Abstract
We have previously developed a telomerase-specific replicating adenovirus expressing GFP (OBP-401), which can selectively label tumors in vivo with GFP. Intraperitoneal (i.p.) injection of OBP-401 specifically labeled peritoneal tumors with GFP, enabling fluorescence visualization of the disseminated disease and real-time fluorescence surgical navigation. However, the technical problems with removing all cancer cells still remain, even with fluorescence-guided surgery. In this study, we report imaging of tumor recurrence after fluorescence-guided surgery of tumors labeled in vivo with the telomerase-dependent, GFP-containing adenovirus OBP-401.. Recurrent tumor nodules brightly expressed GFP, indicating that initial OBP-401-GFP labeling of peritoneal disease was genetically stable, such that proliferating residual cancer cells still express GFP. In situ tumor labeling with a genetic reporter has important advantages over antibody and other non-genetic labeling of tumors, since residual disease remains labeled during recurrence and can be further resected under fluorescence guidance.
Key words: green fluorescent protein, adenovirus, cancer labeling, in situ, fluorescence-guided surgery, recurrence, detection
Introduction
Green fluorescent protein (GFP) serves as a very bright genetic reporter to detect metastatic cancer in mouse models.1–3 Initially, cancer cells were transduced in vitro with GFP using various types of genetic vectors and then implanted in mouse models. Potential clinical application of GFP became possible when it was demonstrated that retroviruses containing GFP could label disseminated cancer in situ in mouse models.4 Subsequently, selective in vivo GFP labeling of tumors was performed with OBP-401, a replicating adenovirus5,6 that contains a replication cassette with the human telomerase reverse transcriptase (hTERT) promoter driving the expression of the viral E1 genes and the inserted GFP gene. Virus replication and, hence, GFP gene expression occur only in the presence of an active telomerase, i.e., in malignant tissue.6 The OBP-401 virus was first tested by injection directly into HT-29 human colon tumors, orthotopically implanted into the rectum in BALB/c nu/nu mice. Subsequent para-aortic lymph node metastasis was observed by laparotomy under fluorescence.6 We then developed a major enhancement of cancer surgical navigation in orthotopic mouse models of cancer using in vivo selective fluorescent tumor labeling with OBP-401 GFP. Bright GFP fluorescence clearly illuminated the tumor boundaries and facilitated detection of the smallest disseminated disease lesions.7
Fluorescence-guided surgical navigation with tumors labeled in vivo with OAP-401 GFP was demonstrated in nude mouse models that represent difficult surgical challenges for the resection of widely disseminated cancer. HCT-116, a model of intraperitoneal disseminated human colon cancer, was labeled by virus injection into the peritoneal cavity. A549, a model of pleural dissemination of human lung cancer, was labeled by OBP-401 virus administered into the pleural cavity. Only the malignant tissue fluoresced brightly in both models. Further, we showed that OBP-401 could visualize liver metastases by tumor-specific expression of the GFP gene after portal venous or i.v. administration. Selective metastatic tumor labeling with GFP and killing by systemic administration of telomerase-dependent adenoviruses suggested that liver metastasis is also a candidate for fluorescence-guided surgery.8
However, even fluorescence-guided surgery may still result in residual disease. The present report demonstrates proliferating residual disease remains stably labeled with OBP-401 GFP, which is readily detected for further resection, suggesting that genetic-reporter labeling of tumors has advantages over non-genetic labeling of tumors for fluorescence-guided surgery.
Results and Discussion
Labeling peritoneal carcinomatosis with OBP-401-GFP.
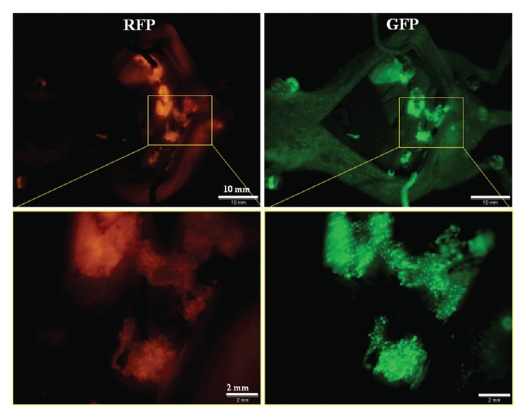
Peritoneal carcinomatosis was induced in the abdominal cavity of nude mice by i.p. implantation of HCT-116-RFP human colorectal cancer cells. Twelve days after implantation, 1 × 108 PFU OBP-401 were injected intraperitoneally. Disseminated HCT-116-RFP nodules expressed GFP fluorescence induced by OBP401 as well as the endogenous RFP fluorescence when imaged 5 d later (Fig. 1). RFP fluorescence was essentially coincident with that of GFP, indicating that i.p. injection of OBP-401 efficiently labeled disseminated tumors with GFP.
