Abstract
Fascioliasis is a worldwide zoonotic infection with Fasciola hepatica and Fasciola gigantica. The zoonoses are particularly endemic in sheep-raising countries and are also endemic in Iran. Typical symptoms that may be associated with fascioliasis can be divided by phases of the disease, including the acute or liver phase, the chronic or biliary phase, and ectopic or pharyngeal fascioliasis. Cholestatic symptoms may be absent, and in some cases diagnosis and treatment may be preceded by a long period of abdominal pain, eosinophilia and vague gastrointestinal symptoms. We report a case with epigastric and upper quadrant abdominal pain for the last 4 years, with imaging suggesting cholangiocarcinoma. Considering a new concept of endoscopic ultrasonography, at last F. hepatica was extracted with endoscopic retrograde cholangiography.
Key words: Magnetic resonance cholangiopancreatography, Endoscopic ultrasonography, Endoscopic retrograde cholangiopancreatography, Fasciola hepatica
Introduction
Fasciola hepatica is a trematode liver fluke that infects primarily sheep, goats and cattle. The adult fluke is large, flat, brownish and leaf-shaped, its size being 2.5 × 1 cm. There has been an increase in F. hepatica infections worldwide and it is reported that 2.5 million people have been infected and more than 180 million people are at risk [1]. Migrating metacercariae cause liver parenchymal destruction since migratory tracks undergo necrosis and fibrosis. The amount of liver damage is correlated with the parasite load. Adult flukes can partially obstruct the bile ducts, causing thickening, dilatation and fibrosis of the proximal biliary tree. Ectopic fascioliasis in other sites also results in eosinophilic and mononuclear infiltration with secondary tissue damage.
Many infections in humans are mild, but morbidity can increase with heavier fluke burdens. Typical symptoms that may be associated with fascioliasis can be divided by phases of the disease, including the acute or liver phase, the chronic or biliary phase, and ectopic or pharyngeal fascioliasis. The biliary phase is usually asymptomatic, but the adult flukes can obstruct the common duct. Chronic infection can lead to biliary colic, cholangitis, cholelithiasis and obstructive jaundice. Prolonged and heavy infections can also result in sclerosing cholangitis and biliary cirrhosis. Pain in the epigastrium and right upper quadrant, diarrhea, nausea, vomiting, wasting, hepatomegaly and jaundice can occur. Eosinophilia is a variable finding in this phase of the disease. All serologic tests have good sensitivity, but many have suboptimal specificity and cross-react with other parasitic infections.
Ultrasonography (US) seems to be more useful than computed tomography (CT) in the biliary stage of the disease. Irregular thickening of the common bile duct (CBD) wall and biliary dilatation are the findings visualized by abdominal CT and US that are suspicious for fascioliasis [2]. Invasive techniques, such as percutaneous cholangiography and endoscopic retrograde cholangiopancreatography (ERCP), can reveal some abnormalities, but are generally not required for the diagnosis [3]. Endoscopic ultrasonography (EUS) emerged as a novel modality for diagnosis of biliary stage. ERCP can be employed in both diagnosis and treatment.
Case Report
A 45-year-old female with epigastric and right upper quadrant pain was admitted to Taleghani hospital, Tehran, Iran. There had been intermittent pain attacks radiating to the back, exacerbated after eating, for the last 4 years. Each episode of pain lasted approximately 1 h and decreased spontaneously or after a few days. Sometimes it led to awakening the patient at 2-3 o'clock AM. She stated weight loss of about 10 kg within 4 years. She was symptom-free between episodes and had no complaints of nausea, vomiting or itching. In her past medical history, there was no important medical problem except for appendectomy 10 years earlier. Famotidin 40 mg twice a day and trifluoperazine 1 mg three times a day were given. Physical examination revealed only mild epigastric tenderness. There were not fever, icterus and other significant findings.
Complete blood count showed hematocrit 44%, platelets 277,000/mm3 (normal 100,000-450,000), and white blood cells 5,300/mm3 (normal 4,000-10,000) with 10% eosinophils on peripheral smear. The erythrocyte sedimentation rate was 15 mm/h and C-reactive protein level was 5.92 mg/dl (normal 0-5). Blood chemistry tests were in the normal range. Table 1 presents the patient's paraclinical results.
