Abstract
Background and Aims
Oil palm, an unbranched perennial monocotyledon, possesses a single shoot apical meristem (SAM), which is responsible for the initiation of the entire above-ground structure of the plant. To compare the palm SAM structure with those of other monocots and to study variations in its structure throughout the life of the plant, its organization was characterized from the embryonic stage to that of the reproductive plant.
Methods
SAM structure was studied by a combination of stained histological sections, light and confocal microscopy, and serial section-based three-dimensional reconstructions.
Key Results
The oil palm SAM is characterized by two developmental phases: a juvenile phase with a single tunica-corpus structure displaying a gradual increase in size; and a mature phase characterized by a stable size, a modified shape and an established histological zonation pattern. In mature plants, fluctuations in SAM shape and volume occur, mainly as a consequence of changes in the central zone, possibly in relation to leaf initiation.
Conclusions
Development of the oil palm SAM is characterized by a juvenile to mature phase transition accompanied by establishment of a zonal pattern and modified shape. SAM zonation is dynamic during the plastochron period and displays distinct features compared with other monocots.
Keywords: Shoot apical meristem, oil palm, Elaeis guineensis, Arecaceae meristem, 3-D, development, evo-devo
INTRODUCTION
The post-embryonic development of plants is accomplished through the organogenic activity of the shoot apical meristem (SAM), a group of dividing cells present at the shoot apex. Its activity is highly controlled in order to carry out two processes: its self-maintenance and the formation of lateral organs. Self-maintenance requires the preservation of the meristem body despite the continuous flow of cells through the meristem via cell division activity for the initiation of lateral organs. This depends on the differentiation of groups of cells and their gradual separation from the meristem proper. The vegetative SAM generates a stem, leaves and also axillary shoot meristems, thus contributing to the overall architecture of the plant during its lifetime.
The SAM of gymnosperms and angiosperms is a heterogeneous structure in which three zones can be distinguished based on parameters such as cell size, cell shape, cell position, staining characteristics, cell division pattern, mitotic index assessments and estimated cell cycle length (Foster, 1943; Evert, 2006; Kwiatkowska, 2008). The central zone (CZ) of low mitotic activity is located in the distal region of the SAM, and includes the initial or stem cells, from which are derived the cells of the other two zones. The rib meristem zone (RZ), which is located beneath the CZ and centrally located in the shoot apex, is characterized by higher mitotic activity and is involved in pith development. Finally, the peripheral zone (PZ), which displays the highest mitotic activity of the three SAM zones, surrounds both the CZ and RZ and is the zone of lateral organ initiation at the periphery of the SAM. The PZ has the smallest, most densely staining cells and generally lacks a preferred plane of cell division. The RZ has larger, more vacuolated, less densely staining cells with a preferred plane of cell division resulting in vertical files of cells. Superimposed on this pattern in angiosperms and some gymnosperm species is a tunica-corpus pattern in which the tunica layers have preferentially anticlinal divisions that make these layers distinct from the corpus, in which the cell mass divides into various planes. The CZ corresponds to the corpus and the portion of the tunica overlying the corpus. The SAM is usually a dome-shaped structure, the height, size and overall geometry of which can differ between species. The shape and size of the SAM may change during the plastochron period (i.e. the time between the initiation of two successive leaf primordia). As a consequence, the SAM is often characterized by a minimal area phase and a maximal area phase.
The palm family (Arecaceae; order Arecales) are of particular interest as they have distinctive features compared with other groups within the monocots. A key feature of palms is their arborescent development based entirely on primary growth. This is achieved through the activity of a single vegetative SAM, which initiates the entire above-ground structure throughout the plant's life, in conjunction with the primary thickening activity, which is responsible to a large extent for stem enlargement. Palms are perennial and most often single-stemmed plants, some of which are able to live for more than 100 years. Different phases can be distinguished during the ontogeny of palms: the embryonic phase; the seedling phase; the growth establishment phase (i.e. the extended period of early development with a gradual increase in stem diameter and transition from juvenile to mature leaf shape); and finally the mature phase (i.e. apical growth with a fixed stem diameter), which itself can be subdivided into the vegetative (non-flowering) and reproductive (flowering and fruiting) mature phases (Tomlinson, 1990). The reported differences between the phases are related to changes in the morphology of leaves (from juvenile simple leaves to mature compound leaves), the diameter of the stem and the production of inflorescences. Various studies of SAM structure in palms have been reported (Ball, 1941; Tomlinson, 1990). With the exception of Ball's work in 1941 on species of the genus Phoenix, no detailed descriptions of palm SAM structure in relation to the plant's life cycle have been reported to date.
We describe here a detailed study of anatomical and morphological variations in the shoot apical meristem of the African oil palm (Elaeis guineensis; subfamily Arecoideae) during the various developmental phases of the plant and with respect to the plastochron period. Under favourable climatic conditions, the vegetative shoot meristem is continuously active, producing a new leaf in a spiral phyllotaxy approximately every 2 weeks in mature palms. At the juvenile stage, the plastochron period may be as short as 9 d (Corley and Gray, 1976). Only partial studies of oil palm development have been reported in relation to embryo, leaf, root and reproductive development (Yampolsky, 1922; Beirnaert, 1935; Vallade, 1966a, b; Van Heel et al., 1987; Jourdan et al., 2000; Adam et al., 2005; Jouannic et al., 2007). This is the first study, through histological analysis and serial section-based three-dimensional (3-D) reconstructions, to describe in detail the variations in oil palm SAM structure which occur during development. The results show that two clear phases can be identified during ontogenesis of the SAM: a juvenile phase involving a gradual increase in SAM size without histological zonation; and a mature phase characterized by a stable size and histological zonation. Moreover, for mature plants, we provide evidence to suggest that alterations in SAM shape and volume occur during the plastochron period, mainly as a consequence of variations in CZ size that parallel the development of the leaf primordium.
MATERIALS AND METHODS
Plant materials
Vegetative shoot apex-containing samples were collected from the hearts of oil palm plants at different developmental stages: young seedlings (from early germination to 4-week-old stages; five samples per stage) and 3-month-old palms (ten samples) originating from seed-derived plants (C1001 genotype) grown in the greenhouse in Montpellier (France); and 15-month-old and 10-year-old palms (five samples for each stage) originating from the Coto plantation (ASD, Costa Rica). All palms were of the tenera variety, i.e. obtained from a cross between pisifera and dura palms (Hartley, 1988). Zygotic embryos were extracted from seeds collected at Pobé Experimental Station (INRAB, Benin) 80 and 120 d after pollination (ten samples for each stage).
Fixation of samples
After dissection, sampled material was fixed for 1 h under vacuum and thereafter for 24 h at room temperature in fixation buffer (2 % paraformaldehyde, 1 % glutaraldehyde, 1 % caffeine, 0·1 mol L−1 phosphate buffer, pH 7). Samples were then dehydrated through a graded ethanol series (30, 50, 70, 90 and 100 %, v/v; 1 h per step) and stored at 4 °C.
