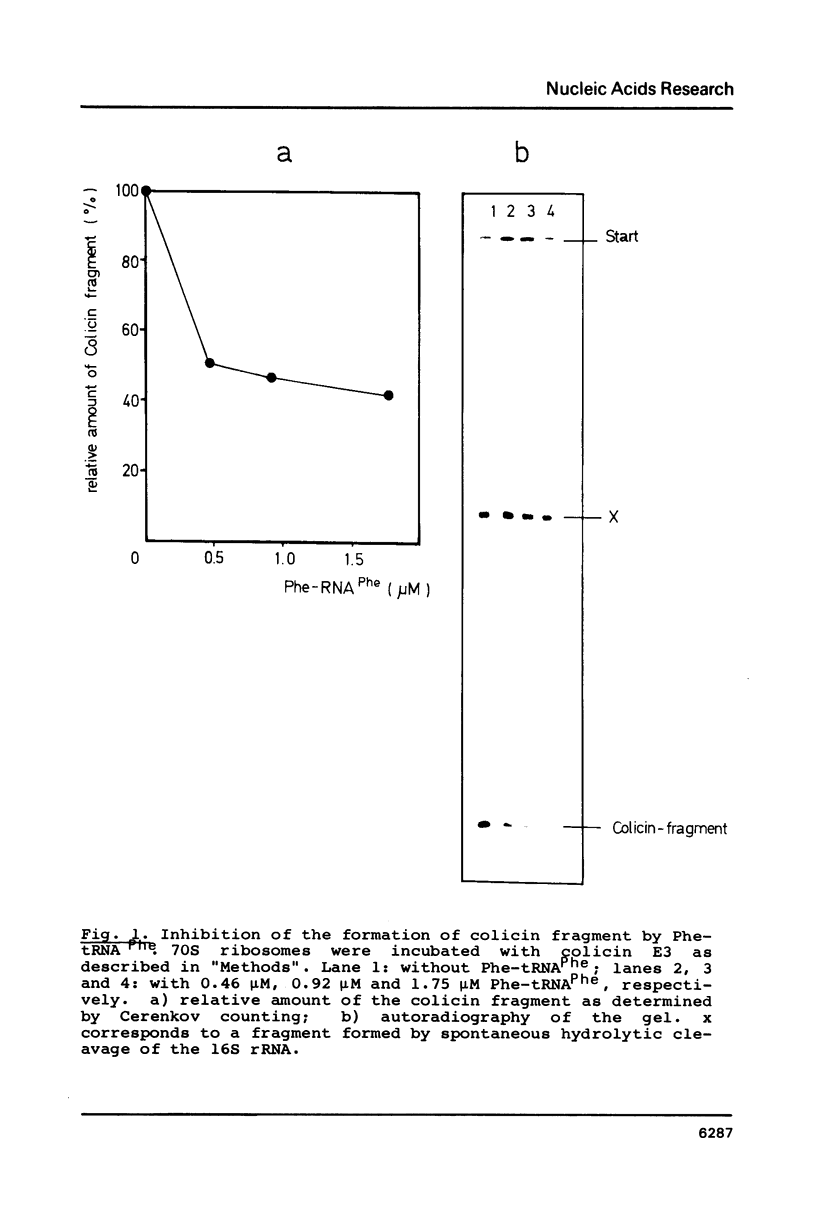
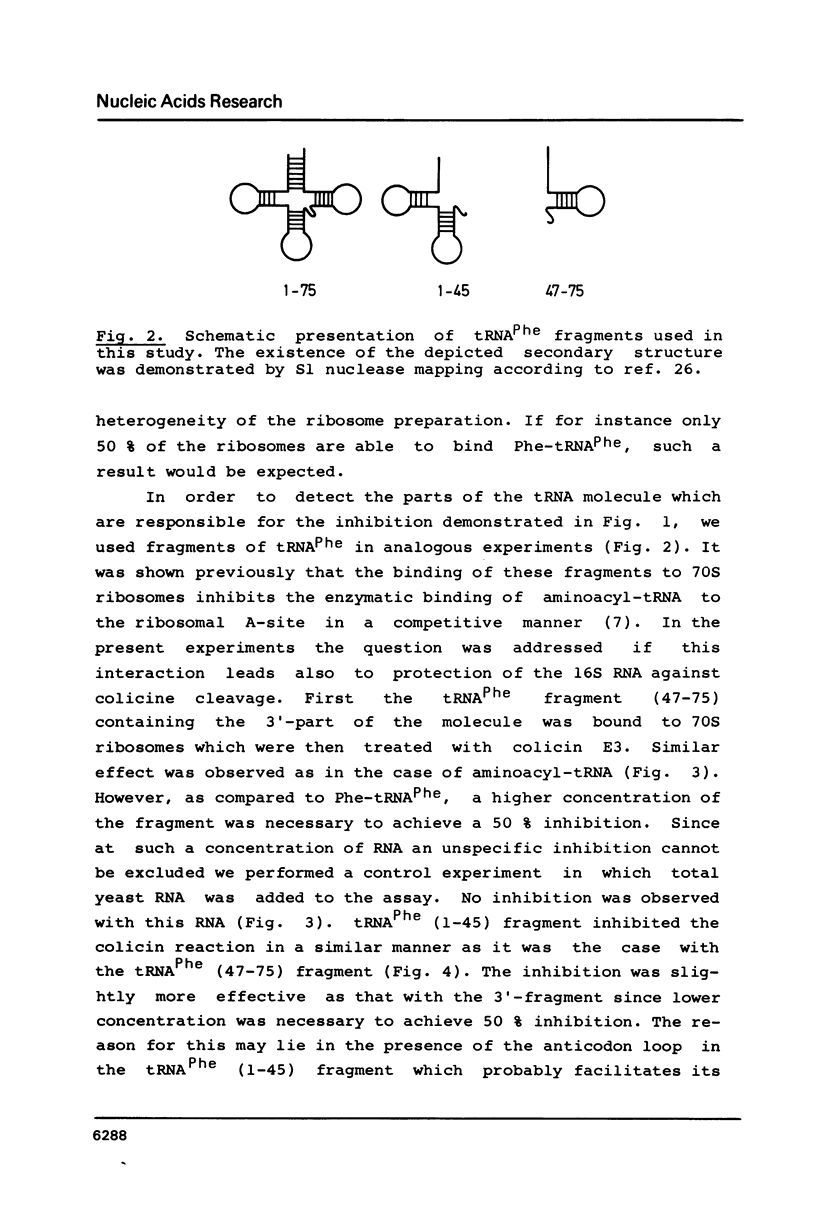
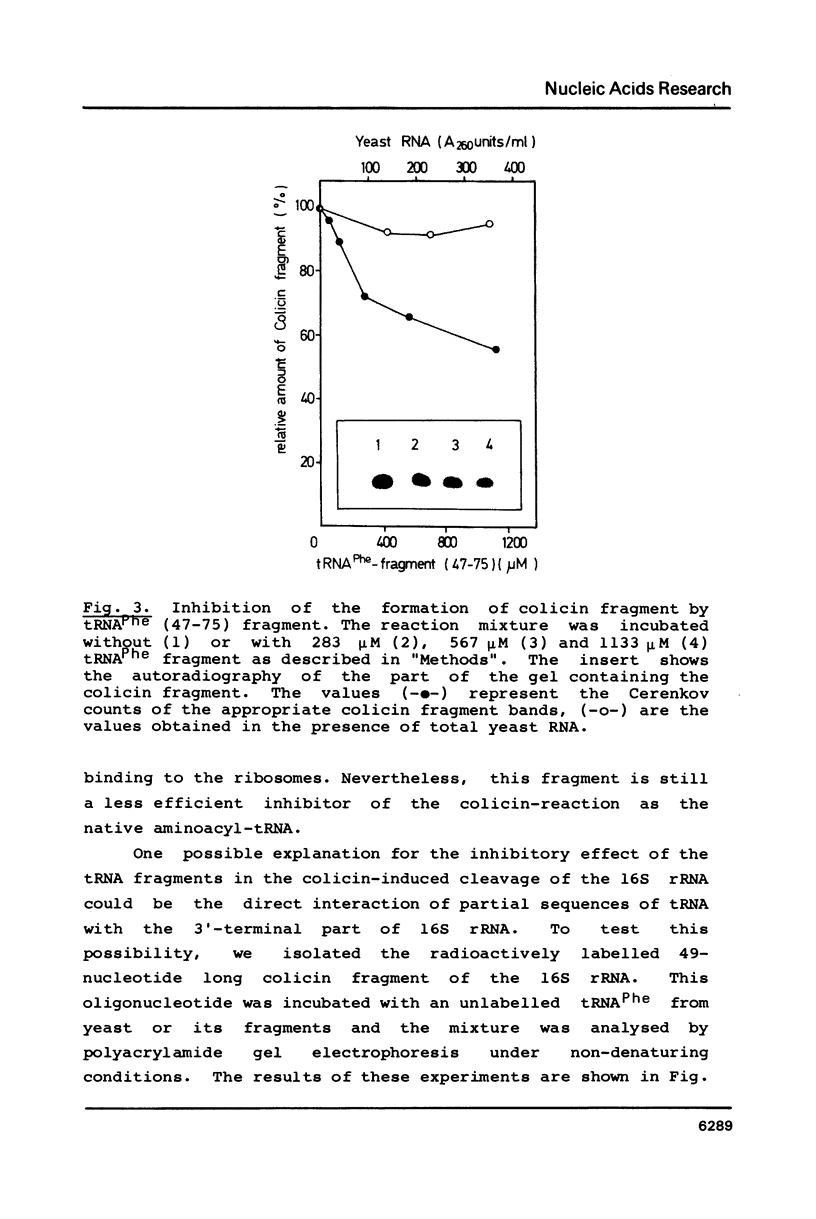
Abstract
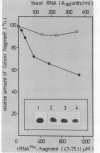
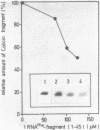
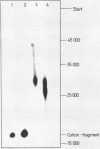
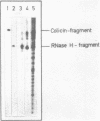
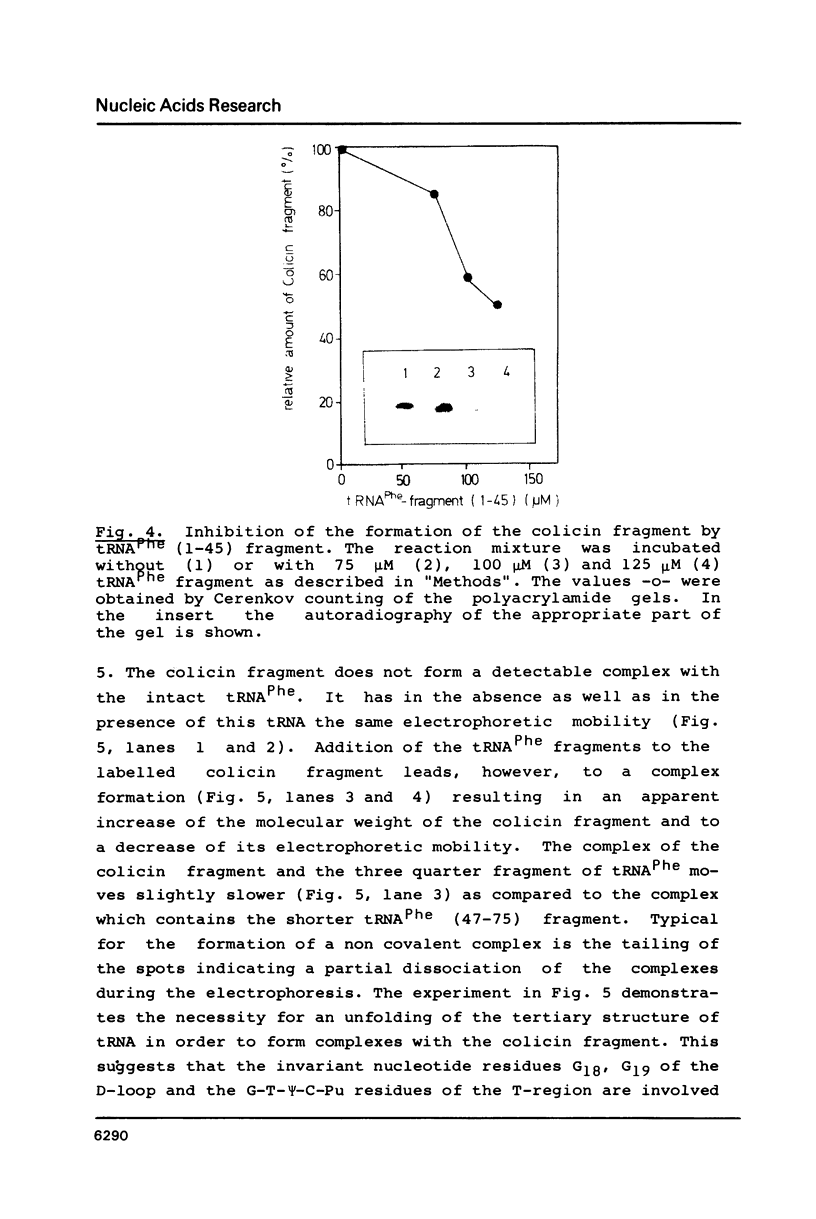
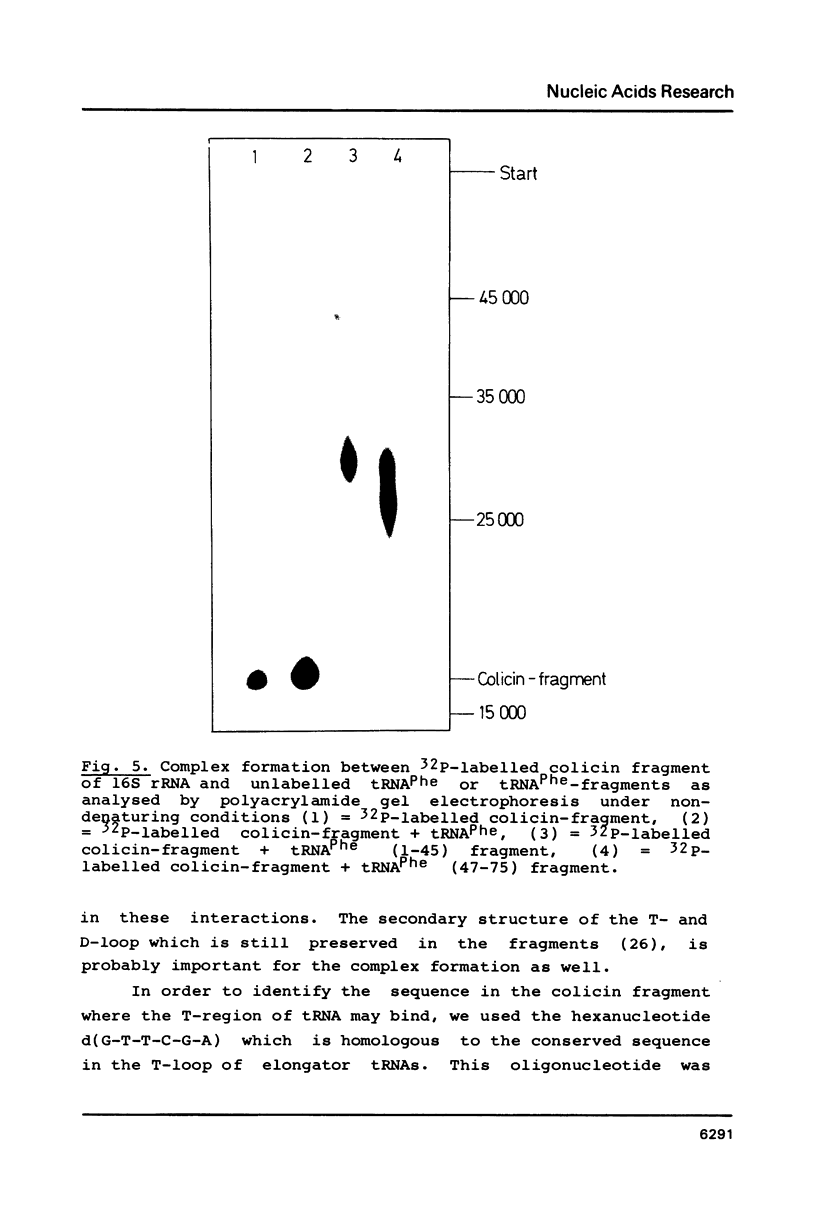
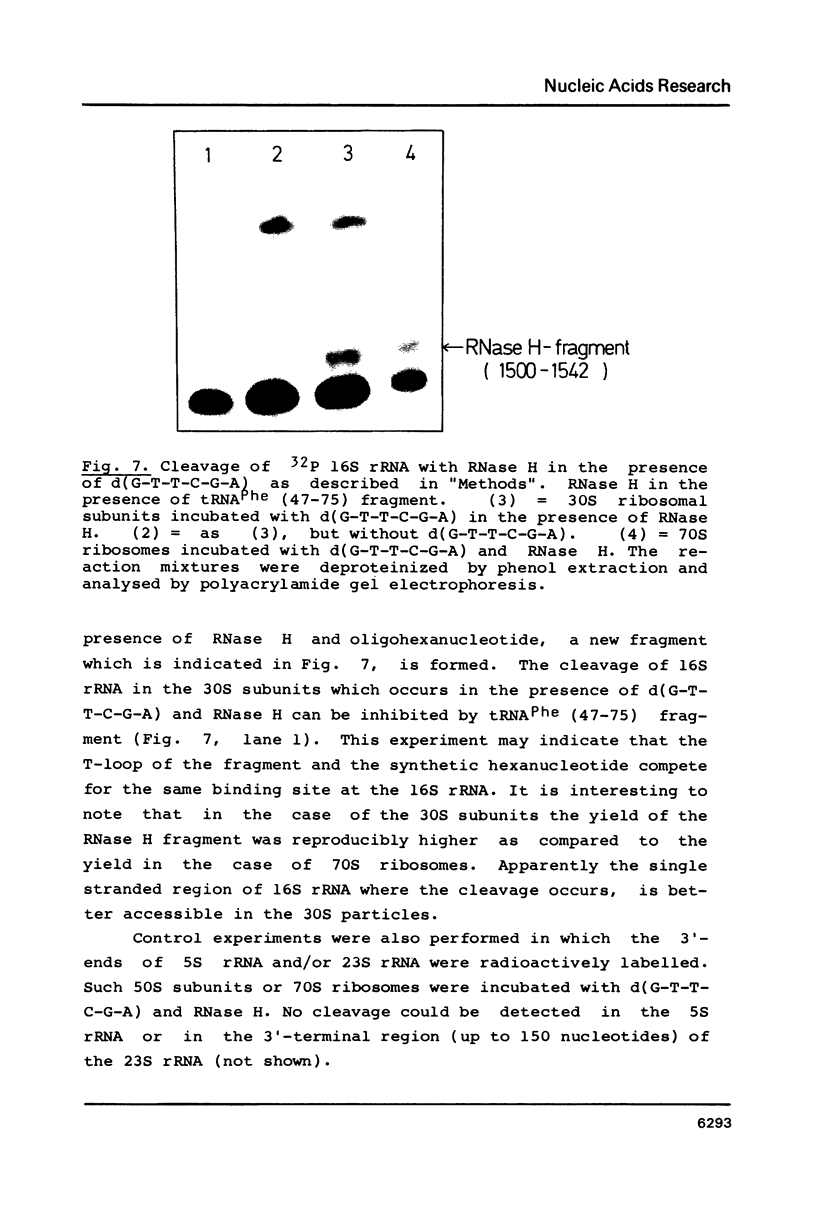
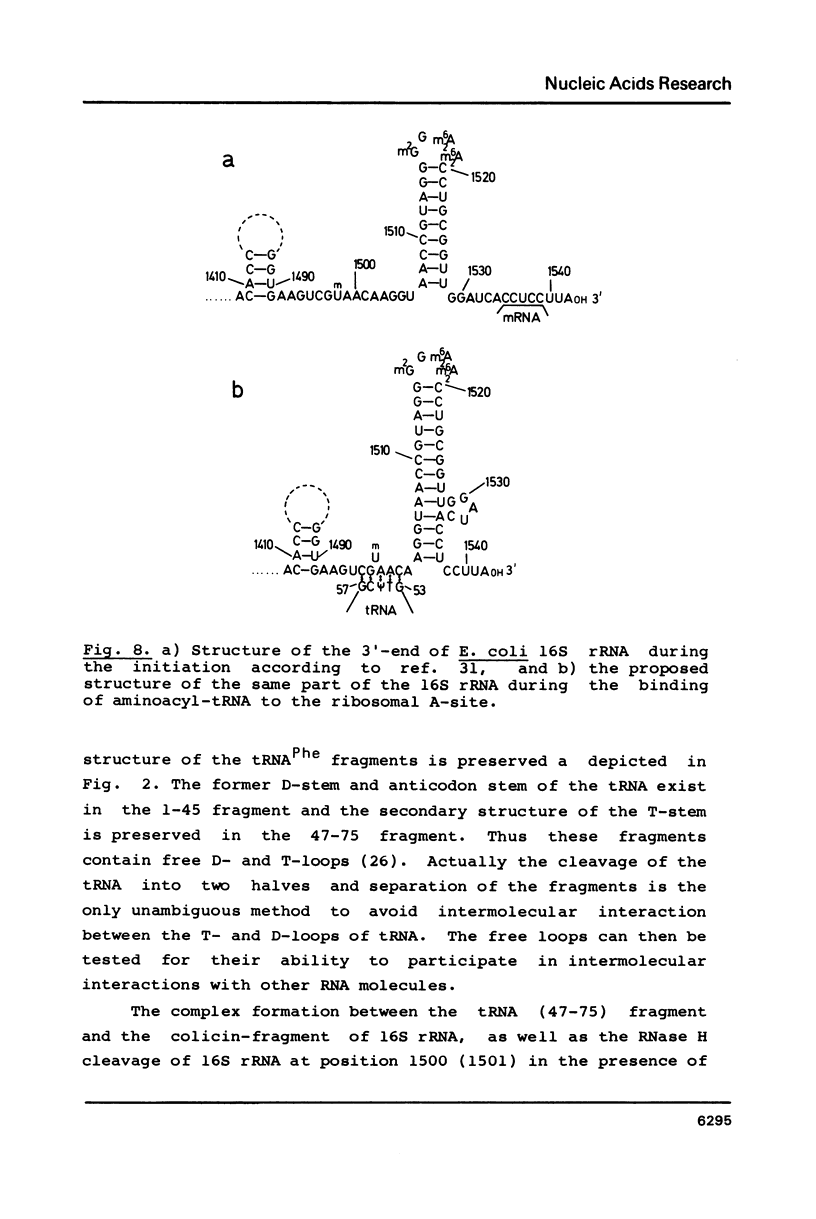
Fragments of tRNA possessing a free TpsiC-loop or a free D-loop form stable complexes with the colicin fragment (1494-1542) of 16S ribosomal RNA from E. coli. The colicin fragment does not bind to tRNA in which the T-loop and the D-loop are involved in tertiary interactions. Colicin cleavage of the 16S rRNA from E. coli is inhibited by aminoacyl-tRNA or tRNA fragments, indicating that a similar interaction may take place on the intact 70S ribosomes. The oligonucleotide d(G-T-T-C-G-A)homologous to the conserved sequence G-T-psi-C-Pu-(m1)A in the TpsiC-region of many elongator tRNAs binds to the conserved sequence U-C-G-mU-A-A-C (1495-1501) of the 16S rRNA. It is suggested that the 3'-end of the 16S rRNA may provide the part of the binding site for the elongator tRNAs on bacterial ribosomes.