Table 1.
Paraclinical results
| Test | Value | Normal range |
|---|---|---|
| White blood cells, /mm3 | 5,300 | 4,000–10,000 |
| Eosinophils, % | 10 | <5 |
| Hematocrit, % | 44 | 40–45 |
| Platelets, /mm3 | 277,000 | 100,000–450,000 |
| C-reactive protein, mg/dl | 5.92 | 0–5 |
| Erythrocyte sedimentation rate, mm/h | 15 | <30 |
| Fasting blood sugar, mg/dl | 92 | 80–116 |
On US examination, the intrahepatic bile ducts were reported to be normal, but ill-defined hypoechoic focus in the inferoposterior area of the right liver lobe was noted. Also, normal gallbladder without stone or sludge, and normal intra- and extrahepatic ducts were seen. Portal vein and CBD diameters were 11 and 5 mm, respectively.
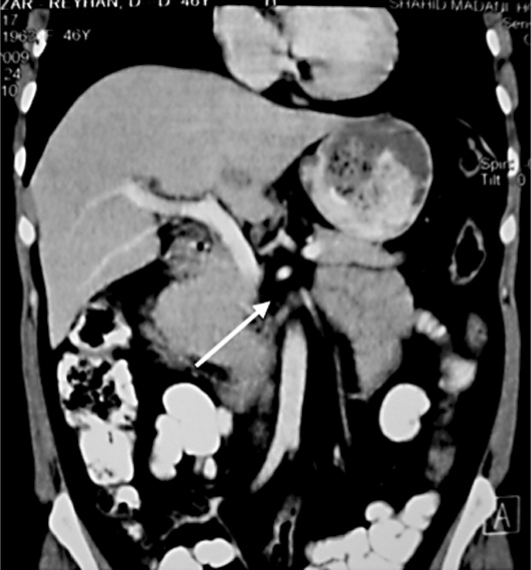
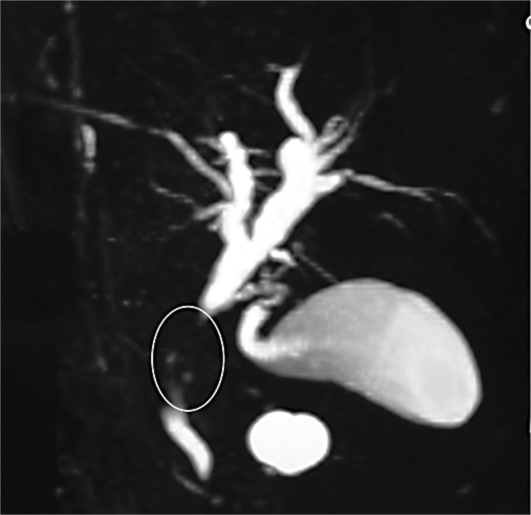
Abdominal CT scan showed mild distension in the intra- and extrahepatic ducts with a suspected space-occupying lesion in the CBD (fig. 1). Figure 2 illustrates the result of magnetic resonance cholangiopancreatography, in which the intrahepatic bile ducts were dilated, common hepatic duct diameter was 9 mm, right main hepatic duct diameter 5 mm and left main hepatic duct diameter 8 mm, the proximal segment of the CBD was not seen (tumor?), and the distal segment of the CBD appeared normal.
Fig. 1.
CT. The arrow points to the filling defects in the CBD.
Fig. 2.
Magnetic resonance cholangiopancreatography shows one stricture in middle part of the CBD (circle).
