Abstract
Symptoms associated with menopause can greatly affect the quality of life for women. Botanical dietary supplements have been viewed by the public as safe and effective despite a lack of evidence indicating a urgent necessity to standardize these supplements chemically and biologically. Seventeen plants were evaluated for estrogenic biological activity using standard assays: competitive estrogen receptor (ER) binding assay for both alpha and beta subtypes, transient transfection of the estrogen response element luciferase plasmid into MCF-7 cells expressing either ER alpha or ER beta, and the Ishikawa alkaline phosphatase induction assay for both estrogenic and antiestrogenic activities. Based on the combination of data pooled from these assays, the following was determined: a) a high rate of false positive activity for the competitive binding assays, b) some extracts had estrogenic activity despite a lack of ability to bind the ER, c) one extract exhibited selective estrogen receptor modulator (SERM) activity, and d) several extracts show additive/synergistic activity. Taken together, these data indicate a need to reprioritize the order in which the bioassays are performed for maximal efficiency of programs involving bioassay-guided fractionation. In addition, possible explanations for the conflicts in the literature over the estrogenicity of Cimicifuga racemosa (black cohosh) are suggested.
Keywords: botanicals, estrogen, estrogen assays, herbal dietary supplements, menopause, selective estrogen receptor modulators
Introduction
Symptoms associated with menopause such as insomnia, loss of libido, vaginal atrophy, depression, and hot flashes can greatly affect the quality of life for women. Many women have used hormone replacement therapy (HRT) to alleviate menopausal symptoms; however, with the publication of the Women’s Health Initiative in 2002, the number of women using HRT has decreased dramatically [1, 2]. Even before the publication of several large studies of HRT, many women have been turning to herbal remedies for the relief of menopausal symptoms [3–5], perhaps because they are viewed as safe [6]. Among the herbal remedies currently being used, many contain phytochemicals that mimic the effects of human estrogen in vitro or in vivo. Such phytochemicals are commonly referred to as phytoestrogens. Unfortunately, few botanicals are chemically and biologically standardized to relevant active compounds using an appropriate mechanism of action [7, 8].
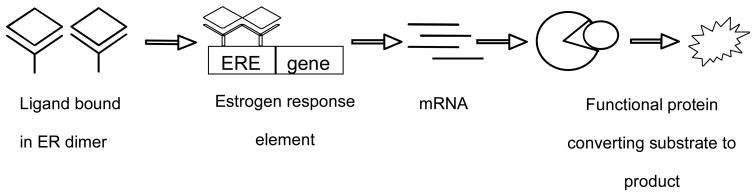
There are a number of different target points along the estrogen signaling pathway. Initially the ligand will bind the estrogen receptor (ER) and the dimerized complex will move from the cytosol of the cell through the nuclear pore and into the nucleus. Once inside the nucleus, the ER-dimer will bind to the estrogen response element (ERE) which is located upstream of estrogen-controlled genes. Once bound, coactivators, corepressors, and transcription factors will bind, and transcription will be initiated. During translation, the mRNA will be converted into protein, and posttranslational modifications will then complete the signaling pathway to produce a functional protein.
The ERα and ERβ [3H]-estradiol competitive binding assay [9], ERE-luciferase (ERE-luc) induction [10] in MCF-7 ERα positive and ERβ positive cell lines, and the Ishikawa alkaline phosphatase assay [11] are assays that target key points in the ER signaling pathway. The general paradigm is that a ligand must bind the estrogen receptor in order to have any downstream effects (Scheme 1); therefore, the competitive binding assay is often utilized as the primary screen [12] to determine if a botanical is further characterized for estrogenic activity. Next, the functional activity, agonist or antagonist, is evaluated using cell-based assays at different points along the pathways. Unfortunately, complicating the matter of characterizing estrogenic activity is the lack of a naturally endogenously expressing ERβ positive cell line [13], which has raised the question of how to show functional activity for ERβ ligands. Cell lines have been stably transfected with ERβ; however, it is clear that the value of an ER stably transfected cell line is not the same as an endogenously expressing cell line [13], since the downstream cellular milieu does not necessarily remain intact. For example, an endogenously expressing ERα cell line will proliferate in the presence of physiological levels of 17β estradiol; whereas, the stably transfected cell line S30 will not grow [14]. However, it is possible to use stable cell lines that are transfected with the estrogen response element-luciferase plasmid [14] since the ERE is the immediate downstream target of the ligand-bound ER.
Scheme 1.
Simplified depiction of the classical estrogen receptor signaling pathway from ligand binding to functional protein.
The purpose of this research was to evaluate seventeen plants (Table 1) using a variety of established cell-free and cell-based estrogenic assays to biologically characterize the botanicals. Since both estrogens and selective serotonin reuptake inhibitors (SSRIs) have been known to alleviate symptoms associated with menopause [15], plants were selected based on a literature search of the Natural Products Alert (NAPRALERT) database for the most widely used remedies for menopausal symptoms, menstrual disorders, or reported estrogenic, serotonergic, anti-steroidogenic, and anti-fertility activity. These plants were obtained, extracted, and evaluated for estrogenic activity in vitro using the ERα and ERβ [3H]-estradiol competitive binding assay, ERE-luc induction in MCF-7 ERα positive and ERβ positive cell lines, and the Ishikawa alkaline phosphatase assay.
Table 1.
Plants Selected for Estrogenic Screening.
| Botanical name | Plant part | Common name | Source | Rational |
|---|---|---|---|---|
| Alisma plantago-aquatica L. | rhizomes | Water Plantain | 1 PureWorld | menopausal symptoms [33] |
| Angelica sinensis (Oliv.) Diels | roots | Dang-Gui | 2 Yin Wall City, Inc | menopausal symptoms; (4, 81) serotonergic [34] |
| Asclepias tuberosa L. | roots | Butterfly Weed | 2 Blessedherbs | contains steroidal compounds [35] |
| Beta vulgaris L. | roots | Beets | 2 local grocery | menopausal symptoms [36] |
| Cimicifuga americana Michaux | roots | Yellow Cohosh | 3 Swain County, NC | chemotaxonomic relationship [37] |
| Cimicifuga racemosa (L.) Nutt. | aerial | Black Cohosh | 3 Sevier County, TN | related plant part |
| Cimicifuga racemosa (L.) Nutt. | roots | Black Cohosh | 3 Sevier County, TN | menopausal symptoms [38–49] serotonergic [22, 50] |
| Cimicifuga rubifolia (Kearney) Kartesz | aerial | Appalachian Bugbane | 3 Hancock County; TN | chemotaxonomic relationship [37] |
| Cornus officinalis Sieb. & Zucc. | fruits | Dogwood | 1 PureWorld | menopausal symptoms [33] |
| Daucus carota L. | roots | Queen Anne’s lace; Carrot | 2 Local grocery store | anti-steroidogenic activity [51]; estrogenic activity [52]; antifertility activity [53] |
| Paeonia moutan Sims | bark | Peony | 1 PureWorld | menopausal symptoms [54] |
| Pueraria lobata (Willd.) Ohwi. | aerial | Kudzu | 3 Evanston, IL | estrogenic activity (98, 99); menopausal symptoms [55]; serotonergic activity [56] |
| Pueraria mirifica Airy, Shaw & Suvatabandhu | bark | Kwao Keur | 1 PureWorld | estrogenic activity [57–60] |
| Valeriana officinalis L. | roots | Valerian | 1 PureWorld | serotonergic activity [61] |
| Viburnum opulus L. | bark | Cramp Bark | 1 PureWorld | smooth muscle antispasmodic [62] |
| Viburnum prunifolium L. | bark | Black Haw | 1 PureWorld | menopausal symptoms [63] |
| Vitex agnus-castus L. | fruits | Chasteberry | 1 PureWorld | menstrual disorders [64–69]; menopausal symptoms [70] |
Provided
Purchased
Collected
Materials and Methods
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) or Fisher Scientific (Itasca, IL) unless stated otherwise.
Plant Material
As indicated in Table 1, Angelica sinensis (roots) was purchased at Yin Wall City, Chicago, IL (2001). Asclepias tuberosa was bought from www.blessedherbs.com. Cimicifuga americana (aerial parts) was collected in Swain County, NC. Cimicifuga racemosa (aerial parts) was collected in Sevier County, TN. Cimicifuga rubifolia (aerial parts) was collected in Hancock County, TN. Pueraria lobata was collected in Evanston, IL. Alisma plantago-aquatica (rhizomes and roots), Cimicifuga racemosa (rhizomes and roots), Cornus officinalis (fruits), Paeonia moutan (bark), Polygonum multiflorum (roots), Pueraria mirifica (bark), Valeriana officinalis (roots), Viburnum opulus (bark), Viburnum prunifolium (bark), and Vitex agnus-castus (berries), were provided by PureWorld Botanicals, now NATUREX (South Hackensack, NJ). Beta vulgaris (roots) and Daucus carota (roots) were purchased from a local grocery store. Voucher specimens have been deposited at the Pharmacognosy Field Station, Department of Medicinal Chemistry and Pharmacognosy, College of Pharmacy, University of Illinois at Chicago, and verified by botanist Dr. D. D. Soejarto.
