F(2,19) = 5.48, p = 0.01), in addition to a main effect of concentration (F(3,57) = 2.74, p = 0.05) but no interaction between the variables (F(6,57) = 1.03, p = 0.41). Planned comparisons revealed significant strain differences at 5% (F(2,19) = 4.4, p = 0.02) and 10% (F(2,19) = 5.06, p = 0.01) with F1 mice showing larger burst sizes relative to both strains (ps < 0.05) at both concentrations, and C57 showing smaller bursts than 129 mice (p = 0.05) with 10% polycose. Overall these findings suggest that C57 show smaller licking bursts for polycose, with F1 mice generally displaying the largest burst size of the three strains.
Fig. 4.
Experiment 2: Polycose consumption: licking microstructure. Licking burst size for (a) 250–500 ms, (b) >500 ms and (c) >1000 ms and number of (d) 250–500 ms, (e) >500 ms and (f) >1000 ms pause criteria for C57 (closed bars), F1 (grey bars) and 129 mice (open bars). Asterisk indicates main effect of strain at selected concentrations.
Burst number
With the 250–500 ms pause criterion (Fig. 4d), two-way strain × concentration ANOVA revealed no effect of strain (F(2,19) = 3.19, p = 0.06), a main effect of concentration (F(3,57) = 7.57, p < 0.0001), and a significant strain × concentra-concentration interaction (F(6,57) = 2.23, p = 0.05). Planned comparisons revealed strain differences at the 10% polycose concentration (F(2,19) = 5.47, p = 0.01) with C57 initiating more bursts compared to both F1 (p < 0.001) and 129 (p < 0.05) strains. With 20% polycose, strain differences were also revealed (F(2,19) = 6.43, p < 0.007) between C57 and all other mice (ps < 0.01). For >500 ms pause criterion (Fig. 4e) strain × concentration ANOVA revealed a main effect of strain only (F(2,19) = 11.78, p < 0.0001), with strain differences at 5–20% polycose concentrations (smallest F-value; 5% polycose, F(2,19) = 4.23, p = 0.02) and C57 differing from all other strains (ps ≤ 0.02). Finally, for the >1000 ms pause criterion (Fig. 4f) strain × concentration ANOVA revealed a main effect of strain (F(2,19) = 16.41, p < 0.0001) and concentration (F(3,57) = 2.64, p < 0.05), with planned individual comparisons revealing a significant strain difference at each concentration (smallest F-value; 2.5% polycose, F(2,19) = 4.3, p = 0.02). In general, these results suggest C57 mice initiated more bursts throughout the consumption session relative to the two other strains.
Summary
Although there were no strain differences in overall polycose consumption, initial lick rates and aspects of lick microstructure differed across strains. Initially, F1 mice showed more licking than C57 mice, but that pattern was reversed by the end of the consumption test session. With the most discriminating pause criterion (>1000 ms), F1 mice showed the largest burst sizes, whereas C57 mice showed the greatest burst numbers.
Experiment 3: sucrose consumption
Total sucrose licks were comparable across the three strains of mice (Fig. 5; right panels). Two-way ANOVA with factors of strain × concentration revealed a main effect of concentration only (F(3,51) = 3.38, p = 0.02), with no strain differences in intake revealed at any concentration (Fs < 1, ps > 0.52).
Fig. 5.
Experiment 3: Total intake and pattern of licking for sucrose. Total licks (right panel) and pattern of licking (left panel) for C57 (closed shapes), F1 (grey shapes) and 129 mice (open shapes) during (a) 2.5%, (b) 5%, (c) 10% and (d) 20% sucrose consumption sessions.
Pattern of licking
Although the three mouse strains appeared to show different patterns of licking at some concentrations of sucrose, this impression was not supported by the overall strain × sucrose concentration (2.5%, 5%, 10% and 20%) × time bin (1–10) ANOVA, which revealed a main effect of sucrose concentration (F(3,51) = 2.98, p < 0.05) and time bin (F(9,153) = 13.79, p < 0.00001), but no effect of strain nor interactions among any of the factors (largest F-value; concentration × bin, F(27,459) = 1.40, p = 0.09).