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baan R. A., Frijmann M., van Knippenberg P. H., Bosch L. Consequences of a specific cleavage in situ of 16-S ribosomal RNA for polypeptide chain elongation. Eur J Biochem. 1978 Jun 1;87(1):137–142. doi: 10.1111/j.1432-1033.1978.tb12359.x. [DOI] [PubMed] [Google Scholar]
- Baan R. A., Naaktgeboren N., van Charldorp R., van Knippenberg P. H., Bosch L. Consequences of a specific cleavage in situ of 16-S ribosomal RNA for polypeptide chain initiation. Eur J Biochem. 1978 Jun 1;87(1):131–136. doi: 10.1111/j.1432-1033.1978.tb12358.x. [DOI] [PubMed] [Google Scholar]
- Baan R. A., van Charldorp R., van Leerdam E., van Knippenberg P. H., Bosch L., de Rooij J. F., van Boom J. H. The 3'-terminus of 16 S ribosomal RNA of Escherichia coli. Isolation and purification of the terminal 49-nucleotide fragment at a milligram scale. FEBS Lett. 1976 Dec 1;71(2):351–355. doi: 10.1016/0014-5793(76)80968-4. [DOI] [PubMed] [Google Scholar]
- Backendorf C., Ravensbergen C. J., Van der Plas J., van Boom J. H., Veeneman G., Van Duin J. Basepairing potential of the 3' terminus of 16S RNA: dependence on the functional state of the 30S subunit and the presence of protein S21. Nucleic Acids Res. 1981 Mar 25;9(6):1425–1444. doi: 10.1093/nar/9.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- England T. E., Bruce A. G., Uhlenbeck O. C. Specific labeling of 3' termini of RNA with T4 RNA ligase. Methods Enzymol. 1980;65(1):65–74. doi: 10.1016/s0076-6879(80)65011-3. [DOI] [PubMed] [Google Scholar]
- Forget B. G., Weissman S. M. Nucleotide sequence of KB cell 5S RNA. Science. 1967 Dec 29;158(3809):1695–1699. doi: 10.1126/science.158.3809.1695. [DOI] [PubMed] [Google Scholar]
- Jelenc P. C. Rapid purification of highly active ribosomes from Escherichia coli. Anal Biochem. 1980 Jul 1;105(2):369–374. doi: 10.1016/0003-2697(80)90472-8. [DOI] [PubMed] [Google Scholar]
- Johnson J. D., Horowitz J. Characterization of ribosomes and RNAs from Mycoplasma hominis. Biochim Biophys Acta. 1971 Oct 14;247(2):262–279. doi: 10.1016/0005-2787(71)90675-7. [DOI] [PubMed] [Google Scholar]
