Abstract
Background and Aims
Secondary somatic embryogenesis has been postulated to occur during induction of peach palm somatic embryogenesis. In the present study this morphogenetic pathway is described and a protocol for the establishment of cycling cultures using a temporary immersion system (TIS) is presented.
Methods
Zygotic embryos were used as explants, and induction of somatic embryogenesis and plantlet growth were compared in TIS and solid culture medium. Light microscopy, scanning electron microscopy (SEM) and transmission electron microscopy (TEM) were used to describe in vitro morphogenesis and accompany morpho-histological alterations during culture.
Key Results
The development of secondary somatic embryos occurs early during the induction of primary somatic embryos. Secondary somatic embryos were observed to develop continually in culture, resulting in non-synchronized development of these somatic embryos. Using these somatic embryos as explants allowed development of cycling cultures. Somatic embryos had high embryogenic potential (65·8 ± 3·0 to 86·2 ± 5·0 %) over the period tested. The use of a TIS greatly improved the number of somatic embryos obtained, as well as subsequent plantlet growth. Histological analyses showed that starch accumulation precedes the development of somatic embryos, and that these cells presented high nucleus/cytoplasm ratios and high mitotic indices, as evidenced by DAPI staining. Morphological and SEM observations revealed clusters of somatic embryos on one part of the explants, while other parts grew further, resulting in callus tissue. A multicellular origin of the secondary somatic embryos is hypothesized. Cells in the vicinity of callus accumulated large amounts of phenolic substances in their vacuoles. TEM revealed that these cells are metabolically very active, with the presence of numerous mitochondria and Golgi apparatuses. Light microscopy and TEM of the embryogenic sector revealed cells with numerous amyloplasts, large nuclei and nucleoli, and numerous plasmodesmata. Plantlets were obtained and after 3 months in culture their growth was significantly better in TIS than on solid culture medium. However, during acclimatization the survival rate of TIS-grown plantlets was lower.
Conclusions
The present study confirms the occurrence of secondary somatic embryos in peach palm and describes a feasible protocol for regeneration of peach palm in vitro. Further optimizations include the use of explants obtained from adult palms and improvement of somatic embryo conversion rates.
Keywords: Bactris gasipaes, tissue culture, somatic embryogenesis, clonal propagation, Picloram
INTRODUCTION
Peach palm (Bactris gasipaes) is a caespitose, multipurpose palm tree that is widely distributed in the lowland humid Neotropics (Mora-Urpí et al., 1997). Although it is listed as an underutilized crop, this species is one of the most useful palms in the Neotropics and the sole palm species that was domesticated for its fruits by Amerindians during the pre-Columbian period (Clement, 1988). Currently, the two major products from peach palm are the fruit, for local consumption, and the heart-of-palm, a gourmet vegetable extracted from the shoot apex and sold commercially worldwide (Clement, 2008). The heart-of-palm market is important in Latin America, and peach palm has numerous advantages for plantation production, such as perennial production from off-shoots, rapid growth rate and the possibility of fresh or minimally processed commercialization. Peach palm is also cultivated in Hawaii, Réunion Island, Indonesia and Malaysia.
Currently, major efforts towards the conservation of peach palm are based on the establishment of field germplasm banks, which are constantly threatened by biotic and abiotic factors, resulting in genetic erosion (Clement, 1997; Clement et al., 2004). Seed banks are not an option because this species has recalcitrant seeds (Bovi et al., 2004). Hence, in vitro conservation is suggested as the most promising technique for this purpose (Mora-Urpí et al., 1997). At the Instituto Nacional de Pesquisas da Amazônia, Manaus, AM, Brazil, for instance, a core collection was created within the main collection and clonal propagation would permit its transfer to other institutions for further studies or use in breeding programmes. The formation of seed orchards with selected genotypes, as well as the large-scale clonal multiplication of selected palms, would also benefit from an in vitro regeneration protocol.
Both organogenesis and somatic embryogenesis pathways have been described for peach palm in vitro regeneration (Arias and Huete, 1983; Stein and Stephens, 1991), although the regeneration rate was limited. Hence, studies on somatic embryogenesis in peach palm were suggested to enhance the regeneration rate. The main factors affecting the induction of somatic embryogenesis from different types of explants have been identified (Steinmacher et al., 2007a, b, c). It was suggested that during the induction of somatic embryos, the development of clusters of somatic embryos could be due to the development of secondary somatic embryos (Steinmacher et al., 2007a), although little evidence was provided. The use of this morphogenetic pathway could be important in improving the regeneration rate of the whole procedure, providing cycling cultures. Cycling cultures can be obtained using cell suspensions or through the induction of secondary somatic embryos, and both have been described in palms (Teixeira et al., 1995; Perez-Nunez et al., 2006; Sané et al., 2006); however, to the best of our knowledge no cycling cultures have been described in peach palm. Embryogenic friable callus, from which cell suspensions could be obtained (e.g. Teixeira et al., 1995; Sané et al., 2006), was observed to occur in peach palm using zygotic embryos as explants (Steinmacher et al., 2007a), but some bottlenecks, such as their apparently random induction and the loss of embryogenic potential after several subcultures, makes secondary somatic embryogenesis the most attractive pathway for scaling-up in vitro regeneration. The use of zygotic embryos as explants in improvement and conservation programmes is inappropriate, although they may serve as an interesting model for peach palm somatic embryogenesis because a relatively high induction rate was observed (Steinmacher et al., 2007a). In addition, the morpho-histological responses from zygotic embryos as explants were very similar to those observed from shoot meristem and leaf sheaths [i.e. first cell division on cells adjacent to the vascular tissue, callus growth through a meristematic zone and multicellular origin of the somatic embryos (Steinmacher et al., 2007a, c)].
Somatic embryogenesis is an example of plant cell totipotency. It has been suggested that morphological and molecular mechanisms underlying plant cell totipotency are different from those controlling pluripotency, observed in the maintenance of root and shoot apices. However, pluripotent and totipotent cells have similar characteristics, including a high nucleus/cytoplasm ratio, a dense cytoplasm and a small fragmented vacuome (Verdeil et al., 2007). Somatic embryogenesis involves the acquisition of embryogenic competence by somatic cells via their dedifferentiation and the reprogramming of their gene expression patterns (Harada, 1999; Feher et al., 2003; Gaj et al., 2005), possibly as a result of profound chromatin remodelling (Verdeil et al., 2007). Secondary somatic embryogenesis is the process by which somatic embryos are formed from pre-existing somatic embryos or using somatic embryos as explants (Raemakers et al., 1995). Usually, somatic embryos, which have higher embryogenic capacity than other explants, have been shown to increase the regeneration rate in several species (Raemakers et al., 1995), and the resulting cultures maintain their morphogenic competence for several years (i.e. in sand grape (Vitis rupestris) (Martinelli et al., 2001), thus increasing the potential regeneration rate several thousand-fold, as shown in coconut (Cocos nucifera) (Perez-Nunez et al., 2006).
Temporary immersion systems (TIS), which involve flooding of plant tissue at regular time intervals, are often used for scaling-up or improving in vitro regeneration protocols and also offer the possibility of automating some culture stages. A semi-automated ‘twin flask’ TIS was initially developed for pineapple (Escalona et al., 1999), and was later shown to improve the regeneration rate and plantlet quality for other plant species (Etienne and Berthouly, 2002). The number of regeneration protocols for other plant species using this system is increasing continually (Niemenak et al., 2008; Sankar-Thomas et al., 2008). Hence, an improved protocol for peach palm regeneration using TIS may be an alternative for the development of cycling cultures.
Parallel to the development of a feasible protocol for peach palm somatic embryogenesis, morpho-histological studies enhance our comprehension of the process, allowing further optimizations and other uses for the protocol. Studies with coconut (Fernando et al., 2003), African oil palm (Elaeis guineensis) (Schwendiman et al., 1988), Juçara palm (Euterpe edulis) (Guerra and Handro, 1998), date palm Phoenix dactylifera (Sané et al., 2006), macauba palm (Acrocomia aculeata) (Moura et al., 2008) and peach palm (Steinmacher et al., 2007a, c) showed that the first events of cell division were always observed in cells adjacent to the vascular tissue, resulting in primary calli or meristematic nodules from which somatic embryos developed.