Subsequently, we analyzed the pattern of licking within each strain. C57 mice showed a slower rate of decay in the intake rate with intermediate sucrose concentrations. ANOVA with factors of concentration and time bin revealed no main effect of concentration (F(3,18) = 2.77, p = 0.07), a main effect of time bin (F(9,54) = 6.95, p < 0.00001), but a significant concentration time bin interaction (F(27,162) = 1.90, p = 0.007). Overall licking was less with 2.5% sucrose than with all other concentrations (smallest F-value; 2.5% vs. 5%, F(1,6) = 7.66, p = 0.03). By contrast, in F1 mice, ANOVA revealed a main effect of time bin only (F(9,54) = 10.13, p < 0.0001), with no significant differences in overall lick rate across any of the concentrations (largest F-value; 2.5% vs. 20%, F(1,6) = 1.09, p = 0.34). Similarly, in 129 mice, only a main effect of time bin was noted (F(9,45) = 2.23, p < 0.05), with no differences in licking across any of the sucrose concentrations (largest F-value; 10% vs. 20% sucrose, F(1,5) = 2.37, p = 0.18). These findings suggest F1 and 129 mice failed to show significant differences in the licking pattern during the consumption of a variety of sucrose concentrations.
Licking microstructure
Burst size
For the 250–500 ms pause criterion (Fig. 6a), two-way ANOVA with strain and concentration as factors revealed no main effects or interactions among any of the variables (largest F-value; strain, F(2,17) = 1.02, p = 0.37). Planned comparisons at each concentration also failed to reveal any strain differences (largest F-value; 2.5% sucrose, F(2,17) = 1.43, p = 0.26). Similarly, two-way ANOVA for >500 ms (Fig. 6b) or >1000 ms criterion (Fig. 6c) revealed a main effect of concentration only for the >1000 ms criterion (F(3,51) = 2.92, p < 0.05). There were no strain differences for these criteria at any concentration of sucrose (Fs < 2.16, ps > 0.14).
Fig. 6.
Experiment 3: Sucrose consumption: licking microstructure. Licking burst size for (a) 250–500 ms, (b) >500 ms and (c) >1000 ms and number of (d) 250–500 ms, (e) >500 ms and (f) >1000 ms pause criteria for C57 (closed bars), F1 (grey bars) and 129 mice (open bars). Asterisk indicates main effect of strain at selected concentrations.
Burst number
The number of bursts initiated differed as a function of strain and pause criteria (Fig. 6d–f). Two-way ANOVA conducted on burst number for the 250–500 ms criterion (Fig. 6d), revealed no effects of strain, concentration or interaction between the factors (largest F-value; concentration, F(3,51) = 1.64, p = 0.19), nor were there any strain effects at any individual concentration (largest F-value; 5% sucrose, F(2,17) = 1.62, p = 0.22). For the >500 ms criterion (Fig. 6e) two-way ANOVA revealed no effects of concentration or interaction (largest F-value; concentration, F(3,51) = 2.45, p = 0.07), with planned comparisons at each concentration revealing no strain differences (largest F-value; 5% sucrose, F(2,17) = 1.39, p = 0.27). Finally, two-way ANOVA conducted on the >1000 ms pause criterion (Fig. 6f) revealed a main effect of concentration only (F(3,51) = 2.45, p < 0.05). Planned comparisons revealed significant strain differences both at 10% (F(2,18) = 3.59, p = 0.04) and 20% sucrose (F(2,18) = 3.38, p = 0.05) with C57 initiating more bursts during consumption at these concentrations relative to all other mice (ps < 0.05).
Summary
Only minor strain differences in sucrose licking were observed. Overall sucrose consumption was similar in the three strains, with only small differences in the patterns of licking across the consumption tests with some concentrations. Although burst size did not differ significantly across the strains at any concentration or pause criterion, when the >1000 ms criterion was used, C57 mice showed significantly more bursts than the other mice at higher sucrose concentrations.