Figure 1.
In situ genetic labeling of disseminated peritoneal carcinomatosis. Red fluorescence indicates HCT-116-RFP-expressing disseminated nodules (left). Peritoneal disseminated HCT116-RFP cells were labeled by GFP after i.p. injection of OBP-401 (right). Fluorescence imaging revealed co-localization of red and green fluorescence.
Stability of OBP-401-GFP expression in tumors.
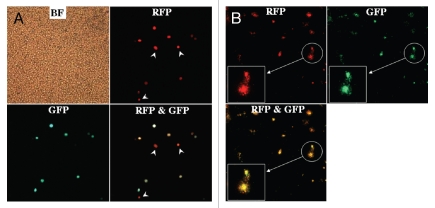
In order to determine stability of GFP expression in OBP-401-labeled tumors, HCT-116-RFP tumors were collected by peritoneal lavage from the abdominal cavity of mice 5 d after OBP-401 administration, put into culture in RPMI 1640 medium supplemented with 10% FBS and observed over time. Eight days after plating (13 d after viral administration), cancer cell colonies expressed both RFP and GFP (Fig. 2). The stability of GFP expression in OBP-401-labeled tumor cells suggests the potential of OBP-401 GFP labeling to detect recurrent tumors after attempted resection.
Figure 2.
Genetic labeling of microscopic tumors. Cells collected by peritoneal lavage from the abdominal cavity of mice 5 d after OBP-401 treatment were plated and cultured with RPMI 1640 medium supplemented with 10% FBS. (A) Plating cells in the peritoneal lavage fluid (5 d after viral administration). Most RFP-expressing cancer cells expressed GFP fluorescence induced by OBP-401 as well, x200 magnification. White arrows, cells unlabeled with GFP. (B) Eight days after plating (i.e., 13 d after viral administration). Cancer cell colonies expressing RFP were observed in the culture dish under fluorescence microscopy. The cancer cells also expressed GFP induced by OBP-401. x40 magnification. Boxes highlight colonies indicated by white circles. Original magnification x100.
Fluorescence-guided resection of disseminated peritoneal tumors labeled with OBP-401 GFP.
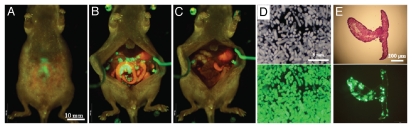
Five days after OBP-401 administration to mice with i.p. HCT-116, laparotomy was performed with the intent to remove all the intra-abdominal cancer using fluorescence-guided navigation under ketamine anesthesia (Fig. 3A and B). OBP-401 labeling and imaging made disseminated cancer nodules visible by GFP fluorescence, and complete resection was attempted (Fig. 3C–E). Tumors were efficiently resected, including those not visible under bright light, as we have previously reported in references 7 and 8.
Figure 3.
Fluorescence-guided resection of tumors labeled with GFP in situ. (A) Peritoneal disseminated nodules were labeled by GFP expression 5 d after OBP-401 virus administration. (B) Laparotomy was performed. (C) Disseminated nodules labeled with GFP were removed under GFP-guided surgical navigation. (D) Disseminated nodules removed under GFP-guided navigation. Top, bright field observation; Bottom, fluorescent detection. (E) Section of disseminated nodules. Top, H&E section; Bottom, frozen section with fluorescence detection.
In vivo detection of recurrent OBP-402-GFP-labeled tumors after fluorescence-guided surgery.
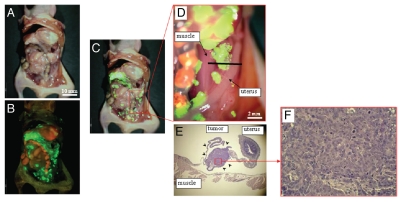
Tumors still recurred after attempted complete resection with fluorescence-guided surgery as visualized by GFP expression (Fig. 4). This result demonstrates that OBP-401 GFP labeling of peritoneal disseminated disease enables detection of tumor recurrence after fluorescence-guided surgery. Thus, OBP-401-GFP labeling is genetically stable, and therefore, proliferating residual disease continues to express GFP.
Figure 4.
In vivo detection of recurrent tumors after fluorescence-guided surgery. (A) Brightfield observation several weeks after fluorescence-guided surgery of OBP-401 GFP-labeled tumors. Disseminated disease re-emerged. (B) Fluorescence observation of field observed by brightfield in (A). (C) Merge of (A and B). The red box outlines a region of (D) below. (D) Detail of the boxed region of (C). Black line indicates the direction of cross-sections. (E) Histologic sections stained with H&E showing that GFP-labeled lesions are recurrent tumor tissues (arrow heads). x40 magnification. (F) Detail of the boxed region of (E). x200 magnification.