In the present report, the occurrence of secondary somatic embryos during the induction of peach palm somatic embryogenesis was confirmed and the applicability of TIS for regeneration of peach palm in vitro was studied using a twin flask system.
MATERIAL AND METHODS
Plant material
Seeds from mature fruits of Bactris gasipaes Kunth, about 4 months after pollination from one selected open-pollinated tree (Mood1) of the Pampa Hermosa landrace grown in a commercial orchard in Ninole, Hawaii, USA, were used as sources of explants. The hard endocarp of the seeds was removed and the kernels (i.e. zygotic embryo enclosed in endosperm) were surface-sterilized by 1 min immersion in 70 % ethanol, followed by 40 min immersion in sodium hypochlorite solution, provided by a solution of 40 % commercial bleach (5 % active chloride), plus one drop of Tween 20 to each 100 mL of this solution (Steinmacher et al., 2007a). Zygotic embryos were aseptically removed under a stereomicroscope.
Culture media and conditions
The zygotic embryos were transferred to Petri dishes containing 30 mL of somatic embryogenesis induction medium: MS (Murashige and Skoog, 1962) salts plus Morel and Wetmore (1951) vitamins, 3 % (w/v) sucrose, 500 mg L−1 glutamine (Duchefa, Haarlem, the Netherlands), 2·5 g L−1 Gelrite (Duchefa), 1 µm AgNO3 and 10 µm Picloram [4-amino-3,5,6-trichloropicolinic acid (Duchefa)] (Steinmacher et al., 2007a). Each Petri dish contained five zygotic embryos that were observed periodically to describe the development of the somatic embryos. After 12 weeks of culture, the somatic embryos were isolated and used as explants for the induction of secondary somatic embryos in two experimental systems: on solid medium and in a temporary immersion system. All the cultures were kept in darkness at 28 °C with a subculture interval of 6 weeks. For culture on solid medium, nine somatic embryos were cultured on 30 mL induction medium as previously described for induction of primary somatic embryogenesis, using 2·5 g L−1 Gelrite (Duchefa) as gelling agent. The TIS used in the present study was based on the ‘twin flasks’ system described by Niemenak et al. (2008). Briefly, the plant compartment consisted of 1-litre glass jars (Weck GmbH u. Co. KG, Wehr, Germany) within which 250–300 mg isolated somatic embryos were cultivated in small baskets made with 150-μm nylon sieves (Laborbedarf-Vertriebs GmbH, Berlin, Germany) attached to the bottom of a 250-mL Kautex polypropylene bottle (Rotilabo® Carl Roth, http://www.carlroth.com). The medium compartment was a 1-litre Schott Duran® (Mainz, Germany) bottle with 250 mL liquid induction culture medium as described above, but without the gelling agent. Every 6 h the medium was air-pumped into the plant compartment for 3 min of contact with the explants. The air was filter-sterilized through an autoclavable 0·2-μm filter (Midisart 2000, Sartorius, Goettingen, Germany). Callus was discarded in each subculture and isolated somatic embryos were further cultured. The induction rate was evaluated at the end of each subculture interval.
The influence of induction conditions on the maturation of somatic embryos was evaluated. After cycling cultures were established, embryogenic cultures obtained from cultures (1) cultivated continually on solid culture medium, (2) cultivated in TIS for 6 weeks and then on solid culture medium for 6 weeks, and (3) cultivated continually in TIS were transferred to maturation conditions. In all treatments, 300–400 mg embryogenic clusters were separated into small clusters of somatic embryos and transferred to Petri dishes containing 30 ml maturation culture medium [MS salts; Morel and Wetmore vitamins; 40 µm 2,4-D (2,4-dichlorophenoxyacetic acid); 10 µm 2-iP [2-isopentyladenine (6-dimethylaminopurine)]; 1·5 g L−1 activated charcoal; 1 g L−1 glutamine, 500 mg L−1 hydrolysed casein and 2·5 g L−1 Gelrite (Steinmacher et al., 2007a)]. The cultures were kept in the dark at 28 °C for 4 weeks. Mature somatic embryos were than isolated and transferred to Petri dishes containing 30 mL conversion culture medium [MS salts; Morel and Wetmore vitamins; 20 µm 2-iP; 0·5 µm NAA (α-naphthaleneacetic acid); adapted from Steinmacher et al. (2007a)]. The cultures were transferred to light conditions (40–60 µmol m−2 s−1 provided by TLD 58 W/840 fluorescent lamps, Philips, Eindhoven, the Netherlands) at 25 °C for 4 weeks. Thereafter, somatic embryos were transferred to Petri dishes containing 30 mL MS salts plus Morel and Wetmore vitamins and 1·5 g L−1 activated charcoal for about 8 weeks until the plantlets reached 1–2 cm.
Plantlets obtained from the cultures induced in TIS and directly transferred to maturation conditions were selected, and their further growth was evaluated on solid culture medium and in TIS. The plantlets were weighed (200–250 mg each) and transferred to jars (Weck; 600 mL) containing 100 mL solid culture medium (eight plantlets per flask) and sealed with plastic film, or 24 plantlets for TIS treatment containing 300 mL of the same basal culture medium, except that the active charcoal was omitted. The same TIS described for induction of secondary somatic embryogenesis was used, but without the baskets. The cultures were kept under light conditions and subcultured at intervals of 4 weeks for 3 months. All the culture media were adjusted to pH 5·8 prior to adding 2·5 g L−1 Gelrite (Duchefa) as gelling agent and were autoclaved at 120 °C for 20 min.
The acclimatization procedure was adapted from that of Steinmacher et al. (2007a), keeping the plantlets in a high-humidity environment for the first 3 weeks. The entire acclimatization step was carried out in a growing room at 28 °C and with 16 h 80–100 µmol m−2 s−1 provided by HP-T Plus Lamps (Philips). To maintain the humidity the plantlets were transferred to plastic trays containing sand and watered with distilled water. The trays were placed inside of another plastic tray containing a transparent cover and this whole acclimatization apparatus was maintained in plastic bags for 3 weeks. After 3 weeks, the plastic bags were removed and the plantlets were watered every other day with distilled water and once a week with 5 mL per plantlet of modified Hoagland's solution. After 3 months survival and rooting of the plantlets were evaluated.
Histology
For light microscopy, samples were fixed in 2 % (w/v) paraformaldehyde/0·1 m phosphate buffer (pH 7·0), dehydrated in an ethanol series [30–100 % (v/v) in water] and embedded in LR White resin (London Resin Co Ltd, London, UK). Gelatine capsules filled with resin and samples were allowed to polymerize at 65 °C overnight. Specimens were cut into 1-μm-thick sections with a glass-knife in a semi-automatic microtome (Reichert Ultracut S, Leica), mounted onto glass slides with a drop of water and fixed over a hot plate (approx. 70 °C). For general histology, Toluidine blue O [0·5 % (w/v) in 0·1 m phosphate buffer] was used. For protein and polysaccharide localization the double-staining technique [with NBB (Naphtol Blue-Black) and PAS (Periodic Acid-Schiff)] or only PAS was used following Fisher (1968). For DAPI (4′-6-diamidino-2-phenylindole) staining, 100 µL of a stock solution (1 mg ml−1 in water) was diluted into 1 mL 0·1 m phosphate buffer (pH 7·0). The sections were incubated in one drop of the dilute solution for 5 min and washed once with 0·1 m phosphate buffer (pH 7·0). After removing the excess washing buffer, the samples were mounted with anti-fading Citifluor (Citifluor Ltd, London, UK) and visualized under UV excitation (excitation 355 nm; emission 450 nm) using an Olympus BH-2 microscope and photographed with a ColorView IIIu (Soft Imaging System, GmbH, Munster, Germany).
For scanning electron microscopy (SEM), the samples were fixed in 3 % (v/v) glutaraldehyde in 0·1 m phosphate buffer (pH 7·0) and dehydrated in an ethanol series [30–100 % (v/v) in water], followed by an acetone series [30, 50 and 100 % (v/v) acetone in ethanol]. The samples were dried to the critical point with liquid CO2 in a CPD 030 critical point dryer (Bal-TEC, Leica, http://www.leica-microsystems.com), affixed to aluminium stubs and coated with gold palladium in a SCD 050 sputter coater (Bal-TEC, Leica). The mounted specimens were examined with a Philips XL 20 scanning electron microscope.