Experiment 4: lecithin consumption
In general, all mice showed increased total licks with higher lecithin concentrations (Fig. 7; right panels). Two-way strain × concentration ANOVA revealed a main effect of concentration only (F(3,63) = 18.54, p < 0.00001). No strain differences were noted at any of the four concentrations (Fs < 1, ps > 0.57).
Fig. 7.
Experiment 4: Total intake and pattern of licking for lecithin. Total licks (right panel) and pattern of licking (left panel) for C57 (closed shapes), F1 (grey shapes) and 129 mice (open shapes) during (a) 1.25%, (b) 2.5%, (c) 3.75% and (d) 5% lecithin consumption sessions.
Pattern of licking
Higher lecithin concentrations resulted in higher initial lick rates, with considerably less licking in C57 than in F1 mice early in the sessions (Fig. 7; left panel). A three-way ANOVA revealed no main effect of strain (F(2,21) = 0.65, p = 0.53), main effects of lecithin concentration (F(3,63) = 18.41, p < 0.00001) and time bin (F(9,189) = 2.02, p = 0.01), and a significant strain × time bin interaction (F(18,189) = 2.02, p = 0.01). Planned comparisons on the significant interaction revealed significant differences between C57 and F1 mice at bin 1 only (F(1,21) = 5.64, p = 0.02). No other differences between any of the strains at any other time bin were noted (largest F-value; bin 1 C57 vs. 129 mice, F(1,21) = 2.55, p = 0.1).
Separate ANOVAs for each strain revealed main effects of concentration (F(3,21) = 10.47, p < 0.001), time bin (F(9,63) = 22.49, p < 0.00001) and a significant interaction between the two variables in C57 mice (F(27,189) = 1.56, p = 0.04). Differences in licking were noted between the highest concentration of lecithin and all other concentrations (smallest F-value; 2.5% vs. 5%, F(1,7) = 10.64, p = 0.01). For F1 mice, main effects of concentration (F(3,21) = 8.98, p < 0.001) and time bin (F(9,63) = 8.90, p < 0.00001), and a significant concentration × time bin interaction were also noted (F(27,189) = 1.56, p < 0.05), with overall lick rates during 5% lecithin differing from all other concentrations (smallest F-value; 3.75% vs. 5%, F(1,7) = 7.58, p = 0.02). Finally, in 129 mice, main effects of concentration (F(3,21) = 3.86, p = 0.02) and time bin (F(9,63) = 21.96, p < 0.00001) and a significant concentration × time bin interaction (F(27,189) = 2.02, p = 0.003) were revealed, with differences in overall licking noted between 1.25% and 5% lecithin (F(1,7) = 17.91, p < 0.01), and 2.5% and 5% (F(1,7) = 11.06, p = 0.01).
Licking microstructure
Burst size
With the 250–500 ms pause criterion (Fig. 8a), a two-way strain × concentration ANOVA revealed only a main effect of concentration (F(3,63) = 3.69, p = 0.01). Planned comparisons revealed no strain differences at any concentration (largest F-value; 3.75% lecithin, F(2,21) = 1.76, p = 0.19). For the >500 ms pause criterion (Fig. 8b) ANOVA revealed a main effect of strain (F(2,21) = 4.57, p = 0.02) and concentration (F(3,63) = 15.63, p < 0.0001) but no interaction between the factors (F(6,63) = 0.86, p = 0.52). Planned comparisons revealed a main effect of strain at 1.25% lecithin (F(2,21) = 3.49, p < 0.05), with significantly smaller bursts in C57 mice than in the other strains (ps ≤ 0.05). A similar analysis for the >1000 ms pause criterion (Fig. 8c) revealed a main effect of strain (F(2,21) = 8.30, p < 0.001) and concentration (F(3,63) = 10.45, p < 0.0001), but no interaction (F(6,63) = 0.38, p = 0.88). Planned comparisons revealed a main effect of strain at each concentration (smallest F-value; 1.25% lecithin, F(2,21) = 4.33, p = 0.02) with C57 mice differing from F1 at all concentrations (ps< 0.05).
