Abstract
Background
The palm family occurs in all tropical and sub-tropical regions of the world. Palms are of high ecological and economical importance, and display complex spatial patterns of species distributions and diversity.
Scope
This review summarizes empirical evidence for factors that determine palm species distributions, community composition and species richness such as the abiotic environment (climate, soil chemistry, hydrology and topography), the biotic environment (vegetation structure and species interactions) and dispersal. The importance of contemporary vs. historical impacts of these factors and the scale at which they function is discussed. Finally a hierarchical scale framework is developed to guide predictor selection for future studies.
Conclusions
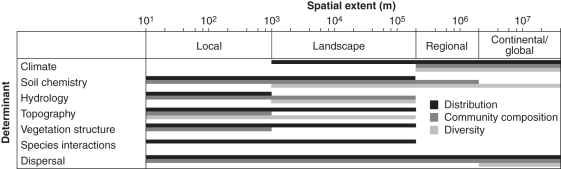
Determinants of palm distributions, composition and richness vary with spatial scale. For species distributions, climate appears to be important at landscape and broader scales, soil, topography and vegetation at landscape and local scales, hydrology at local scales, and dispersal at all scales. For community composition, soil appears important at regional and finer scales, hydrology, topography and vegetation at landscape and local scales, and dispersal again at all scales. For species richness, climate and dispersal appear to be important at continental to global scales, soil at landscape and broader scales, and topography at landscape and finer scales. Some scale–predictor combinations have not been studied or deserve further attention, e.g. climate on regional to finer scales, and hydrology and topography on landscape and broader scales. The importance of biotic interactions – apart from general vegetation structure effects – for the geographic ecology of palms is generally underexplored. Future studies should target scale–predictor combinations and geographic domains not studied yet. To avoid biased inference, one should ideally include at least all predictors previously found important at the spatial scale of investigation.
Keywords: Arecaceae, biotic interactions, climate, dispersal limitation, geographic ecology, hydrology, Palmae, spatial scale, species distributions, species richness, soil, topography
INTRODUCTION
Spatial patterns of species diversity and species distributions are central to ecology and have fascinated naturalists, ecologists and biogeographers for centuries (Humboldt and Bonpland, 1805; MacArthur, 1972; Lomolino et al., 2010). Small-scale studies of distribution patterns have emphasized how species and communities respond to environmental gradients (Whittaker, 1975) and how species diversity is maintained locally (Connell, 1978). More recently, macroecological studies attempt to elucidate general statistical patterns of abundance, distribution, body size and diversity of species across broad scales (Brown and Maurer, 1989). Despite this long-standing interest, understanding what determines the distribution and dynamics of species diversity remains a great challenge (Pennisi, 2005). Geographical studies on species distributions and diversity have provided important insights into the roles played by climate (Hawkins et al., 2003; Currie et al., 2004; Svenning and Skov, 2007), topography and habitat heterogeneity (Kerr and Packer, 1997), dispersal (Svenning et al., 2008b) and biotic interactions (Araújo and Luoto, 2007; Kissling et al., 2007). The importance of biogeographic history (Ricklefs and Schluter, 1993) and the associated need for integration of geographical and evolutionary ecology (Mittelbach et al., 2007) have been highlighted.
Spatial scale is central to geographical ecology (Levin, 1992) because patterns and processes in ecological systems are highly scale dependent (Willis and Whittaker, 2002; Pearson and Dawson, 2003). Two important attributes of spatial scale are grain size (size of an individual sampling unit) and extent (geographical space over which sample units are distributed) (Rahbek, 2005). Both may influence patterns and underlying drivers of species diversity (Qian and Kissling, 2010) which are thought to vary systematically with spatial scale (Condit et al., 2002; Willis and Whittaker, 2002; Pearson and Dawson, 2003). Specific frameworks have been proposed for hierarchical, scale-dependent impacts of different environmental, biotic and historical factors (Willis and Whittaker, 2002; Pearson and Dawson, 2003). However, few empirical studies have examined drivers of species distributions, compositional turnover and species richness across multiple spatial scales (but see Lenoir et al., 2010). The terminology associated with spatial scales is far from unified; for the purpose of this review, we refer to studies based on their extent as local (<1000 m), landscape (1000 m–200 km), regional (200–2000 km), or continental and global scale (>2000 km).
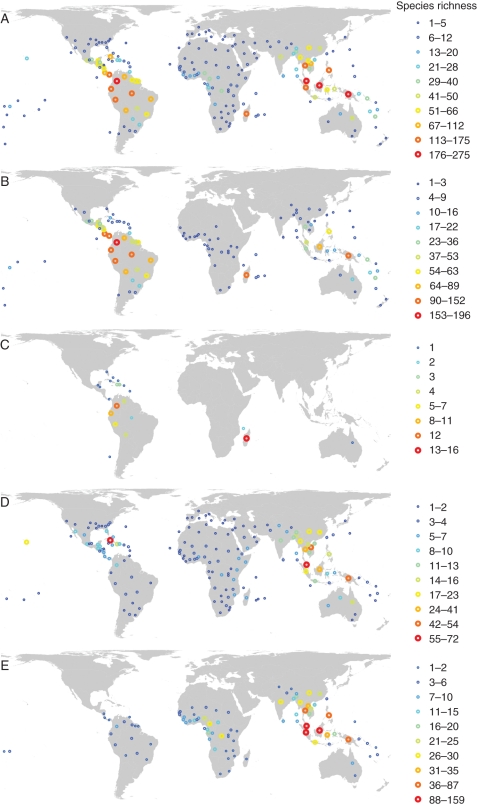
Palms (Arecaceae), characteristic of tropical and sub-tropical regions across the world (Dransfield et al., 2008), are also among the oldest monocotyledonous flowering plants (Janssen and Bremer, 2004) and have a rich fossil record (Harley, 2006). With >2400 species (Govaerts and Dransfield, 2005), palms exhibit an amazing geographic variation in species richness, phylogenetic composition and life forms. At a global scale, the palm family and its five subfamilies show distinct spatial patterns of species richness (Fig. 1). Palms are often abundant in tropical and sub-tropical ecosystems (Pitman et al., 2001; Kahn and de Granville, 1992; Dransfield et al., 2008) and have been so for 80 million years (Wing et al., 1993; Morley, 2000). Palms are a keystone resource for pollinator and frugivore communities (Terborgh, 1986; Zona and Henderson, 1989; Henderson, 2002), and may have shaped the evolution of dependent animal groups (Dominy et al., 2003). Palms are also significant to people, especially rural communities, because they provide construction materials, fabrics, fuel, food, medicine and ornamentals (Balslev and Barfod, 1987; Balick, 1988; Zambrana et al., 2007; de la Torre et al., 2009; Sosnowska and Balslev, 2009). Given their worldwide distribution and their variation in species richness and life forms, palms are an excellent model system for studying what drives the high tropical biodiversity and its geographic variation (Bjorholm et al., 2005, 2006; Svenning et al., 2008a).
Fig. 1.
The global spatial distribution of palm species richness across all species (A) and within subfamilies Arecoideae (B), Ceroxyloideae (C), Coryphoideae (D) and Calamoideae (E). The number of palm species is shown for the mass centroid of each geographic region based on data from the World Checklist of Palms (Govaerts and Dransfield, 2005). Natural breaks classification, Behrmann projection.
Here, we focus on the geographical ecology of palms and review available evidence on palm species diversity drivers. Specifically, we scrutinize studies on the influence of abiotic factors (climate, soil chemistry, hydrology and topography), biotic environment (vegetation structure and species interactions) and dispersal (Fig. 2) on palm species distribution, compositional turnover and species richness across spatial scales. We provide a first synthesis of the many studies that have tested the importance of these factors in a statistical framework, but do not review the existing natural history knowledge, as it has already been subject to several excellent treatments (Kahn and de Granville, 1992; Dransfield et al., 2008): a selection of key studies is presented in the Appendix. We then discuss historical vs. contemporary effects on palm distributions and diversity, and synthesize our current knowledge on how determinants depend on spatial extent. We finally identify current knowledge gaps and important avenues for future research on the geographical ecology of this tropical keystone plant group.
Fig. 2.
Determinants of palm species distribution. (A) Climate: Trachycarpus fortunei invading a warm temperate forest in Switzerland (cf. Walther et al., 2007). (B) Topography: Geonoma undata, a high-altitude species occurring in South and Central America between 1400 and 2400 m a.s.l. (Henderson et al., 1995). (C) Species interactions: seedling of Iriartea deltoidea, half consumed by an unknown herbivore (Peru). (D) Soil: juvenile of Prestoea decurrens on rich, clayey soil in Nicaragua (cf. Clark et al., 1995). (E) Species interactions: interspecific competition between seedlings of the canopy palm Euterpe precatoria (Peru). (F) Dispersal: fruits of Aiphanes weberbaueri clearly adapted to ornithochory (Peru). (G) Hydrology: Bactris riparia, typically found on black water stream margins in South America, here in Peru. (H) Vegetation structure: Thrinax radiata and Coccothrinax argentata growing under a canopy gap in Mexico. Imageo credits: J.-C. Svenning (A), F. Borchsenius (B), M.B. Sørensen (C, E, F), H. Balslev (D, G, H).
ABIOTIC ENVIRONMENT
Climate
Climate plays an important role in determining plant distributions and diversity (Brown, 1995; O'Brien, 1998; Pearson and Dawson, 2003). There is ample empirical evidence for climatic control of species diversity patterns in general (Hawkins et al., 2003) and plant diversity patterns in particular (Kreft and Jetz, 2007), especially on large scales (Willis and Whittaker, 2002). More specifically, measures of water-energy availability such as actual and potential evapotranspiration, annual rainfall and number of wet days emerge as the strongest climatic predictors of plant diversity patterns at broad spatial scales (Gentry, 1988; Clinebell et al., 1995; O'Brien, 1998; Hawkins et al., 2003; Kreft and Jetz, 2007). Here, we provide an overview of studies that have assessed the relationship between climate and palm diversity and distributions. More specifically, we review the influence of climatic variables on (a) the overall geographic range of the palm family; (b) individual palm species distributions; and (c) palm species richness.
Climatic constraints on the global range of the palm family
The global distribution of the palm family nearly coincides with tropical and sub-tropical climates, and only a handful of species are found in warm-temperate regions (Dransfield et al., 2008). Palms have been widely used as palaeo-indicators for warm and humid climates (Greenwood and Wing, 1995; Morley, 2000, 2003; Walther et al., 2007, and references therein) and the clade has been used as a prime example for the tropical conservatism hypothesis (Wiens and Donoghue, 2004). Temperature-related niche conservatism (sensu Wiens et al., 2010) is plausible in the case of palms because palm morphology and physiology seem inherently ill-suited for meso- or microthermal climates. Specifically, the soft and water-rich tissue of palms, their inability to undergo dormancy and their general lack of mechanisms to avoid or tolerate frost are thought to restrict them to megathermal climates (Tomlinson, 2006). Low temperature has indeed been identified as a potential factor limiting the distribution of specific palm species (e.g. Trachycarpus fortunei and Sabal minor) that occur at the northern extreme of the family's range (Tripp and Dexter, 2006; Walther et al., 2007; see below). Given the strong temperature sensitivity of palms, it has been suggested that the expansion of individual species at the distributional limits of the palm family is a good indicator for present-day climate change (Tripp and Dexter, 2006; Walther et al., 2007; Fig. 2A).
Climate and species distributions
The importance of climate for individual palm species distributions within tropical regions has been assessed most comprehensively for African palms (Blach-Overgaard et al., 2009, 2010). Blach-Overgaard and collaborators (2010) found climate to be more important than habitat and human impact in determining the continent-wide distributions of 29 African palm species. Water-related variables are the most influential climatic variables for 25 of the species, whereas the distributions of the remaining four species were most strongly determined by temperature. Most palm species show an overall preference for humid climates, but some are associated with low precipitation (e.g. species in the genus Hyphaene; Blach-Overgaard et al., 2010). The generally low drought tolerance of palms is well illustrated by the dramatic decline of many palm species on Barro Colorado Island in Panama in response to increasing drought in the late 20th century (Condit et al., 1996).
Low temperatures have been found to constrain palm species distributions, e.g. in sub-tropical (Gatti et al., 2008) and temperate regions (Walther et al., 2007). For instance, the pollen record of a tropical American lower montane forest reveals that Dictyocaryum immigrated (most likely from lower altitudes) and then disappeared again (probably migrating upwards) during late-glacial warming (Bush et al., 2004). The absence of Euterpe edulis in the low parts of a topographic gradient in the Brazilian Atlantic forest was explained by the occurrence of freezing due to cold air drainage, damaging the palms (Gatti et al., 2008).
The occurrence of palms is not simply prevented by temperatures below zero, as these are not always lethal (Gatti et al., 2008, and references therein). For instance, the native range of T. fortunei (Fig. 2A) in Asia is limited by a combination of winter temperatures and a subordinate effect of cumulative growing season energy, with the range limit being imposed by frost damage to leaves that cannot be compensated by biomass production in the following growing season (Walther et al., 2007). Other mechanisms, such as detrimental effects of low temperatures on photosynthesis and growth rates, might also restrict palms to the tropics and sub-tropics (Gatti et al., 2008). Palm species distributions may also be sensitive to temperature in the absence of frost; both temperature and precipitation affect the landscape-scale distributions of some abundant canopy palm species in north-eastern Costa Rica (Sesnie et al., 2009).
Besides water availability and temperature, the occurrence of extreme weather events may influence palm distributions. For instance, hurricane damage affected population dynamics, abundance and dominance of Prestoea acuminata (as P. montana) in a forest in Puerto Rico (Frangi and Lugo, 1998).
Climate and species richness
By observation, palms are most diverse in warm and humid regions (e.g. Corner, 1966), but climatic and other determinants of global patterns of palm species diversity have not been quantified yet (see Dransfield et al., 2008). However, the results of several continental-scale macroecological studies indicate that water availability is the strongest determinant of palm species richness in the Americas (Bjorholm et al., 2005, 2006, 2008; Kreft et al., 2006; Svenning et al., 2008a). This relationship is consistent across different grain sizes (1° × 1° to 10° × 10°; Bjorholm et al., 2005), agrees with results for overall plant diversity (Gentry, 1988; Clinebell et al., 1995; O'Brien 1998; Kreft and Jetz, 2007) and confirms the dominance of water-related variables over temperature or energy in megathermal climates (Hawkins et al., 2003). Measures reflecting water-energy dynamics such as actual evapotranspiration also correlate with palm richness on the same spatial scale (Kreft et al., 2006; Svenning et al., 2008a), but mean annual temperature is of negligible importance in tropical America (Bjorholm et al., 2005). Precipitation seasonality is negatively related to the average local (0·25 ha) palm species richness within regions (up to 320 km in extent) of the western Amazon, while annual precipitation and temperature seasonality do not affect richness (Kristiansen et al., 2011). However, Salm and collaborators (2007) found temperature seasonality to be the second most important predictor (after mean annual vapour pressure) of palm species richness in approx. 150 000 km2 large grid cells across Brazil. The highest number of species was found in regions of high humidity (vapour pressure) and low seasonality. Thus, not only water-related variables might be strong determinants of palm richness, but also temperature seasonality or extremes.
The rich palm assemblages in warm and humid parts of the Americas are dominated by species from lineages with a history of high net diversification (the sum of speciation and extinction) (Svenning et al., 2008a). This relationship implies that moist tropical climates have favoured palm diversification, a potential mechanistic explanation for the observed climate richness correlations (Svenning et al., 2008a; see also Mittelbach et al., 2007). This effect may be due to general (higher population sizes, water-energy dynamics or biotic interactions) or palm-specific factors (greater ecological success of palms due to their special morphology and anatomy) (Svenning et al., 2008a). Diversification rates of palms could be higher in such environments because of the tough, late-folded leaves of palms that provide generalized resistance to high herbivory rates (Dominy et al., 2008; Grubb et al., 2008).
The search for richness–environment relationships is complicated by taxon-specific responses. For instance, the four palm subfamilies that occur in tropical America show different richness–environment relationships. Water availability is important in Arecoideae and Calamoideae, whereas species richness patterns in Coryphoideae and Ceroxyloideae are less strongly explained by climate (Bjorholm et al., 2006). Thus, the climate–richness relationships discussed above apply well to the subfamilies that have a long history in this region; given their low richness, the other two contribute little to the overall pattern of palm richness in the Americas (Bjorholm et al., 2006).
Summary: climate
Both individual palm species distributions and patterns of palm species richness are clearly related to current climate. The distribution of the family appears to be limited by temperature extremes, but quantitative analyses are missing at the global scale. Climatic constraints on individual species distributions have been more widely explored, with water-related variables emerging as the most important climatic determinants of the continental-scale distributions of tropical palms. However, large-scale palm species range determinants have only been comprehensively analysed for tropical Africa (Blach-Overgaard et al., 2010). Temperature seasonality and cold have also been found to constrain individual palm species distributions, but more studies exploring the effect of different aspects of low temperatures (see Walther et al., 2007) on palm distributions are desirable. Regarding palm species richness, there is good evidence for water-related climatic factors as the primary determinant of broad-scale patterns. However, no such studies exist on a global scale and for the Palaeotropics. Evidence for climate effects on smaller spatial scales is scarce, possibly because local- to regional-scale studies usually exclude climatic variables. However, considerable climatic variation can occur at smaller scales, especially in mountainous settings (Svenning, 2001a; Svenning et al., 2009; Fig. 3), and including this in analyses of palm diversity patterns could be rewarding (cf. Sesnie et al., 2009).
Soil chemistry
The spatial distributions of many tropical plant species show strong associations with edaphic conditions (Austin et al., 1972; Tuomisto et al., 1995; John et al., 2007), and the importance of different soil types for generating and maintaining local plant diversity in tropical forests has long been hypothesized (e.g. Gentry, 1981). At a regional scale, soil fertility has been demonstrated to be an important factor influencing tropical tree genus composition and functioning (ter Steege et al., 2006). Here, we review effects of soil chemistry and nutrient availability on palm species distributions, community composition and species richness (soil texture is discussed later).