Histological studies, transmission light microscopy and confocal microscopy
For histological analysis, samples were transferred to a 50 % (v/v) ethanol/50 % (v/v) butanol solution for 1 d then to 100 % (v/v) butanol for 2 d. After transfer to a 50 % (v/v) butanol/50 % (v/v) resin (Technovit 7100, Heraeus Kulzer, Wehrheim, Germany) solution for 1 d, samples were embedded in 100 % resin according to the manufacturer's instructions. Blocks were sectioned at 4 µm thickness using an HM 355 S microtome (Microm, Walldorf, Germany). For serial section analysis, individual sections were cut sequentially and collected on glass sides. They were then double stained with PAS stain (periodic acid/Schiff's reagent – CI: 42500, Sigma-Aldrich, Lyon, France) for 10 min at room temperature in the dark) for the detection of insoluble carbohydrate compounds (Clark, 1984) and naphthol blue-black (NBB – CI: 20470, Sigma-Aldrich; 5 min at 50 °C) for the detection of proteins (Fisher, 1968) and permanently mounted after dehydration with Permount and coverslips. Photographs were taken with a Leica DFC300 FX camera in conjunction with a Leica DMRB microscope (Leica, Wetzlar, Germany) and images were processed using Photoshop (http://www.adobe.com; Adobe, France). For confocal microscopy, after fixation, samples were sectioned at 40 µm thickness using an HM650 vibratome (Microm) and thereafter were dehydrated through a graded ethanol series and stored at 4 °C. For confocal microscopy, the sectioned samples were stained using a 100 mg mL−1 propidium iodide (PI) solution in 0·1 mol L−1 phosphate buffer, pH 7, for 10 min and were imaged with a Leica TC5 SP confocal microscope (excitation 488 nm, emission 590–765 nm). Images were processed using the Image J program (http://rsbweb.nih.gov/ij) and assembled using Photoshop. The following measurements of SAM dimension were performed: first, the width at the base of the meristematic dome above the youngest leaf primordium; and secondly, the height from the apical tip of the dome to the level of attachment of the youngest leaf primordium on its adaxial side.
3-D reconstructions and quantifications
3-D reconstructions were performed from three different 10-year-old SAM serial sections stained with PAS and NBB. Images from the 4-μm-thick serial sections were taken using a Leica DFC300 FX camera in conjunction with a Leica DMRB microscope (135, 141 and 92 images for the three series, respectively) and were aligned using ImageJ. The image stacks obtained were optimized manually using a dedicated program named Tomobuilder (http://www.eliis.fr/; Elite Image Software, France). The resulting stacks of aligned images were used to manually define image by image the histological zonation of the shoot apex in conjunction with the ImageJ program and the region of interest manager facilities. The resulting segmentation stacks of the different zones were obtained using a specific macro in the ImageJ program. 3-D views were obtained from these segmentation stacks using the Volocity programme (http://www.improvision.com; Improvision, UK) with a voxel (i.e. volumetric pixel) size unit of 1·28 µm for the width and the height, according to the image pixel resolution, and 4 µm for the depth, according to the thickness of the sections. Quantifications (cell vs. nucleus surface area, cell circularity, grey level of pixels) were performed on the median section of one of the three apices (see supplementary Fig. S1; 30 cells per zone were considered) using the ImageJ program in conjunction with a dedicated macro. Volumes were determined using the Volocity program.
RESULTS
SAM organization during the embryonic phase
To investigate SAM organization during embryogenesis, two developmental stages of the zygotic embryo were analysed: an immature stage (80 d after pollination, DAP) and a mature stage before dormancy (120 DAP).
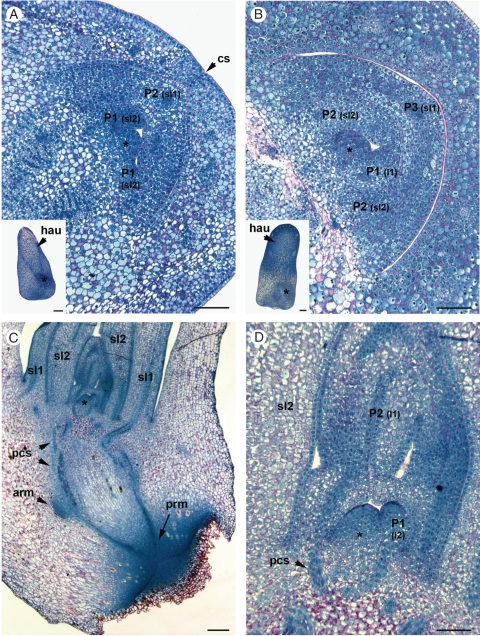
In pyramid-shaped embryos at the 80 DAP stage, the shoot pole is located in the basal periphery of the embryo (see inset to Fig. 1A). The SAM is a dome-shaped body about 60–70 µm in width and 30–35 µm in height (Fig. 1A). The SAM and the first scale leaf (embryonic leaf corresponding to the sheath part only) are already established and the second scale leaf is initiated at the periphery of the meristematic dome (Fig. 1A, see sl1 and sl2). The SAM dome has a single-cell-layer tunica and a corpus of irregularly arranged cells without visible histological zonation. Most of the cells share meristematic features (small isodiametric cells with a high nucleo-cytoplasmic ratio, a central nucleus, small or no detectable vacuoles, deep blue NBB staining associated with high cytoplasmic protein content, mitotic figures, etc.). Mitotic figures are visible in the meristematic dome and in the second scale leaf primordium, indicating zones with high mitotic activity. At the mature stage (120 DAP), the second scale leaf is developed and the first true leaf (or eophyll; i.e. bladed leaf) primordium is already initiated (Fig. 1B, see sl2 and l1). The meristematic dome is slightly larger (82 µm wide and 48 µm high). At this stage, the embryo begins its dormant phase, with arrested organogenesis and an increase in storage protein deposition, as illustrated by the dark blue-stained vacuoles in the cells outside the shoot apex.
Fig. 1.
Shoot apex organization during the embryogenesis and early seedling phases of development. (A) Median longitudinal section of the shoot apex in a zygotic embryo 80 d after pollination (DAP). Inset: a complete longitudinal section of the corresponding zygotic embryo. (B) Median longitudinal section of the shoot apex in a zygotic embryo 120 DAP. Inset: a complete longitudinal section of the corresponding zygotic embryo. (C) Median longitudinal section of a 1-week-old seedling. Note the well-differentiated shoot and root apices. (D) Higher magnification view of (C) in the shoot meristematic dome. The type of organ originating from each primordia is indicated in parentheses in (A), (B) and (D). Abbreviations: *, meristematic dome; arm, axillary root meristem; cs, cotyledonary slit; hau: haustorium (i.e. cotyledon); l1–l2, leaf; P1–P2–P3, primordia; pcs, procambium strand; prm, primary root meristem; sl1–sl2, scale leaf. Scale bars: (C, inset in A, inset in B) = 250 µm; (A, B, D) = 100 µm.