Extraction
Sequential percolation was used to extract the botanicals as follows: Plant material (200 g each) except A. sinesis was minced and macerated in petroleum ether (PE, 600 mL) overnight, and percolated exhaustively with the same solvent (total 8 L). The marc was macerated in dichloromethane (CH2Cl2, 600 mL) overnight, and percolated exhaustively with CH2Cl2 (8 L). Finally, the marc was macerated in 75% ethanol (EtOH, 600 mL) overnight, and percolated exhaustively with of 75% EtOH (8 L). The PE, CH2Cl2, and 75% EtOH percolates were combined separately, and the solvents were removed in vacuo to yield respective extracts of different polarity.
The minced sample of A. sinensis (2 kg) was macerated in CH3OH (2 L, 24 h) and percolated exhaustively with CH3OH (6 L). The percolates were combined and the solvent evaporated in vacuo to yield the CH3OH extract. The CH3OH extract was dissolved in 15% aqueous CH3OH and partitioned with petroleum ether (3 × 1 L). The aqueous-methanol partition was evaporated in vacuo to remove CH3OH, and the remaining aqueous partition was successively partitioned with CHCl3 (3 × 1 L) and n-butanol (BuOH, 3 × 1 L). Removal of the solvent yielded the petroleum ether, CHCl3, BuOH, and H2O soluble partition.
ERα and ERβ competitive binding assays
The competitive ERα and ERβ binding assays were used with tritiated estradiol based on the method of Obourn et al. [9] with minor modifications [16], to determine in vitro binding affinities of the substrates with the receptors. The reaction mixture consisted of sample in DMSO (5 μL), pure human recombinant diluted ERα and ERβ (0.5 pmol, 5 μL) in ER binding buffer, “Hot Mix” [400 nM, 5 μL prepared fresh using 95 Ci/mmol [3H] estradiol, diluted in 1:1 ethanol:ER binding buffer; obtained from NEN Life Science Products (Boston, MA)], and ER binding buffer (85 μL). The incubation was carried out at room temperature for 2 h before a hydroxyappatite slurry (HAPs, 50%, 100 μL) was added. The tubes were incubated on ice for 15 min with vortexing every 5 min. The appropriate ER wash buffer was added (1 mL), and the tubes were vortexed before centrifuging at 10,000 × g for 1 min. The supernatant was discarded, and this wash step was repeated three times. The HAPs pellet containing the ligand-receptor complex was resuspended in ethanol (200 μL) and transferred to scintillation vials. An additional volume of ethanol (200 μL) was used to rinse the centrifuge tube. Cytoscint [4 mL/vial; ICN (Costa Mesa, CA)] was added, and the radioactivity was counted using a Beckman LS 5801 liquid scintillation counter (Schaumburg, IL). The percent inhibition of [3H] estradiol binding to each ER was determined using Equation 1. The binding capability (percent) of the sample was calculated in comparison with that of estradiol (50 nM, 90%).
| Equation 1 |
Cell Culture Conditions
The Ishikawa cell line was provided by R. B. Hochberg (Yale University, New Haven, CT) and was maintained in Dulbecco’s Modified Eagle medium (DMEM/F12) containing sodium pyruvate (1%), non-essential amino acids (NEAA, 1%), glutamax-1 (1%), insulin (0.05%), and heat-inactivated fetal bovine serum (FBS, 10%). MCF-7 WS8 cells were provided by V. C. Jordan (Fox Chase Cancer Center) and were grown in RPMI 1640 media containing glutamax-1 (1%), NEAA (1%), insulin (0.05%), and heat-inactivated FBS (5%). The MCF-7 C4-12-5 ERβ positive stable cell line ([17] referred to as MCF-7 ERβ) was provided by D. B. Lubahn (University of Missouri) and was grown in MEM (catalogue number 3024) supplemented with stripped calf bovine serum (CBS, 5%), Pen/Strep (2%), insulin (6 ng/mL), sodium carbonate (2.2 g/L), HEPES (1.25 M, 8 mL), glutamax (1%), and G418S (50 mg/mL stock, 6 mL). Stripped serum was prepared by incubating the serum with acetone-washed activated charcoal (100 mg/mL) at 4 °C for 30 min, and centrifuged at 4,000 RPM for 15 min at 4 °C. This step was repeated in triplicate. DMSO concentrations for all cell culture assays were below 0.1%.
Induction of alkaline phosphatase in cultured Ishikawa cells
The procedure of Pisha et al. [11] with minor modifications [16] was used. Ishikawa cells (1.5 × 104 cells/190 μL/well) were preincubated in 96-well plates overnight in estrogen-free medium. Test samples (20 μg/mL final concentration in DMSO) were added to the cells in a total volume (200 μL media/well) were incubated at 37 °C for 4 days. For the determination of antiestrogenic activity, media used to dilute the test samples was supplemented with estradiol (2 nM). The induction plates were processed by washing the plates with PBS and adding Triton × 100 (0.01%, 50 μL) in Tris buffer (pH 9.8, 0.1 M). Plates were subjected to a freeze thaw (−80 °C for at least 24 h before warming to 37 °C). An aliquot (150 μL) of p-nitrophenylphosphate (phosphatase substrate, 1 mg/mL) in Tris buffer (pH. 9.8, 0.1 M) was added to each well. The enzyme activity was measured by reading the release of p-nitrophenol at 405 nm every 15 s with a 10 s shake between readings for 16 readings using a Power Wave 200 microplate scanning spectrophotometer (Bio-Tek Instruments, Winooski, VT). The maximal slopes of the lines generated by the kinetic readings were calculated. Estrogenic induction was calculated using Equation 2, and for antiestrogenic determination, the percent induction as compared with the background induction control was calculated using Equation 3.
| Equation 2 |
| Equation 3 |
Measure of ERE activation
The Dual-Luciferase Reporter Assay System from Promega (Madison, WI) was used to evaluate the functional formation of the ER-ERE complex and luciferase protein expression. Both MCF-7 WS8 and MCF-7 ERβ cell lines were cultured in estrogen-free media 96 h before transfection. The cells were transfected with the pERE-luciferase plasmid (2 μg), which contains three copies of the Xenopus laevis vitellogenin A2 ERE upstream of fire fly luciferase (a gift from Dr. V.C. Jordan). To normalize transfection efficiency, pRL-TK plasmid (1 μg, Promega) was co-transfected. Cells (5 × 106) in serum-free media were transfected by electroporation in a 0.4 cm cuvette (Bio-Rad Laboratories) at a voltage (0.320 kV) and a high capacitance (950 μF) in a GenePulser X-cell (Bio-Rad Laboratories). The cells were resuspended in estrogen-free media, transferred to 6-well plates immediately after electroporation, and incubated overnight. The cells were treated with the extracts for 24 h. The luciferase activities in the cell lysates were measured using the Dual-Luciferase Reporter Assay System from Promega (Madison, WI) with a FLUOstar OPTIMA (BMG LABTECH, Durham, NC).
Results
ER alpha and ER beta Competitive Assay
The plant extracts were screened in the ERα and ERβ competitive assay at 200 μg/mL (n = 5, two independent measurements). Extracts where the mean percent inhibitory activity was within one standard deviation of 50% or greater were considered active (Table 2). Six plants were active in the ERα assay Fig. (1): the petroleum ether extracts of A. sinensis (81%), A. tuberosa (69%), C. racemosa (aerial parts; 55%), V. officinalis (43%), the petroleum ether and the dichloromethane extracts of C. rubifolia (56% and 51%, respectively), and the dichloromethane extract of P. lobata (63%). There were thirteen plant extracts that bound the ERβ Fig. (1): the 75% ethanol and the dichloromethane extracts of P. lobata (63 and 45%, respectively), the petroleum ether extract and chloroform partition of A. sinensis (99 and 46%, respectively), the petroleum ether extracts of A. plantago-aquatica (51%), A. tuberosa (85%), B. vulgaris (44%), C. americana (47%), C. officinalis (48%), C. racemosa (aerial parts; 64%), C. rubifolia (66%), P. moutan (60%), P. multiflorum (BC 268; 50%), V. officinalis (81%), and V. agnus-castus (68%).
Table 2.