Soil chemistry and species distributions
At a local scale, distribution and abundance of several tropical American palm species are related to soil factors such as clay and aluminium content and nutrient concentrations (Svenning, 2001a, and references therein). In the western Amazon, palms that are restricted to either poor or rich soils in one area (Iquitos) have the same preference in another area (Pebas, approx. 200 km away) (Ruokolainen and Vormisto, 2000). Different species of canopy palms respond differently to edaphic gradients in a tropical forest in Costa Rica (Clark et al., 1995). Soil fertility affects the local-scale distribution of the African savannah palm Borassus aethiopum (Barot and Gignoux, 2003). On a continental scale, soil type has a negligible impact on the distributions of most African palms (Blach-Overgaard et al., 2009, 2010), except for dry climate species. For these species, soil types probably represent an effect of hydrology as palms occurring in dry climates are strongly dependent on ground water (Blach-Overgaard et al., 2010; see also Dransfield et al., 2008).
Soil chemistry and community composition
If different palm species are favoured by different soils (Clark et al., 1995; Svenning, 2001a, and references therein) edaphic gradients can be expected to cause palm community turnover. At a local scale in Amazonian Ecuador, turnover of palm community composition is related to a range of chemical soil characteristics, including exchangeable cations and aluminium content (Poulsen et al., 2006). However, soil characteristics co-vary with elevation at that locality and can thus not be separated from other elevation-dependent factors such as hydrology (Poulsen et al., 2006). The relationship between soil types and local-scale palm community composition within a lowland rain forest in the western Amazon has also been attributed to differences in soil fertility (Vormisto et al., 2000). On landscape to regional scales, palm community composition is related to soil fertility in lowland terra firme rain forest in the western Amazon (Vormisto et al., 2004a), montane forests in Panama (Andersen et al., 2010) and lowland to montane forests in Costa Rica (Sesnie et al., 2009). Other studies in Amazonian terra firme and restinga forests found only a weak effect of soil chemistry (Normand et al., 2006; Costa et al., 2009).
Soil chemistry and species richness
Soil fertility has been identified as the second most important environmental predictor of palm species richness across tropical America (Bjorholm et al., 2006). Species richness of all palms as well as that of Arecoideae and Calamoideae increases with soil fertility, whereas there is no effect on richness in Coryphoideae and Ceroxyloideae (Bjorholm et al., 2006). The importance of soil fertility (together with water availability) was interpreted as a positive effect of ecosystem productivity on species richness (Bjorholm et al., 2006), where elevated diversification rates (Svenning et al., 2008a) might be the mechanistic link. There is also some evidence for soil–richness relationships at smaller spatial scales. Higher local and regional diversity in the Iquitos–Pebas region (Peru) compared with the Yasuní region (Ecuador) has been attributed to differences in soil fertility and soil types (Vormisto et al., 2004b; Montufar and Pintaud, 2006). Interestingly, the region with the poorer soils (Iquitos–Pebas) supports more species, but the situation is complicated by this region's wider variety of soil types (Vormisto et al., 2004b). However, sites with poorer soil within this region also support more species (Vormisto et al., 2004b), and palm species richness in a tropical forest in Costa Rica decreases with soil fertility (Clark et al., 1995). This was interpreted as support for the hypothesis that less productive (=less fertile) sites are less dominated by superior competitors (Clark et al., 1995).
Summary: soil chemistry
From local to continental scales, several palm species respond in their distribution and abundance to soil conditions (e.g. Clark et al., 1995; Svenning, 2001a; Blach-Overgaard et al., 2010). However, at continental scales, the available evidence suggests that soils are not of particular importance in determining individual palm species distributions in most cases (Blach-Overgaard et al., 2010). Turnover of palm community composition along soil fertility gradients has been demonstrated at local to regional scales (Vormisto et al., 2004a; Poulsen et al., 2006; Andersen et al., 2010), confirming that palm species respond individually to the availability of soil nutrients. However, findings differ slightly as to which factors are most important (Vormisto et al., 2004a; Poulsen et al., 2006; Andersen et al. 2010), and not all studies support an important role for soil conditions in palm community turnover (Normand et al., 2006; Costa et al., 2009). Finally, soil fertility also correlates with local- to continental-scale patterns of palm species richness (e.g. Bjorholm et al., 2006). Soil–richness relationships, however, appear to be scale dependent and reverse their direction between regional and continental scales (see Vormisto et al., 2004b; Bjorholm et al., 2006). Moreover, soil chemistry may interact with other environmental factors including topography, hydrology and vegetation structure (Fig. 3). These aspects warrant further investigation.
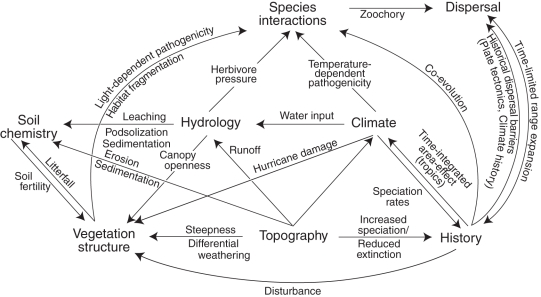
Fig. 3.
Potential interactions between different determinants of palm species distributions, community composition and species richness as discussed in this review (not exhaustive).
Hydrology
Hydrological conditions affect plant species distributions and diversity through flooding and vertical/lateral drainage (Silvertown et al., 1999). In particular, drainage is often related to soil texture. Soil texture characterizes the bulk density, surface area and pore space of soils and affects the water-holding capacity and hydraulic conductivity (Palm et al., 2007). In this section, we review studies that examine associations between hydrology (flooding and drainage/soil texture) and palm species distributions, community composition and species richness.
Hydrology and species distributions
Some palm species prefer swampy habitats [e.g. Metroxylon sagu (Dransfield et al., 2008) and Mauritia flexuosa (Henderson et al., 1995)]. The importance of hydrology for local palm species distributions has been recognized since Kahn's studies of Amazonian palms (e.g. Kahn and de Castro, 1985; Kahn, 1987; Kahn and de Granville, 1992). Several studies (Svenning, 2001a, and references therein; Montufar and Pintaud, 2006) have examined how the distribution and abundance of individual palm species depend on flooding and/or drainage. Many palms are associated with either well-drained or poorly drained soils (Kahn, 1987; Henderson et al., 1995; Fig. 2G). Mechanisms behind the impact of flooding on palm distributions are mainly related to seed germination and seedling survival (Losos, 1995; Pacheco, 2001; Svenning, 2001a).
Within habitats of uniform inundation regime, fine-scale distributions of palms can depend on heterogeneity in soil moisture and drainage (see also Svenning, 2001a). For instance, five out of 23 palms respond individually to poor drainage in Amazonian terra firme forest (Svenning, 1999a), and in an Amazonian floodplain the relationship between abundance and elevation above stream level surface is species specific (Scariot et al., 1989). Similarly, in restinga forests in Peru Aphandra natalia favours dry, high-lying and flat sites presumably due to hydrological differences (Boll et al., 2005). Geonoma brevispatha has a unimodal response to soil moisture in the understorey of a swamp forest in south-eastern Brazil (Souza and Martins, 2004).
Hydrology and community composition
Hydrology also affects palm community composition. As expected from individual species distributions, differences in palm community composition are largest between well-drained and poorly drained soils (Balslev et al., 1987; Kahn, 1987; Normand et al., 2006). In adjacent forests with different hydrology in Ecuador, palms in the terra firme made up 17 % of the individuals and 11 % of the basal area compared with 30 % of individuals and 19 % of the basal area in the floodplain forest (Balslev et al., 1987). On the upper Ucayali in Peru, palm community composition varies significantly along a gradient from the floodplain, over high terraces to terra firme and low hills (Balslev et al., 2010). In non-inundated tropical forests, hydrological differences also affect palm community composition at both local and regional scales. On a local scale in restinga palm communities, soil moisture explained more compositional variation than any other measured environmental variable (Normand et al., 2006). Soil texture explains palm community composition on a local scale in western Amazon terra firme forest (Poulsen et al., 2006). However, in this location, texture correlates with elevation and is thus possibly confounded with other elevation-dependent determinants of palm community composition (Fig. 3). There are also studies that found no relationship between drainage and palm community composition on the local scale (Svenning, 1999a; Amazonian Ecuador). On a landscape scale, palm community composition in a site in the Brazilian Amazon depends strongly on clay content of the soil, and distance to watercourses explained 43 % of the compositional variation in bottomland understorey palm communities (Costa et al., 2009). At landscape to regional scales, differences in soil texture (percentage clay, silt and sand) are strongly associated with palm compositional turnover in western Amazon terra firme rain forests (Vormisto et al. 2004a). Likewise, soil texture significantly explains canopy palm community composition on a landscape scale in north-eastern Costa Rica (Sesnie et al., 2009).
Hydrology and species richness
A couple of studies have compared palm species richness between wetland forests (seasonally inundated floodplains and permanently waterlogged swamps) and geographically proximal terra firme forest in the Amazon basin. At a site in the central Amazon, species richness is three times higher on well-drained soils (terra firme) than on seasonally flooded soils (Kahn and de Castro, 1985). Similarly, in the lower Ucayali valley in the western Amazon, palm communities in wetland forests (including both floodplains and swamps) are less species rich than communities in neighbouring terra firme forests (Kahn and Mejia, 1990, 1991). Floodplain palm communities in the Pastaza fan in northern Peru are less diverse than palm communities in adjacent restinga forest (Normand et al., 2006). In Ecuador a terra firme forest has almost twice as many species (24) as a floodplain forest (14), but they made up the same percentage of all tree species over 10 cm diameter at breast height (Balslev et al., 1987). Kristiansen and collaborators (2011) found a strong effect of habitat on local palm species richness in sites distributed across western Amazonia, putatively driven by the difference between inundated and non-inundated habitats. These findings indicate that the inundation regime is an important determinant of palm species richness on a landscape scale, at least in the presence of large hydrological variation as is the case in Amazonia.
Summary: hydrology
Flooding and drainage have strong effects on local- to landscape-scale distribution of palms as well as on palm species richness on a landscape scale. At this point it is not sufficiently understood to what degree these effects are direct (e.g. anaerobic stress and drought) or mediated by vegetation structure and light availability, notably reflecting a more open canopy in flooded areas (Kahn and de Castro, 1985; Scariot et al., 1989; Svenning, 2000a; Fig. 3). Interestingly, some species show different hydrological preferences in different parts of their global distribution [e.g. Iriartea deltoidea and Socratea exorrhiza (Svenning, 2001a, and references therein) and Oenocarpus bataua (Montufar and Pintaud, 2006)]. A potential explanation could be that light availability is the primary determinant, with its relationship to hydrology being location specific (Svenning, 2001a). Intraspecific variation in the hydrological niche offers an alternative explanation (Montufar and Pintaud, 2006). There is also some evidence that different growth forms (e.g. canopy, sub-canopy or understorey palms) might be differentially affected by hydrology (Kahn and de Castro, 1985; Svenning, 2000a), but this needs more thorough testing. Concerning diversity, palm communities in wetlands are less species rich than palm communities of non-inundated forests, at least in Amazonia. Hydrology may affect palms in concert with other aspects of the environment (Fig. 3); for instance, differential herbivore pressure has also been postulated to drive palm–hydrology associations (Pacheco, 2001), but this again requires further investigation.
Topography
Topography strongly influences the distribution of vegetation and plant species (Merriam, 1890; Whittaker, 1960; Coblentz and Riitters, 2004), and broad-scale patterns of plant species diversity are related to topographic heterogeneity (Kreft and Jetz, 2007). Topography affects plant distributions indirectly by modulating other environmental factors such as soil conditions, hydrology, wind exposure, temperature and fog frequency, as well as forest structure and dynamics (Svenning, 2001a; Fig. 3). At large spatial scales, topography may also relate to historical effects, e.g. when species are still associated with their glacial refugia in mountains (Svenning and Skov, 2007). Here we focus on the effects of topography on overall palm abundance (across species), individual palm species distributions, community composition and species richness.
Topography and overall abundance
At landscape scales, topography correlates with overall palm abundance. For instance, the density of canopy palms in a forest in Costa Rica peaks on crests and steep slopes (Clark et al., 1995). Similarly, palm density is highest on crests of topographical sequences in wet Amazonian forests (Kahn and de Castro 1985; Kahn, 1987). Overall palm abundance decreases with elevation on a landscape scale in a seasonally dry forest in southern Amazonia (Salm et al. 2007), and palms are abundant only in the lower parts of an approx. 2500 m altitudinal transect in Costa Rica (Lieberman et al., 1996). A high frequency of gaps favouring palm recruitment offers an explanation for the high palm density in steep and rugged terrain (Kahn and de Castro, 1985; Kahn, 1987; Clark et al., 1995). The lower abundance of palms on steep slopes compared with crests may be explained by high water runoff rendering those sites too dry for palms (Kahn, 1987; Fig. 3). The high abundance of palms in low-lying sites can also be attributed to high moisture availability (Salm et al., 2007).
Topography and species distributions
Individual local- to landscape-scale topographic preferences have been documented for many palm species in tropical America (Svenning, 2001a; Montufar and Pintaud, 2006), and on broad scales species distributions are strongly related to altitude (Borchsenius and Skov, 1997; Fig. 2B). At a local scale, the abundance of palm species varies individually with topographic position in both Amazonian lowland rain forests (Kahn, 1987) and seasonally dry forest (Salm et al., 2007). In Amazonian Ecuador, the distribution of ten of 23 palm species depends on topographic position, with absolute altitude and inclination being less important (Svenning, 1999a). In contrast, altitude and aspect strongly influence palm species distributions in a wet lower montane forest in Ecuador (Svenning, 2001b). As topography influences species' performance indirectly through its correlation with other environmental variables (Fig. 3), its influence can be locality specific and difficult to interpret (Vormisto et al., 2004b; see below). Importantly, topographic effects need not even reflect abiotic factors. On a local scale in Belize, for example, the density of Astrocaryum mexicanum differs significantly between slopes and flats; germination experiments, however, revealed that this pattern is unrelated to abiotic factors and can only be explained with differences in seed disperser abundance (Klinger and Rejmánek, 2010).
Topography and community composition
If palm species respond individually to topographic position, palm community composition should also be related to topography. Indeed, at a local scale in an Ecuadorian montane rain forest, palm species composition correlates strongly with topographic position (Svenning et al., 2009). Likewise, in lowland Amazonian Ecuador, palm compositional similarity is significantly correlated with topographic dissimilarity even when controlling for geographic distance and canopy structure (Svenning, 1999a). Elevation is the best predictor of palm compositional similarity in a terra firme rain forest in Amazonian Ecuador, where it is strongly correlated with soil variables (Poulsen et al., 2006). A canopy palm community studied on a landscape scale in Costa Rica shows significant turnover along a long altitudinal gradient (40–1200 m a.s.l.; Sesnie et al., 2009). However, within-plot topographic heterogeneity does not affect palm community composition on a landscape to regional scale in western Amazon terra firme and restinga forest (Vormisto et al., 2004a; Normand et al., 2006). Moreover, relative elevation and inclination are only weak (but significant) predictors of local-scale palm community composition in these restinga forests (Normand et al., 2006), while slope has no effect on palm community composition at a landscape scale in non-inundated rain forest in the Brazilian Amazon (Costa et al., 2009). The latter results indicate that topography does not always affect palm community composition well at all spatial scales, especially in lowland areas.
Topography and species richness
In New Guinea and some adjacent islands, palm species and genus richness decrease strongly and linearly with elevation (Bachman et al., 2004). About half of this effect is explained by area, with more area being available at low elevations due to the conical geometry of mountains. The remaining variation is well accounted for by a mid-domain effect, predicting that random placement of altitudinal ranges would result in the highest richness at mid-elevations (Bachman et al., 2004). While environmental correlates were not included in this study, its results suggest that landscape geometry alone might account for palm richness patterns in some settings. At a continental scale in the Americas, topographic heterogeneity is relatively unimportant in determining palm species richness (Bjorholm et al., 2005; Kreft et al., 2006). However, altitudinal range is mainly relevant for the richness of range-restricted species, perhaps reflecting a diversification history signature (Kreft et al., 2006). Local topographic heterogeneity has a negligible effect on local palm species richness across the western Amazon (Kristiansen et al., 2011). However, within some regions this variable was found to be important, in particular in the low-lying Pastaza fan where small-scale topography causes an influential hydrological gradient (Kristiansen et al., 2011).
On a landscape scale, palm species richness decreases with elevation in seasonally dry Amazonian forest (Salm et al., 2007) and along an approx. 2500 m altitudinal transect in Costa Rica (Lieberman et al., 1996). Conversely, Poulsen and collaborators (2006) found the highest species richness of palms at the highest elevations at a local scale in terra firme Amazonian rain forest. These differences probably reflect differences in spatial scale and topographic gradient length.
Summary: topography
Topography affects palm distributions and community composition at local and landscape scales, possibly through the effects of hydrology, forest dynamics and soil (Fig. 3). The modulating effect of topography on climate seems to be particularly relevant in mountains (Svenning, 2001b; Gatti et al., 2008; Svenning et al., 2009). At broader spatial scales, topographic heterogeneity appears to be of little importance as a determinant of palm species richness (Bjorholm et al., 2005; Kristiansen et al., 2011), except for range-restricted species (Kreft et al., 2006). As topography only indirectly influences palm species distributions and community composition, its effects will be prone to vary geographically, depending on the peculiarities of each area (Vormisto et al., 2004a). Hence, it will be important to measure directly the environmental factors of direct consequence for palms that are modulated by topography.
BIOTIC ENVIRONMENT
Biotic interactions play a key role for the structure and functioning of communities and ecosystems, and may influence species distributions and diversity (Thomson, 2005). Biotic effects on a focal species can be mediated by the surrounding vegetation (e.g. canopy gaps) due to influences on microclimate and light availability (Schnitzer and Carson, 2001; Carson and Schnitzer, 2008). Direct species interactions among plants (e.g. competition and facilitation), between plants and animals (e.g. herbivory, predation, pollination and frugivory), and between plants and pathogens can have a strong influence on species co-occurrence and community structure (Grace and Tilman, 1990; Herrera and Pellmyr, 2002; Ricklefs, 2010a, b). We here review available evidence on the influence of (a) vegetation structure, and (b) species (plant–plant, animal–plant and pathogen–plant) interactions on the distribution of palm species, community composition and species richness. We treat seed dispersal by animals in a separate section (see below) because dispersal is fundamentally different from other biotic interactions as it does not influence the success of an individual in a given spot, but determines whether the spot is reached in the first place.