SAM organization during the seedling phase
The longitudinal section shown in Fig. 1C illustrates the organization of the two apices 1 week after germination. At this stage the plumule and radicle have not emerged. The shoot apex is characterized by the two developed scale leaves (sl1 and sl2 in Fig. 1C), the developing first eophyll primordium and the primordium of the second eophyll (l1 and l2 in Fig. 1D). As for the embryonic stages, the meristematic dome consists of cells with meristematic features with a progressive vacuolization towards the base of the dome. The meristematic dome was larger than in the embryonic stages (152 µm wide and 113 µm high). A comparison of different SAMs at the same developmental stage but at different time points of the plastochron period (based on the developmental stage of the youngest leaf primordium) did not reveal any changes, either in geometry (shape and size) or in the inner organization of the meristematic dome (data not shown). This organization was also compared with that of 2-, 3- and 4-week-old seedlings, but in each of these cases the shoot organization was similar without dramatic changes in the size of the meristematic dome (data not shown).
SAM organization during the early establishment growth phase
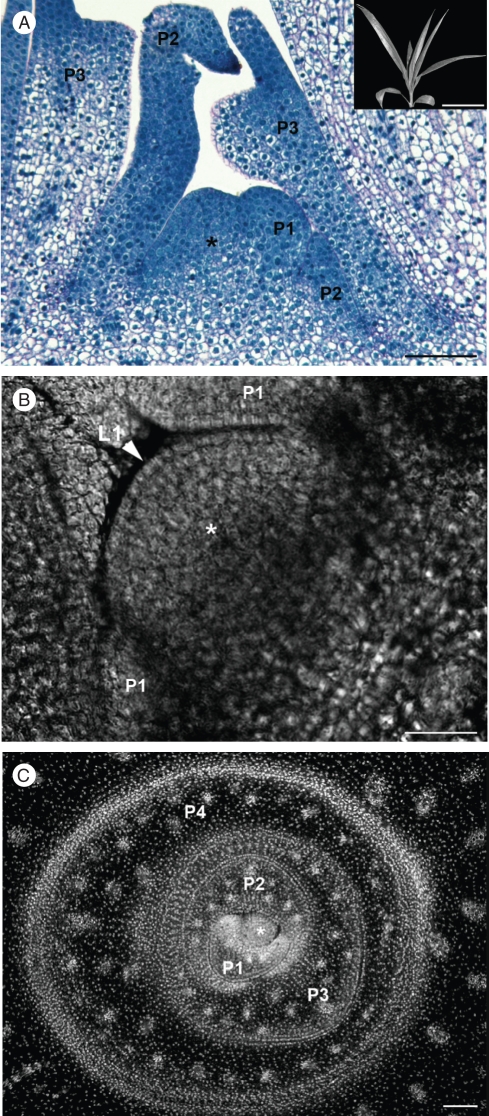
The SAM structure in juvenile plants was analysed using 3-month-old plants. The plants were 25 cm in height without pinnate or bifid emerged leaves (see inset to Fig. 2A). At this stage, plants were in their early establishment growth phase and lacking inflorescence initiation. A closer view of the SAM showed that the meristematic dome had the same geometry and inner organization (i.e. tunica-corpus pattern without histological zonation) as in the younger stages (i.e. embryos and plantlets; Fig. 2A) but with an increased size (179 µm wide and 147 µm high). Similarly, cells in the dome and the youngest primordium shared the same features (small and dense without a vacuole and with a high nucleo-cytoplasmic ratio; Fig. 2A). PI staining in conjunction with confocal microscopy clearly showed the presence of a single-cell-layer tunica and no clear difference in nuclear structure inside the meristematic dome (Fig. 2B). In a transverse section of the upper region of the apex including the meristematic dome, PI staining of nuclei in conjunction with confocal microscopy illustrated the high nuclear density of the meristematic dome and of the youngest leaf primordium, as well as the angular arrangement of the developing leaves (i.e. spiral phyllotaxis; Fig. 2C).
Fig. 2.
Shoot apex organization during the early establishment growth phase. (A) Shoot apex median longitudinal section stained with PAS-NBB from a 3-month-old plant. Inset: the corresponding plant used for the shoot apex preparation. (B, C) Confocal microscopic views of longitudinal (B) and transverse (C) sections of shoot apices stained with propidium iodide from 3-month-old plants. Abbreviations: *, meristematic dome; L1, tunica cell layer; P1–P2–P3, leaf primordia. Scale bars: (A, C) = 100 µm; (B) = 50 µm; (inset in A) = 10 cm.
Shoot apex organization during the mature reproductive phase
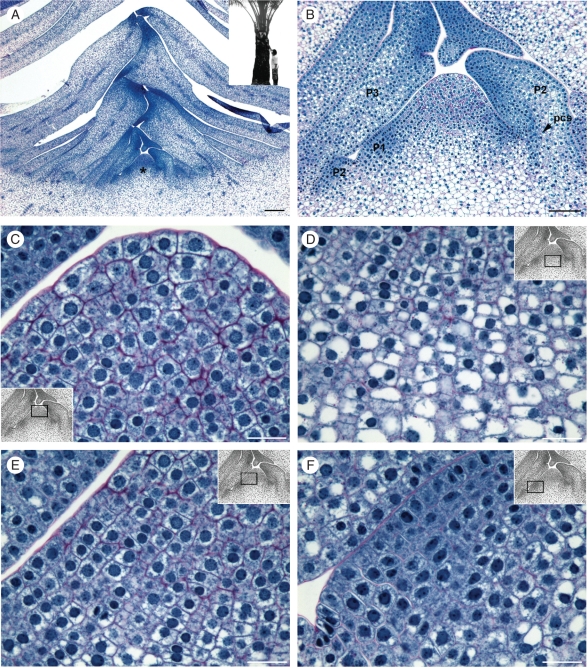
Shoot apices from 10-year-old plants were analysed. The plants used were about 5 m high with approx. 25 expanded leaves (see inset to Fig. 3A). At this stage, under tropical humid climatic conditions, the palms are mature with regular inflorescence production, an established stem width and regular leaf production, at an average of one or two leaves per month, indicating regular activity of the SAM.
Fig. 3.
