Abstract
Metastasis of gastrointestinal stromal tumor (GIST) into the central nervous system is extremely rare. We report a patient with synchronous GIST and brain metastasis. At disease onset, there was left hemiplegia and ptosis of the right eyelids. Resection cytology of the brain tumor was reported as metastasis of GIST. After positron emission tomography examination, another tumor in the small bowel was discovered, which suggested a small bowel GIST associated with intracranial metastasis. Immunohistochemical analysis of the intestinal tumor specimen obtained by double balloon endoscopy showed a pattern similar to the brain tumor, with the tumors subsequently identified as intracranial metastases of jejunal GIST. After surgical resection of one brain tumor, the patient underwent whole brain radiation therapy followed by treatment with imatinib mesylate (Gleevec; Novartis Pharma, Basel, Switzerland). Mutational analysis of the original intestinal tumor revealed there were no gene alterations in KIT or PDGFRα. Since the results indicated the treatment had no apparent effect on either of the tumors, and because ileus developed due to an intestinal primary tumor, the patient underwent surgical resection of the intestinal lesion. However, the patient's condition gradually worsen and she subsequently died 4 months after the initial treatment.
Key words: Gastrointestinal stromal tumor, Brain metastasis, Mutational analysis
Introduction
Gastrointestinal stromal tumors (GISTs) are the most common type of mesenchymal tumors in the gastrointestinal tract [1]. Their biological behavior correlates with clinicopathological parameters such as mitotic rate, tumor size and tumor location, as well as with the molecular alterations of KIT and PDGFR [2]. Imatinib is a potent, specific inhibitor of both the KIT and PDGFR receptor tyrosine kinase. The drug has been approved for the treatment of advanced unresectable or metastatic GIST. Since being approved for use in treatment, imatinib has dramatically improved the outcomes of advanced GIST patients [3]. Most metastasis arises in the liver and peritoneum, which is the result of hematogenous spread and peritoneal seeding, respectively. Disease outside the abdomen is uncommon and primarily seen in advanced cases only [2]. In addition, metastasis of GIST to the central nervous system is also extremely rare [4]. We report a rare case of intracranial metastasis from jejunal GIST. A PubMed database search only found 7 reported cases of brain metastases from primary GIST. Here, we report a summary of the relevant literature that is currently available.
Case Report
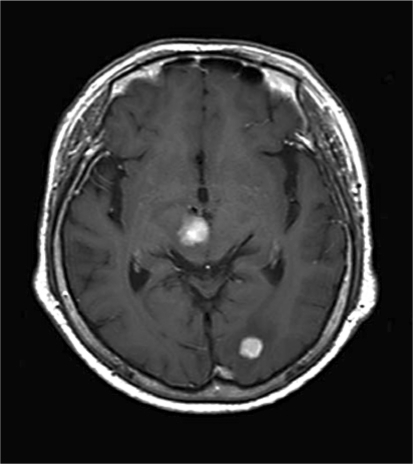
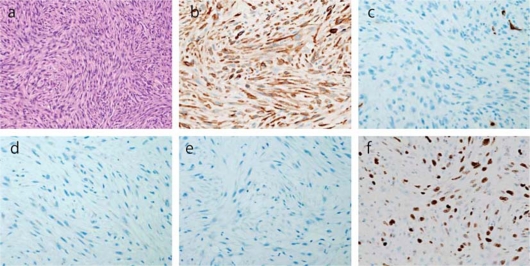
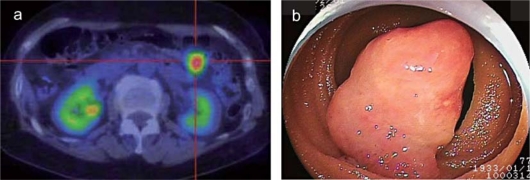
A 77-year-old woman presented in December 2009 with left hemiplegia and ptosis of her right eyelids. Magnetic resonance imaging (MRI) of the brain revealed a 24 mm tumor in her right cerebral peduncle and a 20 mm tumor in her left occipital lobe, which was associated with brain edema (fig. 1). On the basis of the radiologic features of the computed tomography (CT) and MRI procedures, she was initially diagnosed as having a malignant lymphoma in her brain. Subsequently, the tumor in her left occipital lobe was completely resected. Histopathological examination of the hematoxylin and eosin specimen revealed a spindle cell tumor. Since immunohistochemistry was positive for c-kit and negative for CD34, desmin and S-100 (fig. 2), she was diagnosed with metastasis of GIST. After counting the proliferating cells that were immunostained by Ki-67 (MIB-1) antibody, her MIB-1 index was determined to be approximately 20% (fig. 2). A positron emission tomography computed tomography (PET-CT) examination revealed the presence of another tumor in the small bowel (fig. 3a), which suggested small bowel GIST associated with intracranial metastasis. Since capsule endoscopy revealed a tumor in the proximal jejunum, oral endoscopy using the double balloon