For transmission electron microscopy (TEM), samples were fixed in 4 % (v/v) glutaraldehyde in 0·1 m cacodylate buffer (pH 7·0) for 2 h at room temperature. Thereafter the samples were transferred to fresh fixative solution and maintained overnight at 4 °C. The samples were then rinsed in the same buffer without glutaraldehyde three times (15 min each) and post-fixed in 1 % (v/v) OsO4 in 0·1 m cacodylate buffer at 4 °C for 2 h. After rinsing in cacodylate buffer, the samples were dehydrated in a graded acetone series and embedded in Spurr's resin. Ultrathin sections (80–100 nm) were cut with an Ultracut E ultramicrotome (Reichert-Jung, Vienna, Austria), collected over a Formvar-coated copper grid, and stained with 1 % uranyl acetate and 0·1 % lead citrate for 10 min each.
Statistical analysis
A completely randomized design was used for all experiments. For the experiment of induction of secondary somatic embryogenesis, three repetitions comprising three Petri dishes each were evaluated for solid culture medium or three repetitions comprising two flasks for TIS. The data were subjected to ANOVA and when necessary the SNK test was used to compare means using STATISTICA v.6 (StatSoft, Inc.). The variables evaluated were the percentage of callus, spongy tissue development and induction rate of secondary somatic embryos. The percentage induction was additionally separated into three embryogenic-capacity categories [low (<5 somatic embryos), medium (5–15) and high (>15)] because during SEM it was difficult to count the exact number of somatic embryos.
For plantlet growth, the experiment was conducted in a completely randomized design containing five replications, with each replication represented by one flask with solid or liquid culture medium. The variables evaluated were plantlet fresh weight, total number of plantlets and plantlet height (with three classes: <3·5 cm, 3·5–6·5 cm, >6·5 cm). For plantlet acclimatization, the final survival rate (%), average height (cm), rooting rate (%) and average number of roots per plantlet were evaluated. The results were subjected to ANOVA as above.
RESULTS
Induction of primary somatic embryogenesis
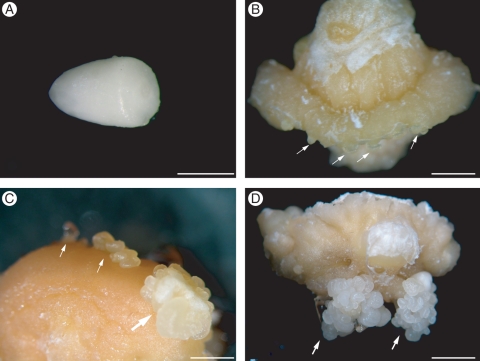
Zygotic embryos (Fig. 1A) cultivated on Picloram-enriched culture medium showed swelling and callus growth during the first week of culture. Globular structures arising from callus were observed within 4–6 weeks of culture (Fig. 1B). These globular structures were whitish and usually only a few isolated globular structures were observed on each explant at this time. Development of additional somatic embryos occurred exactly in the sectors where the globular structures first appeared (Fig. 1C), resulting in clusters of somatic embryos at the end of 12 weeks of culture. These clusters of somatic embryos were observed on all parts of the callus, but usually clusters in contact with the culture medium developed more somatic embryos (Fig. 1D). Approximately 40 % of the explants developed somatic embryos.
Fig. 1.
Induction of somatic embryogenesis from peach palm zygotic embryos. (A) Mature zygotic embryo of peach palm used as explant. (B) Initial development of globular structures resembling somatic embryos on the callus (arrows) after 4–6 weeks of culture. (C) Further development of globular somatic embryos (arrows) where somatic embryos had previously developed. (D) Development of a cluster of somatic embryos (arrows) on the callus after 6 weeks of culture. Scale bars: (A) = 1 mm, (B) = 3 mm, (C, D) = 2 mm.
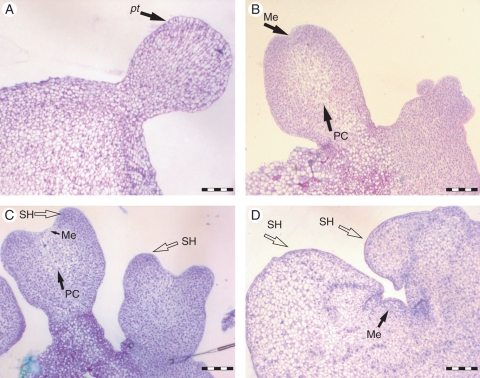
Histological techniques were used to describe the sequence of development of individually selected somatic embryos. Globular structures at the onset of polarization (i.e. elongated cells) and the presence of a well-delimited protoderm were the first clearly distinguishable stages of somatic embryo development in culture (Fig. 2A). Further development included their elongation, development of procambium and differentiation of the shoot meristem pole (Fig. 2B). Somatic embryos transferred to maturation conditions had well-developed procambium and a developing sheath base around the shoot meristem (Fig. 2C). After the somatic embryos were transferred to conversion conditions, their development included a well-differentiated shoot meristem completely enclosed by the sheath base (Fig. 2D).
Fig. 2.
Histological analyses of the development of peach palm somatic embryogenesis. (A) Globular somatic embryo with well-developed protodermis (pt). (B) Elongated somatic embryos showing the initial differentiation of the procambium (PC) and shoot meristem (Me). (C) Mature somatic embryo revealing complete development of the procambium as well as the sheath base (SH). (D) Somatic embryo in conversion conditions revealing a well-formed shoot meristem enclosed by the sheath base. Scale bars = 200 µm.
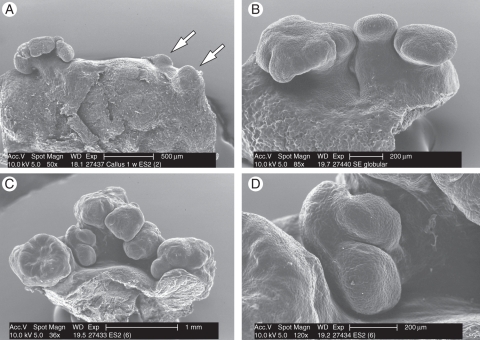
SEM revealed the initial development of the isolated globular structures (Fig. 3A), which resulted in the development of small clusters of somatic embryos (Fig. 3B). Alterations in the morphology of the somatic embryos were observed in particular, but not exclusively, in the sector where the sheath base resulted in a mushroom-like structure (Fig. 3B). From these somatic embryos, secondary somatic embryos developed, resulting in clusters of somatic embryos (Fig. 3C). This confirms that the development of clusters of somatic embryos in peach palm is due to the development of secondary somatic embryos. In the clusters of somatic embryos, several developmental stages could be observed, revealing non-synchronized development. This was due to the fact that secondary somatic embryos are continually produced in these conditions, where secondary somatic embryos arose also from globular somatic embryos (Fig. 3D), and that primary somatic embryos also developed at different points on a callus at different times.
Fig. 3.
Scanning electron microscopy analyses during the induction of peach palm somatic embryos. (A) Initial development of isolated globular structures (arrows). (B) Small clusters of primary somatic embryos. (C) Development of secondary somatic embryos, resulting in a cluster of somatic embryos. (D) Globular somatic embryo revealing the development of secondary somatic embryos. Scale bars: (A) = 500 µm, (C) = 1 mm, (B, D) = 200 µm.
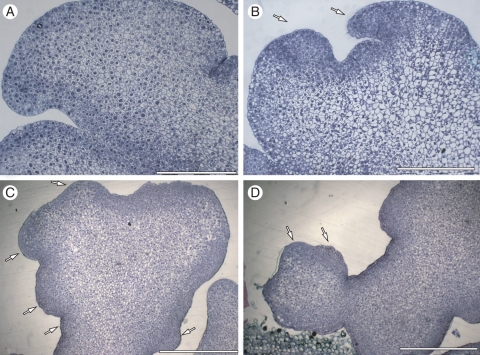
Histological analyses revealed that the sub-epidermal cell layers had a more intense reaction to Toluidine blue (Fig. 4A). No suspensor-like tissue was observed and the somatic embryos had a broad basal area fused to the maternal tissue, but no vascular connections with the explant were observed by light microscopy. As somatic embryo development progressed, intense staining was observed in the sheath base region (Fig. 4B) from where new somatic embryos arose (Fig. 4C). As with SEM analysis, light histology analysis revealed the initial development of secondary somatic embryos from globular somatic embryos (Fig. 4D).