Shoot apex organization in a mature reproductive oil palm. (A) Shoot apex median longitudinal section from a 10-year-old plant. Inset: the corresponding plant used for shoot apex preparation. (B) Higher magnification view of (A) in the meristematic dome region. (C–F) Higher magnification views of the different histological zones of the meristematic dome shown in (B). (C) The central zone at the top of the meristematic dome – note the large cells with thick cell wall and central uncondensed nuclei. (D) The rib meristem zone and the parenchyma beneath the meristematic dome – note the progressive vacuolization and cell size enhancement. (E) The peripheral zone in the left part of the longitudinal section – note the anticlinal files of cells with a thinner cell wall and progressive vacuolization. (F) P1 leaf primordium at the early stage of protrusion – note the dense cytoplasm and nuclei, and limited vacuolization. The insets in (C–F) indicate the corresponding region of the SAM. Abbreviations: *, meristematic dome; P1–P2–P3, leaf primordia. Scale bars: (A) = 250 µm; (B) = 100 µm; (C–F) = 25 µm.
Meristematic dome and histological zonation
The SAM and young leaf primordia are located in a depression surrounding the stem tissue (Fig. 3A). This depression results from primary thickening activity characterized by extensive cell files resulting from polarized cell divisions at the base of the expanding leaf primordia (data not shown). Cells with meristematic features are observed in the dome, but also in the developing leaf primordia (Fig. 3A, B). The longitudinal section shown in Fig. 3B reveals a highly organized dome-shaped shoot meristem, approx. 387 µm wide and 245 µm high. The site of leaf primordium initiation is visible in the form of a bulge at the base of the dome (P1 in Fig. 3B) and is pressed against the adaxial surface of the previously initiated leaf primordium (P2 in Fig. 3B). The SAM is composed of a single-cell-layer tunica with an anticlinal cell division plane and a corpus with different cell types. In addition to the leaf primordium initiation zone, three other zones were defined within the SAM dome on the basis of their distinct cellular features (cell size, cell shape, nucleo-cytoplasmic ratio, vacuolization, etc.; see Figs 3B–F and 4), their location inside the dome and by analogy with the SAM organization of other species (Barton, 1998; Evert, 2006): the CZ, the RZ and the PZ. The distinct cellular features of the mature meristematic dome were observed in both the corpus and the single-cell-layered tunica. A number of different parameters were quantified for the cells of each zone in both the tunica and the corpus (Table 1; see Supplementary Data Fig. S1 for details, available online).
Table 1.
Cell features in the different histological zones of the SAM
| CZ | PZ | RZ | P1 | |
|---|---|---|---|---|
| Cell surface area (μm2) | 213·54 ± 61·68 | 135·15 ± 28·06 | 209·25 ± 56·79 | 155·76 ± 34·04 |
| Cell circularity | 0·81 ± 0·07 | 0·81 ± 0·05 | 0·82 ± 0·07 | 0·78 ± 0·05 |
| Cell grey level | 131·27 ± 8·34 | 151·56 ± 12·78 | 158·10 ± 12·99 | 111·87 ± 9·73 |
| Nuclei surface area (μm2) | 50·43 ± 8·93 | 38·56 ± 8·36 | 38·09 ± 7·39 | 44·50 ± 9·17 |
| Nuclei circularity | 0·94 ± 0·01 | 0·94 ± 0·04 | 0·92 ± 0·02 | 0·91 ± 0·04 |
| Nuclei grey level | 85·11 ± 11·77 | 73·59 ± 13·00 | 90·13 ± 24·10 | 65·40 ± 11·77 |
| N/C ratio | 0·33 ± 0·08 | 0·41 ± 0·09 | 0·24 ± 0·07 | 0·41 ± 0·10 |
Circularity, surface areas and grey levels were calculated from the longitudinal cross-section of the shoot apex from a 10-year-old plant, using the ImageJ program in conjunction with the images in Fig. S1 (Supplementary Data, available online). Values are mean ± s.d. (n = 30 cells per zone). A circularity value of 1 corresponds to a perfect circle. As the value approaches 0, it indicates an increasingly elongated polygon. Grey levels correspond to means of grey levels in cell or nuclei (0, black; 255, white).
Abbreviations: CZ, central zone; PZ, peripheral zone; RZ, rib meristem-like zone; P1, emerging leaf primordium; N/C ratio, nucleo-cytoplasmic surface ratio.
At the top of the dome, the CZ was characterized by large cells, central nuclei, low mitotic activity (i.e. rare mitotic figures) and small but clearly observable vacuoles (Fig. 3B, C). The cells in this zone displayed an average surface area of approx. 214 µm2, a nucleo-cytoplasmic surface area ratio of 0·33 and nearly circular nuclei (Table 1). They were also characterized by a generally deep pink coloration of their cell walls with PAS staining compared with other cells of the meristematic dome, indicating that they were enriched in polysaccharides and/or they possess a larger cell wall compared with other cells of the dome region (Fig. 3C).
The other two zones of the meristematic dome were located beneath the CZ. One, defined as the RZ, was located in the central part of the meristematic dome. It was characterized by the presence of large polyhedral cells with an average surface of 209 µm2 and a low nucleo-cytoplasmic ratio associated with a large vacuole pushing the cytoplasm and nuclei to the periphery of the cells (Fig. 3B, D; Table 1). In the uppermost region of the RZ, periclinal cell divisions were seen to dominate, leading to vertical files of cells. The third zone was defined as the PZ and corresponded to the peripheral regions of the dome beneath the CZ (Fig. 3B, E). The cells in this region were smaller, with an average surface area of 135 µm2, a thinner cell-wall structure, small vacuoles and a higher nucleo-cytoplasmic surface area ratio compared with the cells of the CZ and the RZ (Table 1). These cells also contained nuclei with deeper NBB blue staining compared with the other zones (see grey levels in Table 1), suggesting higher protein content. Mitotic figures were observable in this zone. The most striking feature was the existence of distal–proximal files of cells from the CZ to the leaf primordium initiation site resulting from an orderly arrangement of cell division in this region.
At the basal periphery of the dome, in connection with the PZ, is the site of leaf primordium development (Fig. 3B, F). At the stage shown, it consisted of cells with features intermediate between those of the CZ and PZ with an average cell surface area of approx. 156 µm2 (Table 1). The main specific features observed for cells of this zone were first the presence of numerous tiny vacuoles and a dense deep blue NBB-stained cytoplasm (see grey level in Table 1) and secondly a large number of mitotic figures. The enlargement of this zone is associated with anticlinal divisions of the protoderm and both anticlinal and periclinal divisions in the cell layers beneath the protoderm.
Analysis of a plant during its late establishment growth phase (15-month-old plant approx. 1·50 m high) revealed that this SAM geometry and its histological zonation are already established (see Supplementary Data Fig. S2, available online), with a similar size and a less deep depression of the shoot apex compared with 10-year-old plants.