- Johnson R. A., Walseth T. F. The enzymatic preparation of [alpha-32P]ATP, [alpha-32P]GTP, [32P]cAMP, and [32P]cGMP, and their use in the assay of adenylate and guanylate cyclases and cyclic nucleotide phosphodiesterases. Adv Cyclic Nucleotide Res. 1979;10:135–167. [PubMed] [Google Scholar]
- Kaufmann Y., Zamir A. The effect of tRNA derivatives bound with natural or synthetic mRNA on the interaction of Escherichia coli ribosomes with colicin E3. Eur J Biochem. 1975 May 6;53(2):599–603. doi: 10.1111/j.1432-1033.1975.tb04103.x. [DOI] [PubMed] [Google Scholar]
- Kim S. H., Sussman J. L., Suddath F. L., Quigley G. J., McPherson A., Wang A. H., Seeman N. C., RICH A. The general structure of transfer RNA molecules. Proc Natl Acad Sci U S A. 1974 Dec;71(12):4970–4974. doi: 10.1073/pnas.71.12.4970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug A., Robertus J. D., Ladner J. E., Brown R. S., Finch J. T. Conservation of the molecular structure of yeast phenylalanine transfer RNA in two crystal forms. Proc Natl Acad Sci U S A. 1974 Sep;71(9):3711–3715. doi: 10.1073/pnas.71.9.3711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mankin A. S., Skripkin E. A., Chichkova N. V., Kopylov A. M., Bogdanov A. A. An enzymatic approach for localization of