Fig. 8.
Experiment 4: Lecithin consumption: licking microstructure. Licking burst size for (a) 250–500 ms, (b) >500 ms and (c) >1000 ms and number of (d) 250–500 ms, (e) >500 ms and (f) >1000 ms pause criteria for C57 (closed bars), F1 (grey bars) and 129 mice (open bars). Asterisk indicates main effect of strain at selected concentrations.
Burst number
While there was an apparent trend for C57 mice generally to initiate more bursts when the 250–500 ms pause criterion was used (Fig. 8d), two-way ANOVA conducted on these data revealed only a main effect of concentration (F(3,63) = 5.74, p < 0.01), with no effects of strain at any concentration (largest F-value; 2.5% lecithin, F(2,21) = 2.6, p = 0.09). Similarly, only the main effects of concentration were revealed for both >500 ms (F(3,63) = 12.54, p < 0.00001) and >1000 ms (F(3,63) = 16.82, p < 0.00001) criteria (Fig. 8e, f), with no strain differences noted at any concentration (largest F-value; >500 ms criterion 3.75% lecithin, F(2,21) = 2.09, p = 0.15).
Summary
Although there were no strain differences in overall lecithin licking, C57 mice showed substantially less licking early in the consumption tests. Similarly, C57 mice showed smaller burst sizes than the other strains, whereas the strains did not differ in the numbers of bursts initiated.
Discussion
The primary purpose of the current series of experiments was to examine burst size and number in three strains of mice, using a variety of tastants that differed in their caloric and nutritive properties. We also compared three pause criteria previously used to define licking bursts in rats (Davis, 1996; Davis & Perez, 1993; Davis & Smith, 1992; Spector et al., 1998).
Our results with water replicated previous findings (Boughter et al., 2007; Dotson & Spector, 2005), with C57 mice displaying a larger ILI during continuous licking and initiating more bursts in the session relative to 129 and F1 strains. These latter strains typically displayed more licks within a burst when compared to C57 mice, though these strain differences in burst size and number were least apparent with the 250–500 ms pause criterion. Similarly, this criterion was generally least effective at delineating strain differences in burst size and number with the other tastants. Increasing the pause criterion to >500 ms resulted in more noticeable strain differences with polycose and lecithin compared with the 250–500 ms criterion. However, the >1000 ms was most effective for establishing strain differences in burst size and/or number for all tested tastants. Furthermore, this criterion was also most effective for determining significant effects of tastant concentration on size and number of bursts. Collectively, these findings suggest that the brief interruptions in licking (i.e., 250–500 ms) that may occur with lateral tongue movements (Grill & Norgren, 1978; Smith & Davis, 1992) are least effective in determining the differences in licking microstructure in mice.
Strain differences in lick patterns and microstructure depended on the tastant, its concentration, as well as the aforementioned pause criteria. During polycose consumption tests, the strain differences in burst size and number were comparable to those seen with water. F1 mice displayed higher lick rates at the start of each session and larger bursts of licking throughout. Both of these parameters have been suggested to reflect stimulus palatability (Smith, 2001). The voracious feeding noted in F1 mice was followed by a steeper slope of decline in ingestion rate relative to C57 and 129 groups. The difference between the groups would be expected because the elevations in initial licking would be followed by increased negative feedback, for example, from a swift accumulation of fluid in the gastrointestinal tract. By contrast, since the initial rate of licking was comparable between C57 and 129 mice, the strain differences in licking in the latter stages of the consumption session might reflect differential sensitivity to inhibitory feedback. Notably, the increased burst number in C57 strain might also suggest reduced sensitivity to post-ingestive consequences of this tastant (Davis & Smith, 1992); however, the basis of these strain differences remains to be clarified and could include some learned modulation of preparatory responses associated with the delivery of reward (e.g., approaching the food cup), or natural differences in native licking style (Experiment 1; Dotson & Spector, 2005).