Fig. 4.
Histological analyses of the development of peach palm somatic embryos stained with Touludine blue. (A) Histological analysis of peach palm primary somatic embryos revealing intense cell division on sub-epidermal cells. (B) Developed somatic embryo showing intense staining in the sheath base region (arrows). (C) Development of secondary embryos (arrows) from elongated somatic embryo. (D) Initial development of secondary somatic embryos from globular somatic embryos (arrows). Scale bars: (A, B) = 200 µm, (C, D) = 500 µm.
Induction of secondary somatic embryogenesis and plantlet regeneration
Peach palm somatic embryos were used as explants and different morphological responses were observed, including spongy tissue, callus and somatic embryo development. Picloram-enriched culture medium was effective in inducing secondary somatic embryogenesis and on average peach palm somatic embryos had elevated embryogenic capacity, ranging from 65·8 to 86·2 % of secondary somatic embryo development in all conditions (Table 1). Statistical differences (P < 0·05) were observed for the total rate of induction only in the first cycle (Table 1). A decreasing rate of embryogenic capacity was observed on solid culture medium for this group: 16·3 ± 2·7 % of the explants showed more than 15 somatic embryos per explant in the first cycle, whereas after four cycles (6 weeks each) only 2·6 ± 0·9 % of the explants could be classified as high capacity. On TIS, 48·8–64·2 % of the explants showed high embryogenic capacity (Table 1), without statistical differences between these values, but with significant differences (P < 0·05) compared with solid culture medium. Transfer of somatic embryos from TIS to solid culture medium showed high embryogenic capacity (86·2 %), with most explants showing more than 15 somatic embryos (63·5 %), without differences with the TIS treatment. No development of spongy tissue was observed in TIS, while callus development was significantly (P < 0·05) higher in TIS than on solid culture medium, varying from 15·3 to 21·4 % (Table 1).
Table 1.
Percentage of induction of secondary somatic embryogenesis of peach palm in different culture conditions and during different cycles of 6 weeks each
| Callus | Spongy tissue | Development of secondary somatic embryos |
||||
|---|---|---|---|---|---|---|
| Total | Low | Medium | High | |||
| 1st solid | 7·7 ± 2·1bc | 26·2 ± 2·9b | 66·2 ± 3·1b | 14·4 ± 1·7b | 35·5 ± 2·0a | 16·3 ± 2·7b |
| 2nd solid | 7·6 ± 2·8bc | 26·2 ± 2·4b | 65·8 ± 3·0b | 21·8 ± 2·4b | 37·8 ± 4·3a | 6·2 ± 2·0bc |
| 3rd solid | 1·3 ± 0·5c | 11·1 ± 4·2a | 86·6 ± 4·1a | 35·5 ± 6·0a | 41·3 ± 8·6 a | 9·7 ± 4·5bc |
| 4th solid | 0·0 ± 0·0c | 31·1 ± 1·8b | 70·2 ± 2·3ab | 40·8 ± 2·5a | 26·6 ± 2·5ab | 2·6 ± 0·9c |
| 2nd TIS | 21·4 ± 2·7a | 0·0 ± 0·0c | 78·6 ± 2·7ab | 16·5 ± 2·2b | 13·3 ± 2·9b | 48·8 ± 4·5a |
| 3rd TIS | 16·0 ± 3·1ab | 0·0 ± 0·0c | 83·9 ± 3·0a | 13·4 ± 2·3b | 19·5 ± 4·2ab | 50·9 ± 3·0a |
| 4th TIS | 15·3 ± 3·5ab | 0·0 ± 0·0c | 84·6 ± 3·5a | 9·7 ± 1·0b | 10·7 ± 3·7b | 64·2 ± 5·8a |
| TIS/Solid | 9·3 ± 2·4bc | 0·4 ± 0·4c | 86·2 ± 5·0a | 4·9 ± 2·7b | 13·7 ± 2·6b | 63·5 ± 6·7a |
Data are means ± s.e. Means followed by different lower-case letters in the column represent statistical differences identified by SNK analysis (P < 0·05).
Upon transferring somatic embryo clusters to maturation conditions for 4 weeks, differences among culture conditions were observed. The highest increase in fresh weight was observed for clusters of somatic embryos cultivated exclusively on solid culture medium (Table 2). This increase was due mainly to the development of a spongy haustorial-like tissue. Embryogenic clusters from TIS or TIS/solid medium had high numbers of mature somatic embryos after 4 weeks in maturation conditions, without statistical differences between these treatments (Table 2). The conversion capacity of somatic embryos was around 30 % (data not shown) and plantlets were obtained.
Table 2.
Maturation of peach palm secondary somatic embryos on maturation culture medium
| Treatment | Initial weight (mg f. wt) | 30 days culture on maturation medium |
|
|---|---|---|---|
| Increment (g) | Somatic embryos (100-mg explant) | ||
| Solid | 343 ± 9·3 | 6·89 ± 0·74b | 0·0 ± 0·0b |
| TIS | 349 ± 13·7 | 3·92 ± 1·27a | 62·1 ± 16·2a |
| TIS/Solid | 370 ± 12·2 | 3·96 ± 1·03a | 44·9 ± 7·8a |
Data are means ± s.e. Means followed by different lower-case letters in the column represent statistical differences identified by SNK analysis (P < 0·05).
Small plantlets (200–250 mg each) were cultivated on solid culture medium or transferred to TIS. The final fresh weight of the plantlets was higher in TIS than on solid culture medium (Table 3). Plantlet height was also influenced by culture conditions. After 3 months of culture on solid culture medium, no plantlets taller than 6·5 cm were observed, while in TIS 51·1 ± 11·4 % of the plantlets were taller than 6·5 cm (Table 3). Additionally, newly formed shoots were observed in TIS as well as on solid culture medium; it is not clear if these shoots developed from fused somatic embryos or from the development of off-shoots, as peach palm is caespitose. Root development was observed only occasionally during the culture period.
Table 3.
Comparison of TIS and solid culture medium on subsequent peach palm plantlet growth
| Treatment | Initial weight (mg f. wt) | Final weight (g f. wt) | Total no. | Class of plantlet height (%) |
||
|---|---|---|---|---|---|---|
| <3·5 cm | 3·5–6·5 cm | >6·5 cm | ||||
| TIS | 213 ± 2 | 1·34 ± 0·2 | 177 | 16·9 ± 5·2 | 32·0 ± 7·8 | 51·1 ± 11·4 |
| Solid | 215 ± 1 | 0·97 ± 0·1 | 54 | 41·6 ± 8·1 | 58·4 ± 8·1 | 0·0 ± 0·0 |
Data are means ± s.e.
Upon transferring the regenerated plantlets to acclimatization conditions, 65 % of TIS-grown plantlets and 97 % of solid culture medium plantlets survived after 3 months. On the other hand, plantlet height, rooting rate and number of roots per plantlet were significantly higher in plantlets from TIS than from solid culture medium (Table 4). Successful rooting of TIS-grown plantlets (75·1 %) was much higher than those grown on solid culture medium (12·5 %) and all plantlets were allowed to grow further.
Table 4.
Peach palm plantlet growth and survival rate after 3 months of acclimatization
| Treatment | No. of plantlets | Survival (%) | Height (cm) | Rooting (%) | Roots per plantlet |
|---|---|---|---|---|---|
| TIS | 133 | 65b | 11·2a | 75·1a | 2·3a |
| Solid | 36 | 97a | 6·6b | 12·5b | 1·3b |
Means followed by different lower-case letters in the column represent statistical differences identified by ANOVA (P < 0·05).
Morpho-histological aspects of secondary somatic embryo development
Using somatic embryos as explants resulted in secondary somatic embryos developed directly on the explants. As observed during the induction of primary somatic embryogenesis, secondary somatic embryos developed frequently, but not exclusively, from the sheath base and somatic embryos appeared with a broad basal area fused to maternal tissue without vascular connections to the explant tissue. Once somatic embryogenesis was triggered, continual development of somatic embryos was again observed in all tested conditions, resulting in clusters of somatic embryos.