technique was carried out. The proximal jejunum demonstrated a 3 cm oval-shaped mass which was consistent with the findings obtained by capsule endoscopy (fig. 3b). Subsequent pathological examination found a malignant spindle cell tumor with morphology that was similar to that of the brain tumor. Immunohistochemical staining demonstrated that the tumor cells were positive for c-kit while negative for CD34, desmin and S-100. This was consistent with the findings for the brain tumor. Therefore, the patient was diagnosed as having a primary jejunal GIST associated with brain metastasis. Mutational analysis of the intestinal tumor was carried out and no mutations were found in the KIT exons 11, 13, 17, 19 or for PDGFRα. Postoperatively, the patient underwent whole brain radiation therapy in conjunction with administration of imatinib mesylate 400 mg/day in February 2010. Unfortunately, she suffered from leucopenia 1 month after initiation of imatinib methylate administration and the drug had to be discontinued. The patient remained well until the end of March 2010, when she began to complain of abdominal pain. CT examination revealed the development of ileus due to the jejunal primary tumor, leading to surgical resection of the intestinal lesion. Observation during the surgery revealed that the tumor in the proximal jejunum had not changed in size compared to the size observed during the previous double balloon endoscopy. Although there was minor enlargement of her right cerebral peduncle tumor, brain MRI at that time showed no apparent recurrence in the left occipital lobe where tumor resection had been performed. The patient's condition gradually worsened and she died 4 months after the initial treatment.
Fig. 1.
Axial MRI of the brain shows solid tumors in the right cerebral peduncle and in the left occipital lobe with surrounding cerebral edema.
Fig. 2.
Tumor cells stained with hematoxylin and eosin at 100× magnification (a). Spindle-shaped cells with oval nuclei are arranged in storiform architecture. Under higher magnification, 10/high power field nuclear differentiations were observed. Brown-staining spindle cells are positive for KIT (b) and negative for CD34 (c), desmin (d) and S-100 (e). Approximately 20% were MIB-1-labeled proliferating cells (f).
Fig. 3.
a PET-CT showed there was an abnormal uptake, with a standardized uptake value maximum of 6.8 in the proximal jejunum. b Double balloon endoscopy identified an oval-shaped mass approximately 3 cm in diameter at the proximal jejunum.
Discussion
GISTs are the most common mesenchymal neoplasms in the gastrointestinal tract. GISTs arise from the precursor cells of the interstitial cells of Cajal and express KIT tyrosine kinase. Most GISTs express Kit (94%), CD34 (82%), with 78% of GISTs positive for both KIT and CD34 [1]. Activating mutations of KIT or PDGFRα are found in the vast majority of GISTs, and the mutational status of these oncoproteins can be predictive of the clinical response to imatinib therapy [1, 3, 5]. Although KIT exon 11 is the most common mutation found in GISTs (66.9%), other exons harboring mutations, such as exon 9 (18.1%) and exons 13 and 17 (1.6%), have also been described [3, 6]. The response and sensitivity to imatinib in GISTs that lack KIT mutations has been reported to be due to the PDGFRα mutations [5]. It has also been shown that the reason why imatinib mesylate is not effective in treating metastatic GIST in the brain is because of the presence of the blood brain barrier. Lower concentrations of imatinib mesylate in the central nervous system have been detected in both mice [7] and humans [8]. In addition, a small population of GISTs have been discovered that lack both the KIT and PDGFRα mutations [5], and when these populations are treated with imatinib, a very poor response is observed [3]. Consistent with these reports, our patient also exhibited no mutations in either the KIT exon 7, 9, 11, 13 or PDGFRα, and she was resistant to imatinib mesylate treatment.