The strain differences in licking and its microstructure were at least partly dependent on the tastant under investigation. In contrast to previous reports (e.g., Zukerman, Glendinning, Margolskee, & Sclafani, 2009) lick rates were surprisingly lower for sucrose than for polycose. While we are unsure on the nature of this difference, it may be relevant that across strains, the mice tested with sucrose in the current experiment weighed less than the mice tested with polycose. Furthermore, unlike with polycose, both 129 and F1 mice appeared insensitive to changes in sucrose concentration, as the overall pattern of licking for these strains did not differ significantly across concentrations. Since the taste response to sugars is mediated in part by T1R3 receptor proteins (Montmayeur & Matsunami, 2002; Treesukosol, Blonde, & Spector, 2009; Zhao et al., 2003), it is possible that mutations to the Tas1r3 gene led to an insensitivity to changes in the pattern of licking in 129 and F1 strains. Previous reports also suggest reduced lick rates for sugars in mice with targeted deletion of the T1R3 subunit (Treesukosol et al., 2009; Zukerman et al., 2009) or allelic mutations to the Tas1r3 allele (Inoue et al., 2007). One possible difference between these previous lick rate results and the absence of a strain effect on initial lick rate in Experiment 3 might reflect the procedural and analytical variations between the studies (e.g., use of brief access 5-s trials and/or use of lick rates standardized to water). It is also possible that all mice were able to detect olfactory cues for sucrose (Rhinehart-Doty, Schumm, Smith, & Smith, 1994; Uebayashi, Hatanaka, Kanemura, & Tonosaki, 2001), contributing to initial orosensory positive feedback (Smith, 2001). Interestingly the number of pauses differed between C57 mice and both other strains, even though initial intake rates were comparable for all mice. Thus, C57 mice may have been less sensitive to the accumulation of sucrose in the gastrointestinal tract, which might reflect T1R3-mediated glucose absorption in intestinal enteroendocrine cells (Mace, Affleck, Patel, & Kellett, 2007; Margolskee et al., 2007).
Lecithin consumption also revealed differences in licking behavior. F1 and 129 mice showed similar licking profiles; avid initial consumption with increases in tastant concentration, followed by a steep slope of decline in lick rate. By contrast, C57 mice showed attenuated initial lick rates, and unlike the previous two tastants, showed little evidence of maintained lick rates during the later stages of the consumption session. Consistent with this latter finding, the number of bursts initiated did not differ as a function of strain. Lecithin is a lipid material composed of inositol and choline, and is used extensively as a supplement in the food industry. The basis for the strain differences in licking for lecithin requires future examination, however, it is notable that this tastant causes an increase in brain-acetylcholine levels (Cohen & Wurtman, 1976). Therefore, there may be some relation between the reduced consumption of lecithin by C57 mice, and this strain's deficiencies in cholinergic activity in hypothalamic feeding areas and frontal regions (Bentivoglio, Altavista, Granata, & Albanese, 1994; Mandel, Ayad, Hermetet, & Ebel, 1974).
The current experiments highlight the importance for examining microstructural variables of intake. For instance, while intake levels for 10% polycose were similar, the different strains of mice displayed substantial differences in licking microstructure. C57 mice showed reduced rates of initial intake, shorter bursts of licking, offset by initiating more licking bursts than all other mice. By contrast, both F1 and 129 mice showed larger bursts of licking relative to C57 strain. Had overall intake been the sole variable under investigation, we would have erroneously concluded similar ingestive behavior in these three strains of mice.