Screening results in ER Binding, ERE-luciferase, and Alkaline Phosphatase Assays.
| Plant or sample name | Extract Type | 1 Binding ER alpha | 2,3 ERE-luc ER alpha | 3,4 ALP Estrogenic | 3,4 ALP Antiestrogenic | 3,4 SRB Cytotoxicity | 1 Binding ER beta | 2, 3 ERE-luc ER beta |
|---|---|---|---|---|---|---|---|---|
| 17 β estradiol | Control | 95 ± 1 | 21 ± 7 | 100 ± 10 | −10 ± 8 | 4 ± 2 | 90 ± 1 | 7 ± 3 |
| 4-hydroxytamoxifen | Control | 96 ± 1 | 1 ± 0.1 | 0 ± 5 | 94 ± 1 | 2 ± 1 | 94 ± 1 | 1 ± 0.2 |
| DMSO | Control | 0 | 1 ± 0.2 | 1 ± 2 | 0 ± 5 | 1 ± 2 | 0 | 1 ± 0.2 |
|
| ||||||||
| Alisma plantago-aquatica | Petroleum ether | 31 ± 5 | 0.9 ± 0.4 | −7.3 ± 4.2 | −1.5 ± 13.3 | 10.7 ± 16.2 | 51 ± 7 | 2.3 ± 1.9 |
|
| ||||||||
| Angelica sinensis | Petroleum ether | 581 ± 10 | 9.7 ± 3.5 | −4.7 ± 6.2 | 48.7 ± 9.5 | 16.4 ± 13.6 | 99 ± 9 | 1.1 ± 0.4 |
| Chloroform | 19 ± 9 | 5.9 ± 3 | 12.3 ± 4.7 | 19.4 ± 9.8 | 30.6 ± 8 | 46 ± 6 | 0.8 ± 0.5 | |
| Butanol | 4 ± 3 | 9.5 ± 2.2 | 12.4 ± 8.2 | −16.3 ± 4.5 | 3.3 ± 15.2 | 0 ± 2 | 1.2 ± 0.2 | |
|
| ||||||||
| Asclepias tuberosa | Petroleum ether | 69 ± 7 | 1.1 ± 0.4 | −17.9 ± 15.5 | −9.1 ± 21 | −19.9 ± 9.8 | 85 ± 5 | 1.1 ± 0.8 |
| Dichloromethane | 16 ± 9 | 4.1 ± 3.1 | −26.5 ± 4.5 | 114.6 ± 6.7 | 106.1 ± 5.3 | 32 ± 10 | 10.7 ± 0. | |
| 75% ethanol | 0 ± 2 | 3.9 ± 4.3 | −10.3 ± 8.9 | 110.1 ± 8.5 | 99.1 ± 3.6 | 5 ± 8 | 1.3 ± 1.1 | |
|
| ||||||||
| Beta vulgaris | Petroleum ether | 35 ± 10 | 4.7 ± 1.9 | −2.4 ± 17.4 | 4.4 ± 14.8 | −15.9 ± 29.4 | 44 ± 7 | 1.1 ± 0.3 |
| Dichloromethane | 33 ± 11 | 3.2 ± 2 | −2.3 ± 5.5 | 43.7 ± 8.6 | −20.2 ± 30.9 | 30 ± 5 | 1.1 ± 0.1 | |
|
| ||||||||
| Cimicifuga americana | Petroleum ether | 37 ± 8 | 1.6 ± 0.7 | −5.8 ± 14.4 | 27.1 ± 27.5 | −3.7 ± 37 | 47 ± 10 | 1.4 ± 0.3 |
| Dichloromethane | 31 ± 5 | 2.8 ± 1.1 | −16.2 ± 14.9 | 79.2 ± 19.5 | 5.8 ± 32.1 | 21 ± 9 | 1.4 ± 0.6 | |
|
| ||||||||
| Cimicifuga racemosa | DCM extract | 34 ± 7 | 6.6 ± 3.2 | −2.5 ± 1.3 | 3.5 ± 8.7 | 8.3 ± 5.2 | 45 ± 3 | 1.1 ± 0.4 |
| 75% EtOH | 21 ± 5 | 5.8 ± 1.8 | 4.3 ± 5.3 | −48.4 ± 4.9 | 0.1 ± 5.7 | 23 ± 3 | 1 ± 0.2 | |
| PE extract | 55 ± 8 | 3.2 ± 1.5 | −4.5 ± 4.8 | 21.3 ± 7.2 | 4.8 ± 10.9 | 64 ± 8 | 1 ± 0.5 | |
|
| ||||||||
| Cimicifuga rubifolia | Petroleum ether | 56 ± 2 | 2.5 ± 2 | −13.1 ± 12.4 | 33.2 ± 17 | −23.2 ± 25 | 66 ± 6 | 1.1 ± 0.5 |
| Dichloromethane | 51 ± 5 | 7.4 ± 1.1 | 0.3 ± 15.9 | 21 ± 14.2 | 4.4 ± 19.8 | 40 ± 8 | 0.8 ± 0.3 | |
|
| ||||||||
| Cornus officinalis | Petroleum ether | 26 ± 16 | 24.3 ± 6.6 | 2.6 ± 5.9 | 41.8 ± 4.1 | 11.5 ± 3.4 | 48 ± 4 | 1.5 ± 0.5 |
| Dichloromethane | 0 ± 4 | 2.5 ± 0.7 | −2.2 ± 4.8 | 24.9 ± 5.2 | 30.6 ± 19.1 | 0 ± 2 | 1.3 ± 0.3 | |
|
| ||||||||
| Daucus carota | Petroleum ether | 21 ± 6 | 12.3 ± 5.1 | −6.8 ± 11.2 | −26.6 ± 14.6 | −18.7 ± 19.1 | 36 ± 6 | 0.9 ± 0.5 |
| Dichloromethane | 22 ± 5 | 5.4 ± 1.8 | −5.7 ± 8 | −11.5 ± 16.1 | 13.7 ± 14.1 | 26 ± 6 | 0.7 ± 0.2 | |
|
| ||||||||
| Paeonia moutan | Petroleum ether | 21 ± 5 | 2.8 ± 1 | −9.7 ± 11.4 | 8.7 ± 16.7 | 7.7 ± 19.7 | 60 ± 6 | 2 ± 1.7 |
|
| ||||||||
| Polygonum multiflorum | Petroleum ether | 15 ± 8 | 21.7 ± 8.5 | 6.5 ± 1.8 | 1.2 ± 3.3 | 7.6 ± 14.3 | 50 ± 9 | 1.4 ± 0.7 |
| Dichloromethane | 30 ± 7 | 25.8 ± 11.2 | 12.3 ± 3.4 | 0.4 ± 12.3 | 12.7 ± 17.8 | 36 ± 13 | 3 ± 2.5 | |
|
| ||||||||
| Pueraria lobata | Petroleum ether | 32 ± 2 | 10.3 ± 2.8 | −10.8 ± 12.6 | 46.9 ± 18.9 | 12.7 ± 30 | 36 ± 2 | 1.5 ± 1.4 |
| Dichloromethane | 63 ± 6 | 24.5 ± 9.9 | −0.3 ± 25.6 | 36 ± 32.6 | −3.3 ± 5.5 | 45 ± 19 | 1.2 ± 0.5 | |
| 75% ethanol | 17 ± 16 | 26.9 ± 12.7 | −1.3 ± 13.8 | 28.9 ± 40.7 | 7.6 ± 50.1 | 63 ± 3 | 3.5 ± 1.3 | |
|
| ||||||||
| Pueraria mirifica | Petroleum ether | 12 ± 8 | 6 N.T. | −2.7 ± 2.6 | 58.5 ± 37 | 100.6 ± 38.8 | 18 ± 12 | N.T. |
| Dichloromethane | 12 ± 5 | 25.7 ± 9.5 | −4.2 ± 1.1 | 101.1 ± 8.6 | 115 ± 2.2 | 19 ± 11 | 3.2 ± 1.6 | |
| 75% ethanol | 18 ± 7 | 28 ± 8.6 | −1.4 ± 4.8 | 52.2 ± 61 | 60.4 ± 62.2 | 31 ± 8 | 2.4 ± 1.3 | |
|
| ||||||||
| Valeriana officinalis | Petroleum ether | 43 ± 11 | 7.2 ± 3.1 | −7.8 ± 7.6 | 33.7 ± 16.8 | 12.5 ± 19.3 | 81 ± 4 | 1.3 ± 0.8 |
| Dichloromethane | 13 ± 12 | 5.9 ± 1.6 | −8.5 ± 7.7 | 19.4 ± 8.2 | −7.6 ± 21 | 13 ± 6 | 0.7 ± 0.1 | |
|
| ||||||||
| Viburnum prunifolium | Dichloromethane | 3 ± 6 | 0.7 ± 0.5 | −3.5 ± 3 | 68.5 ± 6.3 | 42 ± 4.4 | 3 ± 4 | 1 ± 0 |
|
| ||||||||
| Vitex agnus-castus | Petroleum ether | 37 ± 6 | 9.5 ± 2.5 | −1.1 ± 1.6 | 43.5 ± 3.3 | 39.1 ± 8.3 | 68 ± 9 | 1.1 ± 0.7 |
| Dichloromethane | 13 ± 3 | 9.6 ± 6.6 | −2.3 ± 1.5 | 94.5 ± 7.2 | 112.5 ± 8.8 | 20 ± 19 | 0.8 ± 0.3 | |
Percent binding at 200 μg/mL.