Variations of mature SAM structure
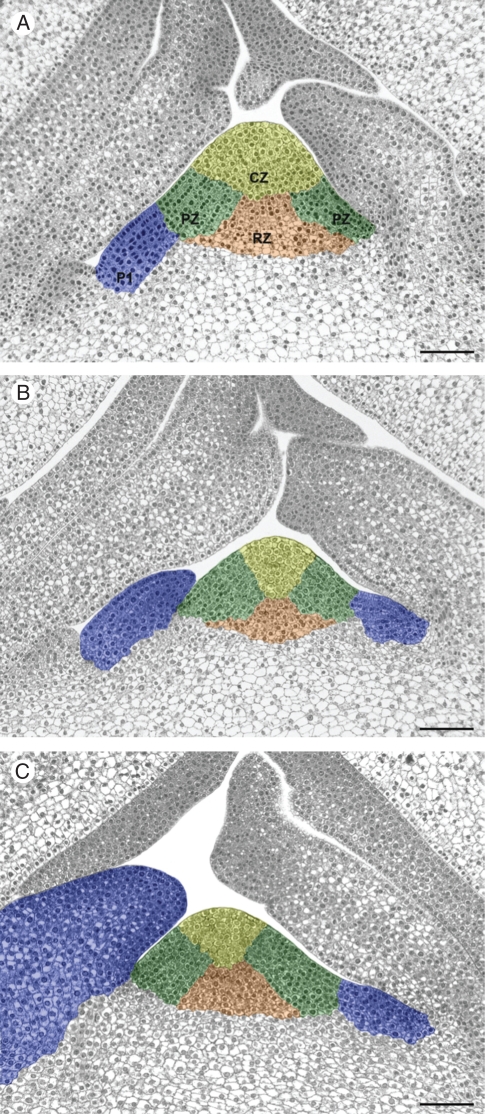
Series of longitudinal sections were obtained for three different shoot meristems. They revealed structural variations of the meristematic dome, which may be related to plastochronic changes associated with the developmental stage of the leaf primordium P1 (Fig. 4). The histological zones illustrated were defined as mentioned above: the CZ at the top of the meristematic dome and the RZ and PZ beneath the CZ (Fig. 4). The SAM shown in Fig. 4A (designated Mer1) is the same as that detailed in Fig. 3. Analysis of the two other SAMs (Mer2 and Mer3) revealed older developmental stages of the leaf primordium P1 that were distinct from each other (Fig. 4B, C). In the latter cases, the meristematic domes were approx. 195 µm in height for both with a basal width of about 342 and 390 µm for Mer2 and Mer3, respectively. In comparison, Mer1 had a more elongated conical shape with a higher height/width ratio (245 µm high, 387 µm wide). Moreover, this shape variation was observed to be associated with an altered surface area of the different histological zones in the median sections (Fig. 4). A higher CZ surface area and a lower PZ surface area were observed in Mer1 and a lower CZ surface area and higher PZ surface area in Mer3. On the basis of our analyses of the longitudinal sections, it can be inferred that the overall shape of the mature meristematic dome varies in relation to the fluctuating pattern of histological zonation.
Fig. 4.
Structural variation in the mature shoot apical meristem. (A–C) Median longitudinal sections of shoot apices from three different 10-year-old plants showing the P1 leaf primordium at different developmental stages. Histological zonation is indicated as follows: the central zone in yellow (CZ); the peripheral zone in green (PZ); the rib meristem-like zone in orange (RZ); the P1 leaf primordia in blue (P1). Scale bars = 100 µm.
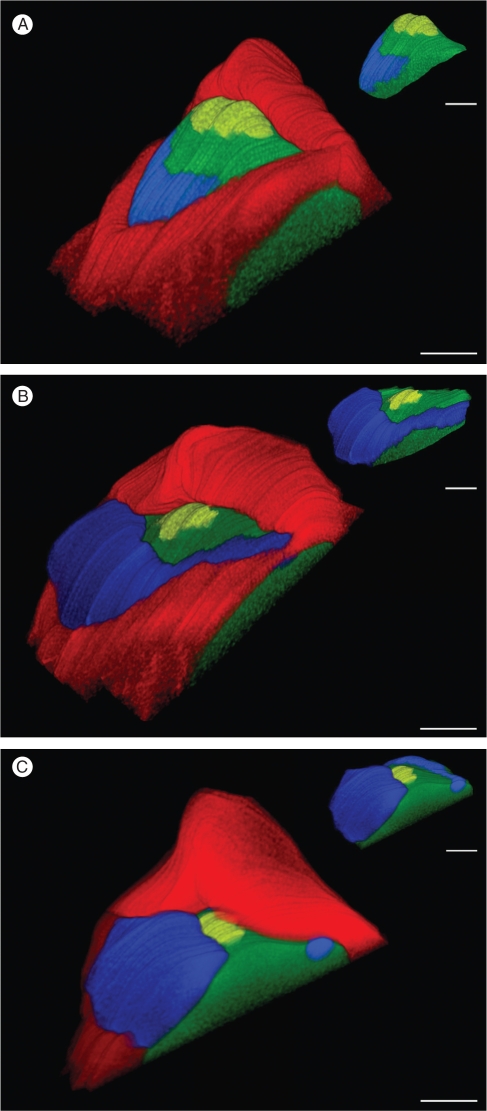
To view these variations more precisely, 3-D reconstructions were undertaken using the corresponding serial sections (Fig. 5). Four different regions were delimited for the reconstructions based on histological features as defined previously: one region corresponding to the CZ, two regions corresponding to the leaf primordia P1 and P2, respectively, and one region corresponding to the PZ and RZ. Based on the 3-D reconstructions obtained, the volumes of the meristematic dome and CZ were calculated (Table 2). In a similar way to the surface area differences observed in the longitudinal sections, variations in the volume of the meristematic dome were noted between the three samples. It should be borne in mind that the volume of the dome of Mer3 was an underestimate, as part of the meristematic dome was missing in the series of sections of Mer3 used for the reconstruction (see Fig. 5C). The most important tendency observed was the fluctuation in CZ volume: in Mer1, the CZ volume represented 27 % of the total volume of the dome as compared with Mer2 in which the CZ corresponded to 20 % of the dome's volume. The percentage CZ volume calculated for Mer3 should be even lower, as the meristematic dome was not complete in the corresponding 3-D reconstruction. Overall, the 3-D reconstructions clearly illustrated the variations in dome shape between the three samples, with a more elongated conical shape for Mer1 compared with the other two SAMs analysed.
Fig. 5.
3-D reconstructions of mature shoot apical meristems. (A–C) Reconstructions of mature shoot apical meristems from sections of the three shoot apices from 10-year-old plants shown in Fig. 4. Histological zonation is indicated as follows: the central zone in yellow, the peripheral and the rib meristem-like zones in green, and the leaf primordia P1 and P2 in blue and red, respectively. The top right views correspond to the 3-D reconstructions without the leaf primordium P2. Scale bars = 100 µm.
Table 2.