While GIST frequently metastasizes to both the liver and peritoneum, metastasis outside the abdomen is very uncommon [2]. There have also been very few reports in the literature regarding concurrent GIST and brain metastasis. A current review of the literature found only 7 case reports including the current case [4, 9, 10, 11, 12, 13]. To verify our present observations, we compared the findings presented for all of the reported cases in the literature (table 1). In all cases, patients underwent administration of imatinib mesylate, with only 1 out of the 7 cases exhibiting a response to the chemotherapy [12]. However, since most of these cases primarily involved brain metastasis, the molecular features were not described [11]. Mutational analysis was described in case report 5, in which a mutation in exon 9 of KIT was detected in the original jejunal tumor, the liver and the cerebral metastases. It is well known that GISTs with different mutations will show different clinical characteristics. However, the prognostic significance of KIT mutations remains controversial, as Cho et al. [14] reported that KIT-mutation-positive GISTs exhibit more frequent liver metastases and a higher mortality than KIT-mutation-negative GISTs. Braconi et al. [6] found in 104 patients that 74% of the exon 11 KIT mutations were associated with metastasis, as compared to only 5% of the wild-type GISTs. PDGFRα mutations rarely result in metastatic disease [6], and thus, to the best of our knowledge, this is the first report that found GIST occurring without any KIT or PDGFRα mutations associated with the intracranial metastasis. Recently, it has been shown that the transcription factor ETV1 is necessary for the survival and growth of GIST cells in vitro and in vivo [15]. Since all GIST samples and GIST cell lines express ETV1, this transcription factor might very well be a new biomarker and therapeutic target for GIST. Thus, in the absence of mutations in either KIT or PDGFRα, as was seen in our case, other factors such as ETV1 might be involved in the promotion and metastasis of GIST.
Table 1.
Metastatic GISTs in the central nervous system (CNS)
| No. | Age/gender | Primary |
CNS |
Treatment for CNS tumor |
Mutational status | Response of CNS tumor | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
| site | size (cm) | site | size (cm) | ||||||
| 1 | 75/M | mesentery | ND | hemisphere | (infiltrative) | imatinib 800 mg/day | NA | CR | Brooks et al. [12], 2002 |
| 2 | 47/M | jejunum | 7.5 | left parasagittal lesion | ND | total resection, imatinib 400–800 mg/day | exon 9 | PD | Hughes et al. [9], 2004 |
| 3 | 68/F | perisacral area | ND | right parietal lobe | 3 | total resection, imatinib | NA | SD | Kaku et al. [10], 2006 |
| 4 | 42/M | mesentery | 8×6 | right parietal lobe | 3.5 | total resection, radiotherapy 60 Gy, imatinib 600 mg/day | NA | PD | Puri et al. [4], 2006 |
| 5 | 49/F | mesentery | ND | left eye, brain | 0.24 | imatinib 400 mg/day | NA | PD | Gentile et al. [13], 2008 |
| 6 | 54/F | esophagus | 11 | left frontal lobe | 5 | complete resection, imatinib 400 mg/day | exon 11 | SD | Hamada et al. [11], 2010 |
| 7 | 77/M | jejunum | 3 | right cerebral | 2.4 | total resection of left occipital lobe tumor, radiation therapy 39 Gy, imatinib 400 mg/day | no mutation in KIT, PDGFRα | PD | our case, 2011 |
| peduncle left occipital lobe | 2 | ||||||||
ND = Not determined; NA = no analysis; CR = complete response; PD = progressive disease; SD = stable disease.
Being able to elucidate the mechanism of tumorigenesis and to identify the responsible gene in GISTs that exhibit no detectable mutations of KIT or PDGFRα may lead to new insights into the nature of GIST and potentially a novel diagnosis and therapy. In summary, we histologically confirmed the brain metastasis of small bowel GIST in the current case, which is a rare and particularly unusual manifestation of this tumor.