While burst size and number are thought to reflect dissociable components of ingestive behavior (Davis & Smith, 1992; Smith, 2001; Spector et al., 1998), other factors are known to influence these parameters. Davis (1996) examined the probability distributions of bouts of licking, showing the occurrence of a burst size of 250–500 ms duration to be under the control of a Poisson process (Wickens, 1984), whereby the probability of a burst of licking occurring was equally likely at the start as it was at the end of the session. Using a larger pause criterion (>500 ms) the duration of long pauses was mediated by a separate Poisson process, increasing as meal progressed (Davis, 1996). Similarly, when a >1000 ms pause criterion was used and the sequence of burst size and number was evaluated based on meal progression, significant decreases in burst size and increases in pause duration were revealed (Spector et al., 1998). Thus, particularly with longer pause criteria, the satiation influence of tastant intake has an effect on burst size. Furthermore, food deprivation might be expected to potentiate outcome tastant palatability and reduce post-ingestive inhibition, but its influences over burst size and number occur only at high and low sucrose concentrations, respectively (Davis & Perez, 1993). Finally, as previously cited, it is possible that the differences in licking behavior might reflect differential sensitivity to the rewarding properties of each tastant and/or natural differences in native licking style between the strains.
Collectively our findings suggest the use of the >1000 ms criterion for defining a pause in an otherwise steady stream of licking in mice. In general, our results are consistent with previous reports indicating reduced burst size but increased number of licking bursts in C57 mice compared to all other strains (Boughter et al., 2007; Dotson & Spector, 2005). We now extend this assessment to include other tastants that differ in their nutritive properties. The use of this microstructural assessment of ingestive behavior allows the potential to evaluate a variety of influences that are known to mediate intake including pre- and post-ingestive factors. Measures that reflect pre-ingestive influences typically maintain food intake via signals that include excitation of gustatory, olfactory and trigeminal receptors; whereas measures associated with post-ingestive influences typically signal meal termination. This negative feedback is influenced by humoral and neural stimuli generated by food acting on chemosensors in the small intestine and stomach (Smith, 2001). Furthermore, the identification of neuropeptides that regulate orexigenic and anorexigenic components of feeding provides an opportunity to examine the molecular basis of food intake. Several studies suggest a distinct role for neuropeptides in licking microstructure and ingestive behavior (Aja, Schwartz, Kuhar, & Moran, 2001; Baird et al., 2006, 2008; Zukerman et al., 2009). Combined with the use of mouse genetics and pharmacological studies, microstructural analyses will surely be useful for elucidating the etiology of food intake and associated disorders.
Acknowledgements
Supported by NCRR grant P40-RR-017688 and NIMH grant R01-MH-60179.
References
- Aja S, Schwartz GJ, Kuhar MJ, Moran TH. Intracerebroventricular CART peptide reduces rat ingestive behavior and alters licking microstructure. American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 2001;280(6):R1613–R1619. doi: 10.1152/ajpregu.2001.280.6.R1613. [DOI] [PubMed] [Google Scholar]
- Baird JP, Rios C, Gray NE, Walsh CE, Fischer SG, Pecora AL. Effects of melanin-concentrating hormone on licking microstructure and brief-access taste responses. American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 2006;291(5):R1265–R1274. doi: 10.1152/ajpregu.00143.2006. [DOI] [PubMed] [Google Scholar]