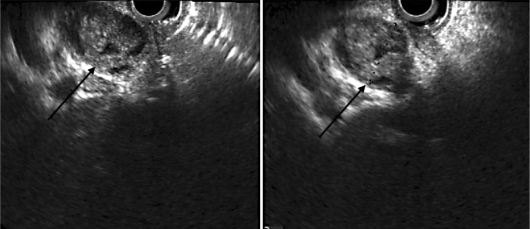
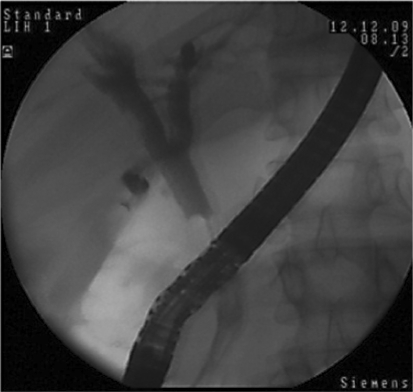
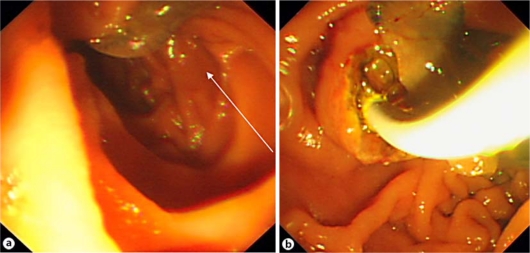
The patient underwent EUS in our center. The CBD was 19 mm long, and a worm was seen in the distal CBD. One enlarged lymph node was reported next to the CBD with mixed echo. EUS showed a mixed echoic circular mass lesion (fig. 3) suggesting ascariasis. ERCP was performed for a precise diagnosis and therapeutic intervention. After selective cannulation, the CBD was filled with contrast agent as seen in figure 4. Slight dilatation of the extrahepatic bile ducts and some floating, oval-shaped filling defects were noted. The intrahepatic bile ducts were proved to be normal. After sphincterotomy, two F. hepatica worms were extracted by balloon (fig. 5). After removing the worms (fig. 6), patient's symptoms gradually improved.
Fig. 3.
EUS view of the CBD. The arrows point to the mixed echoic circular mass lesion.
Fig. 4.
Slight enlargement and filling defect on ERCP.
Fig. 5.
F. hepatica in the duodenum. a The arrow points to the body of F. hepatica. b F. hepatica being removed through the papilla.
Fig. 6.
The adult F. hepatica flukes measuring approximately 2.5-3 × 1-1.5 cm.
Discussion
The adult fluke lives in the common and hepatic bile ducts of the human or animal host [4]. Its eggs are oval, yellow-brown, and measure approximately 130-150 × 60-90 μm. F. hepatica is a common pathogen, particularly in sheep and cattle, causing ‘liver rot’. It occurs mainly in sheep-rearing areas of temperate climates, particularly in certain parts of Central and South America, Europe, China, Africa and the Middle East [5]. In Iran, Gilan, Ardabil, Khuzestan and Kerman are most affected [6]. Sporadic cases have also been reported from the United States. Humans most commonly acquire infection by eating watercress grown in sheep-raising areas [5]. Other freshwater plants may also transmit infection, including water lettuce, mint (Chenopodiaceae), alfalfa, and parsley. Humans are also occasionally infected by drinking unboiled contaminated water containing viable metacercariae [7].
Outbreaks of infection have been described in Gilan [6]. The life span of adult F. hepatica flukes within humans is not known but is estimated to be 9-13 years. The number of adult flukes that reach the biliary tree and fully develop to sexual maturity is usually small. The degree of animal infection and the incidence of human infection rise during wet years because of an increased number of snails and longer survival of encysted cercariae [8]. Many infections in humans are mild, but morbidity can increase with heavier fluke burdens [9]. Typical symptoms that may be associated with fascioliasis can be divided by phases of the disease, including the acute or liver phase, the chronic or biliary phase, and ectopic or pharyngeal fascioliasis.
The early phase of migration of parasites through the liver is often associated with fever, right upper quadrant pain and hepatomegaly. Symptoms usually begin within 6-12 weeks of the ingestion of metacercariae. Other possible symptoms include anorexia, nausea, vomiting, myalgia, cough and urticaria. Jaundice is occasionally observed. Sometimes this phase is complicated by hemobilia or subcapsular hematomas of the liver [7]. Acute symptoms tend to disappear after a few weeks to months (usually 6 weeks). However, in very heavy infections, extensive liver parenchymal necrosis can result.