References
- 1.Hirota S, Isozaki K, Moriyama Y, Hashimoto K, Nishida T, Ishiguro S, Kawano K, Hanada M, Kurata A, Takeda M, Muhammad Tunio G, Matsuzawa Y, Kanakura Y, Shinomura Y, Kitamura Y. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 2.Kroep JR, Bovee JV, van der Molen AJ, Hogendoorn PC, Gelderblom H. Extra-abdominal subcutaneous metastasis of a gastrointestinal stromal tumor: report of a case and a review of the literature. J Cutan Pathol. 2009;36:565–569. doi: 10.1111/j.1600-0560.2008.01067.x. [DOI] [PubMed] [Google Scholar]
- 3.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813–3825. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 4.Puri T, Gunabushanam G, Malik M, Goyal S, Das AK, Julka PK, Rath GK. Mesenteric gastrointestinal stromal tumour presenting as intracranial space occupying lesion. World J Surg Oncol. 2006;4:78. doi: 10.1186/1477-7819-4-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heinrich MC, Corless CL, Duensing A, McGreevey L, Chen CJ, Joseph N, Singer S, Griffith DJ, Haley A, Town A, Demetri GD, Fletcher CD, Fletcher JA. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 6.Braconi C, Bracci R, Bearzi I, Bianchi F, Costagliola A, Catalani R, Mandolesi A, Ranaldi R, Galizia E, Cascinu S, Rossi G, Giustini L, Latini L, Valeri N, Cellerino R. Kit and PDGFRalpha mutations in 104 patients with gastrointestinal stromal tumors (GISTs): a population-based study. Ann Oncol. 2008;19:706–710. doi: 10.1093/annonc/mdm503. [DOI] [PubMed] [Google Scholar]
- 7.Wolff NC, Richardson JA, Egorin M, RL Ilaria., Jr The CNS is a sanctuary for leukemic cells in mice receiving imatinib mesylate for Bcr/Abl-induced leukemia. Blood. 2003;101:5010–5013. doi: 10.1182/blood-2002-10-3059. [DOI] [PubMed] [Google Scholar]
- 8.Petzer AL, Gunsilius E, Hayes M, Stockhammer G, Duba HC, Schneller F, Grunewald K, Poewe W, Gastl G. Low concentrations of STI571 in the cerebrospinal fluid: a case report. Br J Haematol. 2002;117:623–625. doi: 10.1046/j.1365-2141.2002.03523.x. [DOI] [PubMed] [Google Scholar]
- 9.Hughes B, Yip D, Goldstein D, Waring P, Beshay V, Chong G. Cerebral relapse of metastatic gastrointestinal stromal tumor during treatment with imatinib mesylate: case report. BMC Cancer. 2004;4:74. doi: 10.1186/1471-2407-4-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaku S, Tanaka T, Ohtuka T, Seki K, Sawauchi S, Numoto RT, Murakami S, Komine K, Abe T. Perisacral gastrointestinal stromal tumor with intracranial metastasis. Case report. Neurol Med Chir (Tokyo) 2006;46:254–257. doi: 10.2176/nmc.46.254. [DOI] [PubMed] [Google Scholar]
- 11.Hamada S, Itami A, Watanabe G, Nakayama S, Tanaka E, Hojo M, Yoshizawa A, Hirota S, Sakai Y. Intracranial metastasis from an esophageal gastrointestinal stromal tumor. Intern Med. 2010;49:781–785. doi: 10.2169/internalmedicine.49.3124. [DOI] [PubMed] [Google Scholar]
- 12.Brooks BJ, Bani JC, Fletcher CD, Demeteri GD. Challenges in oncology. Case 4. Response of metastatic gastrointestinal stromal tumor including CNS involvement to imatinib mesylate (STI-571) J Clin Oncol. 2002;20:870–872. doi: 10.1200/JCO.2002.20.3.870. [DOI] [PubMed] [Google Scholar]
- 13.Gentile CM, Lombardi AA, Croxatto JO. Choroidal metastasis from gastrointestinal stromal tumour: a case report. Br J Ophthalmol. 2008;92:156–157. doi: 10.1136/bjo.2007.118489. [DOI] [PubMed] [Google Scholar]
- 14.Cho S, Kitadai Y, Yoshida S, Tanaka S, Yoshihara M, Yoshida K, Chayama K. Deletion of the kit gene is associated with liver metastasis and poor prognosis in patients with gastrointestinal stromal tumor in the stomach. Int J Oncol. 2006;28:1361–1367. [PubMed] [Google Scholar]
- 15.Chi P, Chen Y, Zhang L, Guo X, Wongvipat J, Shamu T, Fletcher JA, Dewell S, Maki RG, Zheng D, Antonescu CR, Allis CD, Sawyers CL. ETV1 is a lineage survival factor that cooperates with kit in gastrointestinal stromal tumours. Nature. 2010;467:849–853. doi: 10.1038/nature09409. [DOI] [PMC free article] [PubMed] [Google Scholar]