Fold induction where DMSO is 1.
Tested at 20 μg/mL.
Percent induction (estrogenic), inhibition (antiestrogenic), or cytotoxic.
Bold face-type indicates extract with assay activity.
Not tested due to the limited quantities available.
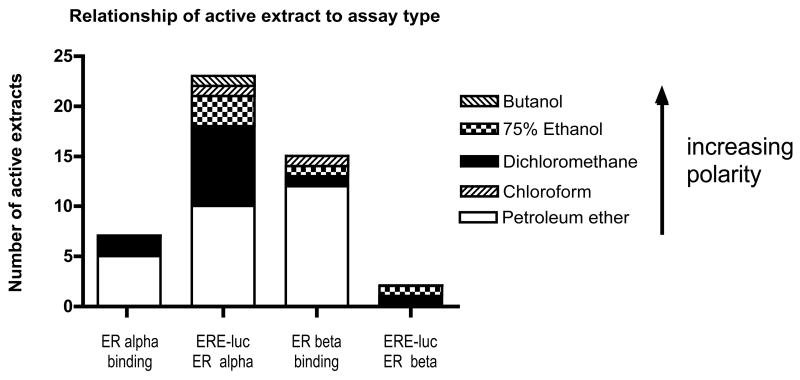
Figure 1.
Bar chart representation of the frequency of extracts with activity in either ER alpha binding or ER beta binding assays and ERE-luc ER alpha or ERE-luc ER beta positive cell lines.
Estrogenic Alkaline Phosphatase Induction in the Ishikawa Cell Line
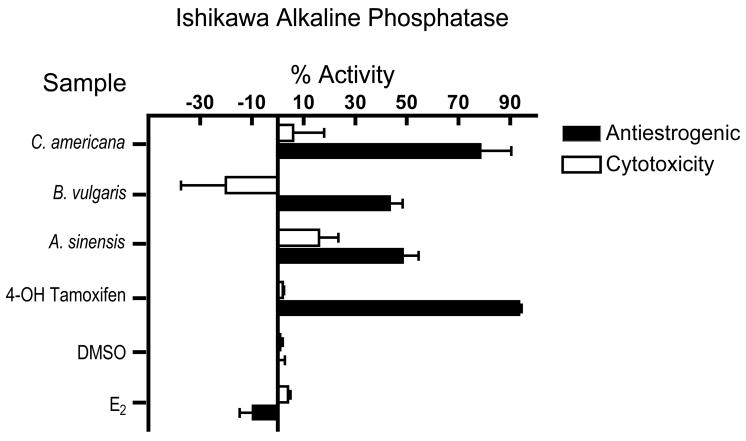
At the tested concentration of 20 μg/mL (n = 3 independent times in triplicate), the petroleum ether extract of A. sinensis (49%), and the dichloromethane extracts of B. vulgaris (44%), C. americana (79%) had antiestrogenic activity that did not appear to be caused by cytotoxicity Fig. (2). Cytotoxicity can have a negative impact on the results of the alkaline phosphatase assays when it is above 20%, as well as on the ERE-luc assay. In the estrogenic ALP assay, cytotoxicity was apparent at 20 μg/mL for the dichloromethane extracts of A. tuberosa (106%), C. officinalis (31%), P. multiflorum (BC 286; 31%), P. mirifica (115%), V. prunifolium (42%), and V. agnus-castus (112%), the petroleum ether extracts of V. agnus-castus (39%), P. mirifica (100%), and C. officinalis (31%), the 75% ethanol extracts of A. tuberosa (99%), P. mirifica (60%), and the chloroform partition of A. sinensis (31%). In the ERE-luc assay ERα positive cell line, cytotoxicity was apparent in all of the A. tuberosa treated samples as indicated by the high standard deviation and low value for the transfection control vector, pRL-TK.
Figure 2.
Antiestrogenic and cytotoxic graph of active samples and controls. The petroleum ether extract of A. sinensis, and the dichloromethane extracts of B. vulgaris and C. americana had antiestrogenic activity without apparent cytotoxicity in the Ishikawa alkaline phosphatase inhibition assay when tested at 20 μg/mL. Samples were tested at least three independent times in triplicate. Samples were considered active if they were within one standard deviation or greater of 50% for the antiestrogenic assay. Samples with greater than 20% cytotoxicity are know to interfere with the accuracy of the antiestrogenic assay by causing a false positive result.
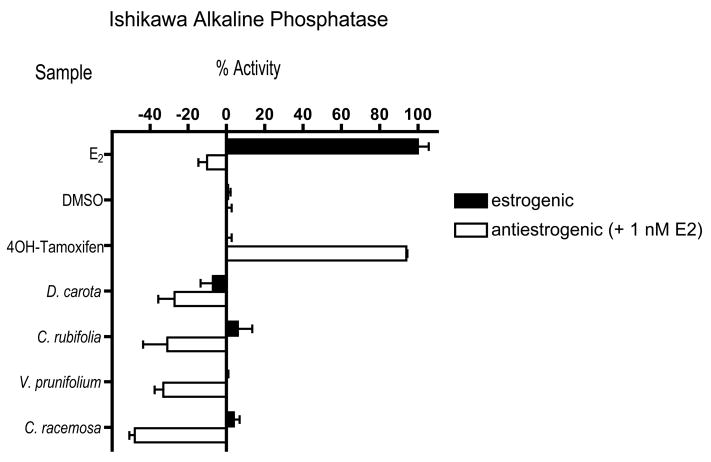
Interestingly, some extracts when combined with estradiol appear to have a greater estrogenic response than the extract alone Fig. (3). The 75% ethanol extract of C. racemosa, aerial parts had a −48% antiestrogenic response indicating that more alkaline phosphatase enzyme was induced when compared to the extract without 17β estradiol (4%). Also, the 75% ethanol extract of V. prunifolium also had a similar effect when combined with 17β estradiol (−33%) compared to the extract alone (0%). This may also be true for the 75% ethanol extract of C. rubifolia (−31%) and the petroleum ether extract of D. carota (−27%), but the standard deviations for both samples were large (22 and 15, respectively).
Figure 3.
Samples with possible synergistic activity when combined with 17β estradiol in the Ishikawa alkaline phosphatase assay. Samples were treated alone to determine the inherent estrogenic activity of the sample, or in combination with 1 nM E2 to determine if there was an antiestrogenic effect of the samples. 17β Estradiol shows the stereotypical estrogenic response alone and is therefore estrogenic, while 4-hydroxytamoxifen shows a stereotypical antiestrogenic response when combined with 1 nM E2, but it is not estrogenic alone. The 75% ethanol extract of C. racemosa (aerial parts) was not estrogenic alone, but when combined with 1 nM E2 was actually more estrogenic than when E2 is tested in the presence of 1 nM E2. The 75% ethanol extract of V. prunifolium and C. rubifolia, and the petroleum ether extract of D. carota are also displayed. Samples were tested in triplicate at least three independent times, and are represented as averages ± standard deviation.
ERE-luciferase Induction in ER alpha and ER beta Positive Cell Lines
Following the ERα and ERβ competitive assay, the transient transfection of MCF-7 WS8 or MCF-7 C4-12-5 ERβ+ cell lines with the ERE-luciferase plasmid was used to help confirm functional activity of the extracts that had activity in the isolated ER competitive assay. The cells were treated with extracts (20 μg/mL) for 24 h, and the activity was first normalized for transfection efficiency using the pRL-TK vector, and then normalizing to the DMSO control (DMSO = 1). Extracts with > two–fold induction were considered active (Table 1; n ≥ 3).