Deduced volumes of the mature SAMs on the basis of 3-D reconstructions
| Histological zone | Voxel count | Volume (μm3) | % ZC vs. Dome | |
|---|---|---|---|---|
| Mer1 | Dome | 1064 898 | 6978 915 | |
| ZC | 294 462 | 1929 786 | 27·65 | |
| Mer2 | Dome | 939 926 | 6159 899 | |
| ZC | 196 129 | 1285 351 | 20·87 | |
| Mer3 | Dome | 765 490 | 5016 715 | |
| ZC | 153 457 | 1005 696 | 20·05 |
Abbreviations: CZ, central zone; Dome, whole meristematic dome. Voxel count is the number of voxels (i.e. volumetric pixels) in a defined 3-D zone. Volumes were calculated according to the voxel size of the reconstructions (1·28 µm for the width and height according to the image resolution, 4 µm for the depth according to the thickness of the sections). Mer1, mer2 and mer3 correspond to SAM reconstructions shown in Fig. 4A, B and C, respectively.
Although we cannot completely exclude the possibility that our results reflect inter-plant variability in mature SAM structure, we hypothesize that the observed SAM size/shape differences and related variations in histological zone proportions are linked to the developmental progression of the youngest leaf primordium during the plastochron period, as seen in the 3-D reconstructions.
DISCUSSION
Ontogenic variation of SAM organisation in oil palm
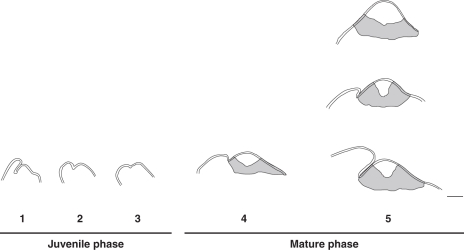
Our study revealed variations in SAM structure in terms of both volume and histological zonation during oil palm development (Fig. 6). During the growth and increase in diameter and height of the oil palm shoot from the embryonic to the mature state, the shoot apical dome increases in size from about 60 µm width in the embryo to 400 µm in the reproductive phase of the plant, corresponding to an approx. seven-fold increase in volume. The shoot apical dome size increases to a large extent and in a gradual fashion between the embryonic and early establishment phases and is nearly stable during the late establishment phase (from 15-month-old to 10-year-old plants). The second key feature is the alteration, during the life cycle of the plant, of the overall shape of the SAM, which is linked to changes in the spatial organization of leaf primordium initiation (i.e. the position of the organogenic region in relation to the apical region). The last main feature is the appearance of zonation during the early establishment phase of development in parallel with the stabilization of SAM size. Before histological zonation becomes apparent, SAM organization can be summarized as being a single-cell-layered tunica and a corpus of irregularly arranged cells sharing the same histological features. In a similar way to oil palm, histological zonation also appears during the early establishment phase in Phoenix canariensis and seems to be associated with the stabilization of SAM size (Ball, 1941).
Fig. 6.
Ontogenic and plastochronic variations in the oil palm SAM. Outlines of median longitudinal sections of the SAM and leaf primordium P1 at different developmental stages: embryo (1); germinating plantlet (2); 3-month-old plant (3); 10-month-old plant (4); and 10-year-old-plant (5). The three different mature SAMs observed from 10-year-old plants are represented in (5). The tunica is indicated by the two outlines. The central zone and peripheral and rib meristem-like zones are indicated in light and dark grey, respectively, in (4) and (5). Scale bar = 100 µm.
In this light, the development of the palm SAM can be divided into two main phases (Fig. 6): a juvenile phase with a tunica-corpus organization and a mature phase with a histological zonation superimposed on the tunica-corpus organization, with a transition operating between 3 and 10 months after germination, during the establishment phase of palm development, which is characterized by a gradual extension of stem diameter and a transition from the juvenile to the mature leaf shape. Thus, in addition to the observable morphological features (increase in height and stem diameter; juvenile vs. mature leaf shape), the transition from the juvenile to the adult phase in oil palm is also associated with a modification of SAM structure, with the mature phase of the SAM occurring before the mature phase of the plant.
The stage of plant development at which zonation is established in the SAM can vary among species (Evert, 2006). The late establishment of zonation in the palm SAM is not observed in species of the Poales, such as rice and maize. One hypothesis is that this might reflect a difference between perennial and annual monocotyledonous species, with a longer juvenile phase in perennial plants.
Mature oil palm SAM structure in comparison with other monocot species
In comparison with the large size of the plant at its mature stage, the dome-shaped SAM of oil palm is small with a width ranging from 350 to 400 µm. Nevertheless, various studies of SAM morphology in monocots have suggested that the size of the apex is correlated with mature stem diameter, with apex width ranging from 95 µm for Acorus species (plants of herbaceous habit in the Acorales) to 1300 µm for Xanthorrhoea species (tree-like plants in the order Asparagales) (Martin and Tucker, 1985). The histological organization of the mature SAM has been detailed or reported in at least eight other palm species from different genera and subfamilies: Phoenix, Washingtonia, Trachycarpus and Corypha from the subfamily Coryphoideae (Ball, 1941; Tomlinson, 1990), Juania from the subfamily Ceroxyloideae (Tomlinson, 1990), and rattan genera Calamus and Korthalsia (Calamoideae subfamily) (Tomlinson, 1990). The SAM width measured for the other palm species studied ranged from 200 to 550 µm, making them not dissimilar to mature oil palm, with the exception of the rattan species characterized by a smaller SAM (less than 100 µm wide). Histological zonation of the SAM was observable for these species with some differences compared with oil palm. The main difference that can be noted between oil palm and the other palm species is the unclear distinction between the PZ and the RZ, with orderly organized cell divisions observed in the entire region beneath the CZ in the species of the Coryphoideae studied (Ball, 1941). The latter are also characterized by a SAM located in a depression at the apex (i.e. a concave apex) resulting from high primary thickening activity in these large-stemmed species. In contrast, in climbing palms of the rattan genera Calamus and Korthalsia, the shoot apex organization differs in that there is a convex apex, with the presence of an orderly organized RZ in the SAM, in contrast to the PZ. This organization is similar to that observed in monocot species such as maize. Together, these observations illustrate a relationship between the apex structure (concave vs. convex) and the primary thickening activity (i.e. stem enlargement). Importantly, stem enlargement could also be associated with cell division patterns of the corpus (PZ and MZ), even if the SAM does not contribute significantly to the primary thickening activity.
The other difference observed between the different palm species concerns the tunica, which is single-layered in oil palm and in the Phoenix, Juania and rattan species, and two-cell-layered in Washingtonia, Trachycarpus and Corypha. This indicates that the number of cell layers in the tunica does not correlate with the phylogenetic relationships of the palm species studied, nor with SAM size. In a similar way, the number of cell layers in the tunica has been found to be variable in other monocot orders and shows no phylogenetic pattern, as illustrated by the Poales (Brown et al., 1957).