The biliary phase is usually asymptomatic, but the adult flukes can obstruct the CBD. Chronic infection can lead to biliary colic, cholangitis, cholelithiasis, and obstructive jaundice. Prolonged and heavy infections can also result in sclerosing cholangitis and biliary cirrhosis. Pain in the epigastrium and right upper quadrant, diarrhea, nausea, vomiting, wasting, hepatomegaly and jaundice can occur. Eosinophilia is a variable finding in this phase of the disease. The diagnosis can be made by finding characteristic ova in feces, duodenal aspirates, or bile specimens; negative fecal specimens do not exclude the diagnosis. It is often difficult to distinguish between the eggs of F. hepatica, Fasciola gigantica and the intestinal fluke Fasciolopsis buski. The diagnosis may also be made during surgery or endoscopy for biliary obstruction when adult flukes are found in the biliary tree [10]. Serology usually becomes positive during the early phase of migration through the liver, and thus is useful in diagnosing early symptoms to the appearance of eggs in the feces prior. It is also particularly useful in ectopic disease. ELISA-based assays, especially the ones that use genus-specific antigens, have largely replaced all of the other techniques because they are sensitive, rapid and quantitative [11, 12].
The most useful imaging technique up to now has been CT scanning of the liver, which may show characteristic hypodense nodules or tortuous tracks resulting from migration of the parasite through the liver [13, 14]. EUS, cholangiography and ERCP are more useful in the biliary stage of infection and may show mobile flukes in the bile ducts and gallbladder, often associated with lithiasis; irregular thickening of the CBD wall may be noted [15]. It should be emphasized that EUS as a noninvasive modality in equivocal magnetic resonance cholangiopancreatography has an excellent accuracy. Similarly a NIH consensus conference has recommended the use of ERCP only when the clinical probability for choledocholithiasis is high (i.e. when the need for therapeutic intervention is likely). For diagnosis of choledocholithiasis alone, EUS and magnetic resonance cholangiopancreatography are equal in accuracy to ERCP [16]. Magnetic resonance imaging has also been used for making the diagnosis but has no apparent advantage over CT or US [13].
A combination of the symptoms of absolute eosinophilia and right upper quadrant pain should bring to mind the possibility of F. hepatica infection. The biliary stage usually presents with intermittent right upper quadrant pain with or without cholangitis or cholestasis. Eosinophilia can also be detected [2]. In our case the clinical presentation was epigastric and right upper quadrant pain lasting 4 years with intermittent pain attacks radiating to the back, difficult to tolerate and exacerbated after eating. The patient was assessed as a case of right upper quadrant and epigastric pain several times with no clear outcome. At clinical presentation, there were no biliary obstruction symptoms (jaundice, cholangitis, …) or pancreatitis, in comparison to other cases in the literature [1, 2, 14, 17, 18]. Besides, there was no increase in liver function tests and acute phase reactants. Imaging studies such as abdominal sonography and CT were equivocal (or misleading to tumor). EUS defined the lesion clearly, advising ERCP. F. hepatica was removed and fell to the gut after successful sphincterotomy. CT and US may also be used in follow-up to evaluate the efficacy of medical therapy [14]. ERCP is particularly effective in the biliary stage and images must be carefully examined to rule out other possible causes producing irregularity and thickening of the CBD wall [17].
Sometimes, the diagnosis of fascioliasis is difficult, as seen in the present case. Fascioliasis must be considered on differential diagnosis of abdominal pain without biliary symptoms, especially in patients who come from endemic areas.