In the MCF-7 WS8 (ERα positive) cell line, the petroleum ether, chloroform, and butanol partitions of A. sinensis (9.7, 5.9, and 9.5, respectively), the petroleum ether, dichloromethane, and 75% ethanol extracts of P. lobata (10.3, 24.5, and 26.9, respectively), the dichloromethane and 75% ethanol extracts of C. racemosa (aerial parts; 6.6 and 5.8, respectively) and P. mirifica (25.7 and 28.0, respectively), the petroleum ether and dichloromethane extracts of D. carota (12.3 and 5.4, respectively), P. multiflorum (BC 268: 21.7 and 25.8; BC 286: 15.1 and 16, respectively), V. officinalis (7.2 and 5.9), and V. agnus-castus (9.5 and 9.6, respectively), the petroleum ether extracts of B. vulgaris (4.7), C. rubifolia (7.4), C. officinalis (24.3) were active Fig. (1). All extracts of A. tuberosa were cytotoxic at 20 μg/mL, which caused some false positive activity. In the MCF-7 ERβ cell line, none of the petroleum ether extracts were active. The 75% ethanol extract of P. lobata (3.5) and the dichloromethane extracts of A. tuberosa (10.7) and P. mirifica (3.2) had activity Fig. (1).
Discussion
The UIC/NIH Center for Botanical Dietary Supplements Research (the Center) was established in the fall of 1999 to address issues of standardization, quality, safety, and efficacy of botanical dietary supplements. Using a multidisciplinary strategy to achieve its basic and clinical research objectives, the Center has focused on botanicals with potential benefits for women’s health. Based on these data, over time a paradigm shift has occurred within the Center in utilizing the assays to provide the most meaningful data. For example, during the initial years when testing botanicals in the Center, the competitive estrogen receptor binding assay was primarily performed, and then the induction of alkaline phosphatase in Ishikawa cells was used to determine if the botanicals were acting as antagonists or agonists. The logical thought was that botanicals that were not active in the competitive binding assay would not be active in the alkaline phosphatase assay. However, recently increased evidence of non-classical estrogen receptor signaling has been more fully understood [18–20]. Additionally, the Center has identified botanicals that do not bind to the isolated receptor, but exhibited activity in the cell-based assays. Furthermore, C. racemosa (roots) is an example of a botanical that questioned this paradigm since it was not estrogenic either in vitro or in vivo [21, 22]; however, it is one of the most popular botanicals used by menopausal women. Alternative mechanisms such as the serotonergic [22] and opiod [23] pathways have since been implicated.
In order to identify plants that might have a beneficial effect on symptoms associated with menopause, a NAPRALERT search was conducted to select plants with previously reported hormonal or neurotransmitter activity. Among the plants selected, nine plants had previously been reported to be useful for the relief of menopause symptoms, and four plants were specified as having estrogenic activity. In general, to cover the whole range of polarity for phytoconstituents three extracts of each plant, petroleum ether, dichloromethane, and 75% ethanol, were prepared and tested in the isolated ERα and ERβ competitive assay, ERE-luc fold-induction in both ERα and ERβ positive breast cancer cell lines, and the induction of alkaline phosphatase in the Ishikawa endometrial cell line. The extracts were also tested in a secondary assay for cytotoxicity. Due to the nature of in vitro assays we have had to define for the purpose of evaluating these data the terms “false positive” and “false negative”. False positive activity is attributed to a mechanism assumed to be independent of the tested mechanism such as non-specific binding of lipophilic material to an isolated receptor which blocks the tritiated-estradiol from specifically binding to the binding pocket. A false negative result has been defined to mean a lack of activity which is attributed to a mechanism assumed to be independent of the tested mechanism such as a sample that is cytotoxic rather than specifically inhibits a specific enzymatic reaction in the cell.
The initial biological characterization of the extracts indicated that many of the petroleum ether extracts had activity in the ER binding assays, particularly in the ERβ assay, where twelve of the fifteen active extracts were petroleum ether extracts Fig. (1). Petroleum ether extracts are known to contain fatty acids, which have been reported in the literature to non-specifically bind isolated receptors and/or may only have weak estrogenic activity [24]. This hypothesis was supported by the observation that, it was not surprising when only one extract, the 75% ethanol extract of P. lobata, which had activity in the ERβ competitive assay also had activity in the ERE-luc assay in the ERβ positive cell line Fig. (1), an observation consistent with other reported literature [25, 26]. In the ERα assays, there was a correlation between ERα competitive binding activity and ERE-luc assay for the petroleum ether extract of A. sinensis (81% binding affinity and 9.7 fold induction) and the dichloromethane extracts of C. rubifolia, and P. lobata. For the latter two extracts, there was relatively weak activity (51% and 63%, respectively) in the ERα competitive binding assay, but relatively strong fold-induction (7.4 and 25, respectively) at one-tenth the concentration in the ERE-luc assay.
However, what was unexpected was the number of extracts that did not bind the receptor, but did have activity in the ERE-luc assay Fig. (1). In the ERβ assays, A. tuberosa and P. lobata were the only plants that did not have activity in the competitive ERβ binding assay while having activity in the ERE-luc assay in ERβ cells. In the ERα assays, this was also the case for the tested chloroform and butanol partitions of A. sinensis and certain extracts of C. racemosa, aerial parts, C. officinalis, D. carota, P. multiflorum, P. lobata, P. mirifica, V. officinalis, and V. agnus-castus. One important example is A. sinensis, which did not have ERα isolated receptor competitive binding activity for any of the partitions except for petroleum ether, but showed potent activity in the ERE-luc assay for both the chloroform and butanol partitions. While the petroleum ether extracts generally did not have functional activity, A. sinensis did have functional activity in the ERE-luc assay and antiestrogenic activity in the Ishikawa alkaline phosphatase assay. Angelica sinensis is one of the most commonly used herbs in China for relief of PMS and menopause; however, the mechanism of action has not clearly been identified. One reason might be the interesting pattern of activity where it can have receptor binding activity, ERE-luc and antiestrogenic activity as in the case of the petroleum ether extract, but it also might have components that are not ligands for the receptor, but still have activity in the ERE-luc assay.
Classically, extracts that do not bind the estrogen receptor would not be thought to be active in the ERE-luc assay, which is generally used to identify ligand-ER complexes that bind the ERE causing gene transcription. The disconnection between the binding to the estrogen receptor and the activation of the ERE has raised many hypotheses. One is that these plant extracts do not bind to the ER, but instead use a non-classical estrogen pathway that phosphorylates the ER [27]. The non-liganded, phosphorylated ER has been demonstrated to bind the ERE and recruit transcription factors. This could explain why it has been challenging to explain the mechanism of action for plants that have been traditionally used by women, but when the extracts were scientifically tested, they do not contain ER ligands. Another hypothesis is that the extract contains ligands that do not competitively bind to the ER, but instead bind to a second binding site [28]. The full implication of this situation with different structures has yet to be explored, but does raise some interesting possibilities. These hypotheses raise the concern that women taking extracts that are considered to be “non-estrogenic” due to health concerns of hormone-dependent cancers might still be at risk. A third explanation might be metabolism of botanical compounds in the cells to estrogenic products. It has previously been shown that biochanin A, a compound found in Trifolium pratense, does not inherently have estrogenic activity, but can be metabolically converted in the MCF-7 WS8 cell line to the estrogenic compound, genistein [16]. Similarly, isoxanthohumol, a compound found in Humulus lupulus, can also be metabolized to 8-prenylnaringenin, a potent phytoestrogen [16, 29].
In the Ishikawa assay, none of the extracts had agonist activity, but some had antiestrogenic activity that appeared to be unrelated to cytotoxicity or direct inhibition of alkaline phosphatase. At 20 μg/mL the petroleum ether partition of A. sinensis was the only sample that had ERα activity in all three assays. In fact, A. sinensis might be considered a true SERM since it was an agonist in the ERE-luc assay, but had antagonistic activity in the Ishikawa assay. Angelica sinensis may also have selective tissue effects since the antiestrogenic Ishikawa assay was in an endometrial cell line, while the ERE-luc was in a breast cancer cell line. Other extracts that appear to have antiestrogenic activity in the ALP assay were the dichloromethane extracts of B. vulgaris and C. americana. It is noteworthy that the ethnobotanical use of B. vulgaris has generally been attributed to its vitamin content rather than the presence of antiestrogenic compound(s). Cimicifuga americana has not been reported in the literature as being used to alleviate any hormone-related symptoms; however, it is taxonomically closely related to C. racemosa.
There were some unexpected results in the alkaline phosphatase assay. Some extracts, had large negative values in the antagonist ALP assay. This initially was overlooked, but after carefully review of the data, and multiple independent repetitions of the assay, the values were determined to be reproducible. The antagonist assay was designed to identify compounds that blocked the effect of estrogen. When this occurs, the ALP enzyme is not produced, and substrate is not converted to product. Therefore, a score of 100% in this assay would indicate complete blockage of the estrogenic activity, and 0% would indicate that the estrogenic activity led to the ALP enzyme converting substrate to product. When the data go into the negative range, it might indicate a synergistic effect with estrogen where even more ALP is produced causing faster conversion of substrate to product Fig. (3).