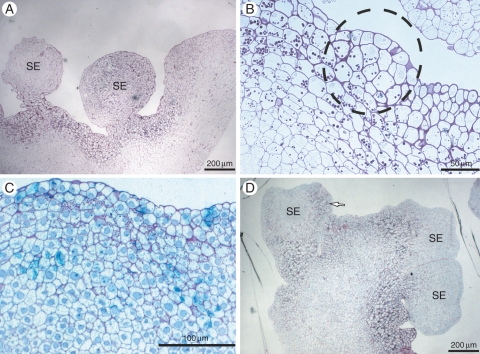
Histochemical analyses revealed that starch accumulation generally precedes the development of somatic embryos (Fig. 5A). A multicellular origin, involving niches of sub-epidermal and epidermal cells (Fig. 5B), is hypothesized for these somatic embryos. However, in cultures on solid culture medium only a few cells showed the presence of amyloplasts (Fig. 5C), whereas in cultures from TIS most subepidermal cells showed starch accumulation. As development of somatic embryos progressed, starch accumulation was observed only in the basal area of the somatic embryos or later on those sectors where other somatic embryos would develop (Fig. 5D). However, starch accumulation could not be systematically correlated with cell embryogenic capacity, as in culture sub-epidermal cells also accumulated large amounts of starch without developing into somatic embryos, especially in TIS conditions.
Fig. 5.
Histochemical analyses during the development of peach palm secondary somatic embryos (SE). (A) Cluster of somatic embryos from TIS showing high starch accumulation. (B) Possible origin of somatic embryos involving sub-epidermal and epidermal cells (circle). (C) Samples cultured only on solid culture medium. (D) Further development of somatic embryos showing specific starch accumulation in those sectors where other somatic embryos would develop (arrow). Scale bars: (A) = 200 µm, (B) =50 µm, (C) = 100 µm, (D) = 200 µm.
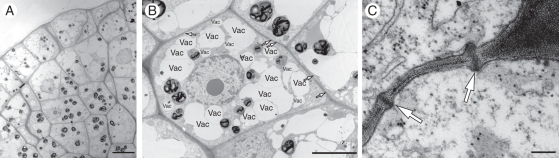
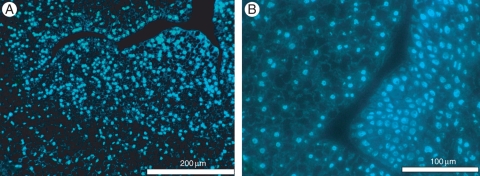
Ultrastructural analyses of the embryogenic sector revealed small starch granules in the cells of the protodermis, while sub-epidermal cells had larger amyloplasts that were also more abundant and well distributed in the cells (Fig. 6A). The cells contained numerous small vacuoles and a large central nucleus with prominent nucleolus (Fig. 6B), no cell-wall thickening, and plasmodesma was often observed connecting the cells (Fig. 6C). These results together suggest the multicellular origin of the somatic embryos and epidermal and sub-epidermal cells for the origin of the secondary somatic embryos. Also in this embryogenic sector, mitotic events could be observed by DAPI staining and a higher nucleus/cytoplasm ratio was observed in epidermal and sub-epidermal cells compared with those that would result in callus growth (Fig. 7A). More numerous mitotic events were observed in the sub-epidermal cell layer and these cells also had centrally located nuclei with one or two nucleoli (Fig. 7B).
Fig. 6.
Ultrastructural analyses of peach palm embryogenic cells. (A) Embryogenic sector including epidermal and sub-epidermal cells. (B) Example of a cell of the embryogenic sector contained numerous small vacuoles (Vac) and numerous plasmodesma (arrows). (C) Aspect of the plasmodesma. Scale bars: (A) = 10 µm, (B) = 5 µm, (C) = 200 nm.
Fig. 7.
Mitotic events in the embryogenic sector revealed by DAPI staining. (A) Higher nucleus/cytoplasm ratio observed in sub-epidermal cells. (B) Mitotic events during the initial development of peach palm secondary somatic embryos. Scale bars: (A) = 200 µm, (B) = 100 µm.
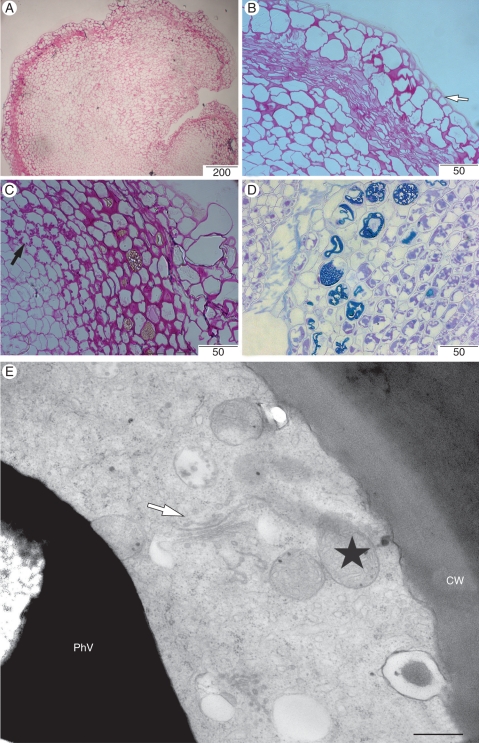
Histological analyses of callus revealed the presence of a specific zone of small cells (Fig. 8A), as well as the presence of an epidermis-like layer (Fig. 8B). Only a few amyloplasts were observed in some areas of the callus (Fig. 8C). In the area where the embryogenic sector was in contact with the callus sector, an intense metachromatic reaction with Toluidine blue O was observed in the vacuoles (Fig. 8D) of a specific layer of cells usually associated with the development of somatic embryos. This metachromatic reaction indicates the accumulation of large amounts of phenolic substances in the vacuoles of the cells. Ultrastructural analysis of these phenol-storing cells revealed the presence of a large vacuole containing electron-dense substances, numerous mitochondria, small amyloplasts, plastids also containing electron-dense substances and Golgi complexes (Fig. 8E), all suggesting metabolically active cells.
Fig. 8.
Histological and ultrastructural aspects of the callus sector. (A) General view of callus stained with PAS reagent. (B) Detailed view of A showing an epidermis-like cell layer (arrow). (C) Presence of amyloplast in the callus sector (black arrow). (D) Histological section of the callus sector in contact with the embryogenic sector revealing the accumulation of phenolic substances in the cells. (E) Detailed view of the phenol-storing cells showing numerous mitochondria (stars), and Golgi complex (arrow). Scale bars: (A) = 200 µm, (B, C) = 50 µm, (D) = 500 µm, (E) = 500 nm.
DISCUSSION
The occurrence of secondary somatic embryogenesis during the induction of peach palm somatic embryogenesis, as previously hypothesized (Steinmacher et al., 2007a), was confirmed on auxin-enriched culture medium with light microscopy and SEM in the present study. The occurrence of secondary somatic embryos in primary culture conditions was observed in Bermuda grass (Li and Qu, 2002) and the constant requirement of plant growth regulators for the development of somatic embryos has been described in several monocot species [i.e. banana (Khalil et al., 2002); see also the review by Raemakers et al. (1995)]. In the present study, the use of this morphogenetic pathway also allowed the establishment of an effective protocol for the induction of secondary somatic embryogenesis and permitted the development of a cycling culture.