oligodeoxyribonucleotide binding sites on RNA. Application to studying rRNA topography. FEBS Lett. 1981 Aug 31;131(2):253–256. doi: 10.1016/0014-5793(81)80378-x. [DOI] [PubMed] [Google Scholar]
- McCoy M. I., Lubben T. H., Gumport R. I. The purification of nuclease-free T4-RNA ligase. Biochim Biophys Acta. 1979 Mar 28;562(1):149–161. doi: 10.1016/0005-2787(79)90134-5. [DOI] [PubMed] [Google Scholar]
- Moras D., Comarmond M. B., Fischer J., Weiss R., Thierry J. C., Ebel J. P., Giegé R. Crystal structure of yeast tRNAAsp. Nature. 1980 Dec 25;288(5792):669–674. doi: 10.1038/288669a0. [DOI] [PubMed] [Google Scholar]
- Pace B., Matthews E. A., Johnson K. D., Cantor C. R., Pace N. R. Conserved 5S rRNA complement to tRNA is not required for protein synthesis. Proc Natl Acad Sci U S A. 1982 Jan;79(1):36–40. doi: 10.1073/pnas.79.1.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter D., Erdmann V. A., Sprinzl M. A new transfer RNA fragment reaction: Tp psi pCpGp bound to a ribosome-messenger RNA complex induces the synthesis of guanosine tetra- and pentaphosphates. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3226–3229. doi: 10.1073/pnas.71.8.3226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riehl N., Giegé R., Ebel J. P., Ehresmann B. Effect of elongation factor Tu on the conformation of phenylalanyl-tRNAPhe. FEBS Lett. 1983 Apr 5;154(1):42–46. doi: 10.1016/0014-5793(83)80871-0. [DOI] [PubMed] [Google Scholar]
- Sander G. Mechanism of action of colicin E3. Effect on ribosomal elongation-factor-dependent reactions. Eur J Biochem. 1977 May 16;75(2):523–531. doi: 10.1111/j.1432-1033.1977.tb11553.x. [DOI] [PubMed] [Google Scholar]
- Schneider D., Solfert R., von der Haar F. Large scale purification of tRNA ser , tRNA tyr and tRNA phe from Baker's yeast. Hoppe Seylers Z Physiol Chem. 1972 Aug;353(8):1330–1336. doi: 10.1515/bchm2.1972.353.2.1330. [DOI] [PubMed] [Google Scholar]
- Schwarz U., Menzel H. M., Gassen H. G. Codon-dependent rearrangement of the three-dimensional structure of phenylalanine tRNA, exposing the T-psi-C-G sequence for binding to the 50S ribosomal subunit. Biochemistry. 1976 Jun 1;15(11):2484–2490. doi: 10.1021/bi00656a035. [DOI] [PubMed] [Google Scholar]