Four plants had negative values in the antagonist ALP assay indicating a possible synergistic activity: the 75% ethanol extracts of C. racemosa (aerial parts), C. rubifolia, and V. prunifolium, and the petroleum ether extracts of D. carota. The activity was most prevalent in the 75% ethanol extract of C. racemosa (aerial parts). The extract alone at 20 μg/mL was not estrogenic in the Ishikawa assay (4.3%); however, when combined with estrogen it had a −48% activity in the antiestrogenic ALP assay. These data might be interpreted as the extract combined with estrogen induced enough ALP enzyme to convert substrate to product twice as fast as estrogen alone. This may have occurred by the extract working through a non-estrogen pathway that upregulates the ER, such as the progesterone pathway. A striking observation was that this extract also had activity in the ERE-luc assay (5.8 fold-induction) in the ERα positive cell line, but did not bind to the estrogen receptor (24%). Investigation of the contribution of aerial parts to the biological profile of adulterated C. racemosa preparations may also contribute to the resolution of the conflict in the literature revolving around the estrogenic activity of C. racemosa [30, 31]. While completely unexplored in the literature, the biological activities of the aerial parts of C. racemosa clearly deserve further investigation.
Cytotoxicity can play a large role in the false positive and false negative interpretation of cell based assays. In the ALP assays, cytotoxicity could cause a false negative in the agonist assay, and similarly, cytotoxicity can cause a false positive in the antagonist ALP assay where a lack of enzyme is really caused by a lack of cell viability. At 20 μg/mL, the dichloromethane and the 75% ethanol extracts of A. tuberosa were completely cytotoxic in the Ishikawa cell line, and was the likely cause of the large standard deviations in the ERE-luc assay. The vector control for A. tuberosa in the ERE-luc assay was low for a few of the replicates compared with other samples tested in parallel. This indicates that the cells were not able to utilize the control vector, because the cells were not viable rather than due to poor transfection efficiency, which is what the control vector is supposed to indicate. This resulted in dividing by a small denominator, which resulted in a large product with a large standard deviation. The dichloromethane and the 75% ethanol extracts had activity in the ERE-luc in ERα positive cells, and the dichloromethane extract had activity in the ERβ positive cells. When the values for the transfection control vector were evaluated, data were consistent with other plant extracts that did not have cytotoxicity. Another case where cytotoxicity was a factor was for P. mirifica, which was completely cytotoxic for the petroleum ether and dichloromethane extracts, and 60% cytotoxic for the 75% ethanol extract when tested in the Ishikawa cell line. While P. mirifica has been reported to be estrogenic in the literature [32], a different and more gentle extraction procedure was used in the present study and the lack of estrogenicity may be a result of the cytotoxicity, extraction procedure, or a combination of both. Of interest was that, while the extracts were toxic in the four-day assays, they did not appear to have a significant effect on the 24-hour assays.
In summary, based on these findings, the Center has moved toward cell-based assays for evaluating the hormonal activities of botanicals. Employing a diverse panel of bioassays, several extracts have been identified which do not conform to the classical estrogen receptor signaling pathway as they do not bind the estrogen receptor, but appear to have cell-based activity in the ERE-luc assay. Extracts that are not inherently estrogenic, but appear to increase the estrogenic activity in the presence of estrogen have also been identified. Finally, the synergistic activity opens avenues that need to be explored concerning the overall biological activity of the plant extracts and the value of multiple bioassays in characterizing the plant activities.
Supplementary Material
Acknowledgments
This work was supported, in part, by grant P50 AT00155 provided jointly by the National Center for Complementary and Alternative Medicine (NCCAM), the Office of Dietary Supplements (ODS), the Office for Research on Women’s Health (ORWH), and the National Institute of General Medicine NIGMS) of the National Institutes of Health (NIH). C.R.O. is grateful for a Ruther L. Kirschstein NCCAM Predoctoral fellowship F31 AT 24232. The contents of this paper are solely the responsibility of the authors and do not necessarily represent the official views of NIH.
List of abbreviations
- ER
estrogen receptor
- HRT
hormone replacement therapy
- ERE
estrogen response element
- ERE-luc
ERE-luciferase
- SSRIs
selective serotonin reuptake inhibitors
- NAPRALERT
Natural Products Alert
- SERMs
selective estrogen receptor modulators
- PE
petroleum ether
- DMEM/F12
Dublecco’s Modified Eagle medium
- NEAAs
non-essential amino acids
- FBS
fetal bovine serum
- CBS
calf bovine serum
- HAPs
hydroxyappatitie slurry
Cited Literature
- 1.Hersh AL, Stefanick ML, Stafford RS. National use of postmenopausal hormone therapy: annual trends and response to recent evidence. JAMA. 2004;291:47–53. doi: 10.1001/jama.291.1.47. [DOI] [PubMed] [Google Scholar]
- 2.Haas JS, Kaplan CP, Gerstenberger EP, Kerlikowske K. Changes in the use of postmenopausal hormone therapy after the publication of clinical trial results. Ann Intern Med. 2004;140:184–188. doi: 10.7326/0003-4819-140-3-200402030-00009. [DOI] [PubMed] [Google Scholar]
- 3.Murkies AL, Wilcox G, Davis SR. Clinical review 92: Phytoestrogens. Clin Endocrinol Metab. 1998;83:297–303. doi: 10.1210/jcem.83.2.4577. [DOI] [PubMed] [Google Scholar]
- 4.Setchell KD. Phytoestrogens: the biochemistry, physiology, and implications for human health of soy isoflavones. Am J Clin Nutr. 1998;68:1333S–1346S. doi: 10.1093/ajcn/68.6.1333S. [DOI] [PubMed] [Google Scholar]
- 5.Setchell KD, Cassidy A. Dietary isoflavones: biological effects and relevance to human health. J Nutr. 1999;129:758S–767S. doi: 10.1093/jn/129.3.758S. [DOI] [PubMed] [Google Scholar]
- 6.Anonymous. What are bioidentical hormones. Harvard Women’s Health Watch. 2006;13:1–3. [PubMed] [Google Scholar]
- 7.Calixto JB. Efficacy, safety, quality control, marketing and regulatory guidelines for herbal medicines (phytotherapeutic agents) Braz J Med Biol Res. 2000;33:179–189. doi: 10.1590/s0100-879x2000000200004. [DOI] [PubMed] [Google Scholar]
- 8.van Breemen RB, Fong HHS, Farnsworth NR. The Role of Quality Assurance and Standardization in the Safety of Botanical Dietary Supplements. Chem Res Toxicol. 2007;20:577–582. doi: 10.1021/tx7000493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Obourn JD, Koszewski NJ, Notides AC. Hormone- and DNA-binding mechanisms of the recombinant human estrogen receptor. Biochemistry. 1993;32:6229–6236. doi: 10.1021/bi00075a016. [DOI] [PubMed] [Google Scholar]
- 10.Catherino WH, Jordan VC. Increasing the number of tandem estrogen response elements increases the estrogenic activity of a tamoxifen analogue. Cancer Letters. 1995;92:39–47. doi: 10.1016/0304-3835(95)03755-l. [DOI] [PubMed] [Google Scholar]
- 11.Pisha E, Pezzuto JM. Cell-based assay for the determination of estrogenic and anti-estrogenic activities. Methods in Cell Science:An Official Journal of the Society for In Vitro Biology. 1997;19:37–43. [Google Scholar]