References
- 1.Gulsen M, Savas MC, Koruk M, Kadayifci A, Demirci F. Fascioliasis: a report of five cases presenting with common bile duct obstruction. Neth J Med. 2006;64:17–19. [PubMed] [Google Scholar]
- 2.Aksoy DY, Kerimoglu U, Oto A, et al. Infection with Fasciola hepatica. Clin Microbiol Infect. 2005;11:859–861. doi: 10.1111/j.1469-0691.2005.01254.x. [DOI] [PubMed] [Google Scholar]
- 3.Condomines J, Rene-Espinet JM, Espinos-Perez JC, Vilardell F. Percutaneous cholangiography in the diagnosis of hepatic fascioliasis. Am J Gastroenterol. 1985;80:384–386. [PubMed] [Google Scholar]
- 4.Haseeb AN, el-Shazly AM, Arafa MA, Morsy AT. A review on fascioliasis in Egypt. J Egypt Soc Parasitol. 2002;32:317–354. [PubMed] [Google Scholar]
- 5.S Mas-Coma. Epidemiology of fascioliasis in human endemic areas. J Helminthol. 2005;79:207–216. doi: 10.1079/joh2005296. [DOI] [PubMed] [Google Scholar]
- 6.Salahimoghadam A. Epidemiology of human fascioliasis in Iran. J Med Univ Kerman. 2009;4:385–398. [Google Scholar]
- 7.Chan CW, Lam SK. Diseases caused by liver flukes and cholangiocarcinoma. Baillieres Clin Gastroenterol. 1987;1:297–318. doi: 10.1016/0950-3528(87)90006-6. [DOI] [PubMed] [Google Scholar]
- 8.Arjona R, Riancho JA, Aguado JM, et al. Fascioliasis in developed countries: a review of classic and aberrant forms of the disease. Medicine (Baltimore) 1995;74:13–23. doi: 10.1097/00005792-199501000-00002. [DOI] [PubMed] [Google Scholar]
- 9.Adachi S, Kotani K, Shimizu T, et al. Asymptomatic fascioliasis. Intern Med. 2005;44:1013–1015. doi: 10.2169/internalmedicine.44.1013. [DOI] [PubMed] [Google Scholar]
- 10.Leder K, Weller PF: Liver flukes: fascioliasis. Uptodate, September 2009, 17.3.
- 11.Jones EA, Kay JM, Milligan HP, Owens D. Massive infection with Fasciola hepatica in man. Am J Med. 1977;63:836–842. doi: 10.1016/0002-9343(77)90171-1. [DOI] [PubMed] [Google Scholar]
- 12.Espino AM, Diaz A, Perez A, Finlay CM. Dynamics of antigenemia and coproantigens during a human Fasciola hepatica outbreak. J Clin Microbiol. 1998;36:2723–2726. doi: 10.1128/jcm.36.9.2723-2726.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Van Beers B, Pringot J, Geubel A, et al. Hepatobiliary fascioliasis: noninvasive imaging findings. Radiology. 1990;174:809–810. doi: 10.1148/radiology.174.3.2406786. [DOI] [PubMed] [Google Scholar]
- 14.Dauchy FA, Vincendeau P, Lifermann F. Eight cases of fascioliasis: clinical and microbiological features. Med Mal Infect. 2006;36:42–46. doi: 10.1016/j.medmal.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Sezgin O, Altintas E, Disibeyaz S, et al. Hepatobiliary fascioliasis: clinical and radiologic features and endoscopic management. J Clin Gastroenterol. 2004;38:285–291. doi: 10.1097/00004836-200403000-00017. [DOI] [PubMed] [Google Scholar]
- 16.Wang DQH, Afdhal NH. Gallstone disease. In: M Feldman, LS Friedman, LJ Brandt., editors. Sleisenger and Fordtran's Gastrointestinal and Liver Disease, ed 9. New York: Saunders; 2010. pp. 1110–1111. [Google Scholar]
- 17.Echenique-Elizondo M, Amondarain J, Liron de Robles C. Fascioliasis: an exceptional cause of pancreatitis. JOP. 2005;6:36–39. [PubMed] [Google Scholar]
- 18.Dobrucali A, Yigitbasi R, Erzin Y, Sunamak O, Polat E, Yakar H. Fasciola hepatica infestation as a very rare cause of extrahepatic cholestasis. World J Gastroenterol. 2004;10:3076–3077. doi: 10.3748/wjg.v10.i20.3076. [DOI] [PMC free article] [PubMed] [Google Scholar]