Tsien's laboratory has developed a method to label and visualize tumors during surgery using activatable cell-penetrating pep-tides (ACPPs), in which the fluorescently labeled, polycationic cell-penetrating peptide (CPP) is coupled via a cleavable linker to a neutralizing peptide. Upon exposure to proteases expressed by tumors, the linker is cleaved, dissociating the inhibitory peptide and allowing the CPP to bind to and enter tumor cells. Animals whose tumors were resected with ACPPD guidance had better long-term tumor-free survival and overall survival than animals whose tumors were resected with traditional brightfield illumination only.9
Another approach to tumor labeling and fluorescence-guided surgery is with the use of labeled tumor-specific antibodies. A monoclonal antibody specific for CA19-9 was conjugated to a green fluorophore and delivered to tumor-bearing mice as a single intravenous (IV) dose. Intravital fluorescence imaging was used to localize metastatic pancreatic cancer in orthotopic mouse models 24 h after antibody administration. Using fluorescence imaging, the primary tumor was clearly visible at laparotomy as were small metastases in the liver and spleen and on the peritoneum. The metastatic tumors, which were nearly impossible to see using standard brightfield imaging, demonstrated clear fluorescence under LED light excitation.10
We have also previously investigated the use of fluorophorelabeled anti-carcinoembryonic antigen (CEA) monoclonal antibody to aid in cancer visualization in nude mouse models of human colorectal and pancreatic cancer. Anti-CEA was conjugated with a green fluorophore. Subcutaneous, orthotopic primary and metastatic human pancreatic and colorectal tumors were easily visualized with fluorescence imaging after administration of conjugated anti-CEA. The fluorescence signal was detectable 30 min after systemic antibody delivery and remained present for 2 weeks, with minimal in vivo photobleaching after exposure to standard operating room lighting. Fluorescent anti-CEA administration improved ability to resect the labeled tumors under fluorescence guidance.11
Neither the ACPP nor the labeled monoclonal antibodies described above involves genetic labeling of cancer cells, and recurrence would, therefore, not be detectable. In the present study, we selectively and efficiently labeled tumors with a genetic reporter, GFP, using a telomerase-dependent adenovirus OBP-401. We demonstrated that tumors recurred after fluorescence-guided surgery and maintained GFP expression. Therefore, the detection of recurrence and future metastasis is possible with OBP-401 GFP labeling, since recurrent cancer cells stably express GFP, which is not possible with non-genetic labeling of tumors.
In clinical studies performed with OBP-401, circulating tumor cells (CTC) obtained from cancer patients were labeled with OBP-401 GFP ex vivo. OBP-401-GFP labeling greatly increased the detection of CTC.12 Other targets for in vivo GFP labeling could include, for example, breast cancer emboli.13 Specific labeling by GFP of cancer stem cells is also a promising approach.14 Labeling of cancer stem cells is especially important, since at least some stem cells can now be imaged non-invasively.15 The present report suggests the clinical potential of OBP-401 GFP labeling to improve the surgical outcome of cancer.
Materials and Methods
Recombinant adenovirus.
Telomerase-specific replication-selective adenovirus OBP-401, containing the GFP gene under the control of the CMV promoter, with the hTERT promoter driving the E1A and E1B genes, was constructed and produced as previously described in references 5 and 6.
Cell culture.
The human colorectal cancer cell line HCT-116 was cultured in RPMI 1640 medium supplemented with 10% FBS.
Production of red fluorescent protein (RFP) retroviral vector.
For RFP retrovirus production, the HindIII/NotI fragment from pDsRed2 (Clontech) containing the full-length RFP cDNA, was inserted into the HindIII/NotI site of pLNCX2 (Clontech) containing the neomycin-resistance gene. PT67, an NIH3T3-derived packaging cell line (Clontech) expressing the viral envelope, was cultured in DMEM supplemented with 10% FBS. For vector production, PT67 packaging cells, at 70% confluence, were incubated with a precipitated mixture of LipofectAMINE reagent (Life Technologies) and saturating amounts of pLNCX2-DsRed2 plasmid for 18 h. Fresh medium was replenished at this time. The cells were examined by fluorescence microscopy 48 h post-transduction. For selection of a clone producing high amounts of RFP retroviral vector (PT67-DsRed2), the cells were cultured in the presence of 200 to 1,000 µg/ml G418 (Life Technologies) for 7 d. The isolated packaging cell clone was termed PT67-DSRed2.16
RFP gene transduction of cancer cells.