- 12.Soto AM, Maffini Maricel V, Schaeberle Cheryl M, Sonnenschein C. Strengths and weaknesses of in vitro assays for estrogenic and androgenic activity. Best Practice and Research Clinical Endocrinology and Metabolism. 2006;20:15–33. doi: 10.1016/j.beem.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 13.Mueller SO. Overview of in vitro tools to assess the estogenic and antiestrogenic activity of phytoestrogens. Journal of Chromatography B: Analytical Technologies in the Biomedical & Life Sciences. 2002;777:155–165. doi: 10.1016/s1570-0232(02)00282-9. [DOI] [PubMed] [Google Scholar]
- 14.Tonetti DA, Rubenstein R, DeLeon M, Zhao H, Pappas SG, Bentrem DJ, Chen B, Constantinou A, Craig Jordan V. Stable transfection of an estrogen receptor beta cDNA isoform into MDA-MB-231 breast cancer cells. Journal of Steroid Biochemistry & Molecular Biology. 2003;87:47–55. doi: 10.1016/j.jsbmb.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 15.Nelson HD, Vesco KK, Haney E, Fu R, Nedrow A, Miller J, Nicolaidis C, Walker M, Humphrey L. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057–2071. doi: 10.1001/jama.295.17.2057. [DOI] [PubMed] [Google Scholar]
- 16.Overk CR, Yao P, Chadwick LR, Nikolic D, Sun Y, Cuendet MA, Deng Y, Hedayat AS, Pauli GF, Farnsworth NR, van Breemen RB, Bolton JL. Comparison of the in vitro estrogenic activities of compounds from hops (Humulus lupulus) and red clover (Trifolium pratense) J Agric Food Chem. 2005;53:6246–6253. doi: 10.1021/jf050448p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oesterreich Steffi, Zhang Ping, Guler Rebecca L, Sun Xiuhua, Curran Edward M, Welshons Wade V, Osborne C Kent, Lee Adrian V. Re-expression of Estrogen Receptor alpha in Estrogen Receptor alpha-negative MCF-7 Cells Restores both Estrogen and Insulin-like Growth Factor-mediated Signaling and Growth. Cancer Res. 2001;61:5771–5777. [PubMed] [Google Scholar]
- 18.Kelly Martin J, Qiu Jian, Wagner Edward J, Ronnekleiv Oline J. Rapid effects of estrogen on G protein-coupled receptor activation of potassium channels in the central nervous system (CNS) J Steroid Biochem Mol Biol. 2003;83:187–193. doi: 10.1016/s0960-0760(02)00249-2. [DOI] [PubMed] [Google Scholar]
- 19.Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol. 2002;80:231–238. doi: 10.1016/s0960-0760(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 20.Edwards Dean P. Regulation of signal transduction pathways by estrogen and progesterone. Annu Rev Physiol. 2005;67:335–376. doi: 10.1146/annurev.physiol.67.040403.120151. [DOI] [PubMed] [Google Scholar]
- 21.Liu J, Burdette JE, Xu H, Gu C, van Breemen RB, Bhat KP, Booth N, Constantinou AI, Pezzuto JM, Fong HH, Farnsworth NR, Bolton JL. Evaluation of estrogenic activity of plant extracts for the potential treatment of menopausal symptoms. J Agric Food Chem. 2001;49:2472–2479. doi: 10.1021/jf0014157. [DOI] [PubMed] [Google Scholar]
- 22.Burdette JE, Liu J, Chen SN, Fabricant DS, Piersen CE, Barker EL, Pezzuto JM, Mesecar A, Van Breemen RB, Farnsworth NR, Bolton JL. Black cohosh acts as a mixed competitive ligand and partial agonist of the serotonin receptor. J Agric Food Chem. 2003;51:5661–5670. doi: 10.1021/jf034264r. [DOI] [PubMed] [Google Scholar]
- 23.Rhyu MR, Lu J, Webster DE, Fabricant DS, Farnsworth NR, Wang ZJ. Black cohosh (Actaea racemosa, Cimicifuga racemosa) behaves as a mixed competitive ligand and partial agonist at the human mu opiate receptor. J Agric Food Chem. 2006;54:9852–9857. doi: 10.1021/jf062808u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J, Burdette JE, Sun Y, Deng S, Schlecht SM, Zheng W, Nikolic D, Mahady G, van Breemen RB, Fong HH, Pezzuto JM, Bolton JL, Farnsworth NR. Isolation of linoleic acid as an estrogenic compound from the fruits of Vitex agnus-castus L. (chaste-berry) Phytomedicine. 2004;11:18–23. doi: 10.1078/0944-7113-00331. [DOI] [PubMed] [Google Scholar]
- 25.Boue SM, Wiese TE, Nehls S, Burow ME, Elliott S, Carter-Wientjes CH, Shih BY, McLachlan JA, Cleveland TE. Evaluation of the estrogenic effects of legume extracts containing phytoestrogens. J Agric Food Chem. 2003;51:2193–2199. doi: 10.1021/jf021114s. [DOI] [PubMed] [Google Scholar]
- 26.Zhang CZ, Wang SX, Zhang Y, Chen JP, Liang XM. In vitro estrogenic activities of Chinese medicinal plants traditionally used for the management of menopausal symptoms. J Ethnopharmacol. 2005;98:295–300. doi: 10.1016/j.jep.2005.01.033. [DOI] [PubMed] [Google Scholar]
- 27.Pettersson K, Gustafsson JA. Role of estrogen receptor beta in estrogen action. Annu Rev Physiol. 2001;63:165–192. doi: 10.1146/annurev.physiol.63.1.165. [DOI] [PubMed] [Google Scholar]
- 28.Wang Y, Chirgadze NY, Briggs SL, Khan S, Jensen EV, Burris TP. A second binding site for hydroxytamoxifen within the coactivator-binding groove of estrogen receptor beta. Proc Natl Acad Sci U S A. 2006;103:9908–9911. doi: 10.1073/pnas.0510596103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nikolic D, Li Y, Chadwick LR, Pauli GF, van Breemen RB. Metabolism of xanthohumol and isoxanthohumol, prenylated flavonoids from hops (Humulus lupulus L.), by human liver microsomes. J Mass Spectrom. 2005;40:289–299. doi: 10.1002/jms.753. [DOI] [PubMed] [Google Scholar]
- 30.Piersen CE. Phytoestrogens in botanical dietary supplements: implications for cancer. Integr Cancer Ther. 2003;2:120–138. doi: 10.1177/1534735403002002004. [DOI] [PubMed] [Google Scholar]
- 31.Kronenberg F, Fugh-Berman A. Complementary and alternative medicine for menopausal symptoms: a review of randomized, controlled trials. Ann Intern Med. 2002;137:805–813. doi: 10.7326/0003-4819-137-10-200211190-00009. [DOI] [PubMed] [Google Scholar]
- 32.Cherdshewasart W, Subtang S, Dahlan W. Major isoflavonoid contents of the phytoestrogen rich-herb Pueraria mirifica in comparison with Pueraria lobata. J Pharm Biomed Anal. 2007;43:428–434. doi: 10.1016/j.jpba.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 33.Liang R, Chen MR, Xu X. Effect of dandi tablet on blood lipids and sex hormones in women of postmenopausal stage. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2003;23:593–595. [PubMed] [Google Scholar]
- 34.Deng S, Chen SN, Yao P, Nikolic D, van Breemen RB, Bolton JL, Fong HH, Farnsworth NR, Pauli GF. Serotonergic activity-guided phytochemical investigation of the roots of Angelica sinensis. J Nat Prod. 2006;69:536–541. doi: 10.1021/np050301s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abe F, Yamauchi T. An androstane bioside and 3′-thiazolidinone derivatives of doubly-linked cardenolide glycosides from the roots of Asclepias tuberosa. Chem Pharm Bull. 2000;48:991–993. doi: 10.1248/cpb.48.991. [DOI] [PubMed] [Google Scholar]
- 36.Rosso R, Brema F, Porcile GF, Santi L. Antitumoral activity of calusterone in advanced mammary carcinoma (author’s transl) Tumori. 1976;62:79–84. doi: 10.1177/030089167606200108. [DOI] [PubMed] [Google Scholar]
- 37.He K, Pauli GF, Zheng B, Wang H, Bai N, Peng T, Roller M, Zheng Q. Cimicifuga species identification by high performance liquid chromatography-photodiode array/mass spectrometric/evaporative light scattering detection for quality control of black cohosh products. J Chromatogr. 2006;1112:241–254. doi: 10.1016/j.chroma.2006.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garita-Hernandez M, Calzado MA, Caballero FJ, Macho A, Muänoz E, Meier B, Brattstrèom A, Fiebich BL, Appel K. The growth inhibitory activity of the Cimicifuga racemosa extract Ze 450 is mediated through estrogen and progesterone receptors-independent pathways. Planta Med. 2006;72:317–323. doi: 10.1055/s-2005-916233. [DOI] [PubMed] [Google Scholar]