Vegetation structure
Vegetation structure reflects the role of plants (particularly trees) as ecosystem engineers (Jones et al., 1994), causing heterogeneity in environmental factors such as light availability, litter fall and microclimate (Svenning, 2001a). In tropical forests, light gradients strongly influence species distributions through competition for light and shade tolerances (Carson and Schnitzer, 2008). Spatiotemporal dynamics such as small-scale gap dynamics (Schnitzer and Carson, 2001) or large-scale differences in disturbance regimes (Laurance et al., 2006; ter Steege et al., 2006) influence plant species distributions, and may contribute to maintenance of tree species richness in tropical forests (Ricklefs, 1977; Denslow, 1987). Here, we focus on the effects of vegetation on palm species distributions, community composition and species richness. We particularly address canopy gaps, forest structure and disturbance, and landscape-level implications of fragmentation and edge effects.
Vegetation structure and species distributions
Several palms prefer an open canopy, possibly due to increased light availability (Svenning, 2001a; Fig. 2H). Clumped occurrence of rattans in Sulawesi was attributed to tree-fall gaps (Siebert, 2005). In an Andean rain forest, adults of two of five palms prefer gaps (Svenning, 2001b). In terra firme rain forest in lowland Ecuador, three of six palm species are distributed non-randomly with respect to canopy openness (Svenning, 2000b). In Belize, the climbing Desmoncus orthacanthos has the highest densities at sites with high light intensity (Siebert, 2000), and even highly shade-adapted species (Geonoma macrostachys) may depend on small canopy gaps to maintain their population (Svenning, 2002a). However, the density of the understorey palm G. brevispatha in a Brazilian swamp forest is unrelated to canopy openness (Souza and Martins, 2004), probably reflecting that this species, belonging to a genus of shade-tolerant palms, is not light limited beneath the open swamp forest canopy (see Scariot et al., 1989).
Light demands of palms may differ between ontogenetic stages (Svenning, 2001a). More specifically, it has been hypothesized that large canopy palms can reach the adult stage only in tree-fall gaps because their light requirements increase through ontogeny (Kahn, 1986; Salm et al., 2005; cf. Svenning, 1999b). Central to this idea is that stem development is energy demanding and can only be accomplished at high light intensities (Kahn, 1986; Svenning, 2000a; Salm et al., 2005). The same logic may apply to caulescent mid-storey palms (Homeier et al., 2002). In line herewith, the canopy palm Attalea maripa is more abundant in forests with more open canopy and more disturbance in seasonally dry Amazonian forest (Salm, 2005). In a pre-montane rain forest in Costa Rica, juveniles of Cryosophila warscewiczii are distributed randomly, while adults are spatially clumped, interpreted to reflect recruitment in canopy gaps (Homeier et al., 2002; see also Svenning, 2001a). In an Amazonian lowland rain forest, the two most abundant canopy palms (I. deltoidea and O. bataua) have increasing association with light gaps through ontogeny (Svenning, 1999b).
Other studies have related the occurrence of palm species to forest structure or disturbance. In a lowland moist forest in Panama, C. warscewiczii, Attalaea butyracea and Astrocaryum standleyanum are associated with secondary forest, and S. exorrhiza with old-growth forest (Svenning et al., 2004). In another Panamanian locality, S. exorrhiza, Oenocarpus mapora and Bactris coloradonis are strongly associated with old-growth forest (Dalle et al., 2002). Svenning (1998) interpreted the increased presence of two Chamaedorea species in disturbed parts of an Andean forest as a consequence of increased light availability. The presence and abundance of three out of 23 palm species is significantly related to canopy height in Amazonian terra firme rain forest, with two preferring low canopy and one high canopy (Svenning, 1999a).
Population density of the understorey palm A. mexicanum is low in small rain forest fragments in southern Mexico (Arroyo-Rodríguez et al., 2007). This finding was attributed to edge effects, notably an increase in tree falls, but also disturbance of pollinator and disperser mutualisms (Arroyo-Rodríguez et al., 2007). However, while abundance of pollinators of A. mexicanum was negatively related to fragment size at the same locality, there was no effect on fruit set (Aguirre and Dirzo, 2008). In Brazilian Atlantic Forest, fragmentation increases post-dispersal predation of Syagrus romanzoffiana seeds by squirrels (Fleury and Galetti, 2006). In two rain forest fragments in western Ecuador, two out of six palm species were more abundant in the interior, less disturbed parts (Baez and Balslev, 2007). In fragments of the Brazilian Atlantic forest, the understorey palm Attalea humilis had no consistent response to distance from the forest edge (Souza and Martins, 2002).
Vegetation structure and community composition
In line with the findings above that usually only few palm species in a community respond to canopy heterogeneity or light availability (e.g. Svenning 1999a, 2000b, 2001b), no strong associations between these variables and palm community composition have been identified. There were weak (but significant) correlations of palm community composition with canopy height at a local scale in terra firme rain forest in lowland Amazonian Ecuador (Svenning, 1999a) and terrace forest in the Peruvian Amazon (Normand et al., 2006). In a terra firme rain forest in central Brazilian Amazon, neither canopy openness nor the abundance of non-palm forest trees is related to palm species composition at local scales (Cintra et al., 2005). Similarly, on a landscape scale, understorey palm community composition in a Panamanian montane forest was unrelated to light availability (Andersen et al., 2010).
Vegetation structure and species richness
Spatial patterns of palm species richness could be affected by vegetation structure, but evidence is scarce. In a local-scale study in Amazonian terra firme rain forest, canopy openness and the abundance of non-palm forest trees had little power to explain palm species richness (Cintra et al., 2005). In two rain forest fragments in Ecuador, Baez and Balslev (2007) found fragment-specific responses of palm species richness to distance from the forest edge, potentially reflecting changes in forest structure. Several studies have assessed the impact of forest fragment size on palm species richness. In Costa Rica, Wang (2008) found no correlation between palm species richness and fragment size, but found seedling density differences indicating potential long-term effects. Palm species richness did not vary with size among 10-year-old forest fragments in the Brazilian Amazon (Scariot, 1999). Species richness of palm seedlings increased during secondary forest regeneration in Costa Rica over 5 years (Capers et al., 2005), accompanying a gradual change from pioneer shrubs and lianas to shade-tolerant palms and canopy trees. In the same locality, old secondary forests were dominated by recruiting mature forest canopy palms (Norden et al., 2009), demonstrating that palm species richness is reduced in disturbed and early successional vegetation. Climbing and arborescent multistemmed palms appear to depend on open forest conditions (Kahn and de Castro, 1985; Scariot et al., 1989; Svenning, 2001a; Siebert, 2005). In Sulawesi, rattan diversity peaks at high elevations, possibly due to a gradient in canopy openness (Siebert, 2005). However, thorough quantitative analyses of relationships between species richness of particular palm growth forms to vegetation structure are lacking.
Summary: vegetation structure
Local gradients in canopy openness and light clearly affect distributions of some palm species. However, often only a small proportion of species show such relationships, mirrored in a weak response of palm community composition to these factors. Part of the problem may be difficulties with characterizing small-scale light variation, or, alternatively, effects of canopy heterogeneity may not be reflected in fine-scale distributions due to spatiotemporal source–sink dynamics (Svenning, 2002a). Notably, canopy structure in tropical forests is highly dynamic, obscuring associations of long-lived organisms such as palms with light conditions. Furthermore, light demand may change through ontogeny (e.g. Kahn, 1986; Svenning, 1999b, 2000a, 2001a; Salm et al., 2005). Overall models of palm recruitment (Kahn, 1986; Salm et al., 2005; see also Svenning, 1999b) may be of restricted validity, and further studies of the relationship between palm species distributions and vegetation structure during all ontogenetic stages are needed. Finally, only a few studies have addressed palm species richness in relation to forest structure, and no general effect of forest fragmentation on palm diversity has been documented. Several studies show that landscape-scale palm species distributions can be affected by disturbance history and canopy structure. Geographic differences in canopy dynamics at even larger scales may affect palm species distributions, but this remains untested.
Species interactions
Besides vegetation structure, plant performance and community structure can be strongly affected by plant–plant interactions such as competition and facilitation (Grace and Tilman, 1990; Bengtsson et al., 1994), plant–animal interactions such as herbivory, granivory or frugivory (the latter is not treated here, see section ‘Dispersal’), and pollination (Herrera and Pellmyr, 2002), as well as plant–pathogen interactions (Bradley et al., 2008). At local scales, there is ample evidence that species distributions depend on competition for resources (within and among plant species; Case et al., 2005; Stoll and Bergius, 2005) and facilitative interactions (Callaway and Walker, 1997; Callaway et al., 2002). Herbivores can influence the distribution of plants, potentially in interaction with the abiotic environment (Fine et al., 2004). Pests and pathogens (including granivores) are thought to strongly influence the fine-scale distribution of plants in the tropics, favouring local species coexistence (Janzen, 1970, 1971) and potentially interacting with climate to contribute also to large-scale diversity gradients (Givnish, 1999). Finally, pollinators are a key component of the maintenance of forest plant diversity (Bawa, 1990) and may influence plant distribution patterns (Pellissier et al., 2010). We here review the available evidence on the role that plant–plant, plant–animal and plant–pathogen interactions play in determining the distribution, community composition and species richness of palms.
Species interactions and distributions
Few studies have targeted species interactions among palms or between palms and other plants. In the savannah palm B. aethiopum, competitive interactions between juveniles and competitive effects of adults on juveniles have been found (Barot and Gignoux, 2003). In the Atlantic coastal plains of Brazil, the tank bromeliad Quesnelia arvensis reduces recruitment of E. edulis by trapping its seeds (Brancalion et al., 2009). This is thought to be beneficial for the bromeliad as the two species compete for light and resources. A number of studies provide evidence that palms may limit the recruitment and abundance of tree saplings (e.g. Peters et al., 2004; Wang and Augspurger, 2004); hence, it is also conceivable that such effects may exist between palms, but this has not been studied.
Among tropical rain forest plants, palms stand out by having very tough leaves (Dominy et al., 2008), which may reduce herbivore pressure on palms compared with dicots and other monocots (Grubb et al., 2008). However, palms are not immune to herbivores (Fig. 2C), and discoveries of fossil palm remains with dinosaur bones (Manchester et al., 2010) and in dinosaur coprolites (Prasad et al., 2005) suggest that palms were part of the diet of large herbivores in the late Cretaceous. Nevertheless, few studies have addressed the effects of present-day herbivores on palm distributions. Herbivores were hypothesized to affect palm distributions in interaction with flooding (Pacheco, 2001) but, although seed germination and seedling survival were enhanced by protection against herbivores in two palms, this effect was not related to inundation (Pacheco, 2001). Herbivore pressure by introduced ungulates caused population decline in Ptychosperma macarthurii (syn. Ptychosperma bleeseri) in monsoonal rain forest in Australia (Liddle et al., 2006), indicating that herbivores can influence the occurrence of a given palm species even at a landscape scale. In south-eastern Brazil, herbivore attacks of a butterfly larva on E. edulis are not density dependent, perhaps reflecting that larvae occur at a constant density due to territorial behaviour of adults (da Silva Matos, 2000).
The presence and abundance of granivores can strongly affect palm recruitment. The density of Astrocaryum murumuru seedlings in a 2 km transect in south-eastern Peru increased strongly during a 12 year period of absence of white-lipped peccaries (Tayassu pecari), and reverted to the original level when the animal re-appeared (Silman et al., 2003). Similarly, recruitment of E. edulis is much lower on an island with unnaturally high levels of seed predation by agoutis than in forests with natural population sizes of this granivore (Fadini et al., 2009). Thus, mammals can exert major controls on palm recruitment at a landscape scale. The distribution of A. murumuru seedlings was also influenced by peccaries at finer spatial scales, possibly reflecting their foraging behaviour (Silman et al., 2003). Furthermore, negative density dependence due to seed predators has been observed in palms (Janzen, 1971). Predation of palm seeds is highest close to adults in several different ecosystems (Fragoso, 1997; Wehncke et al., 2009; Álvarez-Loayza et al., 2011). Although invertebrates might be the primary agents of density-dependent seed predation, at least some beetles do not conform to this pattern (Dracxler et al., 2011).
Evidence on the impact of pathogens on palm species distributions is scarce. One study indicates that a specific palm–pathogen interaction in the Amazon depends on temperature, with a potential effect on the palm's distribution (Thompson et al., 2010). Locally, the same pathogen (a fungus) is pathogenous in canopy gaps, but protects the palm from herbivores when growing in the forest understorey (Álvarez-Loayza et al., 2011). The fine-scale distribution of the palm appears to be shaped by a complex interaction between vegetation structure, fungal pathogens and invertebrate herbivores (Álvarez-Loayza et al., 2011; Fig. 3).
A wide range of insect taxa are associated with palm pollination (Henderson, 1986). However, how particular pollinators influence palm species distributions remains largely unclear. It has been suggested that the pollination mode has implications for the altitudinal distribution of species/genera in South America. Beetle pollination is thought to be a lowland phenomenon while high-altitude taxa are predominantly pollinated by flies and bees (Borchsenius, 1993). Adaptation to different pollinators has been suggested to allow the coexistence of closely related palm species that would otherwise be prone to hybridization (Borchsenius, 1997). Thus, pollinator diversity (allowing such differential adaptations) may be important for both species distributions and diversity at a given place. At a local scale, pollination (by bats) strongly limits fruit initiation in the understorey palm Calyptrogyne ghiesbreghtiana in Central America (Cunningham, 1995, 1996), indicating that specific plant–pollinator interactions have the potential to influence palm species distributions. In other understorey palms, pollination is by wind, and exclusion of animal pollinators has no effect on seed production (Otero-Arnaiz and Oyama, 2001). For the double coconut Lodoicea maldivica it has been suggested that wind pollination prevents genetic differentiation between populations on different islands of the Seychelles, in spite of seed dispersal being negligible (Fleischer-Dogley et al., 2011).
Species interactions, community composition and species richness
We are unaware of studies that relate palm community composition or palm species richness to plant–plant, plant–animal or plant–pathogen interactions.
Summary: species interactions
In spite of their potential relevance, the effects of plant–plant, plant–animal and plant–pathogen interactions on palm species distributions have rarely been investigated and the few existing studies mostly cover local scales. In terms of intraspecific competition, spatial patterns have been documented that suggest self-thinning in palms (Fig. 2E), but direct studies (e.g. experimental tests) are lacking. To our knowledge, interspecific competition between palms has never been addressed empirically. A promising approach in the future for detecting signatures of palm–palm interactions in species distribution data could be to analyse the phylogenetic structure of palm communities (e.g. Webb et al., 2002). Alternatively, one could use the presence of conspecifics and non-conspecifics as predictor variable(s) to examine the signature of intra- and interspecific interactions such as competition and facilitation in spatial distribution data (e.g. Wiegand et al., 2006) or spatial demographic data. Widespread positive effects of non-conspecifics and negative effects of conspecifics have been reported from spatial demographic studies of tropical tree communities (Peters, 2003). The best studied aspects of palm–animal interactions are clearly the effects of granivores, and to a lesser degree herbivores and pathogens, on the fine-scale distribution of palms. The landscape-scale presence or abundance of mammalian seed predators affects palm recruitment in some circumstances. Quantitative evidence on potential relationships between palm distributions and the presence, abundance and distribution of their pollinators is lacking. A potential obstacle is the limited distributional data for insect pollinators and even more so potential pathogens. Studies on palm seed mortality caused by granivores are logistically difficult, but may in the future benefit from new techniques such as X-ray scanning of seeds (Brancalion et al., 2011). Given the various ways in which palms are interacting with animals, exploring the implications of those interactions for palm species distribution and diversity is a promising field for future research.
DISPERSAL
Due to physical barriers or spatiotemporal constraints on dispersal, species often do not occur everywhere where the environment is suitable (Svenning and Skov, 2004; Gaston, 2009; Paul et al., 2009). This is particularly evident at large spatial scales (e.g. Tuomisto et al., 2003; Svenning and Skov, 2004), but availability of seeds also affects species occurrence and abundance at finer spatial scales (Turnbull et al., 2000; Svenning and Wright, 2005). Along with demographic stochasticity, the fundamental role of dispersal is reflected in neutral biodiversity theory (Bell, 2001; Hubbell, 2001). The importance of spatially restricted dispersal (‘dispersal assembly’) is often contrasted with species-specific relationships to the abiotic or biotic environment (‘niche assembly’) to explain spatial patterns of species diversity (e.g. Condit et al., 2002; Tuomisto et al., 2003). Dispersal is an essential element of metacommunity theory (Leibold et al., 2004) and the species pool hypothesis (Zobel, 1997), the latter stating that local species richness is limited by the number of available species that are both adapted to the local environment and present in the region. The probability that a species reaches a given place is conditioned by a range of factors including dispersal mode (cf. Howe and Smallwood, 1982), occurrence and abundance of dispersers, and dispersal barriers. As palms are predominantly animal dispersed (Zona and Henderson, 1989), frugivory and dispersal by vertebrates (Herrera, 2002) play a special role. We here review the available evidence on the role of dispersal in determining the distribution, community composition and species richness of palms.
Dispersal and species distributions
An important role for dispersal limitation is obvious from the global distribution of palm species and clades (Fig. 1). Only very few palms (e.g. Raphia taedigera and Cocos nucifera) occur naturally in more than one of the global centres of palm diversity: tropical America, Africa and Indo-Malaya. Many higher taxa are also constrained to these regions; 11 of the 28 tribes are confined to tropical America and 12 to the Palaeotropics, and of the remaining clades, only two (Lepidocaryeae and Trachycarpeae) attain high diversity in both tropical America and the Old World (Dransfield et al., 2008). The differentiation between Africa and the Indo-Pacific region is less pronounced. Two tribes (Podococceae and Sclerospermeae) and two sub-tribes (Ancistrophyllinae and Raphiinae) are endemic to Africa, but tribes Borasseae and Phoeniceae are species rich in both Africa and the Indo-Pacific region. Seven of the Palaeotropical clades diversified in the latter region and are absent from Africa, and an eighth clade, the diverse Calameae, follows this pattern, with the exception of one African species. Taken together, these patterns imply that continental isolation played a prominent role in the diversification of palms, and still poses a formidable constraint on species distributions. Invasions by palms after introduction to new continental areas (e.g. Indo-Malayan and Australasian species in Africa and the New World) provide direct evidence of dispersal limitation at broad spatial scales (Sunderland and Morakinyo, 2002; Svenning, 2002b; Dawson et al., 2008).