- Baird JP, Rios C, Loveland JL, Beck J, Tran A, Mahoney CE. Effects of hindbrain melanin-concentrating hormone and neuropeptide Y administration on licking for water, saccharin, and sucrose solutions. American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 2008;294(2):R329–R343. doi: 10.1152/ajpregu.00611.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentivoglio AR, Altavista MC, Granata R, Albanese A. Genetically determined cholinergic deficiency in the forebrain of C57BL/6 mice. Brain Research. 1994;637(1–2):181–189. doi: 10.1016/0006-8993(94)91231-9. [DOI] [PubMed] [Google Scholar]
- Boughter JD, Jr., Baird JP, Bryant J, St John SJ, Heck D. C57BL/6J and DBA/2J mice vary in lick rate and ingestive microstructure. Genes, Brain, and Behavior. 2007;6(7):619–627. doi: 10.1111/j.1601-183X.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- Cohen EL, Wurtman RJ. Brain acetylcholine: control by dietary choline. Science. 1976;191(4227):561–562. doi: 10.1126/science.1251187. [DOI] [PubMed] [Google Scholar]
- Davis JD. Microstructural analysis of the ingestive behavior of the rat ingesting polycose. Physiology and Behavior. 1996;60(6):1557–1563. doi: 10.1016/s0031-9384(96)00321-6. [DOI] [PubMed] [Google Scholar]
- Davis JD, Perez MC. Food deprivation- and palatability-induced micro-structural changes in ingestive behavior. American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 1993;264(1 Pt 2):R97–R103. doi: 10.1152/ajpregu.1993.264.1.R97. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behavioral Neuroscience. 1992;106(1):217–228. [PubMed] [Google Scholar]
- Dotson CD, Spector AC. Drinking spout orifice size affects licking behavior in inbred mice. Physiology and Behavior. 2005;85(5):655–661. doi: 10.1016/j.physbeh.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Fintini D, Alba M, Schally AV, Bowers CY, Parlow AF, Salvatori R. Effects of combined long-term treatment with a growth hormone-releasing hormone analogue and a growth hormone secretagogue in the growth hormone-releasing hormone knock out mouse. Neuroendocrinology. 2005;82(3–4):198–207. doi: 10.1159/000092520. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Chyou S, Lin I, Onishi M, Patel P, Zheng KH. Initial licking responses of mice to sweeteners: effects of tas1r3 polymorphisms. Chemical Senses. 2005;30(7):601–614. doi: 10.1093/chemse/bji054. [DOI] [PubMed] [Google Scholar]
- Glendinning JI, Feld N, Goodman L, Bayor R. Contribution of orosensory stimulation to strain differences in oil intake by mice. Physiology and Behavior. 2008;95(3):476–483. doi: 10.1016/j.physbeh.2008.07.017. [DOI] [PubMed] [Google Scholar]
- Grill HJ, Norgren R. The taste reactivity test. I. Mimetic responses to gustatory stimuli in neurologically normal rats. Brain Research. 1978;143(2):263–279. doi: 10.1016/0006-8993(78)90568-1. [DOI] [PubMed] [Google Scholar]
- Horowitz GP, Stephan FK, Smith JC, Whitney G. Genetic and environmental variability in lick rates of mice. Physiology and Behavior. 1977;19(4):493–496. doi: 10.1016/0031-9384(77)90224-4. [DOI] [PubMed] [Google Scholar]
- Inoue M, Glendinning JI, Theodorides ML, Harkness S, Li X, Bosak N, et al. Allelic variation of the Tas1r3 taste receptor gene selectively affects taste responses to sweeteners: evidence from 129.B6-Tas1r3 congenic mice. Physiological Genomics. 2007;32(1):82–94. doi: 10.1152/physiolgenomics.00161.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakaye B, Adamantidis A, Coumans B, Grisar T. Promoter characterization of the mouse melanin-concentrating hormone receptor 1. Biochimica Biophysica Acta. 2004;1678(1):1–6. doi: 10.1016/j.bbaexp.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Mace OJ, Affleck J, Patel N, Kellett GL. Sweet taste receptors in rat small intestine stimulate glucose absorption through apical GLUT2. Journal of Physiology. 2007;582(Pt 1):379–392. doi: 10.1113/jphysiol.2007.130906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandel P, Ayad G, Hermetet JC, Ebel A. Correlation between choline acetyltransferase activity and learning ability in different mice strains and their offspring. Brain Research. 1974;72(1):65–70. doi: 10.1016/0006-8993(74)90650-7. [DOI] [PubMed] [Google Scholar]