- 39.Nappi RE, Malavasi B, Brundu B, Facchinetti F. Efficacy of Cimicifuga racemosa on climacteric complaints: a randomized study versus low-dose transdermal estradiol. Gynecol Endocrinol. 2005;20:30–35. doi: 10.1080/09513590400020922. [DOI] [PubMed] [Google Scholar]
- 40.Mahady GB. Black cohosh (Actaea/Cimicifuga racemosa): review of the clinical data for safety and efficacy in menopausal symptoms. Treat Endocrinol. 2005;4:177–184. doi: 10.2165/00024677-200504030-00006. [DOI] [PubMed] [Google Scholar]
- 41.Huntley A. The safety of black cohosh (Actaea racemosa, Cimicifuga racemosa) Expert Opin Drug Saf. 2004;3:615–623. doi: 10.1517/14740338.3.6.615. [DOI] [PubMed] [Google Scholar]
- 42.Dog TL, Powell KL, Weisman SM. Critical evaluation of the safety of Cimicifuga racemosa in menopause symptom relief. Menopause. 2003;10:299–313. doi: 10.1097/01.GME.0000056039.51813.21. [DOI] [PubMed] [Google Scholar]
- 43.Borrelli F, Izzo AA, Ernst E. Pharmacological effects of Cimicifuga racemosa. Life Sci. 2003;73:1215–1229. doi: 10.1016/s0024-3205(03)00378-3. [DOI] [PubMed] [Google Scholar]
- 44.Anonymous. Cimicifuga racemosa. Monograph Altern Med Rev. 2003;8:186–189. [PubMed] [Google Scholar]
- 45.Popp M, Schenk R, Abel G. Cultivation of Cimicifuga racemosa (L.) nuttal and quality of CR extract BNO 1055. Maturitas. 2003;44:S1–7. doi: 10.1016/s0378-5122(02)00343-2. [DOI] [PubMed] [Google Scholar]
- 46.Borrelli F, Ernst E. Cimicifuga racemosa: a systematic review of its clinical efficacy. Eur J Clin Pharmacol. 2002;58:235–241. doi: 10.1007/s00228-002-0457-2. [DOI] [PubMed] [Google Scholar]
- 47.Pepping J. Black cohosh: Cimicifuga racemosa. Am J Health Syst Pharm. 1999;56:1400–1402. doi: 10.1093/ajhp/56.14.1400. [DOI] [PubMed] [Google Scholar]
- 48.Lieberman S. A review of the effectiveness of Cimicifuga racemosa (black cohosh) for the symptoms of menopause. J Womens Health. 1998;7:525–529. doi: 10.1089/jwh.1998.7.525. [DOI] [PubMed] [Google Scholar]
- 49.Dèuker EM, Kopanski L, Jarry H, Wuttke W. Effects of extracts from Cimicifuga racemosa on gonadotropin release in menopausal women and ovariectomized rats. Planta Med. 1991;57:420–424. doi: 10.1055/s-2006-960139. [DOI] [PubMed] [Google Scholar]
- 50.Fabricant DS, Nikolic D, Lankin DC, Chen SN, Jaki BU, Krunic A, van Breemen RB, Fong HH, Farnsworth NR, Pauli GF. Cimipronidine, a cyclic guanidine alkaloid from Cimicifuga racemosa. J Nat Prod. 2005;68:1266–1270. doi: 10.1021/np050066d. [DOI] [PubMed] [Google Scholar]
- 51.Majumder PK, Dasgupta S, Mukhopadhaya RK, Mazumdar UK, Gupta M. Anti-steroidogenic activity of the petroleum ether extract and fraction 5 (fatty acids) of carrot (Daucus carota L.) seeds in mouse ovary. J Ethnopharmacol. 1997;57:209–212. doi: 10.1016/s0378-8741(97)00056-1. [DOI] [PubMed] [Google Scholar]
- 52.Sharma MM, Lal G, Jacob D. Estrogenic and pregnancy interceptory effects of carrot daucus carota seeds. Indian J Exp Biol. 1976;14:506–508. [PubMed] [Google Scholar]
- 53.Kapoor M, Garg SK, Mathur VS. Anthiovulatory activity of five indigenous plants in rabbits. Indian J Med Res. 1974;62:1225–1227. [PubMed] [Google Scholar]
- 54.Miller-Martini DM, Chan RY, Ip NY, Sheu SJ, Wong YH. A reporter gene assay for the detection of phytoestrogens in traditional Chinese medicine. Phytother Res. 2001;15:487–492. doi: 10.1002/ptr.767. [DOI] [PubMed] [Google Scholar]
- 55.Woo J, Lau E, Ho SC, Cheng F, Chan C, Chan AS, Haines CJ, Chan TY, Li M, Sham A. Comparison of Pueraria lobata with hormone replacement therapy in treating the adverse health consequences of menopause. Menopause. 2003;10:352–361. doi: 10.1097/01.GME.0000054764.94658.33. [DOI] [PubMed] [Google Scholar]
- 56.Chueh FS, Chang CP, Chio CC, Lin MT. Puerarin acts through brain serotonergic mechanisms to induce thermal effects. J Pharmacol Sci. 2004;96:420–427. doi: 10.1254/jphs.fp0040424. [DOI] [PubMed] [Google Scholar]
- 57.Chansakaow S, Ishikawa T, Seki H, Sekine K, Okada M, Chaichantipyuth C. Identification of deoxymiroestrol as the actual rejuvenating principle of “Kwao Keur”, Pueraria mirifica. The known miroestrol may be an artifact. J Nat Prod. 2000;63:173–175. doi: 10.1021/np990547v. [DOI] [PubMed] [Google Scholar]
- 58.Malaivijitnond S, Kiatthaipipat P, Cherdshewasart W, Watanabe G, Taya K. Different effects of Pueraria mirifica, a herb containing phytoestrogens, on LH and FSH secretion in gonadectomized female and male rats. J Pharmacol Sci. 2004;96:428–435. doi: 10.1254/jphs.fpj04029x. [DOI] [PubMed] [Google Scholar]
- 59.Trisomboon H, Malaivijitnond S, Watanabe G, Taya K. Estrogenic effects of Pueraria mirifica on the menstrual cycle and hormone-related ovarian functions in cyclic female cynomolgus monkeys. J Pharmacol Sci. 2004;94:51–59. doi: 10.1254/jphs.94.51. [DOI] [PubMed] [Google Scholar]
- 60.Cherdshewasart W, Cheewasopit W, Picha P. The differential anti-proliferation effect of white (Pueraria mirifica), red (Butea superba), and black (Mucuna collettii) Kwao Krua plants on the growth of MCF-7 cells. J Ethnopharmacol. 2004;93:255–260. doi: 10.1016/j.jep.2004.03.041. [DOI] [PubMed] [Google Scholar]
- 61.Dietz BM, Mahady GB, Pauli GF, Farnsworth NR. Valerian extract and valerenic acid are partial agonists of the 5-HT5a receptor in vitro. Brain Res Mol Brain Res. 2005;138:191–197. doi: 10.1016/j.molbrainres.2005.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nicholson JA, Darby TD, Jarboe CH. Viopudial, a hypotensive and smooth muscle antispasmodic from Viburnum opulus. Proc Soc Exp Biol Med. 1972;140:457–461. doi: 10.3181/00379727-140-36479. [DOI] [PubMed] [Google Scholar]
- 63.Xu H, Fabricant DS, Piersen CE, Bolton JL, Pezzuto JM, Fong H, Totura S, Farnsworth NR, Constantinou AI. A preliminary RAPD-PCR analysis of Cimicifuga species and other botanicals used for women’s health. Phytomedicine. 2002;9:757–762. doi: 10.1078/094471102321621403. [DOI] [PubMed] [Google Scholar]
- 64.Daniele C, Thompson Coon J, Pittler MH, Ernst E. Vitex agnus castus: a systematic review of adverse events. Drug Saf. 2005;28:319–332. doi: 10.2165/00002018-200528040-00004. [DOI] [PubMed] [Google Scholar]
- 65.Wuttke W, Jarry H, Christoffel V, Spengler B, Seidlovâa-Wuttke D. Chaste tree (Vitex agnus-castus)--pharmacology and clinical indications. Phytomedicine. 2003;10:348–357. doi: 10.1078/094471103322004866. [DOI] [PubMed] [Google Scholar]
- 66.Atmaca M, Kumru S, Tezcan E. Fluoxetine versus Vitex agnus castus extract in the treatment of premenstrual dysphoric disorder. Hum Psychopharmacol. 2003;18:191–195. doi: 10.1002/hup.470. [DOI] [PubMed] [Google Scholar]
- 67.Schellenberg R. Treatment for the premenstrual syndrome with agnus castus fruit extract: prospective, randomised, placebo controlled study. Brit Med J. 2001;322:134–137. doi: 10.1136/bmj.322.7279.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berger D, Schaffner W, Schrader E, Meier B, Brattstrèom A. Efficacy of Vitex agnus castus L. extract Ze 440 in patients with pre-menstrual syndrome (PMS) Arch Gynecol Obstet. 2000;264:150–153. doi: 10.1007/s004040000123. [DOI] [PubMed] [Google Scholar]
- 69.Loch EG, Selle H, Boblitz N. Treatment of premenstrual syndrome with a phytopharmaceutical formulation containing Vitex agnus castus. J Womens Health Gend Based Med. 2000;9:315–320. doi: 10.1089/152460900318515. [DOI] [PubMed] [Google Scholar]
- 70.Chopin Lucks B. Vitex agnus castus essential oil and menopausal balance: a research update [Complementary Therapies in Nursing and Midwifery 8 (2003) 148–154] Complement Ther Nurs Midwifery. 2003;9:157–160. doi: 10.1016/S1353-6117(03)00020-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.