In peach palm induction of both primary and secondary somatic embryos was achieved with Picloram-enriched culture medium, confirming that this as an effective auxin analogue for peach palm somatic embryogenesis, as previously demonstrated (Valverde et al., 1987; Steinmacher et al., 2007a, b, c). This auxin analogue also induced embryogenic competence in African oil palm (Teixeira et al., 1995) and was the most suitable auxin source for somatic embryogenesis in arecanut palm (Areca catechu, Karun et al., 2004) and macauba palm (Moura et al., 2008). Auxins have been shown to act like a molecular glue binding to its TIR1 receptor and promoting ubiquitin-dependent degradation of Aux/IAA repressor proteins, activating the auxin response elements (Guilfoyle, 2007; Tan et al., 2007). Naturally occurring auxin (indol acetic acid – IAA) and synthesized auxin analogues (i.e. NAA and 2,4-D) showed the same activity (Guilfoyle, 2007; Tan et al., 2007). It is thought that auxin analogues also have a dual role during the induction of somatic embryogenesis, one related to auxin signalling and the other to a stress component (Feher et al., 2003) that also changes the endogenous content of auxins (Jiménez, 2005). Picloram is thought to have a stronger auxin effect compared with other auxin analogues (i.e. 2,4-D) and this observation is supported by the fact that germinating Arabidopsis seeds in Picloram-enriched culture medium mimicked an auxin over-producing mutant (sur2) (Delarue et al., 1998). However, other differences between 2,4-D and Picloram in signalling cascades may also exist, as a mutation in one receptor altered the response to Picloram but not to 2,4-D and IAA (Walsh et al., 2006).
Secondary somatic embryogenesis has already been proved to be an excellent morphogenetic process for several species, such as coconut (Perez-Nunez et al., 2006), cassava (Manihot esculenta) (Groll et al., 2001) and cacao (Theobroma cacao) (Maximova et al., 2002). It has been shown that somatic embryos, especially during the first developmental stages, have higher embryogenic capacity than other explants (Raemakers et al., 1995). This could be attributed to the presence of higher levels of somatic embryogenesis-related transcripts, such as the somatic embryogenesis-related kinase (SERK) and leafy cotyledon (LEC), which are still being expressed at the first developmental stages of somatic embryos (Schmidt et al., 1997; Kwaaitaal and de Vries, 2007; Alemanno et al., 2008; Sharma et al., 2008).
The combination of a secondary somatic embryogenesis pathway with TIS also increases propagation efficiency in several species, for example rubber (Hevea brasiliensis) (Etienne et al., 1997), coffee (Albarran et al., 2005) and cacao (Niemenak et al., 2008). To the best of our knowledge, however, there are no conclusive reports on the use of TIS and secondary somatic embryogenesis in palms. In our study, efficient cycling cultures could be established only on TIS, while on solid culture medium a decreasing rate of highly embryogenically capable cultures and an increasing rate of spongy tissue development were observed. Other advantages of TIS could be related to the absence of nutrient gradients (as in solid culture medium), nutrient uptake without permanent hypoxia and the frequent renewal of the in vitro atmosphere, where ethylene accumulates (Etienne and Berthouly, 2002). The presence of silver nitrate, a known blocker of ethylene perception, was shown to greatly increase somatic embryogenesis of peach palm (Steinmacher et al., 2007a).
Stress is an important factor related to the acquisition of embryogenic competence (Pasternak et al., 2002) and the temporary flooding in TIS could create different forms of stress, such as temporary hypoxia, which is sensed by the cells as a sort of stress triggering short-term metabolic adaptations. During hypoxia plant cells adopt a carbohydrate-conserving response to reserve starch and hexose sugars for use in aerobic metabolism upon recovery from the stress (Fukao and Bailey-Serres, 2004). This strategy also decreases oxygen consumption and improves plant re-growth after reoxygenation (Geigenberger, 2003). Therefore, it is tempting to suggest that peach palm explants adopted such an adaptation strategy, because, according to our histological analysis, greater starch accumulation was observed in TIS-cultivated explants. Additionally, in the present study, greater starch accumulation was observed in the embryogenic sector in particular, while in the callus sector starch was rarely observed. Starch accumulation is considered a marker for embryogenic capacity in several systems, including oil palm and coconut (Schwendiman et al., 1988; Verdeil et al., 2001). However, in peach palm starch accumulation could not be systematically correlated to somatic embryogenesis, as some cells accumulated starch without developing into somatic embryos, as also observed in rattan somatic embryogenesis (Goh et al., 2001), while in pineapple-guava (Feijoa sellowiana) non-embryogenic tissue accumulated much more starch (Canhoto et al., 1996). Therefore, it remains to be determined if starch accumulation is more related to hypoxia or other metabolic pathways than to the specific development of somatic embryos. Even in well-oxygenated surroundings, plant tissues that have high metabolic activity can become hypoxic, especially those that lack large intercellular air spaces or contain cells that are poorly vacuolated (Geigenberger, 2003), characteristics similar to those observed in the present study.
Histological analyses revealed cells with embryogenic characteristics, such as small cells with small vacuoles, large nuclei and dense cytoplasm. Such cells were usually found as niches of sub-epidermal and epidermal tissue. In coconut somatic embryogenesis, sub-epidermal embryogenic cells also had a dense cytoplasm (Saenz et al., 2006). At the onset of somatic embryo development, DAPI staining also revealed that these cells were at different stages of division or with centrally located nuclei with one or two nucleoli, while in the callus sector much less signal was observed and the nuclei were usually in the periphery of the cells. This agrees with the observation that cells able to produce somatic embryos are mitotically more active than non-embryogenic cells (Pasternak et al., 2002).
Somatic embryos may originate from either unicellular or multicellular pathways and, in our study, niches of epidermal and sub-epidermal cells participated in the formation of peach palm secondary somatic embryos. Ultrastructural analyses revealed that peach palm somatic embryogenesis follows a pathway similar to that described for pineapple-guava (Canhoto et al., 1996), in which embryogenic cells were connected by plasmodesmata through their cell walls. Additionally, peach palm somatic embryos also appeared with a broad basal area in contact to maternal tissue, but without vascular connection with the maternal tissue, and histological analyses showed no co-ordinated cell division during the initial developmental steps. All these characteristics suggest the multicellular origin of the somatic embryos (Quiroz-Figueroa et al., 2006). In coconut, both multicellular and unicellular pathways have been observed (Verdeil et al., 2001; Fernando et al., 2003), although plantlets have been regenerated mostly from somatic embryos with a multicellular origin (Perera et al., 2007).
Histological analyses of the callus sector revealed a sector of phenol-rich cells exactly on the border of the callus, especially in the sectors where somatic embryos developed. Similar results were also observed in pineapple-guava, and these phenol-storing cells were hypothesized to form a barrier between the somatic embryos and the mother tissue during induction and development of somatic embryos (Reis et al., 2008). Blockage of symplastic transport is also thought to be a major driver of morphological alterations (Pfluger and Zambryski, 2001) and it is possible that for the development of somatic embryos symplastic isolation is required also at the multicellular level. Such isolation can occur physically through the thickening of the cell wall and closing of the plasmodesmata by deposition of callose (Dubois et al., 1990; Verdeil et al., 2001), as well as by deposition of phenolic and lipophilic substances on cell walls (Pedroso et al., 1995), or through the development of barrier cells (i.e. phenol-storing cells) between somatic embryos and mother tissue (Reis et al., 2008), as observed in the present study. As these phenol-storing cells are metabolically very active, with the presence of numerous mitochondria and Golgi apparatuses, their specific roles during the development of somatic embryos remain to be elucidated.
In the present study, somatic embryos were transferred to maturation conditions prior to conversion. These steps can still be considered as a bottleneck to a successful protocol, as some mature somatic embryos were fused or no mature somatic embryos were observed from those cultures induced only on solid culture medium. Additionally, a relatively low (around 30 %) conversion rate was observed and the regenerated plantlets had deficient root development in vitro. Previous studies of acclimatization of peach palm plantlets suggested that the in vitro-grown roots were not functional (Arias, 1985) and they were removed, allowing new roots to develop during the acclimatization step (Steinmacher et al., 2007a). Plantlets produced in TIS had lower survival rates than those from solid culture medium; however, TIS-grown plantlets showed enhanced growth and higher rooting rate, suggesting physiologically better plantlets. Among the other vantages of TIS, the ventilation of the culture containers may also result in plantlets that are more capable of growing in ex vitro conditions. In coconut, increased capacity of in vitro-grown plants to control water loss was related to the ventilation of the flasks (Talavera et al., 2001). This suggests that TIS could be an alternative technique for the growth of other palm species.