At a continental scale, species distribution models of African palms (Blach-Overgaard et al., 2009, 2010) provide strong evidence of dispersal limitation. Environment-based models overpredicted distributions in most cases (Blach-Overgaard et al., 2009, 2010), but inclusion of spatial constraints improved the predictions considerably (Blach-Overgaard et al., 2010). This effect is consistent with dispersal limitation, as many spatially restricted distributions agree with known dispersal barriers or time-limited expansion of species from glacial refugia (Blach-Overgaard et al., 2010). Naturalizations after introductions beyond the range of a species within a biogeographic region further provide direct evidence of dispersal limitation at the continental scale (e.g. Svenning, 2002b). Time-limited expansion has also been shown at a regional scale for the north-east South American understorey palm Astrocaryum sciophilum. The distribution and demographic structure of this species suggest that it is still expanding after past disturbances (Charles-Dominique et al., 2003).
At a local scale, the frequently observed spatial aggregation of palm individuals has been attributed to strong dispersal limitation. For example, in an Andean forest, four of five palm species had patchy distributions, also at the adult stage, and young plants were more frequent near adult conspecifics (Svenning, 2001b). Young and mature Aphandra natalia were strongly clumped in western Amazon terrace forests independently of environmental conditions, and the presence of adults was a good predictor for the presence of juveniles of this large-seeded, rodent-dispersed palm (Boll et al., 2005). Patchy distributions of seedlings were found in Euterpe precatoria, I. deltoidea and C. warscewicziana in a pre-montane rain forest in Costa Rica (Homeier et al., 2002). The interpretation of such patterns as a consequence of dispersal limitation is also supported by a study showing that recruitment of the mid-storey palm O. mapora is strongly limited by seed availability (Svenning and Wright, 2005).
However, there is increasing evidence against short, but isotropic, dispersal distances as a universal explanation for the fine-scale aggregation of palm individuals. Some studies indicate that dispersal is important for escaping seed predation, which seems to be most severe beneath (fruiting) adult trees under many circumstances (Galetti et al., 2006; de Almeida and Galetti, 2007; Pinto et al., 2009). A patchy distribution was shown for the undergrowth palm A. humilis in fragments of the Brazilian Atlantic forest, but different ontogenetic stages do not co-occur more often than expected by chance (Souza and Martins, 2002). In this case it was suggested that seedling clusters are generated by scatter-hoarding rodents, while undispersed seeds below adult plants are removed by seed predators (Souza and Martins, 2002). In a tropical lowland rain forest in northern Brazil, Fragoso (1997) also demonstrated that the very patchy distribution of A. maripa is due to a complex interplay of dispersers and seed predators. Here, recruitment beneath adult trees is prevented by beetle larvae and foraging animals, but tapirs (Tapirus terrestris) remove seeds directly after fruit fall, before beetle infestation is possible, and drop viable seeds at sites repeatedly used for defecation. These sites also appear to be less frequently visited by foraging animals such as peccaries, granting higher seedling survival (Fragoso, 1997). Moreover, tapirs possibly remove larvae from already infested fruits by digestion (Fragoso, 1997; but see Quiroga-Castro and Roldán, 2001). Genetic studies have also provided insights into the spatial pattern of seed dispersal and recruitment. The seedlings of I. deltoidea in secondary forest in Costa Rica (Sezen et al., 2009) are aggregated around adults, but parentage analysis revealed that >83 % of them were offspring of adult palms located further away, with dispersal distances of >50 m for the majority of seeds (Sezen et al., 2009). Here, clumping around adults can be explained by the behaviour of frugivorous birds, which are highly mobile but stay most of the time close to fruiting trees (Sezen et al., 2009). These examples indicate that neither clumping of seedlings per se nor the association of patches with conspecific adults necessarily indicates dispersal limitation from parent trees, but instead may also represent more complex dispersal patterns. Moreover, it must be noted that a patchy environment can also produce aggregated distributions (Homeier et al., 2002; Barot and Gignoux, 2003).
The interactions between palms, dispersers and seed predators are complex and involve different degrees of specialization. Seed predators can act as efficient dispersers of palm species (e.g. scatter-hoarding rodents; Galetti et al., 2006). Although most frugivores do not specialize on a particular plant species (Herrera, 2002), the dispersal of some palms can be tightly bound to, and strongly influenced by, specific animal dispersers (Zona and Henderson, 1989; Galetti et al., 2008, 2010). For instance, the pacu fish (Piaractus mesopotamicus) in the Pantanal of Brazil seems to be especially important in dispersing the seeds of the palm Bactris glaucescens (Galetti et al., 2008). The abundance of A. mexicanum on a local scale in Belize is strongly related to the distribution of the granivore Heteromys desmarestianus which is the palm's only effective disperser in the area, and dispersal strongly enhances seed germination (Klinger and Rejmánek, 2010). Similarly, the abundance of specific mammalian dispersers such as agoutis (Dasyprocta spp.) may strongly influence the spatial dispersal pattern of several palm species (de Almeida and Galetti, 2007; Galetti et al., 2010), and the absence of these scatter-hoarding rodents can even cause a collapse of dispersal (Galetti et al., 2006). To the degree that successful establishment depends on dispersal away from the mother plant (cf. Galetti et al., 2006; de Almeida and Galetti, 2007), disperser loss will also affect population trends and, in the longer run, species distributions. Hence, the presence or absence of certain dispersers may influence the fine-scale distribution of palms (de Almeida and Galetti, 2007; see also Donatti et al., 2009), but could also have consequences at larger spatial and temporal scales. For instance, the extinction of frugivorous megafauna in the Pleistocene might have had dramatic impacts on the distributions of many palm species (Janzen and Martin, 1982), but how exactly these megafaunal extinctions have changed seed dispersal patterns, geographic ranges and population structures of palm species remains unclear (Guimarães et al. 2008; Hansen and Galetti, 2009).
While dispersal is usually thought to determine which environmentally suitable areas are colonized by a species and which are not, dispersal can also lead to the presence of a species in locations where populations would not be self-sustaining (mass effect; Shmida and Ellner, 1984). From the population structure of 20 palm species occurring along an altitudinal transect in the Bolivian Andes, Kessler (2000) inferred the existence of an upslope-directed mass effect. The fact that eight of the species occur only as juveniles in the upper part of their distribution was attributed to dispersal (by oilbirds, Steatornis caripensis) of those palms to altitudes where they were able to germinate, but not to reach maturity.
Dispersal and community composition
Besides individual palm species distributions, dispersal patterns can also influence the compositional turnover of palm assemblages. Compositional similarity of palm assemblages in 1° × 1° grid cells in the New World decays strongly (exponentially) with geographic distance, both on a bicontinental scale and within smaller (approx. 1·25 × 106 km2) regions (Bjorholm et al., 2008). The variation in compositional dissimilarity is consistently much better explained by geographic distance than by environmental distance, indicating a prominent role of dispersal limitation (Bjorholm et al., 2008). This conclusion is supported by the finding that distance decay is strongest in environmentally complex and geographically fragmented regions (Bjorholm et al., 2008).
Distance decay of compositional similarity in palms at landscape to regional scales is well documented, indicating dispersal limitation. In the western Amazon, pure spatial distance explains as much as 40 % of the compositional variation between 21 palm assemblages, even when environmental variables, including soil and topography, are taken into account (landscape to regional scale; Vormisto et al., 2004a). Similarly, palm community compositional similarity decays with geographic distance in restinga forests in the Pastaza fan at landscape to regional scales (Normand et al., 2006). Understorey palm composition is significantly correlated (r2 approx. 0·7) with geographic distance (landscape scale) in a Panamanian montane forest, but in this case the relationship becomes non-significant when controlling for soil calcium (Andersen et al., 2010). Similarity in canopy palm species composition correlates with geographic distance at a landscape scale in north-eastern Costa Rica but, when accounting for environmental factors (elevation and soil magnesium), the correlation disappears (Sesnie et al., 2009).
Palm community composition has also been related to spatial distance at local scales. Svenning and collaborators (2009) found palm composition to be locality specific when comparing three areas in a montane rain forest, Ecuador. Although the influence of unmeasured ecological determinants (soil and historical disturbance) could not be ruled out, the authors suggested local dispersal limitation as an explanation. In a local-scale analysis of restinga palm communities (Normand et al., 2006), community composition is significantly related to geographic distance, but explains only a minor fraction of variance independently from environmental factors. Within a terra firme rain forest in the Brazilian Amazon, landscape-scale dissimilarity in palm community composition was by and large not significantly correlated to geographic distance, which was interpreted as evidence against dispersal limitation (Costa et al., 2009).
Dispersal limitation and species richness
Effects of dispersal limitation – in terms of secular migration (Lomolino et al., 2010) at the clade level – are probably reflected in the results of Bjorholm and collaborators (2006), who demonstrated that lineage history plays a role for the present-day richness patterns of the different palm subfamilies in the Americas. Richness of Coryphoideae, Ceroxyloideae and Calamoideae (tribe Lepidocaryeae) is more spatially structured than richness in Arecoideae or the whole palm family, being biased towards either northern or southern latitudes. This was interpreted as signatures of diversification around ancestral points of arrival in tropical America. Worldwide maps of subfamily richness (Fig. 1B–E) show a similar geographic bias in Coryphoideae and Lepidocaryeae, suggesting consistent dynamics at a global scale.
Summary: dispersal
Dispersal limitation influences palm species distributions at all scales, reflected by high correlations between compositional similarity and geographic distance (especially at broad scales) as well as stochastic variation in species composition (especially at small scales, e.g. Normand et al., 2006). At large scales, the distributions of palm species and clades are shaped by dispersal barriers [at least in the form of oceans (Bjorholm et al., 2008), but also within continents (Blach-Overgaard et al., 2010)] and time-limited dispersal (Charles-Dominique et al., 2003; Blach-Overgaard et al., 2010). At local scales, the behaviour of animal dispersers affects the distribution of palm individuals (Fragoso, 1997; de Almeida and Galetti, 2007). Two mechanisms seem to be particularly important: (1) the majority of seeds are moved only a few metres away from the mother plant (Fragoso, 1997; Wehncke et al., 2009) and (2) seeds are deposited (defecated, regurgitated and scatter-hoarded) by frugivores in a non-random way (Fragoso, 1997; Kessler, 2000; Sezen et al., 2009). The abiotic or biotic environment indirectly affects palm dispersal processes by influencing the distribution, abundance and behaviour of the dispersers (see also Svenning, 2001a), but also by defining dispersal barriers (Wiens and Graham, 2005). The strength of the barriers determines how strongly palm community composition is controlled by dispersal limitation (Bjorholm et al., 2008). On the other hand, dispersal can partially overcome environmental range constraints by mass effects (Kessler, 2000), although probably mainly on smaller scales. Dispersal processes may be tightly integrated with the environment, further complicating the search for simple mechanisms behind palm distributions and diversity patterns.
HISTORICAL VS. CONTEMPORARY EFFECTS
Present-day species distributions and diversity patterns depend not only on the contemporary environment, but also on past events. After a change in environmental conditions or an evolutionary event, it takes time until equilibrium with environmental conditions is reached by dispersal or diversification. Here we review the available evidence of historical imprints in present-day patterns of palm species distributions and diversity by examining effects of (a) climate history and dispersal limitation; (b) time and diversification rates; (c) the historical distribution of landmasses; and (d) the historical effects at finer spatial scales.
Climate history and broad-scale dispersal limitation
The climate history of a region together with dispersal limitation may strongly influence present-day diversity patterns. For instance, the distributions of some palm species in Africa and South America suggest that they are still expanding from ice age refugia (Charles-Dominique et al., 2003; Blach-Overgaard et al., 2010). At a global scale, tropical Africa has fewer palms than other tropical regions (Fig. 1A). This diversity anomaly has been attributed to extinctions in the Palaeogene (Pan et al., 2006) and maybe later (cf. Trénel et al., 2007; Cuenca et al., 2008) caused by climate change (Morley, 2000; see also Dransfield et al., 2008). These ancient diversity losses have not been compensated by diversification or immigration from other continents, confirming that long-term climate stability of biota can be an important determinant of diversity patterns (Dynesius and Jansson, 2000; Stropp et al., 2009). In the western Amazon, regional palm species richness was significantly lower in regions with lower historical habitat stability (Kristiansen et al., 2011). Positive effects of historical climate fluctuations on diversity have also been hypothesized, but have not been confirmed with phylogenetic evidence. For instance, the idea that Pleistocene climatic oscillations have fostered diversification in tropical America by means of range fragmentation/allopatric speciation (Haffer, 1969) has been rejected for Phytelephas (Trénel et al., 2007; Barfod et al., 2010) and Aiphanes (Eiserhardt et al., 2011), in line with findings for other groups of organisms (Rull, 2008).
Time and diversification rates
A relationship between diversity and time for diversification has been suggested for the tropical American understorey palm genus Geonoma (Roncal et al., 2011a). Regions that have long been inhabited by Geonoma show a higher diversity compared with more recently colonized regions, independent of environmental differences. Interestingly, this relationship was also found when diversity was measured as species richness in 1° × 1° grid cells, demonstrating that historical factors not only impact broad-scale diversity patterns, but potentially translate to finer scales (species pool effect; Zobel, 1997). Evidence for a species pool effect was also found by Kristiansen and collaborators (2011) who described a strong relationship between local and regional palm species richness in the western Amazon, indicating that regional differences in diversification history may be reflected in palm richness at a local scale (Kristiansen et al., 2011). In a study on New World palms, Svenning and collaborators (2008a) suggested that a time-integrated area effect in combination with phylogenetic climate niche conservatism could have increased net diversification of palms in equatorial-warm climates. According to this explanation, long-term net diversification rates are highest in those environments that have been widespread and stably present during the whole period over which modern palm diversity has evolved. The historical position and extent of moist-tropical climates thus appear to have influenced present-day palm species richness patterns (Svenning et al., 2008a).
The historical distribution of landmasses
The distribution of larger palm clades reflects, to a certain extent, the historical configuration of landmasses. Against the background of recent divergence time estimates, the break up of Gondwana has played a smaller role for the diversification history of palms than previously expected (cf. Dransfield et al., 2008). Several cases of putative Gondwana vicariance have been tested, and refuted, with molecular age estimates (Gunn, 2004; Trénel et al., 2007; Cuenca et al., 2008; see also Eiserhardt et al., 2011). Instead, the existence of migration pathways between continents in geological time (Morley, 2003) has been invoked to explain disjunct distribution patterns in tribes Ceroxyleae (Trénel et al., 2007), Chamaedoreae (Cuenca et al., 2008), Cocoseae (Gunn, 2004) and Geonomateae (Roncal et al., 2010). The historical separation of landmasses has visible impacts on the distribution of palm species and clades at the interchange areas of Central America and Southeast Asia. In tropical America, several clades are entirely or almost confined to one side of the Panamanian Isthmus (Henderson et al., 1995; Dransfield et al., 2008). A northern hemisphere (Laurasian) bias is especially obvious for subfamily Coryphoideae, which is most diverse in Central America and the Caribbean, reflecting its boreotropical invasion route (Bjorholm et al., 2006; Figs 1D, 2H). A similar pattern is also seen in certain lower taxa such as the genus Chamaedorea (Cuenca et al., 2008). In Southeast Asia, palm distribution patterns are prominently shaped by the long separation of the Sunda and Sahul shelves (Baker and Couvreur, 2011). Many clades are confined or biased to either of the shelves, and palm diversity as a whole is clearly bimodal with strikingly low diversities in Wallacea (Dransfield et al., 2008). These patterns cannot be explained with contemporary environmental conditions and suggest that the historical configuration of landmasses is important in shaping palm distribution patterns.
Historical effects at smaller spatiotemporal scales
While historical effects are often discussed for large-scale distributions and diversity patterns, they may well occur at much finer spatial scales and shorter time scales. However, examples for palms are few. Importantly, historical human impacts can influence palm species distributions at landscape and smaller scales. For example, Clark and collaborators (1995) found an anomaly in the landscape scale distribution of I. deltoidea that is unrelated to edaphic or topographic conditions. However, the absence of the palm in one part of the study area can be convincingly explained with past harvesting of this useful palm (Clark et al., 1995; see Zambrana et al., 2007 for the utility of this species). After a historical disturbance, re-colonization of secondary forest by palms can stretch over decades (Capers et al., 2005; Norden et al., 2009) or longer, and some palm species are associated with either disturbed or non-disturbed forest even after >100 years of succession (Dalle et al., 2002; Svenning et al., 2004). Such associations may reflect re-colonization lags for some species, but for others reflect effects of persistent vegetation structure differences, e.g. favouring some palms in secondary forests (Dalle et al., 2002; Svenning et al., 2004).
Summary: history
There is ample evidence for imprints of historical events on palm distribution and diversity, especially at broad spatial scales. However, inferring the relative importance of ‘historical anomalies’ in diversity and distribution patterns is challenging because information on past environmental conditions is much harder to obtain than measures of the contemporary environment. Where such information is available, historical factors have proven to be important determinants of palm species distributions (e.g. Blach-Overgaard et al., 2010) and diversity (e.g. Roncal et al., 2011).
PALMS AND SPATIAL SCALE
The effects of different abiotic and biotic environmental factors and dispersal on palm diversity and distributions have not been explored comprehensively across spatial scales. Available evidence suggests that some determinants are more influential on some scales than on others (Fig. 4). Individual species distributions, community composition and species richness are not necessarily explained by the same determinants, and their respective relevant predictors vary with scale in different ways. None of this is unexpected in the light of ecological theory and empirical evidence from other groups of organisms, but the range of scales on which some determinants are important deviates from previous suggestions (Willis and Whittaker, 2002; Pearson and Dawson, 2003).
Fig. 4.
Overview showing which processes (left column) have been shown to influence palm distributions, community composition and palm diversity, as indicated, on different scales (top row). Numbers show the upper and lower extents of the scale domains in metres.