Thus, mature palm SAMs are highly organized with a histological zonation pattern defined by cells with different features reflecting different cell activities/identities but which can differ to some extent between species, especially with regard to the distinction between the PZ and the RZ. The most notable feature of histological zonation seen in palms compared with other monocot species is the presence of a PZ with highly organized cell division patterns, which separate spatially the CZ and the initiation sites of the leaf primordium. This zone is similar to that observed in some gymnosperm species displaying a so-called ‘transition zone’ with a highly organized cell division pattern (Evert, 2006). To our knowledge, this kind of organization has not been reported for any other monocot species.
SAM changes during the plastochron period
As a consequence of the limited accessibility of the oil palm SAM structure (embedded in leaf primordia and developing leaves), scanning electron microscopy (Kwiatkowska and Dumais, 2003), confocal microscopy (Laufs et al., 1998; Reddy et al., 2004) or optical coherence microscopy (Reeves et al., 2002) and tomography techniques such as X-ray computed tomography (Kaminuma et al., 2008) and optical projection tomography (Sharpe et al., 2002; Lee et al., 2006) were not feasible strategies to obtain a global 3-D reconstruction of the oil palm SAM, even with the younger developmental stages. An alternative approach using serial sections of the wheat and the bulrush millet SAMs has, however, been used previously to obtain 3-D reconstructions based on manual drawings of the histological structures. This method produced good representations of the 3-D structure but was limited to one angle of view as defined by the drawing (Metcalf et al., 1975; Williams and Langer, 1975; Williams et al., 1975). For this study, we obtained computer-assisted 3-D reconstructions from series of microtome-derived sections, this approach having the advantage that histological stains can be viewed readily, a large number of images can be aligned easily and different structures can be traced and placed together in a single 3-D view that can be animated (Fiala, 2005). Finally, the volume of the different structures can be calculated. To our knowledge, this paper provides the first documented example of such an approach being applied to the SAM of a perennial monocotyledonous species.
Our study suggests that the overall shape, volume and histological zonation pattern of the meristematic dome are dynamic and oscillate in relation to the organogenic activity of the SAM. The maximal volume of the SAM (for which there was a characteristic elongated conical shape) was observed at the very early stage of leaf primordium protrusion (leaf buttress stage), CZ volume also being maximal at this stage. During later stages of leaf primordium development, the volume of the meristematic dome decreases concomitantly with changes in its shape (towards a less elongated conical form) and a decrease in the CZ proportion within the dome (from 27 to 20 %). Later, the development of the leaf primordium continues in association with an increase in dome width and a decrease in the CZ proportion within the dome. These observations corroborate with those made on other species showing plastochronic changes in which maximal volume precedes initiation of the leaf primordium and minimal volume coincides with the early stages of leaf primordium (Evert, 2006).
Although the dynamics of SAM structure during the plastochron period have yet to be detailed for other palms, we can speculate that the patterns seen here will also apply to other species in the family. Global size and geometry changes during the plastochron period have been reported for a number of different monocot and eudicot species; the same cannot be said, however, for the phenomenon of major variations in inner cell type proportions described here (Smith and Rogan, 1979; Martin and Tucker, 1985). Indeed, obvious changes in CZ size during the plastochron period have not to our knowledge been reported to date for any other monocot species. In species such as maize or rice, in which the SAM is elevated considerably above the leaf primordium initiation site, plastochronic changes in size are not observed (Evert, 2006). This situation contrasts with that of the oil palm SAM, which displays plastochronic changes but which also possesses a SAM which is elevated with respect to the organogenic region. These size variations which characterize the mature palm SAM may be in some way related to the relatively large volume of the SAM compared with the other species studied. In this context, it is interesting to note that no variations in oil palm SAM size were observed during the plastochron period in the juvenile phase. This suggests a link between SAM plastochronic variations on the one hand and histological zonation and/or SAM size on the other. To support these views, it would be interesting to study the dynamics of the SAM in other species possessing a large SAM.
Conclusions
Eudicot and monocot species differ significantly in the functioning of their SAMs in relation to the mechanisms involved in leaf primordium founder cell recruitment, which in turn affects leaf shape (Poethig and Szymkowiak, 1995; Nardmann et al., 2004; Evert, 2006). Moreover, this diversity in SAM structure and function exists within the monocot group itself. Because of their perennial character and their specific features such as compound leaf development and primary growth-based stem thickening, palms are distinct from other monocots and of great interest in an evo-devo context. As shown here, oil palm SAM ontogenesis is characterized by a juvenile phase and a mature phase, with histological zonation specific to the mature phase. A distinct feature of mature oil palm SAM zonation is the presence of a large PZ with orderly cell files, which may act as a transition zone between the CZ and the sites of leaf primordium initiation, as observed in some gymnosperm species. Plastochronic changes seem to occur in the mature oil palm SAM with variations in CZ volume fluctuating in parallel with the early development of the leaf primordium. We showed recently that compound leaf formation in oil palm seems to be associated with a KNOX-dependent pathway as in other compound-leaved plant species, even though the mechanism of leaflet formation differs from the canonical blastozone fractionation mechanism (Jouannic et al., 2007). In the same way, in an evo-devo context, it will be interesting to characterize the expression patterns in palms of orthologues to genes that regulate SAM function in other species, so as to understand better the evolution of this process in angiosperms.
SUPPLEMENTARY DATA
ACKNOWLEDGEMENTS
We gratefully acknowledge the generous support of colleagues at INRAB (Pobé, Benin) and ASD (Coto, Costa Rica) for providing plant material. We thank Axel Labeyrie, Bruno Nouy (PalmElit, France) and David Cros (CIRAD, France) for logistical help. We are grateful to Fabienne Morcillo and Thierry Beulé (CIRAD, UMR DIAPC, France) for collecting zygotic embryo samples. Finally, we would like to thank Patrick Laufs, Barry Tomlinson and Darleen A. DeMason for critical reading of the manuscript. This work was supported by institutional funding from IRD and CIRAD.