In conclusion, the occurrence of secondary somatic embryos during the induction of somatic embryogenesis in peach palm was confirmed. This morphogenetic pathway allowed the development of a protocol suitable for in vitro multiplication of peach palm using a TIS and cycling cultures. Although zygotic embryos were used as explants, somatic embryogenesis was already obtained from inflorescences and shoot meristems (Steinmacher et al., 2007b, c), making this protocol useable for selected genotypes. In fact, a pilot project is underway for the mass propagation of selected peach palm genotypes from Hawaii. The maturation and conversion conditions in this protocol must also be improved, possibly using storage proteins as a quality marker. The use of a TIS may also be an interesting strategy for the scaling-up of other palm tree in vitro protocols.
ACKNOWLEDGEMENTS
We thank Mr John Mood (Honolulu, Hawaii, USA), considered as a partner in this project, for continually sending plant material. Thanks also to Prof. Charles Clement for critical reading of the manuscript. The assistance from Mr Detlef Böhm, Mrs Karen Dehn and Mrs Elke Woelken is also highly appreciated. D.A.S. thanks the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) Brasília, Brazil, and DAAD (Deutscher Akademischer Austausch Dienst) Bonn, Germany, for financial support for the development of this study.
LITERATURE CITED
- Albarran J, Bertrand B, Lartaud M, Etienne H. Cycle characteristics in a temporary immersion bioreactor affect regeneration, morphology, water and mineral status of coffee (Coffea arabica) somatic embryos. Plant Cell Tissue and Organ Culture. 2005;81:27–36. [Google Scholar]
- Alemanno L, Devic M, Niemenak N, et al. Characterization of leafy cotyledon1-like during embryogenesis in Theobroma cacao L. Planta. 2008;227:853–866. doi: 10.1007/s00425-007-0662-4. [DOI] [PubMed] [Google Scholar]
- Arias O. Propagación vegetativa por cultivo de tejidos del pejibaye (Bactris gasipaes H.B.K.) Asbana. 1985;24:24–27. [Google Scholar]
- Arias O, Huete F. Vegetative propagation in vitro of pejibaye (Bactris gasipaes HBK) Turrialba. 1983;33:103–108. [Google Scholar]
- Bovi MLA, Martins CC, Spiering SH. Desidratação de sementes de quatro lotes de pupunheira: efeitos sobre a germinação e o vigor. Horticultura Brasileira. 2004;22:109–112. [Google Scholar]
- Canhoto JM, Mesquita JF, Cruz GS. Ultrastructural changes in cotyledons of Pineapple Guava (Myrtaceae) during somatic embryogenesis. Annals of Botany. 1996;78:513–521. [Google Scholar]
- Clement CR. Domestication of the Pejibaye palm (Bactris gasipaes): past and present. Advances in Economic Botany. 1988;6:155–174. [Google Scholar]
- Clement CR. Pupunha: Recursos genéticos, pesquisas realizadas e tecnologias disponíveis. In: Claret de Souza AG, Figueredo dos Santos A, editors. 1° Workshop sobre as Culturas de Cupuaçu e Pupunha da Amazônia. CPAA/EMBRAPA, Manaus, AM: 1997. pp. 33–49. [Google Scholar]
- Clement CR. Peach palm (Bactris gasipaes) In: Janick J, Paull RE, editors. The encyclopedia of fruit and nuts. Wallingford, UK: CABI Publishing; 2008. pp. 93–101. [Google Scholar]
- Clement CR, Weber JC, van Leeuwen J, et al. Why extensive research and development did not promote use of peach palm fruit in Latin America. Agroforestry Systems61–62. 2004:195–206. [Google Scholar]
- Delarue M, Prinsen E, Onckelen HV, Caboche M, Bellini C. Sur2 mutations of Arabidopsis thaliana define a new locus involved in the control of auxin homeostasis. The Plant Journal. 1998;14:603–611. doi: 10.1046/j.1365-313x.1998.00163.x. [DOI] [PubMed] [Google Scholar]
- Dubois T, Guedira M, Dubois J, Vasseur J. Direct somatic embryogenesis in roots of Cichorium – Is callose an early marker? Annals of Botany. 1990;65:539–545. [Google Scholar]
- Escalona M, Lorenzo JC, Gonzalez B, et al. Pineapple (Ananas comosus L-Merr) micropropagation in temporary immersion systems. Plant Cell Reports. 1999;18:743–748. [Google Scholar]
- Etienne H, Berthouly M. Temporary immersion systems in plant micropropagation. Plant Cell Tissue and Organ Culture. 2002;69:215–231. [Google Scholar]
- Etienne H, Lartaud M, Michaux-Ferriére N, Carron M, Berthouly M, Teisson C. Improvement of somatic embryogenesis in Hevea brasiliensis (Müll. Arg.) using the temporary immersion technique. In vitro Cellular and Developmental Biology – Plant. 1997;33:81–87. [Google Scholar]
- Feher A, Pasternak TP, Dudits D. Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue and Organ Culture. 2003;74:201–228. [Google Scholar]
- Fernando SC, Verdeil JL, Hocher V, Weerakoon LK, Hirimburegama K. Histological analysis of plant regeneration from plumule explants of Cocos nucifera. Plant Cell Tissue and Organ Culture. 2003;72:281–284. [Google Scholar]
- Fisher DB. Protein staining of ribboned epon sections for light microscopy. Histochemie. 1968;16:92–96. doi: 10.1007/BF00306214. [DOI] [PubMed] [Google Scholar]
- Fukao T, Bailey-Serres J. Plant responses to hypoxia – is survival a balancing act? Trends in Plant Science. 2004;9:449–456. doi: 10.1016/j.tplants.2004.07.005. [DOI] [PubMed] [Google Scholar]
- Gaj MD, Zhang SB, Harada JJ, Lemaux PG. Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta. 2005;222:977–988. doi: 10.1007/s00425-005-0041-y. [DOI] [PubMed] [Google Scholar]
- Geigenberger P. Response of plant metabolism to too little oxygen. Current Opinion in Plant Biology. 2003;6:247–256. doi: 10.1016/s1369-5266(03)00038-4. [DOI] [PubMed] [Google Scholar]
- Goh DKS, Bon MC, Aliotti F, Escoute J, Ferriere N, Monteuuis O. In vitro somatic embryogenesis in two major rattan species: Calamus merrillii and Calamus subinermis. In vitro Cellular and Developmental Biology – Plant. 2001;37:375–381. [Google Scholar]
- Groll J, Mycock DJ, Gray VM, Laminski S. Secondary somatic embryogenesis of cassava on picloram supplemented media. Plant Cell Tissue and Organ Culture. 2001;65:201–210. [Google Scholar]
- Guerra MP, Handro W. Somatic embryogenesis and plant regeneration in different organs of Euterpe edulis Mart. (Palmae): control and structural features. Journal of Plant Research. 1998;111:65–71. [Google Scholar]
- Guilfoyle T. Plant biology – Sticking with auxin. Nature. 2007;446:621–622. doi: 10.1038/446621a. [DOI] [PubMed] [Google Scholar]
- Harada JJ. Signaling in plant embryogenesis. Current Opinion in Plant Biology. 1999;2:23–27. doi: 10.1016/s1369-5266(99)80005-3. [DOI] [PubMed] [Google Scholar]
- Jiménez VM. Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regulation. 2005;47:91–110. [Google Scholar]
- Karun A, Siril EA, Radha E, Parthasarathy VA. Somatic embryogenesis and plantlet regeneration from leaf and inflorescence explants of arecanut (Areca catechu L.) Current Science. 2004;86:1623–1628. [Google Scholar]
- Khalil SM, Cheah KT, Perez EA, Gaskill DA, Hu JS. Regeneration of banana (Musa spp. AAB cv. Dwarf Brazilian) via secondary somatic embryogenesis. Plant Cell Reports. 2002;20:1128–1134. [Google Scholar]
- Kwaaitaal MA, de Vries SC. The SERK1 gene is expressed in procambium and immature vascular cells. Journal of Experimental Botany. 2007;58:2887–2896. doi: 10.1093/jxb/erm103. [DOI] [PubMed] [Google Scholar]
- Li L, Qu R. In vitro somatic embryogenesis in turf-type bermudagrass: roles of abscisic acid and gibberellic acid, and occurrence of secondary somatic embryogenesis. Plant Breeding. 2002;121:155–158. [Google Scholar]