- Sharp S., DeFranco D., Dingermann T., Farrell P., Söll D. Internal control regions for transcription of eukaryotic tRNA genes. Proc Natl Acad Sci U S A. 1981 Nov;78(11):6657–6661. doi: 10.1073/pnas.78.11.6657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprinzl M., Cramer F. The -C-C-A end of tRNA and its role in protein biosynthesis. Prog Nucleic Acid Res Mol Biol. 1979;22:1–69. doi: 10.1016/s0079-6603(08)60798-9. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Sternbach H., von der Haar F., Cramer F. Enzymatic incorporation of ATP and CTP analogues into the 3' end of tRNA. Eur J Biochem. 1977 Dec;81(3):579–589. doi: 10.1111/j.1432-1033.1977.tb11985.x. [DOI] [PubMed] [Google Scholar]
- Sprinzl M., Wagner T., Lorenz S., Erdmann V. A. Regions of tRNA important for binding to the ribosomal A and P sites. Biochemistry. 1976 Jul 13;15(14):3031–3039. doi: 10.1021/bi00659a015. [DOI] [PubMed] [Google Scholar]
- Van Knippenberg P. H., Van Kimmenade J. M., Heus H. A. Phylogeny of the conserved 3' terminal structure of the RNA of small ribosomal subunits. Nucleic Acids Res. 1984 Mar 26;12(6):2595–2604. doi: 10.1093/nar/12.6.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vournakis J. N., Celantano J., Finn M., Lockard R. E., Mitra T., Pavlakis G., Troutt A., van den Berg M., Wurst R. M. Sequence and structure analysis of end-labeled RNA with nucleases. Gene Amplif Anal. 1981;2:267–298. [PubMed] [Google Scholar]
- Wickstrom E. Nuclease mapping of the secondary structure of the 49-nucleotide 3' terminal cloacin fragment of Escherichia coli 16s RNA and its interactions with initiation factor 3. Nucleic Acids Res. 1983 Apr 11;11(7):2035–2052. doi: 10.1093/nar/11.7.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yarus M., Breeden L. Mutants of Su+7 tRNA include a functional tRNA with an altered T pseudo uracil CG sequence. Cell. 1981 Sep;25(3):815–823. doi: 10.1016/0092-8674(81)90189-6. [DOI] [PubMed] [Google Scholar]
- Zagorska L., Van Duin J., Noller H. F., Pace B., Johnson K. D., Pace N. R. The conserved 5 S rRNA complement to tRNA is not required for translation of natural mRNA. J Biol Chem. 1984 Mar 10;259(5):2798–2802. [PubMed] [Google Scholar]
- Zasloff M. tRNA transport from the nucleus in a eukaryotic cell: carrier-mediated translocation process. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6436–6440. doi: 10.1073/pnas.80.21.6436. [DOI] [PMC free article] [PubMed] [Google Scholar]