Determinants of palm species distributions across spatial scales
On continental to global scales, palm species distributions are strongly constrained by climate and dispersal (Walther et al., 2007; Blach-Overgaard et al., 2010). Soils play a role for some species, but these are dry-climate species and thus likely to be dependent on a high ground water table (Blach-Overgaard et al., 2010). On a regional scale, the scarce evidence suggests a role for climate (Walther et al., 2007) and dispersal limitation (Charles-Dominique et al., 2003), but, given the findings for community composition (see below), it is likely that soils also play a role (Vormisto et al., 2004a). Climate influences palm distributions at global and landscape scales, and therefore also most probably at regional scales (Gatti et al., 2008). Palm species distributions at landscape scale are also influenced by soil fertility (Clark et al., 1995) and topography (Clark et al., 1995; Salm et al., 2007). The well-known associations of many palm species with certain inundation regimes (e.g. Henderson et al., 1995) suggests that hydrology is an important factor at landscape level even if substantial quantitative evidence is lacking. There are some indications that activities of herbivores, seed predators and frugivores influence palm distributions and abundance at a landscape scale. Moreover, vegetation structure (successional stage) appears to be important. At local scales, the distribution of palm individuals can strongly depend on soil fertility (Barot and Gignoux, 2003), hydrology (Svenning, 1999a; Boll et al., 2005) and topography (Svenning, 1999a). Vegetation structure has also been found as a relevant factor at local scales, but its impact differs greatly between species in both intensity and direction (Svenning, 1999a). The scant evidence available suggests that intra- as well as interspecific competition, pests and pathogens (Janzen-Connell effects), and pollinators influence palm distributions at local scales, but comprehensive quantitative evidence is missing. Finally, the behaviour of fruit-eating animals causes complex, non-random patterns of palm species distribution at local and landscape scales (Fragoso, 1997; Sezen et al., 2009), but spatially explicit details of seed dispersal curves of individual palm species are mostly lacking.
Determinants of palm community composition across spatial scales
While current climate emerges as an important determinant of continental-scale compositional turnover, its role is overshadowed by dispersal limitation at this scale (Bjorholm et al., 2008). Dispersal has also been suggested to be the main determinant of palm community composition at regional scales, but soil conditions also seem to play an important role (Vormisto et al., 2004a). Dispersal and soils (Andersen et al., 2010) as well as hydrology (Costa et al., 2009) appear to be of importance at landscape scales. At local scales, soil conditions (Poulsen et al., 2006) and hydrology (Normand et al., 2006), but also topographic position (Svenning, 1999a) and vegetation structure (Svenning, 1999a; Normand et al., 2006), emerge as important determinants of community composition. Dispersal effects are also evident at local scales in the distance decay of floristic similarity, but potentially also in the high fractions of unexplained variation at this scale (Normand et al., 2006). It appears that animal behaviour often creates dispersal patterns that cannot be described by a classical, isotropic dispersal kernel (see, for example, Fragoso, 1997). Such complex behaviour is likely to scramble the relationship between spatial and floristic distance, and is difficult to capture with the commonly used environmental predictors.
Determinants of palm species richness across spatial scales
Several studies have documented an overwhelming importance of climate (water and water-energy) for palm species richness patterns at a continental scale (Bjorholm et al., 2005; Kreft et al., 2006). Additionally, soil fertility and dispersal-related historical processes play a role (Bjorholm et al., 2006). At finer spatial scales, palm species richness has been much less investigated. However, there is some evidence that soil fertility may be important for palm species richness at a regional scale (Vormisto et al., 2004b; Montufar and Pintaud, 2006). Climate might likewise be important at this scale where sufficient variation exists, e.g. in topographically complex landscapes, but this has hardly been investigated. At the landscape scale, the effects of soil (Clark et al., 1995) and topography (Salm et al., 2007) seem to be particularly important for palm richness. An effect of the inundation regime on landscape-scale patterns of palm species richness has also been described (e.g. Kahn and de Castro, 1985), but this relationship needs more rigorous assessment. Topography is the only environmental factor for which a local-scale effect on palm richness has been documented (Poulsen et al., 2006). However, on this scale, environment–richness relationships are clearly underexplored.
Summary: spatial scale
The results summarized here agree overall with general hierarchical frameworks concerning the scale dependence of determinants of species distributions (Pearson and Dawson, 2003) and species richness (Willis and Whittaker, 2002). Some determinants, however, appear to influence palm species distributions over a wider range of scales than suggested by Pearson and Dawson (2003). These authors suggested that climate has a dominant control over species distributions only at the regional scale and above, but Gatti and collaborators (2008) demonstrated that climate can be an efficient range constraint on finer spatial scales, given that climatic gradients are sufficiently steep. Yet, this study concerns low temperature, a climatic factor that is expected to be most relevant close to the palms' latitudinal extremes. The role of fine-scale (regional and below) climatic variation for the distributions of palms in more typical climatic settings is largely unexplored (but see Sesnie et al., 2009). Topography has previously been thought to influence species distributions on landscape to regional scales (Pearson and Dawson, 2003), but can be a strong predictor of local-scale palm distributions as well (e.g. Svenning, 1999a). On the other hand, neither palm species distributions nor assemblage composition have been substantially analysed with respect to topography at regional scales (but see Vormisto et al., 2004a). However, a role for topography, in concert with climate, is implied by the clear altitudinal limits of many species. Soil characteristics, important for species distributions at local to landscape scales (according to Pearson and Dawson, 2003), were also found to be relevant at regional scales for palms (e.g. Vormisto et al., 2004a). Perhaps most importantly, the studies reviewed here suggest a substantial influence of dispersal for palm distributions on all spatial scales (Fig. 4). This runs counter to Pearson and Dawson (2003) who postulated that biotic interactions influence species distributions only at landscape scales and below. It should, however, be noted that dispersal – especially large-scale long-term processes of migration and colonization – never exclusively depends on biotic interactions, but may also involve abiotic dispersal (e.g. hydrochory). Dispersal also depends on the environment as represented by past and present dispersal barriers, and differs from other biotic interactions in that it does not influence survival in, but colonization of, a given place. This may, in part, explain how biotic processes in the case of dispersal can scale up to determine regional and global distributions and diversity patterns. After all, the importance of other biotic interactions mainly at the very fine spatial scales (up to local; Pearson and Dawson, 2003) is confirmed by the palm results.
Although most determinants of palm species distributions, composition and richness apparently are more influential on some scales than on others, cross-scale links are implied by several studies. Measures of range size or commonness often correlate across scales (Cintra et al., 2005; Kristiansen et al., 2009), indicating common determinants. Kristiansen and collaborators (2009) found that the landscape frequency of palm species in the western Amazon (within a 290 × 240 km region) depends on the range of topographical positions they inhabit locally, with topographic generalist species occurring more frequently throughout the region. This implies that the same environmental factors control distributions on both scales, notwithstanding the fact that topography is an indirect predictor and might reflect different environmental variables at different scales. In a similar vein, the size of the continent-wide distribution of palm species was found to correlate positively with measures of edaphic niche breadth and number of utilized habitats, i.e. generalist species tended to be more widespread (Ruokolainen and Vormisto, 2000). These studies suggest a gradual turnover of determinants across scales (Fig. 4).
CONCLUSIONS AND OUTLOOK
A large number of ecological studies have used palms as a model system to investigate determinants of species distributions and diversity patterns. Numerous aspects of the abiotic and biotic environment as well as dispersal have been related to palm species distributions, community composition and species richness on spatial scales from local to global. The effect of all determinants is strongly scale dependent, and different predictors can be important for distributions, composition and richness at a given scale (Fig. 4). Moreover, those determinants might interact in complex ways (Fig. 3). The signatures of past events or processes influencing distributions, composition or richness clearly play a role, too. We suggest that future research should focus on (a) comprehensively exploring determinants across all scales; (b) targeting missing ecosystems or regions; (c) integrating phylogenies with spatial distribution data; (d) integrating new spatial data and methods; and (e) integrating palm geographic ecology with ecosystem and global change research.
Studying determinants across all spatial scales
Not all determinants have been (quantitatively) studied at all scales, and some scale domains are clearly better explored than others (see section ‘Palms and spatial scale’). Palm richness has been studied much more comprehensively at a continental to global scale than at smaller spatial scales, while studies on community composition are numerous at local scales, fewer at landscape to regional scales, and scarce at a continental to global scales. Some scale–predictor combinations are particularly underexplored, or appear promising for further research. For instance, the impact of climate on any aspect of palm diversity has rarely been studied at scales smaller than continental to global (but see Sesnie et al., 2009; Andersen et al., 2010). This is probably due to the a priori assumption, following hierarchical frameworks like those of Pearson and Dawson (2003) or Willis and Whittaker (2002), that climate is not a major determinant at those scales (e.g. Costa et al., 2009). However, there can be strong variation in climate also on smaller scales, caused by topography (Humboldt and Bonpland, 1805) or vegetation structure, potentially exerting an effect on palm distributions (Gatti et al., 2008). An effect of inundation regime on landscape- to regional-scale species distributions and composition has often been assumed, but few studies have addressed this topic quantitatively. This is even more so for the relationship between species richness and hydrology. Understanding topography and its interaction with spatial scale in determining the length and steepness of environmental gradients and their importance for palms could be a main focus of future research. Finally, the importance of some aspects of the biotic environment for palm distributions and diversity patterns is severely understudied; particularly few studies have investigated the importance of competition (especially for resources other than light), pollination or trophic interactions for determining palm distributions.
Although several studies have included a broad range of predictors, usually at least one factor is missing that was shown by other studies to be important at the same scale. Not only can important relationships go unnoticed, but multicollinearity of predictors can lead to premature conclusions on the importance of specific factors (Graham, 2003). Especially for studies investigating the role of dispersal as opposed to the environment, omission of potentially important environmental factors is problematic (Tuomisto and Ruokolainen, 2008). This is evidenced by the many studies finding high spatial autocorrelation of environmental conditions at the scale of study (e.g. Andersen et al., 2010). A similar situation occurs when dealing with topography: many facets of the environment (temperature, soil strata, hydrology, etc.) may vary simultaneously with elevation (e.g. Poulsen et al., 2006). Future studies should aim at including as many as possible of the predictors that have previously been found important at the scale of investigation; we hope that our review may serve as a guide in this process.
Targeting missing ecosystems or regions
Another potential issue for the search for general scale–predictor relationships is the bias of existing studies towards certain regions or ecosystems. More globally comprehensive studies are clearly needed to improve our understanding of what determines palm distributions, community composition and richness not only across scales, but also across regions and ecosystems. Most aspects of palm diversity and distributions are best studied in the Americas, especially in the lowlands of the Amazon basin, whereas continental-scale palm distributions have been studied only for Africa. However, different parts of the world have different histories, which impact the available pool of species (Ricklefs, 1987; Kristiansen et al., 2011). The global centres of palm diversity have strongly divergent biogeographic histories, with long-term stability in South America, severe Tertiary extinctions in Africa, and complex plate tectonics and island dynamics in Southeast Asia (Morley, 2000; Pan et al., 2006; Baker and Couvreur, 2011). This means, in concert with environmental differences and isolation between the regions, that regions are dominated by different clades and life forms. Thus, ecological findings should at least to some degree be corroborated by studies from different biogeographic regions before generalizing them. Notably, extremely few studies have targeted the geographic ecology of palms in the Indo-Pacific region despite its high palm diversity.
Integrating phylogenies with spatial distribution data
One important innovation in spatial ecology is the integration of phylogenetic data. The phylogenetic relatedness of co-occurring species (‘phylogenetic community structure’, Webb et al., 2002; Cavender-Bares et al., 2009; Vamosi et al., 2009) can provide invaluable information on the mechanisms underlying community assembly. In particular, this approach has been used to test for interspecific competition, a mechanism that is otherwise hard to get a handle on (Gotelli, 2000) and poorly documented among palms. Phylogenetic turnover (Graham and Fine, 2008) relates phylogenetic community structure to spatial and environmental gradients, thus providing additional insights into assembly mechanisms. Phylogenetic information is also important when functional relationships are analysed in a spatial context (Diniz-Filho et al., 2007; Kühn et al., 2009; Stephens and Wiens, 2009), based on the same principles as in non-spatial phylogenetic comparative methods (Harvey and Pagel, 1991). Testing the importance of evolutionary rates for diversity patterns (cf. Mittelbach et al., 2007) relies on phylogenetic information, too. One study has already taken this approach in palms, providing a mechanistic explanation for large-scale patterns of palm diversity in the Americas (Svenning et al., 2008a). Finally, the importance of phylogenetic niche conservatism (cf. Wiens et al., 2010) for diversity patterns is increasingly acknowledged (Wiens and Donoghue, 2004; Wiens and Graham, 2005; Buckley et al., 2010; Kozak and Wiens, 2010). The restriction of palms to the tropics and sub-tropics is thought to be due to this phenomenon (Wiens and Donoghue, 2004; Tomlinson, 2006). However, the hypothesis that palms have not radiated into meso- or microthermal climates because they are unable to evolve cold tolerance (tropical conservatism hypothesis) has not been formally tested.
Integrating new spatial data and methods
Remotely sensed data (obtained from aircraft or satellites by means of, for example, photography, radar, laser or microwave techniques) might provide unprecedented insights into distributions and diversity patterns; not only by providing predictor variables, but possibly also by revealing palm distributions per se (Jansen et al., 2008; Blach-Overgaard et al., 2009). The distinctive architecture of palms provides particularly good possibilities for the latter, as was already explored in a classical study on the range limits of Copernicia alba (Rapoport, 1982). Both approaches have been taken successfully in other groups of organisms (e.g. Buermann et al., 2008; Andrew and Ustin, 2009). The need for a better understanding of the geographic ecology of palms on the one hand, and the availability of new data on the other hand, will probably inspire further studies on palm species distributions, community composition and richness.
Integrating palm geographical ecology with ecosystem and global change research
Integrating research on palm ecology with ecosystem and global change studies has great potential. Not only is the geographic distribution of palm species and diversity likely to be influenced by a variety of other organisms, but the opposite is also true. Palms are known to provide keystone resources for frugivores (e.g. Galetti et al., 2001; Genini et al., 2009; Giombini et al., 2009) and may have played this role already in the Palaeogene (Dominy et al., 2003). Palms can also strongly influence vegetation structure and dynamics, e.g. via constraining tree recruitment (Peters et al., 2004; Wang and Augspurger, 2004). Moreover, the presence of certain palm species has been shown to influence animal distributions and behaviour, which in turn might impact other parts of the ecosystem, e.g. plant regeneration (Beck, 2007; Keuroghlian and Eaton, 2009) and soil properties (Young et al., 2010). Hence, palms must be viewed as an integrated part of ecosystems, playing an important role for the functioning of the entire biotic community and its ecosystem services. On the one hand, global change impacts on palm distributions will be partially mediated by changes in the biotic environment that the palms experience. On the other hand, changes in palm distributions and abundance will influence global change effects on other parts of the ecosystem, including the millions of people who depend on palms as crucial resources of construction materials, food, etc. (Balslev and Barfod, 1987; Balick, 1988; Zambrana et al., 2007) as discussed for Hyphaene petersiana by Blach-Overgaard and collaborators (2009). Thus, studying palms and their distribution patterns is clearly relevant from an ecosystem perspective and this plant group that includes many keystone species may be a good model system to better understand the wider consequences of climate and land use change for tropical biodiversity, ecosystems and the associated ecosystem services.
ACKNOWLEDGEMENTS
We are grateful to James Tregear and Jean-Christophe Pintaud for inviting us to submit an article summarizing our research. We are also grateful to our colleagues at the Ecoinformatics and Biodiversity research group, especially Finn Borchsenius, Anders Barfod, Anne Blach Overgaard and Thea Kristiansen, for good discussion and collaboration. We thank John Dransfield, Rafael Govaerts and William Baker (Royal Botanic Gardens, Kew) for making the data of the World Checklist of Palms (Fig. 1) available, and two anonymous reviewers for improving the manuscript with their comments. This work was supported by the Danish Council for Independent Research–Natural Sciences (grant# 272-07-0242 to JCS, #10-083348 to H.B.); the European Community (FP7 grant #212631 to H.B.); and the Villum Kahn Rasmussen Foundation (grant # VKR09b-141 to J.C.S.).