- Margolskee RF, Dyer J, Kokrashvili Z, Salmon KS, Ilegems E, Daly K, et al. T1R3 and gustducin in gut sense sugars to regulate expression of Na+-glucose cotransporter 1. Proceedings of the National Academy of Sciences of United States of America. 2007;104(38):15075–15080. doi: 10.1073/pnas.0706678104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montmayeur JP, Matsunami H. Receptors for bitter and sweet taste. Current Opinion in Neurobiology. 2002;12(4):366–371. doi: 10.1016/s0959-4388(02)00345-8. [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Katakura N. Generation of masticatory rhythm in the brainstem. Neuroscience Research. 1995;23(1):1–19. [PubMed] [Google Scholar]
- Norgren R. Gustatory system. In: Paxino G, editor. The rat nervous system. Academic Press; San Diego, CA: 1995. pp. 751–771. [Google Scholar]
- Rhinehart-Doty JA, Schumm J, Smith JC, Smith GP. A non-taste cue of sucrose in short-term taste tests in rats. Chemical Senses. 1994;19(5):425–431. doi: 10.1093/chemse/19.5.425. [DOI] [PubMed] [Google Scholar]
- Robinson SW, Dinulescu DM, Cone RD. Genetic models of obesity and energy balance in the mouse. Annual Review of Genetics. 2000;34:687–745. doi: 10.1146/annurev.genet.34.1.687. [DOI] [PubMed] [Google Scholar]
- Schoenbaum G, Garmon JW, Setlow B. A novel method for detecting licking behavior during recording of electrophysiological signals from the brain. Journal of Neuroscience Methods. 2001;106(2):139–146. doi: 10.1016/s0165-0270(01)00341-7. [DOI] [PubMed] [Google Scholar]
- Sclafani A. Enhanced sucrose and polycose preference in sweet “sensitive” (C57BL/6J) and “subsensitive” (129P3/J) mice after experience with these saccharides. Physiology and Behavior. 2006;87(4):745–756. doi: 10.1016/j.physbeh.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Sclafani A, Cardieri C, Tucker K, Blusk D, Ackroff K. Intragastric glucose but not fructose conditions robust flavor preferences in rats. American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 1993;265(2 Pt 2):R320–R325. doi: 10.1152/ajpregu.1993.265.2.R320. [DOI] [PubMed] [Google Scholar]
- Smith GP. John Davis and the meanings of licking. Appetite. 2001;36(1):84–92. doi: 10.1006/appe.2000.0371. [DOI] [PubMed] [Google Scholar]
- Spector AC, Klumpp PA, Kaplan JM. Analytical issues in the evaluation of food deprivation and sucrose concentration effects on the microstructure of licking behavior in the rat. Behavioral Neuroscience. 1998;112(3):678–694. doi: 10.1037//0735-7044.112.3.678. [DOI] [PubMed] [Google Scholar]
- Travers SP, Norgren R. Coding the sweet taste in the nucleus of the solitary tract: differential roles for anterior tongue and nasoincisor duct gustatory receptors in the rat. Journal of Neurophysiology. 1991;65(6):1372–1380. doi: 10.1152/jn.1991.65.6.1372. [DOI] [PubMed] [Google Scholar]
- Treesukosol Y, Blonde GD, Spector AC. T1R2 and T1R3 subunits are individually unnecessary for normal affective licking responses to polycose: implications for saccharide taste receptors in mice. American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 2009;296(4):R855–R865. doi: 10.1152/ajpregu.90869.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebayashi H, Hatanaka T, Kanemura F, Tonosaki K. Acute anosmia in the mouse: behavioral discrimination among the four basic taste substances. Physiology and Behavior. 2001;72(3):291–296. doi: 10.1016/s0031-9384(00)00441-8. [DOI] [PubMed] [Google Scholar]
- Wickens TD. Models for behavior. Freeman; San Francisco, CA: 1984. [Google Scholar]
- Zhao GQ, Zhang Y, Hoon MA, Chandrashekar J, Erlenbach I, Ryba NJ, et al. The receptors for mammalian sweet and umami taste. Cell. 2003;115(3):255–266. doi: 10.1016/s0092-8674(03)00844-4. [DOI] [PubMed] [Google Scholar]
- Zukerman S, Glendinning JI, Margolskee RF, Sclafani A. T1R3 taste receptor is critical for sucrose but not polycose taste. American Journal of Physiology: Regulatory Integrative and Comparative Physiology. 2009;296(4):R866–R876. doi: 10.1152/ajpregu.90870.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]