- Martinelli L, Candioli E, Costa D, Poletti V, Rascio N. Morphogenic competence of Vitis rupestris S. secondary somatic embryos with a long culture history. Plant Cell Reports. 2001;20:279–284. [Google Scholar]
- Maximova SN, Alemanno L, Young A, Ferriere N, Traore A, Guiltinan MJ. Efficiency, genotypic variability, and cellular origin of primary and secondary somatic embryogenesis of Theobroma cacao L. In vitro Cellular and Developmental Biology – Plant. 2002;38:252–259. [Google Scholar]
- Mora-Urpí J, Weber JC, Clement CR. Peach-Palm (Bactris gasipaes Kunth) Rome: Institute of Plant Genetics and Crop Plant Research and International Plant Genetic Resources Institute; 1997. Available via http://www.bioversityinternational.org/publications.html . [Google Scholar]
- Morel G, Wetmore RH. Tissue culture of monocotyledons. American Journal of Botany. 1951;38:138–140. [Google Scholar]
- Moura EF, Ventrella MC, Motoike SY, de Sa AQ, Carvalho M, Manfio CE. Histological study of somatic embryogenesis induction on zygotic embryos of macaw palm (Acrocomia aculeata (Jacq.) Lodd. ex Martius) Plant Cell Tissue and Organ Culture. 2008;95:175–184. [Google Scholar]
- Murashige T, Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiologia Plantarum. 1962;15:473–497. [Google Scholar]
- Niemenak N, Saare-Surminski K, Rohsius C, Ndoumou DO, Lieberei R. Regeneration of somatic embryos in Theobroma cacao L. in temporary immersion bioreactor and analyses of free amino acids in different tissues. Plant Cell Reports. 2008;27:667–676. doi: 10.1007/s00299-007-0497-2. [DOI] [PubMed] [Google Scholar]
- Pasternak TP, Prinsen E, Ayaydin F, et al. The role of Auxin, pH, and stress in the activation of embryogenic cell division in leaf protoplast-derived cells of alfalfa. Plant Physiology. 2002;129:1807–1819. doi: 10.1104/pp.000810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedroso MC, Pais MS. Factors controlling somatic embryogenesis – Cell wall changes as an in vivo marker of embryogenic competence. Plant Cell Tissue and Organ Culture. 1995;43:147–154. [Google Scholar]
- Perera PIP, Hocher V, Verdeil JL, Doulbeau S, Yakandawala DMD, Weerakoon LK. Unfertilised ovary: a novel explants for coconut (Cocos nucifera L.) somatic embryogenesis. Plant Cell Reports. 2007;26:21–28. doi: 10.1007/s00299-006-0216-4. [DOI] [PubMed] [Google Scholar]
- Perez-Nunez MT, Chan JL, Saenz L, Gonzalez T, Verdeil JL, Oropeza C. Improved somatic embryogenesis from Cocos nucifera (L.) plumule explants. In vitro Cellular Developmental Biology – Plant. 2006;42:37–43. [Google Scholar]
- Pfluger J, Zambryski PC. Cell growth: the power of symplastic isolation. Current Biology. 2001;11:R436–R439. doi: 10.1016/s0960-9822(01)00254-8. [DOI] [PubMed] [Google Scholar]
- Quiroz-Figueroa FR, Rojas-Herrera R, Galaz-Avalos RM, Loyola-Vargas VM. Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell, Tissue and Organ Culture. 2006;86:285–301. [Google Scholar]
- Raemakers CJJM, Jacobsen E, Visser RGF. Secondary somatic embryogenesis and applications in plant breeding. Euphytica. 1995;81:93–107. [Google Scholar]
- Reis E, Batista MT, Canhoto JM. Effect and analysis of phenolic compounds during somatic embryogenesis induction in Feijoa sellowiana Berg. Protoplasma. 2008;232:193–202. doi: 10.1007/s00709-008-0290-2. [DOI] [PubMed] [Google Scholar]
- Saenz L, Azpeitia A, Chuc-Armendariz B, et al. Morphological and histological changes during somatic embryo formation from coconut plumule explants. In vitro Cellular and Developmental Biology – Plant. 2006;42:19–25. [Google Scholar]
- Sané D, Aberlenc-Bertossi F, Gassama-Dia YK, et al. Histocytological analysis of callogenesis and somatic embryogenesis from cell suspensions of date palm (Phoenix dactylifera) Annals of Botany. 2006;98:301–308. doi: 10.1093/aob/mcl104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sankar-Thomas YD, Saare-Surminski K, Lieberei R. Plant regeneration via somatic embryogenesis of Camptotheca acuminata in temporary immersion system (TIS) Plant Cell Tissue and Organ Culture. 2008;95:163–173. [Google Scholar]
- Schmidt EDL, Guzzo F, Toonen MAJ, de Vries SC. A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development. 1997;124:2049–2062. doi: 10.1242/dev.124.10.2049. [DOI] [PubMed] [Google Scholar]
- Schwendiman J, Pannetier C, Michaux-Ferriere N. Histology of somatic embryogenesis from leaf explants of the oil palm Elaeis guineensis. Annals of Botany. 1988;62:43–52. [Google Scholar]
- Sharma SK, Millam S, Hein I, Bryan GJ. Cloning and molecular characterization of a potato SERK gene transcriptionally induced during initiation of somatic embryogenesis. Planta. 2008;228:319–330. doi: 10.1007/s00425-008-0739-8. [DOI] [PubMed] [Google Scholar]
- Stein M, Stephens C. Effect of 2,4-dichlorophenoxyacetic acid and activated charcoal on somatic embryogenesis of Bactris gasipaes HBK. Turrialba. 1991;41:196–201. [Google Scholar]
- Steinmacher DA, Cangahuala-Inocente GC, Clement CR, Guerra MP. Somatic embryogenesis from peach palm zygotic embryos. In vitro Cellular and Development Biology – Plant. 2007a;43:124–132. [Google Scholar]
- Steinmacher DA, Clement CR, Guerra MP. Somatic embryogenesis from immature peach palm inflorescence explants: towards development of an efficient protocol. Plant Cell, Tissue and Organ Culture. 2007b;89:15–22. [Google Scholar]
- Steinmacher DA, Krohn NG, Dantas ACM, Stefenon VM, Clement CR, Guerra MP. Somatic embryogenesis in peach palm using the thin cell layer technique: induction, morpho-histological aspects and AFLP analysis of somaclonal variation. Annals of Botany. 2007c;100:699–709. doi: 10.1093/aob/mcm153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talavera CR, Espadas FL, Aguilar ML, Maust BE, Oropeza CM, Santamaria JM. The control of leaf water loss by coconut plants cultured in vitro depends on the type of membrane used for ventilation. Journal of Horticultural Science and Biotechnology. 2001;76:569–574. [Google Scholar]
- Tan X, Calderon-Villalobos LIA, Sharon M, et al. Mechanism of auxin perception by the TIR1 ubiquitin ligase. Nature. 2007;446:640–645. doi: 10.1038/nature05731. [DOI] [PubMed] [Google Scholar]
- Teixeira JB, Söndahl MR, Nakamura T, Kirby EG. Establishment of oil palm cell suspensions and plant regeneration. Plant Cell, Tissue and Organ Culture. 1995;40:105–111. [Google Scholar]
- Valverde R, Arias O, Thorpe TA. Picloram-induced somatic embryogenesis in pejibaye palm (Bactris gasipaes HBK) Plant Cell Tissue and Organ Culture. 1987;10:149–156. [Google Scholar]
- Verdeil JL, Hocher V, Huet C, et al. Ultrastructural changes in coconut calli associated with the acquisition of embryogenic competence. Annals of Botany. 2001;88:9–18. [Google Scholar]
- Verdeil JL, Alemanno L, Niemenak N, Tranbarger TJ. Pluripotent versus totipotent plant stem cells: dependence versus autonomy? Trends in Plant Science. 2007;12:245–252. doi: 10.1016/j.tplants.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Walsh TA, Neal R, Merlo AO, et al. Mutations in an auxin receptor homolog AFB5 and in SGT1b confer resistance to synthetic picolinate auxins and not to 2,4-dichlorophenoxyacetic acid or indole-3-acetic acid in Arabidopsis. Plant Physiology. 2006;142:542–552. doi: 10.1104/pp.106.085969. [DOI] [PMC free article] [PubMed] [Google Scholar]