APPENDIX
Studies that provide key findings on determinants of (1) palm species distributions; (2) palm community composition; and (3) palm species richness. The list is aimed to exemplify key studies but is not intended to be exhaustive. Relationships between geographical distance (spatial factors, spatial location) and species distributions, community composition or species richness are generally interpreted to reflect dispersal limitation; see the text for further discussion.
| Determinant(s) | Taxa/ecosystem | Region | Spatial scale | Main finding | Reference |
|---|---|---|---|---|---|
| Palm species distributions | |||||
| Topography, drainage, vegetation | All 23 palm species in a 50 ha plot | South America | Local | Palm species distributions were related to microhabitat variables, mainly topography, but also drainage and canopy height. | Svenning (1999) |
| Soil, human influence | Seven species of canopy and sub-canopy palms | Central America | Local–landscape | Distribution and abundance of five palm species is related to edaphic variation. The distribution of one species is influenced by human harvesting. | Clark et al. (1995) |
| Temperature, topography | Euterpe edulis | South America | Landscape | The distribution of E. edulis is constrained by low temperatures and possibly dew formation (especially occurring in valleys). | Gatti et al. (2008) |
| Climate | Trachycarpus fortunei | Europe, Asia | Regional–continental | There is a strong relationship between minimum winter temperatures (influenced by growing season length) and the native and invasive distribution of this palm. | Walther et al. (2007) |
| Climate, soil, habitat, human impact, space | 29 palm species | Africa | Continental | Climate (especially water availability) and space (possibly reflecting dispersal limitation) are the most important distributional determinants for most palm species. Soil type is only relevant for a few dry-climate species, possibly reflecting hydrology. | Blach-Overgaard et al. (2010) |
| Topography, species traits | 62 palm species | South America | Local/landscape–continental | Palm species abundance at the landscape scale was related to topographic niche breadth, while continental range size correlated with stem height (possibly reflecting species' dispersal potential). | Kristiansen et al. (2009) |
| Palm community composition | |||||
| Soil fertility and texture, topography, spatial distance | All palms; non-inundated lowland rainforest | South America | Local | Palm community composition is related to soil fertility (cations), texture (sand content), elevation and spatial distance. The environmental predictors remain important when spatial distance is taken into account, but not vice versa. | Poulsen et al. (2006) |
| Hydrology, topography, canopy openness, spatial distance | All palms; forest on palaeo-riverine terraces | South America | Local–landscape | At local scale, most variation (approx. 85 %) remains unexplained, with soil moisture being the strongest predictor. At the landscape scale, geographic distance explains most variation (88 %), and composition exhibits links to larger-scale biogeographic patterns (e.g. species with sub-Andean affinities in western local assemblages). | Normand et al. (2006) |
| Soil, precipitation, vegetation, spatial distance | Understorey palms; lower montane forest | Central America | Landscape | Variation in palm community similarity is related to soil properties (especially inorganic nitrogen availability and cation concentration), but also climate and geographic distance. | Andersen et al. (2010) |
| Soil fertility and texture, hydrology, topography, spatial distance | All palms; non-inundated lowland rainforest | South America | Landscape | Palm community composition is strongly related to soil clay content and for a sub-set (understorey palms in bottomlands) also to distance to watercourses. Soil fertility, topography (slope) and spatial distance were not relevant predictors. | Costa et al. (2009) |
| Topography, space | All palms; montane rainforest | South America | Local–landscape | Both topography and spatial location imposed strong controls on palm community composition. | Svenning et al. (2009) |
| Soil, topography, spatial distance | All palms; non-inundated lowland rainforest | South America | Landscape–regional | Palm community composition is more strongly related to geographic distance than to environmental variables. Soil fertility and texture are the only relevant environmental factors. | Vormisto et al. (2004a) |
| Geographic distance, climate, topography, vegetation, soil | All New World palms | Americas | Continental | Geographic distance decay in palm community similarity depends more on geographic distance than on environmental distance. Environmentally complex or geographically fragmented sub-regions exhibit stronger distance decays than more homogenous sub-regions. | Bjorholm et al. (2008) |
| Palm species richness | |||||
| Area, mid-domain effect | All palms in New Guinea | Australasia | Regional | Area and a mid-domain effect explain the majority of variation in palm species richness along an elevational gradient. | Bachman et al. (2004) |
| Climate, topography, vegetation, space | All New World palms | Americas | Continental | Water-related variables (annual rainfall, number of wet days) are the most important environmental predictors for palm species richness, followed by soil fertility. Space (latitude squared) is the dominant spatial variable. | Bjorholm et al. (2005, 2006) |
| Climate, topography | All New World palms | Americas | Continental | Climatic factors related to energy and water availability and productivity determine the species richness of widespread palms, but the species richness of range-restricted palms is to some extent determined by topographical complexity, too. | Kreft et al. (2006) |
| Climate | All Brazilian palms | South America | Continental | Water availability and temperature seasonality are most important in determining palm species richness. | Salm et al. (2007) |
| Climate, topography, net diversification rate | All New World palms | Americas | Continental | Palm species richness increases with net diversification at both deep and shallow phylogenetic levels, and all increase with decreasing (absolute) latitude and increasing energy/temperature and water availability. An increase of species richness with topographic range is linked to recent diversification. | Svenning et al. (2008) |
LITERATURE CITED
- Aguirre A, Dirzo R. Effects of fragmentation on pollinator abundance and fruit set of an abundant understory palm in a Mexican tropical forest. Biological Conservation. 2008;141:375–384. [Google Scholar]
- Álvarez-Loayza P, White JE, Jr, Torres MS, et al. Light converts endosymbiotic fungus to pathogen, influencing seedling survival and niche-space filling of a common tropical tree, Iriartea deltoidea. PLoS ONE. 2011;6:e16386. doi: 10.1371/journal.pone.0016386. doi:10.1371/journal.pone.0016386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Almeida LB, Galetti M. Seed dispersal and spatial distribution of Attalea geraensis (Arecaceae) in two remnants of Cerrado in Southeastern Brazil. Acta Oecologica. 2007;32:180–187. [Google Scholar]
- Andersen KM, Turner BL, Dalling JW. Soil-based habitat partitioning in understorey palms in lower montane tropical forests. Journal of Biogeography. 2010;37:278–292. [Google Scholar]
- Andrew ME, Ustin SL. Habitat suitability modelling of an invasive plant with advanced remote sensing data. Diversity and Distributions. 2009;15:627–640. [Google Scholar]
- Araujo MB, Luoto M. The importance of biotic interactions for modelling species distributions under climate change. Global Ecology and Biogeography. 2007;16:743–753. [Google Scholar]
- Arroyo-Rodríguez V, Aguirre A, Benitez-Malvido J, Mandujano S. Impact of rain forest fragmentation on the population size of a structurally important palm species: Astrocaryum mexicanum at Los Tuxtlas, Mexico. Biological Conservation. 2007;138:198–206. [Google Scholar]
- Austin MP, Ashton PS, Greig-Smith P. The application of quantitative methods to vegetation survey. III. A re-examination of rain forest data from Brunei. Journal of Ecology. 1972;60:305–324. [Google Scholar]
- Bachman S, Baker WJ, Brummitt N, Dransfield J, Moat J. Elevational gradients, area and tropical island diversity: an example from the palms of New Guinea. Ecography. 2004;27:299–310. [Google Scholar]
- Baez S, Balslev H. Edge effects on palm diversity in rain forest fragments in western Ecuador. Biodiversity and Conservation. 2007;16:2201–2211. [Google Scholar]
- Baker WJ, Couvreur TLP. Biogeography and distribution patterns of Southeast Asian palms. In: Gower D, Johnson K, Richardson JE, Rosen B, Rüber L, Williams S, editors. Biotic evolution and environmental change in Southeast Asia. Cambridge: Cambridge University Press (in press); 2011. [Google Scholar]
- Balick MJ. The palm – tree of life: biology, utilization and conservation. New York: The New York Botanical Garden; 1988. [Google Scholar]
- Balslev H, Barfod AS. Ecuadorean palms – an overview. Opera Botanica. 1987;92:1–21. [Google Scholar]
- Balslev H, Luteyn JL, Øllgaard B, Holm-Nielsen LB. Composition and structure of adjacent unflooded and floodplain forest in Amazonian Ecuador. Opera Botanica. 1987;92:37–57. [Google Scholar]
- Balslev H, Eiserhardt W, Kristiansen T, Pedersen D, Grandez C. Palms and palm communities in the upper Ucayali river valley – a little known region in the Amazon basin. PALMS. 2010;54:57–72. [Google Scholar]
- Barfod AS, Trenel P, Borchsenius F. Drivers of diversification in the vegetable ivory palms (Arecaceae: Ceroxyloideae, Phytelepheae) – vicariance or adaptive shifts in niche traits? In. In: Seberg O, Petersen G, Barfod AS, Davis JI, editors. Diversity, phylogeny, and evolution of the monocotyledons. Aarhus: Aarhus University Press; 2010. pp. 225–243. [Google Scholar]
- Barot S, Gignoux J. Neighbourhood analysis in the savanna palm Borassus aethiopum: interplay of intraspecific competition and soil patchiness. Journal of Vegetation Science. 2003;14:79–88. [Google Scholar]
- Bawa KS. Plant–pollinator interactions in tropical rain-forests. Annual Review of Ecology and Systematics. 1990;21:399–422. [Google Scholar]
- Beck H. Synergistic impacts of ungulates and falling palm fronds on saplings in the Amazon. Journal of Tropical Ecology. 2007;23:599–602. [Google Scholar]
- Bell G. Neutral macroecology. Science. 2001;293:2413–2418. doi: 10.1126/science.293.5539.2413. [DOI] [PubMed] [Google Scholar]
- Bengtsson J, Fagerström T, Rydin H. Competition and coexistence in plant communities. Trends in Ecology and Evolution. 1994;9:246–250. doi: 10.1016/0169-5347(94)90289-5. [DOI] [PubMed] [Google Scholar]
- Bjorholm S, Svenning JC, Skov F, Balslev H. Environmental and spatial controls of palm (Arecaceae) species richness across the Americas. Global Ecology and Biogeography. 2005;14:423–429. [Google Scholar]
- Bjorholm S, Svenning JC, Baker WJ, Skov F, Balslev H. Historical legacies in the geographical diversity patterns of New World palm (Arecaceae) subfamilies. Botanical Journal of the Linnean Society. 2006;151:113–125. [Google Scholar]
- Bjorholm S, Svenning J-C, Skov F, Balslev H. To what extent does Tobler's 1st law of geography apply to macroecology? A case study using American palms (Arecaceae) BMC Ecology. 2008;8:11. doi: 10.1186/1472-6785-8-11. doi:10.1186/1472-6785-8-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blach-Overgaard A, Svenning J-C, Balslev H. Climate change sensitivity of the African ivory nut palm, Hyphaene petersiana Klotzsch ex Mart. (Arecaceae) – a keystone species in SE Africa. IOP Conference Series: Earth and Environmental Science. 2009;8:012014. [Google Scholar]
- Blach-Overgaard A, Svenning JC, Dransfield J, Greve M, Balslev H. Determinants of palm species distributions across Africa: the relative roles of climate, non-climatic environmental factors, and spatial constraints. Ecography. 2010;33:380–391. [Google Scholar]
- Boll T, Svenning JC, Vormisto J, Normand S, Grandez C, Balslev H. Spatial distribution and environmental preferences of the piassaba palm Aphandra natalia (Arecaceae) along the Pastaza and Urituyacu rivers in Peru. Forest Ecology and Management. 2005;213:175–183. [Google Scholar]
- Borchsenius F. Flowering biology and insect visitation of three Ecuadorean Aiphanes species. Principes. 1993;37:139–150. [Google Scholar]
- Borchsenius F. Flowering biology of Geonoma irena and G. cuneata var. sodiroi (Arecaceae) Plant Systematics and Evolution. 1997;208:187–196. [Google Scholar]
- Borchsenius F, Skov F. Ecological amplitudes of Ecuadorian palms. Principes. 1997;41:179–183. [Google Scholar]
- Bradley DJ, Gilbert GS, Martiny JBH. Pathogens promote plant diversity through a compensatory response. Ecology Letters. 2008;11:461–469. doi: 10.1111/j.1461-0248.2008.01162.x. [DOI] [PubMed] [Google Scholar]
- Brancalion PHS, Gabriel VdA, Gómez JM. Do terrestrial tank bromeliads in Brazil create safe sites for palm establishment or act as natural traps for its dispersed seeds? Biotropica. 2009;41:3–6. [Google Scholar]
- Brancalion PHS, Rodrigues RR, Novembre ADLC, Gómez JM. Are we misinterpreting seed predation in palms? Biotropica. 2011;43:12–14. [Google Scholar]
- Brown JH. Macroecology. Chicago: The University of Chicago Press; 1995. [Google Scholar]
- Brown JH, Maurer BA. Macroecology – the division of food and space among species on continents. Science. 1989;243:1145–1150. doi: 10.1126/science.243.4895.1145. [DOI] [PubMed] [Google Scholar]
- Buckley LB, Davies TJ, Ackerly DD, et al. Phylogeny, niche conservatism and the latitudinal diversity gradient in mammals. Proceedings of the Royal Society B: Biological Sciences. 2010;277:2131–2138. doi: 10.1098/rspb.2010.0179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buermann W, Saatchi S, Smith TB, et al. Predicting species distributions across the Amazonian and Andean regions using remote sensing data. Journal of Biogeography. 2008;35:1160–1176. [Google Scholar]
- Bush MB, De Oliveira PE, Colinvaux PA, Miller MC, Moreno JE. Amazonian paleoecological histories: one hill, three watersheds. Palaeogeography Palaeoclimatology Palaeoecology. 2004;214:359–393. [Google Scholar]
- Callaway RM, Walker LR. Competition and facilitation: a synthetic approach to interactions in plant communities. Ecology. 1997;78:1958–1965. [Google Scholar]
- Callaway RM, Brooker RW, Choler P, et al. Positive interactions among alpine plants increase with stress. Nature. 2002;417:844–848. doi: 10.1038/nature00812. [DOI] [PubMed] [Google Scholar]
- Capers RS, Chazdon RL, Brenes AR, Alvarado BV. Successional dynamics of woody seedling communities in wet tropical secondary forests. Journal of Ecology. 2005;93:1071–1084. [Google Scholar]
- Carson WP, Schnitzer SA. Tropical forest community ecology. Chichester, UK: Wiley-Blackwell; 2008. [Google Scholar]
- Case TJ, Holt RD, McPeek MA, Keitt TH. The community context of species' borders: ecological and evolutionary perspectives. Oikos. 2005;108:28–46. [Google Scholar]
- Cavender-Bares J, Kozak KH, Fine PVA, Kembel SW. The merging of community ecology and phylogenetic biology. Ecology Letters. 2009;12:693–715. doi: 10.1111/j.1461-0248.2009.01314.x. [DOI] [PubMed] [Google Scholar]
- Charles-Dominique P, Chave J, Dubois MA, De Granville JJ, Riera B, Vezzoli C. Colonization front of the understorey palm Astrocaryum sciophilum in a pristine rain forest of French Guiana. Global Ecology and Biogeography. 2003;12:237–248. [Google Scholar]
- Cintra R, Ximenes AdC, Gondim FR, Kropf MS. Forest spatial heterogeneity and palm richness, abundance and community composition in Terra Firme forest, Central Amazon. Revista Brasileira de Botânica. 2005;28:75–84. [Google Scholar]
- Clark DA, Clark DB, Sandoval R, Castro MV. Edaphic and human effects on landscape-scale distributions of tropical rain-forest palms. Ecology. 1995;76:2581–2594. [Google Scholar]
- Clinebell RR, Phillips OL, Gentry AH, Stark N, Zuuring H. Prediction of Neotropical tree and liana species richness from soil and climatic data. Biodiversity and Conservation. 1995;4:56–90. [Google Scholar]
- Coblentz DD, Riitters KH. Topographic controls on the regional-scale biodiversity of the south-western USA. Journal of Biogeography. 2004;31:1125–1138. [Google Scholar]
- Condit R, Hubbell SP, Foster RB. Changes in tree species abundance in a Neotropical forest: impact of climate change. Journal of Tropical Ecology. 1996;12:231–256. [Google Scholar]
- Condit R, Pitman N, Leigh EG, et al. Beta-diversity in tropical forest trees. Science. 2002;295:666–669. doi: 10.1126/science.1066854. [DOI] [PubMed] [Google Scholar]
- Connell JH. Diversity in tropical rain forests and coral reefs – high diversity of trees and corals is maintained only in a non-equilibrium state. Science. 1978;199:1302–1310. doi: 10.1126/science.199.4335.1302. [DOI] [PubMed] [Google Scholar]
- Corner EJH. The natural history of palms. London: Weiden Feld and Nicolson; 1966. [Google Scholar]
- Costa FRC, Guillaumet JL, Lima AP, Pereira OS. Gradients within gradients: the mesoscale distribution patterns of palms in a central Amazonian forest. Journal of Vegetation Science. 2009;20:69–78. [Google Scholar]
- Cuenca A, Asmussen-Lange CB, Borchsenius F. A dated phylogeny of the palm tribe Chamaedoreeae supports Eocene dispersal between Africa, North and South America. Molecular Phylogenetics and Evolution. 2008;46:760–775. doi: 10.1016/j.ympev.2007.10.010. [DOI] [PubMed] [Google Scholar]
- Cunningham SA. Ecological constraints on fruit initiation by Calyptrogyne ghiesbreghtiana (Arecaceae): floral herbivory, pollen availability, and visitation by pollinating bats. American Journal of Botany. 1995;82:1527–1536. [Google Scholar]
- Cunningham SA. Pollen supply limits fruit initiation by a rain forest understorey palm. Journal of Ecology. 1996;84:185–194. [Google Scholar]