LITERATURE CITED
- Adam H, Jouannic S, Escoute J, Verdeil JL, Duval Y, Tregear JW. Reproductive developmental complexity in the African oil palm (Elaeis guineensis) American Journal of Botany. 2005;92:1836–1852. doi: 10.3732/ajb.92.11.1836. [DOI] [PubMed] [Google Scholar]
- Ball E. The development of the shoot apex and the primary thickening meristem in Phoenix canariensis Chaub., with comparisons to Washingtonia filifere Wats. and Trachycarpus excelsa Wendl. American Journal of Botany. 1941;92:1836–1852. [Google Scholar]
- Barton MK. Cell type specification and self renewal in the vegetative shoot apical meristem. Current Opinion in Plant Biology. 1998;1:37–42. doi: 10.1016/s1369-5266(98)80125-8. [DOI] [PubMed] [Google Scholar]
- Beirnaert A. Introduction à la biologie florale du palmier à huile (Elaeis guineensis Jacquin). Publications de l'Institut National pour l'Etude Agronomique du Congo Belge (I.N.E.A.C.), Brussels, Belgium. Botanical Reviews. 1935;48:1–69. [Google Scholar]
- Brown WV, Heimsch C, Emery WHP. The organisation of the grass shoot apex and systematics. American Journal of Botany. 1957;44:590–595. [Google Scholar]
- Clark G. Staining procedures. 4th edn. Baltimore: Williams & Wilkins; 1984. [Google Scholar]
- Corley RHV, Gray BS. Growth and morphology. In: Corley RHV, Hardon JJ, Wood BJ, editors. Oil palm research. vol. 1. Amsterdam: Elsevier; 1976. pp. 7–21. Developments in crop. [Google Scholar]
- Evert RF. Esau's plant anatomy: meristems, cells, and tissues of the plant body – their structure, function and development. 3. New York: Wiley-Interscience; 2006. [Google Scholar]
- Fiala RC. Reconstruct: a free editor for serial section microscopy. Journal of Microscopy. 2005;218:52–61. doi: 10.1111/j.1365-2818.2005.01466.x. [DOI] [PubMed] [Google Scholar]
- Fisher DB. Protein staining of ribboned epon sections for light microscopy. Histochemie. 1968;16:92–96. doi: 10.1007/BF00306214. [DOI] [PubMed] [Google Scholar]
- Foster AS. Zonal structure and growth of the shoot apex in Microcycas calocoma (Miq.) A. DC. American Journal of Botany. 1943;30:56–73. [Google Scholar]
- Hartley CWS. The oil palm. 3rd edn. London: Longman; 1988. Longman Agriculture Series. [Google Scholar]
- Jourdan C, Michaux-Ferriere N, Perbal G. Root system architecture and gravitropism in the oil palm. Annals of Botany. 2000;85:861–868. doi: 10.1006/anbo.2000.1148. [DOI] [PubMed] [Google Scholar]
- Jouannic S, Collin M, Vidal B, Verdeil JL, Tregear JW. A class I KNOX gene from the palm species Elaeis guineensis (Arecaceae) is associated with meristem function and a distinct mode of leaf dissection. New Phytologist. 2007;174:551–568. doi: 10.1111/j.1469-8137.2007.02020.x. [DOI] [PubMed] [Google Scholar]
- Kaminuma E, Yoshizumi T, Wada T, Matsui M, Toyoda T. Quantitative analysis of heterogeneous spatial distribution of Arabidopsis leaf trichomes using micro X-ray computed tomography. Plant Journal. 2008;56:470–482. doi: 10.1111/j.1365-313X.2008.03609.x. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska D. Flowering and apical meristem growth dynamics. Journal of Experimental Botany. 2008;59:187–201. doi: 10.1093/jxb/erm290. [DOI] [PubMed] [Google Scholar]
- Kwiatkowska D, Dumais J. Growth and morphogenesis at the vegetative shoot apex of Anagallis arvensis L. Journal of Experimental Botany. 2003;54:1585–1595. doi: 10.1093/jxb/erg166. [DOI] [PubMed] [Google Scholar]
- Laufs P, Grandjean O, Jonak C, Kiêu K, Traas J. Cellular parameters of the shoot apical meristem in Arabidopsis. Plant Cell. 1998;10:1375–1390. doi: 10.1105/tpc.10.8.1375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Avondo J, Morrison H, Blot L, Stark M. Visualizing plant development and gene expression in three dimensions using optical projection tomography. Plant Cell. 2006;18:2145–2156. doi: 10.1105/tpc.106.043042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin BF, Tucker SC. Developmental studies in Smilax (Liliaceae). I: Organography and the shoot apex. American Journal of Botany. 1985;72:66–74. [Google Scholar]
- Metcalf RA, Fernandez A, Williams RF. The genesis of form in bulrush millet (Pennisetum americanum (L.) K. Schurn.) Australian Journal of Botany. 1975;23:761–773. [Google Scholar]
- Nardmann J, Ji J, Werr W, Scanlon MJ. The maize duplicate genes narrow sheath1 and narrow sheath2 encode a conserved homeobox gene function in a lateral domain of shoot apical meristems. Development. 2004;131:2827–2839. doi: 10.1242/dev.01164. [DOI] [PubMed] [Google Scholar]
- Poethig RS, Szymkowiak EJ. Clonal analysis of leaf development in maize. Maydica. 1995;40:67–76. [Google Scholar]
- Reddy GV, Heisler MG, Ehrhardt DW, Meyerowitz EM. Real-time lineage analysis reveals oriented cell divisions associated with morphogenesis at the shoot apex of Arabidopsis thaliana. Development. 2004;131:4225–4237. doi: 10.1242/dev.01261. [DOI] [PubMed] [Google Scholar]
- Reeves A, Parsons RL, Hettinger JW, Medford JI. In vivo three-dimensional imaging of plants with optical coherence microscopy. Journal of Microscopy. 2002;208:177–189. doi: 10.1046/j.1365-2818.2002.01086.x. [DOI] [PubMed] [Google Scholar]
- Sharpe J, Ahlgren U, Perry P, et al. Optical projection tomography as a tool for 3-D microscopy and gene expression studies. Science. 2002;296:541–545. doi: 10.1126/science.1068206. [DOI] [PubMed] [Google Scholar]
- Smith DL, Rogan PG. Growth of the shoot apex of Agropyron repens (L.) Beauv. during successive plastochrons. Annals of Botany. 1979;44:27–34. [Google Scholar]
- Tomlinson PB. The structural biology of palms. Oxford: Oxford Science Publications; 1990. [Google Scholar]
- Vallade J. Aspect morphologique et cytologique de l'embryon quiescent d'Elaeis guineensis Jacq. Compte Rendu de l'Académie des Sciences. 1966a;262:856–859. [Google Scholar]
- Vallade J. L'évolution de l'embryon quiescent d'Elaeis guineensis Jacq. au cours de la germination. Compte Rendu de l'Académie des Sciences. 1966b;262:856–859. [Google Scholar]
- Van Heel WA, Breure CJ, Menendez T. The early development of inflorescences and flowers of oil palm (Elaeis guineensis Jacq.) seen through the scanning electron microscope. Blumea. 1987;32:67–78. [Google Scholar]
- Williams RF, Langer RHM. Growth and development of the wheat tiller. II The dynamics of tiller growth. Australian Journal of Botany. 1975;23:745–759. [Google Scholar]
- Williams RF, Shavman BC, Langer RHM. Growth and development of the wheat tiller. I Growth and form of the tiller bud. Australian Journal of Botany. 1975;23:715–743. [Google Scholar]
- Yampolsky C. A contribution to the study of the oil palm Elaeis guineensis Jacq. Bulletin du Jardin Botanique Buitenzorg. 1922;vol V:107–174. série III. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.