- Currie DJ, Mittelbach GG, Cornell HV, et al. Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecology Letters. 2004;7:1121–1134. [Google Scholar]
- Dalle SP, Lopez H, Diaz D, Legendre P, Potvin C. Spatial distribution and habitats of useful plants: an initial assessment for conservation on an indigenous territory, Panama. Biodiversity and Conservation. 2002;11:637–667. [Google Scholar]
- Dawson W, Mndolwa AS, Burslem DFRP, Hulme PE. Assessing the risks of plant invasions arising from collections in tropical botanical gardens. Biodiversity and Conservation. 2008;17:1979–1995. [Google Scholar]
- Denslow JS. Tropical rainforest gaps and tree species diversity. Annual Review of Ecology and Systematics. 1987;18:421–451. [Google Scholar]
- Diniz-Filho JAF, Bini LM, Rodriguez MA, Rangel TFLVB, Hawkins BA. Seeing the forest for the trees: partitioning ecological and phylogenetic components of Bergmann's rule in European Carnivora. Ecography. 2007;30:598–608. [Google Scholar]
- Dominy NJ, Svenning J-C, Li W-H. Historical contingency in the evolution of primate color vision. Journal of Human Evolution. 2003;44:25–45. doi: 10.1016/s0047-2484(02)00167-7. [DOI] [PubMed] [Google Scholar]
- Dominy NJ, Grubb PJ, Jackson RV, et al. In tropical lowland rain forests monocots have tougher leaves than dicots, and include a new kind of tough leaf. Annals of Botany. 2008;101:1363–1377. doi: 10.1093/aob/mcn046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donatti CI, Guimarães PR, Galetti M. Seed dispersal and predation in the endemic Atlantic rainforest palm Astrocaryum aculeatissimum across a gradient of seed disperser abundance. Ecological Research. 2009;24:1187–1195. [Google Scholar]
- Dracxler CM, Pires AS, Fernandez FAS. Invertebrate seed predators are not all the same: seed predation by Bruchine and Scolytine beetles affects palm recruitment in different ways. Biotropica. 2011;43:8–11. [Google Scholar]
- Dransfield J, Uhl NW, Asmussen CB, Baker WJ, Harley MM, Lewis CE. Genera Palmarum. Richmond, UK: Royal Botanic Gardens, Kew; 2008. [Google Scholar]
- Dynesius M, Jansson R. Evolutionary consequences of changes in species' geographical distributions driven by Milankovitch climate oscillations. Proceedings of the National Academy of Sciences, USA. 2000;97:9115–20. doi: 10.1073/pnas.97.16.9115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiserhardt WL, Pintaud J.-C, Asmussen-Lange C, et al. Phylogeny and divergence times of Bactridinae (Arecaceae, Palmae) based on plastid and nuclear DNA sequences. Taxon. 2011;60:485–498. [Google Scholar]
- Fadini RF, Fleury M, Donatti CI, Galetti M. Effects of frugivore impoverishment and seed predators on the recruitment of a keystone palm. Acta Oecologica-International Journal of Ecology. 2009;35:188–196. [Google Scholar]
- Fine PVA, Mesones I, Coley PD. Herbivores promote habitat specialization by trees in Amazonian forests. Science. 2004;305:663–665. doi: 10.1126/science.1098982. [DOI] [PubMed] [Google Scholar]
- Fleischer-Dogley F, Kettle CJ, Edwards PJ, Ghazoul J, Määttänen K, Kaiser-Bunbury CN. Morphological and genetic differentiation in populations of the dispersal-limited coco de mer (Lodoicea maldivica): implications for management and conservation. Diversity and Distributions. 2011;17:235–243. [Google Scholar]
- Fleury M, Galetti M. Forest fragment size and microhabitat effects on palm seed predation. Biological Conservation. 2006;131:1–13. [Google Scholar]
- Fragoso JMV. Tapir-generated seed shadows: scale-dependent patchiness in the Amazon rain forest. Journal of Ecology. 1997;85:519–529. [Google Scholar]
- Frangi JL, Lugo AE. A floodplain palm forest in the Luquillo Mountains of Puerto Rico five years after hurricane Hugo. Biotropica. 1998;30:339–348. [Google Scholar]
- Galetti M, Keuroghlian A, Hanada L, Morato MI. Frugivory and seed dispersal by the lowland tapir (Tapirus terrestris) in southeast Brazil. Biotropica. 2001;33:723–726. [Google Scholar]
- Galetti M, Donatti CI, Pires AS, Guimarães PR, Jordano P. Seed survival and dispersal of an endemic Atlantic forest palm: the combined effects of defaunation and forest fragmentation. Botanical Journal of the Linnean Society. 2006;151:141–149. [Google Scholar]
- Galetti M, Donatti CI, Pizo MA, Giacomini HC. Big fish are the best: seed dispersal of Bactris glaucescens by the pacu fish (Piaractus mesopotamicus) in the Pantanal, Brazil. Biotropica. 2008;40:386–389. [Google Scholar]
- Galetti M, Donatti CI, Steffler C, Genini J, Bovendorp RS, Fleury M. The role of seed mass on the caching decision by agoutis, Dasyprocta leporina (Rodentia: Agoutidae) Zoologia. 2010;27:472–476. [Google Scholar]
- Gaston KJ. Geographic range limits: achieving synthesis. Proceedings of the Royal Society B: Biological Sciences. 2009;276:1395–1406. doi: 10.1098/rspb.2008.1480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti MG, Campanello PI, Montti LF, Goldstein G. Frost resistance in the tropical palm Euterpe edulis and its pattern of distribution in the Atlantic Forest of Argentina. Forest Ecology and Management. 2008;256:633–640. [Google Scholar]
- Genini J, Galetti M, Morellato LPC. Fruiting phenology of palms and trees in an Atlantic rainforest land-bridge island. Flora. 2009;204:131–145. [Google Scholar]
- Gentry AH. Distributional patterns and an additional species of the Passiflora–Vitifolia complex: Amazonian species-diversity due to edaphically differentiated communities. Plant Systematics and Evolution. 1981;137:95–105. [Google Scholar]
- Gentry AH. Changes in plant community diversity and floristic composition on environmental and geographical gradients. Annals of the Missouri Botanical Garden. 1988;75:1–34. [Google Scholar]
- Giombini MI, Bravo SP, Martinez MF. Seed dispersal of the palm Syagrus romanzoffiana by tapirs in the semi-deciduous Atlantic Forest of Argentina. Biotropica. 2009;41:408–413. [Google Scholar]
- Givnish TJ. On the causes of gradients in tropical tree diversity. Journal of Ecology. 1999;87:193–210. [Google Scholar]
- Gotelli NJ. Null model analysis of species co-occurrence patterns. Ecology. 2000;81:2606–2621. [Google Scholar]
- Govaerts R, Dransfield J. World checklist of palms. Richmond: Royal Botanic Gardens Kew; 2005. [Google Scholar]
- Grace JB, Tilman D. Perspectives on plant competition. San Diego: Academic Press; 1990. [DOI] [PubMed] [Google Scholar]
- Graham MH. Confronting multicollinearity in ecological multiple regression. Ecology. 2003;84:2809–2815. [Google Scholar]
- Graham CH, Fine PVA. Phylogenetic beta diversity: linking ecological and evolutionary processes across space in time. Ecology Letters. 2008;11:1265–1277. doi: 10.1111/j.1461-0248.2008.01256.x. [DOI] [PubMed] [Google Scholar]
- Greenwood DR, Wing SL. Eocene continental climates and latitudinal temperature-gradients. Geology. 1995;23:1044–1048. [Google Scholar]
- Grubb PJ, Jackson RV, Barberis IM, et al. Monocot leaves are eaten less than dicot leaves in tropical lowland rain forests: correlations with toughness and leaf presentation. Annals of Botany. 2008;101:1379–1389. doi: 10.1093/aob/mcn047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guimarães PR, Galetti M, Jordano P. Seed dispersal anachronisms: rethinking the fruits extinct megafauna ate. PLoS One. 2008;3:e1745. doi: 10.1371/journal.pone.0001745. doi:10.1371/journal.pone.0001745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunn BF. The phylogeny of the Cocoeae (Arecaceae) with emphasis on Cocos nucifera. Annals of the Missouri Botanical Garden. 2004;91:505–522. [Google Scholar]
- Haffer J. Speciation in amazonian forest birds. Science. 1969;165:131–137. doi: 10.1126/science.165.3889.131. [DOI] [PubMed] [Google Scholar]
- Hansen DM, Galetti M. The forgotten megafauna. Science. 2009;324:42–43. doi: 10.1126/science.1172393. [DOI] [PubMed] [Google Scholar]
- Harley MM. A summary of fossil records for Arecaceae. Botanical Journal of the Linnean Society. 2006;151:39–67. [Google Scholar]
- Harvey PH, Pagel MD. The comparative method in evolutionary biology. Oxford: Oxford University Press; 1991. [Google Scholar]
- Hawkins BA, Field R, Cornell HV, et al. Energy, water, and broad-scale geographic patterns of species richness. Ecology. 2003;84:3105–3117. [Google Scholar]
- Henderson A. A review of pollination studies in the Palmae. Botanical Review. 1986;52:221–259. [Google Scholar]
- Henderson A. Evolution and ecology of palms. Bronx: The New York Botanical Garden Press; 2002. [Google Scholar]
- Henderson A, Galeano G, Bernal R. Field guide to the palms of the Americas. Princeton, NJ: Princeton University Press; 1995. [Google Scholar]
- Herrera CM. Seed dispersal by vertebrates. In: Herrera CM, Pellmyr O, editors. Plant–animal interactions: an evolutionary approach. Malden: Blackwell Science; 2002. pp. 185–208. [Google Scholar]
- Herrera CM, Pellmyr O. Plant–animal interactions: an evolutionary approach. Malden: Blackwell Science; 2002. [Google Scholar]
- Homeier J, Breckle S-W, Dalitz H, Leyers C, Ortiz R. Demography, spatial distribution, and growth of three arborescent palm species in a tropical premontane rain forest in Costa Rica. Ecotropica. 2002;8:239–247. [Google Scholar]
- Howe HF, Smallwood J. Ecology of seed dispersal. Annual Review of Ecology and Systematics. 1982;13:201–228. [Google Scholar]
- Hubbell SP. The unified neutral theory of biodiversity and biogeography. Princeton, NJ: Princeton University Press; 2001. [Google Scholar]
- Humboldt A von, Bonpland A. Essai sur la géographie des plantes; accompagné d'un tableau physique des régions équinoxiales. Paris: Schoell et compagnie; 1805. [Google Scholar]
- Jansen PA, Bohlman SA, Garzon-Lopez CX, Olff H, Muller-Landau HC, Wright SJ. Large-scale spatial variation in palm fruit abundance across a tropical moist forest estimated from high-resolution aerial photographs. Ecography. 2008;31:33–42. [Google Scholar]
- Janssen T, Bremer K. The age of major monocot groups inferred from 800 + rbcL sequences. Botanical Journal of the Linnean Society. 2004;146:385–398. [Google Scholar]
- Janzen DH. Herbivores and number of tree species in tropical forests. American Naturalist. 1970;104:501–528. [Google Scholar]
- Janzen DH. The fate of Scheelea rostrata fruits beneath the parent tree: predispersal attack by bruchids. Principes. 1971;15:89–101. [Google Scholar]
- Janzen DH, Martin PS. Neotropical anachronisms – the fruits the Gomphotheres ate. Science. 1982;215:19–27. doi: 10.1126/science.215.4528.19. [DOI] [PubMed] [Google Scholar]
- John R, Dalling JW, Harms KE, et al. Soil nutrients influence spatial distributions of tropical tree species. Proceedings of the National Academy of Sciences, USA. 2007;104:864–869. doi: 10.1073/pnas.0604666104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones CG, Lawton JH, Shachak M. Organisms as ecosystem engineers. Oikos. 1994;69:373–386. [Google Scholar]
- Kahn F. Life forms of Amazonian palms in relation to forest structure and dynamics. Biotropica. 1986;18:214–218. [Google Scholar]
- Kahn F. The distribution of palms as a function of local topography in Amazonian Terra-Firme forests. Experientia. 1987;43:251–259. [Google Scholar]
- Kahn F, de Castro A. The palm community in a forest of central Amazonia, Brazil. Biotropica. 1985;17:210–216. [Google Scholar]
- Kahn F, de Granville JJ. Palms in forest ecosystems of Amazonia. Berlin: Springer-Verlag; 1992. [Google Scholar]
- Kahn F, Mejia K. Notes on the biology, ecology, and use of a small Amazonian palm. Lepidocaryum tessmannii. Principes. 1987;31:14–19. [Google Scholar]
- Kahn F, Mejia K. Palm commuities in wetland forest ecosystems of Peruvian Amazonia. Forest Ecology and Management. 1990;33–4:169–179. [Google Scholar]
- Kahn F, Mejia K. The palm communities of two ‘terra firme’ forests in Peruvian Amazonia. Principes. 1991;35:22–26. [Google Scholar]
- Kerr JT, Packer L. Habitat heterogeneity as a determinant of mammal species richness in high-energy regions. Nature. 1997;385:252–254. [Google Scholar]
- Kessler M. Upslope-directed mass effect in palms along an Andean elevational gradient: a cause for high diversity at mid-elevations? Biotropica. 2000;32:756–759. [Google Scholar]
- Keuroghlian A, Eaton DP. Removal of palm fruits and ecosystem engineering in palm stands by white-lipped peccaries (Tayassu pecari) and other frugivores in an isolated Atlantic Forest fragment. Biodiversity and Conservation. 2009;18:1733–1750. [Google Scholar]
- Kissling WD, Rahbek C, Böhning-Gaese K. Food plant diversity as broad-scale determinant of avian frugivore richness. Proceedings of the Royal Society B: Biological Sciences. 2007;274:799–808. doi: 10.1098/rspb.2006.0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinger R, Rejmánek M. A strong conditional mutualism limits and enhances seed dispersal and germination of a tropical palm. Oecologia. 2010;162:951–963. doi: 10.1007/s00442-009-1542-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak KH, Wiens JJ. Niche conservatism drives elevational diversity patterns in Appalachian salamanders. American Naturalist. 2010;176:40–54. doi: 10.1086/653031. [DOI] [PubMed] [Google Scholar]
- Kreft H, Jetz W. Global patterns and determinants of vascular plant diversity. Proceedings of the National Academy of Sciences, USA. 2007;104:5925–5930. doi: 10.1073/pnas.0608361104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreft H, Sommer JH, Barthlott W. The significance of geographic range size for spatial diversity patterns in Neotropical palms. Ecography. 2006;29:21–30. [Google Scholar]
- Kristiansen T, Svenning JC, Grandez C, Salo J, Balslev H. Commonness of Amazonian palm (Arecaceae) species: cross-scale links and potential determinants. Acta Oecologica-International Journal of Ecology. 2009;35:554–562. [Google Scholar]
- Kristiansen T, Svenning JC, Pedersen D, Eiserhardt W, Grández C, Balslev H. Local and regional palm (Arecaceae) species richness patterns and their cross-scale determinants in the western Amazon. Journal of Ecology. 2011 (in press). doi:10.1111/j.1365-2745.2011.01834.x. [Google Scholar]
- Kühn I, Nobis MP, Durka W. Combining spatial and phylogenetic eigenvector filtering in trait analysis. Global Ecology and Biogeography. 2009;18:745–758. [Google Scholar]
- Laurance WF, Nascimento HEM, Laurance SG, et al. Rapid decay of tree-community composition in Amazonian forest fragments. Proceedings of the National Academy of Sciences, USA. 2006;103:19010–19014. doi: 10.1073/pnas.0609048103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold MA, Holyoak M, Mouquet N, et al. The metacommunity concept: a framework for multi-scale community ecology. Ecology Letters. 2004;7:601–613. [Google Scholar]
- Lenoir J, Gegout JC, Guisan A, et al. Cross-scale analysis of the region effect on vascular plant species diversity in southern and northern European mountain ranges. Plos One. 2010;5:e15734. doi: 10.1371/journal.pone.0015734. doi:10.1371/journal.pone.0015734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin SA. The problem of pattern and scale in ecology. Ecology. 1992;73:1943–1967. [Google Scholar]
- Liddle DT, Brook BW, Matthews J, Taylor SM, Caley P. Threat and response: a decade of decline in a regionally endangered rainforest palm affected by fire and introduced animals. Biological Conservation. 2006;132:362–375. [Google Scholar]
- Lieberman D, Lieberman M, Peralta R, Hartshorn GS. Tropical forest structure and composition on a large-scale altitudinal gradient in Costa Rica. Journal of Ecology. 1996;84:137–152. [Google Scholar]
- Lomolino MV, Riddle BR, Whittaker RJ, Brown JH. Biogeography. Sunderland, MA: Sinauer Associates; 2010. [Google Scholar]
- Losos E. Habitat specificity of two palm species: experimental transplantation in Amazonian successional forests. Ecology. 1995;76:2595–2606. [Google Scholar]
- MacArthur RH. Geographical ecology. Princeton, NJ: Princeton University Press; 1972. [Google Scholar]
- Manchester SR, Lehman TM, Wheeler EA. Fossil palms (Arecaceae, Coryphoideae) associated with juvenile herbivorous dinosaurs in the upper Cretaceous Aguja formation, Big Bend National Park, Texas. International Journal of Plant Sciences. 2010;171:679–689. [Google Scholar]
- Merriam CH. Results of a biological survey of the San Francisco Mountain region and the desert of the Little Colorado, Arizona. North American Fauna. 1890;3:1–136. [Google Scholar]
- Mittelbach GG, Schemske DW, Cornell HV, et al. Evolution and the latitudinal diversity gradient: speciation, extinction and biogeography. Ecology Letters. 2007;10:315–331. doi: 10.1111/j.1461-0248.2007.01020.x. [DOI] [PubMed] [Google Scholar]
- Montufar R, Pintaud JC. Variation in species composition, abundance and microhabitat preferences among western Amazonian terra firme palm communities. Botanical Journal of the Linnean Society. 2006;151:127–140. [Google Scholar]
- Morley RJ. Origin and evolution of tropical rain forests. Chichester, UK: John Wiley & Sons; 2000. [Google Scholar]
- Morley RJ. Interplate dispersal paths for megathermal angiosperms. Perspectives in Plant Ecology, Evolution and Systematics. 2003;6:5–20. [Google Scholar]
- Norden N, Chazdon RL, Chao A, Jiang YH, Vilchez-Alvarado B. Resilience of tropical rain forests: tree community reassembly in secondary forests. Ecology Letters. 2009;12:385–394. doi: 10.1111/j.1461-0248.2009.01292.x. [DOI] [PubMed] [Google Scholar]
- Normand S, Vormisto J, Svenning JC, Grandez C, Balslev H. Geographical and environmental controls of palm beta diversity in paleo-riverine terrace forests in Amazonian Peru. Plant Ecology. 2006;186:161–176. [Google Scholar]
- O'Brien EM. Water–energy dynamics, climate, and prediction of woody plant species richness: an interim general model. Journal of Biogeography. 1998;25:379–398. [Google Scholar]
- Otero-Arnaiz A, Oyama K. Reproductive phenology, seed-set and pollination in Chamaedorea alternans, an understorey dioecious palm in a rain forest in Mexico. Journal of Tropical Ecology. 2001;17:745–754. [Google Scholar]
- Pacheco MAW. Effects of flooding and herbivores on variation in recruitment of palms between habitats. Journal of Ecology. 2001;89:358–366. [Google Scholar]
- Palm C, Sanchez P, Ahamed S, Awiti A. Soils: a contemporary perspective. Annual Review of Environment and Resources. 2007;32:99–129. [Google Scholar]
- Pan AD, Jacobs BF, Dransfield J, Baker WJ. The fossil history of palms (Arecaceae) in Africa and new records from the Late Oligocene (28–27 Mya) of north-western Ethiopia. Botanical Journal of the Linnean Society. 2006;151:69–81. [Google Scholar]
- Paul JR, Morton C, Taylor CM, Tonsor SJ. Evolutionary time for dispersal limits the extent but not the occupancy of species' potential ranges in the tropical plant genus Psychotria (Rubiaceae) American Naturalist. 2009;173:188–199. doi: 10.1086/595762. [DOI] [PubMed] [Google Scholar]
- Pearson RG, Dawson TP. Predicting the impacts of climate change on the distribution of species: are bioclimate envelope models useful? Global Ecology and Biogeography. 2003;12:361–371. [Google Scholar]
- Pellissier L, Pottier J, Vittoz P, Dubuis A, Guisan A. Spatial pattern of floral morphology: possible insight into the effects of pollinators on plant distributions. Oikos. 2010;119:1805–1813. [Google Scholar]
- Pennisi E. What determines species diversity. Science. 2005;309:90–90. doi: 10.1126/science.309.5731.90. [DOI] [PubMed] [Google Scholar]
- Peters HA. Neighbour-regulated mortality: the influence of positive and negative density dependence on tree populations in species-rich tropical forests. Ecology Letters. 2003;6:757–765. [Google Scholar]
- Peters HA, Pauw A, Silman MR, Terborgh JW. Falling palm fronds structure Amazonian rainforest sapling communities. Proceedings of the Royal Society B: Biological Sciences. 2004;271:S367–S369. doi: 10.1098/rsbl.2004.0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto SRR, Santos AMM, Tabarelli M. Seed predation by rodents and safe sites for large-seeded trees in a fragment of the Brazilian Atlantic forest. Brazilian Journal of Biology. 2009;69:765–773. doi: 10.1590/s1519-69842009000400003. [DOI] [PubMed] [Google Scholar]
- Pitman NCA, Terborgh JW, Silman MR, et al. Dominance and distribution of tree species in upper Amazonian terra firme forests. Ecology. 2001;82:2101–2117. [Google Scholar]
- Poulsen AD, Tuomisto H, Balslev H. Edaphic and floristic variation within a 1-ha plot of lowland Amazonian rain forest. Biotropica. 2006;38:468–478. [Google Scholar]
- Prasad V, Stromberg CAE, Alimohammadian H, Sahni A. Dinosaur coprolites and the early evolution of grasses and grazers. Science. 2005;310:1177–1180. doi: 10.1126/science.1118806. [DOI] [PubMed] [Google Scholar]
- Qian H, Kissling WD. Spatial scale and cross-taxon congruence of terrestrial vertebrate and vascular plant species richness in China. Ecology. 2010;91:1172–1183. doi: 10.1890/09-0620.1. [DOI] [PubMed] [Google Scholar]
- Quiroga-Castro VD, Roldan AI. The fate of Attalea phalerata (Palmae) seeds dispersed to a tapir latrine. Biotropica. 2001;33:472–477. [Google Scholar]
- Rahbek C. The role of spatial scale and the perception of large-scale species-richness patterns. Ecology Letters. 2005;8:224–239. [Google Scholar]
- Rapoport EH. Areography: geographical strategies of species. New York: Pergamon Press; 1982. [Google Scholar]
- Ricklefs RE. Environmental heterogeneity and plant species-diversity: a hypothesis. American Naturalist. 1977;111:376–381. [Google Scholar]
- Ricklefs RE. Community diversity – relative roles of local and regional processes. Science. 1987;235:167–171. doi: 10.1126/science.235.4785.167. [DOI] [PubMed] [Google Scholar]
- Ricklefs RE, Schluter D. Species diversity in ecological communities – historical and geographical perspectives. Chicago: University of Chicago Press; 1993. [Google Scholar]
- Ricklefs RE. Evolutionary diversification, coevolution between populations and their antagonists, and the filling of niche space. Proceedings of the National Academy of Sciences, USA. 2010a;107:1265–1272. doi: 10.1073/pnas.0913626107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklefs RE. Host–pathogen coevolution, secondary sympatry and species diversification. Philosophical Transactions of the Royal Society B: Biological Sciences. 2010b;365:1139–1147. doi: 10.1098/rstb.2009.0279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roncal J, Borchsenius F, Asmussen-Lange CB, Balslev H. Divergence times in the tribe Geonomateae (Arecaceaea) coincide with tertiary geological events. In: Seberg O, Petersen G, Barfod AS, Davis JI, editors. Diversity, phylogeny, and evolution of the monocotyledons. Aarhus: Aarhus University Press; 2010. pp. 245–265. [Google Scholar]
- Roncal J, Blach-Overgaard A, Borchsenius F, Balslev H, Svenning JC. A dated phylogeny complements macroecological analysis to explain the diversity patterns in Geonoma (Arecaceae) Biotropica. 2011;43:324–334. [Google Scholar]
- Rull V. Speciation timing and Neotropical biodiversity: the Tertiary–Quaternary debate in the light of molecular phylogenetic evidence. Molecular Ecology. 2008;17:2722–2729. doi: 10.1111/j.1365-294X.2008.03789.x. [DOI] [PubMed] [Google Scholar]
- Ruokolainen K, Vormisto J. The most widespread Amazonian palms tend to be tall and habitat generalists. Basic and Applied Ecology. 2000;1:97–1008. [Google Scholar]
- Salm R. The importance of forest disturbance for the recruitment of the large arborescent palm Attalea maripa in a seasonally-dry Amazonian forest. Biota Neotropica. 2005;5:1. [Google Scholar]
- Salm R, Jalles-Filho E, Schuck-Paim C. A model for the importance of large arborescent palms in the dynamics of seasonally-dry Amazonian forests. Biota Neotropica. 2005;5:2. [Google Scholar]
- Salm R, Salles NVd, Alonso WJ, Schuck-Paim C. Cross-scale determinants of palm species distribution. Acta Amazonica. 2007;37:17–25. [Google Scholar]
- Scariot A. Forest fragmentation effects on palm diversity in central Amazonia. Journal of Ecology. 1999;87:66–76. [Google Scholar]
- Scariot AO, Oliveira Filho AT, Lleras E. Species richness, density and distribution of palms in an Eastern Amazonian seasonally flooded forest. Principes. 1989;33:172–179. [Google Scholar]
- Schnitzer SA, Carson WP. Treefall gaps and the maintenance of species diversity in a tropical forest. Ecology. 2001;82:913–919. [Google Scholar]
- Sesnie SE, Finegan B, Gessler PE, Ramos Z. Landscape-scale environmental and floristic variation in Costa Rican old-growth rain forest remnants. Biotropica. 2009;41:16–26. [Google Scholar]
- Sezen UU, Chazdon RL, Holsinger KE. Proximity is not a proxy for parentage in an animal-dispersed Neotropical canopy palm. Proceedings of the Royal Society B: Biological Sciences. 2009;276:2037–2044. doi: 10.1098/rspb.2008.1793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmida A, Ellner S. Coexistence of plant-species with similar niches. Vegetatio. 1984;58:29–55. [Google Scholar]
- Siebert SF. Abundance and growth of Desmoncus orthacanthos Mart. (Palmae) in response to light and ramet harvesting in five forest sites in Belize. Forest Ecology and Management. 2000;137:83–90. [Google Scholar]
- Siebert SF. The abundance and distribution of rattan over an elevation gradient in Sulawesi, Indonesia. Forest Ecology and Management. 2005;210:143–158. [Google Scholar]
- Silman MR, Terborgh JW, Kiltie RA. Population regulation of a dominant-rain forest tree by a major seed-predator. Ecology. 2003;84:431–438. [Google Scholar]
- da Silva Matos D. Herbivore and plant demography: a case study in a fragment of semi-deciduous forest in Brazil. Journal of Tropical Ecology. 2000;16:159–165. [Google Scholar]
- Silvertown J, Dodd ME, Gowing DJG, Mountford JO. Hydrologically defined niches reveal a basis for species richness in plant communities. Nature. 1999;400:61–63. [Google Scholar]
- Sosnowska J, Balslev H. American palm-ethnomedicine: a meta-analysis. Journal of Ethnobiology and Ethnomedicine. 2009;5:43. doi: 10.1186/1746-4269-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souza AF, Martins FR. Spatial distribution of an undergrowth palm in fragments of the Brazilian Atlantic Forest. Plant Ecology. 2002;164:141–155. [Google Scholar]
- Souza AF, Martins FR. Microsite specialization and spatial distribution of Geonoma brevispatha, a clonal palm in south-eastern Brazil. Ecological Research. 2004;19:521–532. [Google Scholar]
- Stephens PR, Wiens JJ. Bridging the gap between community ecology and historical biogeography: niche conservatism and community structure in emydid turtles. Molecular Ecology. 2009;18:4664–4679. doi: 10.1111/j.1365-294X.2009.04378.x. [DOI] [PubMed] [Google Scholar]
- Stoll P, Bergius E. Pattern and process: competition causes regular spacing of individuals within plant populations. Journal of Ecology. 2005;93:395–403. [Google Scholar]
- Stropp J, ter Steege H, Malhi Y, Atdn Rainfor. Disentangling regional and local tree diversity in the Amazon. Ecography. 2009;32:46–54. [Google Scholar]
- Sunderland TCH, Morakinyo T. Nypa fruticans, a weed in West Africa. Palms. 2002;46:154–155. [Google Scholar]
- Svenning JC. The effect of land-use on the local distribution of palm species in an Andean rain forest fragment in northwestern Ecuador. Biodiversity and Conservation. 1998;7:1529–1537. [Google Scholar]
- Svenning JC. Microhabitat specialization in a species-rich palm community in Amazonian Ecuador. Journal of Ecology. 1999a;87:55–65. [Google Scholar]
- Svenning JC. Recruitment of tall arborescent palms in the Yasuni National Park, Amazonian Ecuador: are large treefall gaps important? Journal of Tropical Ecology. 1999b;15:355–366. [Google Scholar]
- Svenning JC. Growth strategies of clonal palms (Arecaceae) in a neotropical rainforest, Yasuni, Ecuador. Australian Journal of Botany. 2000a;48:167–178. [Google Scholar]
- Svenning JC. Small canopy gaps influence plant distributions in the rain forest understory. Biotropica. 2000b;32:252–261. [Google Scholar]
- Svenning JC. On the role of microenvironmental heterogeneity in the ecology and diversification of neotropical rain-forest palms (Arecaceae) Botanical Review. 2001a;67:1–53. [Google Scholar]
- Svenning JC. Environmental heterogeneity, recruitment limitation and the mesoscale distribution of palms in a tropical montane rain forest (Maquipucuna, Ecuador) Journal of Tropical Ecology. 2001b;17:97–113. [Google Scholar]
- Svenning JC. Crown illumination limits the population growth rate of a neotropical understorey palm (Geonoma macrostachys, Arecaceae) Plant Ecology. 2002a;159:185–199. [Google Scholar]
- Svenning JC. Non-native ornamental palms invade a secondary tropical forest in Panama. Palms. 2002b;46:81–86. [Google Scholar]
- Svenning JC, Skov F. Limited filling of the potential range in European tree species. Ecology Letters. 2004;7:565–573. [Google Scholar]
- Svenning JC, Skov F. Ice age legacies in the geographical distribution of tree species richness in Europe. Global Ecology and Biogeography. 2007;16:234–245. [Google Scholar]
- Svenning JC, Wright SJ. Seed limitation in a Panamanian forest. Journal of Ecology. 2005;93:853–862. [Google Scholar]
- Svenning JC, Kinner DA, Stallard RF, Engelbrecht BMJ, Wright SJ. Ecological determinism in plant community structure across a tropical forest landscape. Ecology. 2004;85:2526–2538. [Google Scholar]
- Svenning JC, Borchsenius F, Bjorholm S, Balslev H. High tropical net diversification drives the New World latitudinal gradient in palm (Arecaceae) species richness. Journal of Biogeography. 2008a;35:394–406. [Google Scholar]
- Svenning JC, Normand S, Skov F. Postglacial dispersal limitation of widespread forest plant species in nemoral Europe. Ecography. 2008b;31:316–326. [Google Scholar]
- Svenning JC, Harlev D, Sorensen M, Balslev H. Topographic and spatial controls of palm species distributions in a montane rain forest, southern Ecuador. Biodiversity and Conservation. 2009;18:219–228. [Google Scholar]
- ter Steege H, Pitman NC, Phillips OL, et al. Continental-scale patterns of canopy tree composition and function across Amazonia. Nature. 2006;443:444–447. doi: 10.1038/nature05134. [DOI] [PubMed] [Google Scholar]
- Terborgh J. Keystone plant resources in the tropical forest. In: Soule M, editor. Conservation biology: science of scarcity and diversity. Sunderland, MA: Sinauer; 1986. pp. 330–344. [Google Scholar]
- Thompson S, Alvarez-Loayza P, Terborgh J, Katul G. The effects of plant pathogens on tree recruitment in the western Amazon under a projected future climate: a dynamical systems analysis. Journal of Ecology. 2010;98:1434–1446. [Google Scholar]
- Thomson NJ. The geographic mosaic of coevolution. Chicago: University of Chicago Press; 2005. [Google Scholar]
- Tomlinson PB. The uniqueness of palms. Botanical Journal of the Linnean Society. 2006;151:5–14. [Google Scholar]
- de la Torre L, Calvo-Irabien LM, Salazar C, Balslev H, Borchsenius F. Contrasting palm species and use diversity in the Yucatan Peninsula and the Ecuadorian Amazon. Biodiversity and Conservation. 2009;18:2837–2853. [Google Scholar]
- Trénel P, Gustafsson MHG, Baker WJ, Asmussen-Lange CB, Dransfield J, Borchsenius F. Mid-Tertiary dispersal, not Gondwanan vicariance explains distribution patterns in the wax palm subfamily (Ceroxyloideae: Arecaceae) Molecular Phylogenetics and Evolution. 2007;45:272–288. doi: 10.1016/j.ympev.2007.03.018. [DOI] [PubMed] [Google Scholar]
- Tripp EA, Dexter KG. Sabal minor (Arecaceae): a new northern record of palms in eastern North America. Castanea. 2006;71:172–177. [Google Scholar]
- Tuomisto H, Ruokolainen K. Analyzing or explaining beta diversity? Reply. Ecology. 2008;89:3244–3256. doi: 10.1890/08-1247.1. [DOI] [PubMed] [Google Scholar]
- Tuomisto H, Ruokolainen K, Kalliola R, Linna A, Danjoy W, Rodriguez Z. Dissecting Amazonian biodiversity. Science. 1995;269:63–6. doi: 10.1126/science.269.5220.63. [DOI] [PubMed] [Google Scholar]
- Tuomisto H, Ruokolainen K, Yli-Halla M. Dispersal, environment, and floristic variation of western Amazonian forests. Science. 2003;299:241–244. doi: 10.1126/science.1078037. [DOI] [PubMed] [Google Scholar]
- Turnbull LA, Crawley MJ, Rees M. Are plant populations seed-limited? A review of seed sowing experiments. Oikos. 2000;88:225–238. [Google Scholar]
- Vamosi SM, Heard SB, Vamosi JC, Webb CO. Emerging patterns in the comparative analysis of phylogenetic community structure. Molecular Ecology. 2009;18:572–592. doi: 10.1111/j.1365-294X.2008.04001.x. [DOI] [PubMed] [Google Scholar]
- Vormisto J, Phillips OL, Ruokolainen K, Tuomisto H, Vasquez R. A comparison of fine-scale distribution patterns of four plant groups in an Amazonian rainforest. Ecography. 2000;23:349–359. [Google Scholar]
- Vormisto J, Svenning JC, Hall P, Balslev H. Diversity and dominance in palm (Arecaceae) communities in terra firme forests in the western Amazon basin. Journal of Ecology. 2004a;92:577–588. [Google Scholar]
- Vormisto J, Tuomisto H, Oksanen J. Palm distribution patterns in Amazonian rainforests: what is the role of topographic variation? Journal of Vegetation Science. 2004b;15:485–494. [Google Scholar]
- Walther GR, Gritti ES, Berger S, Hickler T, Tang ZY, Sykes MT. Palms tracking climate change. Global Ecology and Biogeography. 2007;16:801–809. [Google Scholar]
- Wang YH. Palm community structure and land cover changes in the San Juan biological corridor, Costa Rica. Biotropica. 2008;40:44–54. [Google Scholar]
- Wang YH, Augspurger C. Dwarf palms and cyclanths strongly reduce Neotropical seedling recruitment. Oikos. 2004;107:619–633. [Google Scholar]
- Webb CO, Ackerly DD, McPeek MA, Donoghue MJ. Phylogenies and community ecology. Annual Review of Ecology and Systematics. 2002;33:475–505. [Google Scholar]
- Wehncke EV, Medellin XL, Ezcurra E. Patterns of frugivory, seed dispersal and predation of blue fan palms (Brahea armata) in oases of northern Baja California. Journal of Arid Environments. 2009;73:773–783. [Google Scholar]
- Whittaker RH. Vegetation of the Siskiyou Mountains, Oregon and California. Ecology Monographs. 1960;30:279–338. doi: 10.1002/ecy.3764. [DOI] [PubMed] [Google Scholar]
- Whittaker RH. Communities and ecosystems. New York: Macmillan; 1975. [Google Scholar]
- Wiegand T, Kissling WD, Cipriotti PA, Aguiar MR. Extending point pattern analysis for objects of finite size and irregular shape. Journal of Ecology. 2006;94:825–837. [Google Scholar]
- Wiens JJ, Donoghue MJ. Historical biogeography, ecology and species richness. Trends in Ecology and Evolution. 2004;19:639–44. doi: 10.1016/j.tree.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Wiens JJ, Graham CH. Niche conservatism: integrating evolution, ecology, and conservation biology. Annual Review of Ecology Evolution and Systematics. 2005;36:519–539. [Google Scholar]
- Wiens JJ, Ackerly DD, Allen AP, et al. Niche conservatism as an emerging principle in ecology and conservation biology. Ecology Letters. 2010;13:1310–1324. doi: 10.1111/j.1461-0248.2010.01515.x. [DOI] [PubMed] [Google Scholar]
- Willis KJ, Whittaker RJ. Species diversity – scale matters. Science. 2002;295:1245–1248. doi: 10.1126/science.1067335. [DOI] [PubMed] [Google Scholar]
- Wing SL, Hickey LJ, Swisher CC. Implications of an exceptional fossil flora for late Cretaceous vegetation. Nature. 1993;363:342–344. [Google Scholar]
- Young HS, Raab TK, McCauley DJ, Briggs AA, Dirzo R. The coconut palm, Cocos nucifera, impacts forest composition and soil characteristics at Palmyra Atoll, Central Pacific. Journal of Vegetation Science. 2010;21:1058–1068. [Google Scholar]
- Zambrana NYP, Byg A, Svenning JC, Moraes M, Grandez C, Balslev H. Diversity of palm uses in the western Amazon. Biodiversity and Conservation. 2007;16:2771–2787. [Google Scholar]
- Zobel M. The relative of species pools in determining plant species richness: an alternative explanation of species coexistence? Trends in Ecology and Evolution. 1997;12:266–269. doi: 10.1016/s0169-5347(97)01096-3. [DOI] [PubMed] [Google Scholar]
- Zona S, Henderson A. A review of animal mediated seed dispersal of palms. Selbyana. 1989;11:6–21. [Google Scholar]