For RFP gene transduction, cancer cells were incubated with a 1:1 precipitated mixture of retroviral supernatants of PT67 cells and RPMI 1640 containing 10% FBS for 72 h. Fresh medium was replenished at this time. Tumor cells were harvested with trypsin/EDTA 72 h post-transduction and subcultured at a ratio of 1:15 into selective medium that contained 200 µg/ml G418. To select brightly fluorescent cells, the level of G418 was increased up to 800 µg/ml in a stepwise manner. RFP-expressing cancer cells were isolated with cloning cylinders using trypsin/EDTA and were amplified by conventional culture methods in the absence of selective agent.16
Mice.
Athymic nude mice were kept in a barrier facility under HEPA filtration and fed with autoclaved laboratory rodent diet. All animal studies were conducted in accordance with the principals and procedures outlined in the National Institute of Health Guide for the Care and Use of Laboratory Animals under Assurance Number A3873-1. All animal procedures were performed under anesthesia using s.c. administration of a ketamine mixture (10 µl ketamine HCL, 7.6 µl xylazine, 2.4 µl acepromazine maleate and 10 µl PBS).
In vivo fluorescence imaging.
An Olympus OV100 Small Animal Imaging System (Olympus Corp., Tokyo, Japan) with macro- and micro-optics was used.17 High-resolution images directly captured on a PC were processed and analyzed with the use of Adobe Photoshop Elements 4.0 software (Adobe).
Peritoneal carcinomatosis model with HCT-116 human colon cancer cells implanted in nude mice.
Nude mice were intraperitoneally (i.p.) injected either with HCT-116 or HCT-116-RFP human colon cancer cells at a density of 3 × 106 in 200 µl PBS. Twelve days after tumor cell inoculation, mice were injected i.p. with OBP-401 at a dose of 1 × 108 PFU in 200 µl PBS. Five days after virus injection, the abdominal cavity was examined by fluorescence imaging, and mice were operated on with fluorescence guidance with the intent to resect all intraabdominal tumor nodules under ketamine-induced anesthesia.
Collection of microscopic tumors from peritoneal lavage fluid of OBP-401-treated mice.
Twelve days after nude mice were i.p. injected with HCT-116-RFP, 1 × 108 PFU OBP-401 were injected intraperitoneally. Five days after virus injection, mice were instilled with 8 ml PBS intraperitoneally. The abdomen was gently massaged, and the peritoneal fluid was carefully aspirated using a 22-gauge needle. Approximately 6 ml peritoneal lavage fluid (PLF) were obtained from most mice. After filtering the PLF with a 40 µm cell strainer (BD, Franklin Lakes, NJ) in order to collect only microscopic tumors and/or cancer cells in the abdominal cavity, 3 ml of PLF were cultured on 6-well tissue culture plates. After incubation for 1 h, supernatants were carefully aspirated and 3 ml RPMI 1640 medium, containing 10% FBS, were added to each well. The cells were further incubated at 37°C in a humidified atmosphere of 5% CO2 and observed under fluorescence microscopy at day 0 and day 8 after collection of PLF.
Acknowledgments
This study was supported in part by National Cancer Institute grant CA132971 and CA142669.
References
- 1.Chishima T, Miyagi Y, Wang X, Yamaoka H, Shimada H, Moossa AR, et al. Cancer invasion and micrometastasis visualized in live tissue by green fluorescent protein expression. Cancer Res. 1997;57:2042–2047. [PubMed] [Google Scholar]
- 2.Yang M, Baranov E, Jiang P, Sun FX, Li XM, Li L, et al. Whole-body optical imaging of green fluorescent protein-expressing tumors and metastases. Proc Natl Acad Sci USA. 2000;97:1206–1211. doi: 10.1073/pnas.97.3.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffman RM. The multiple uses of fluorescent proteins to visualize cancer in vivo. Nat Rev Cancer. 2005;5:796–806. doi: 10.1038/nrc1717. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa S, Yang M, Chishima T, Miyagi Y, Shimada H, Moossa AR, et al. In vivo tumor delivery of the green fluorescent protein gene to report future occurrence of metastasis. Cancer Gene Ther. 2000;7:1336–1340. doi: 10.1038/sj.cgt.0237. [DOI] [PubMed] [Google Scholar]
- 5.Fujiwara T, Kagawa S, Kishimoto H, Endo Y, Hioki M, Ikeda Y, et al. Enhanced antitumor efficacy of telomerase-selective oncolytic adenoviral agent OBP-401 with docetaxel: preclinical evaluation of chemovirotherapy. Int J Cancer. 2006;119:432–440. doi: 10.1002/ijc.21846. [DOI] [PubMed] [Google Scholar]
- 6.Kishimoto H, Kojima T, Watanabe Y, Kagawa S, Fujiwara T, Uno F, et al. In vivo imaging of lymph node metastasis with telomerase-specific replication-selective adenovirus. Nat Med. 2006;12:1213–1219. doi: 10.1038/nm1404. [DOI] [PubMed] [Google Scholar]
- 7.Kishimoto H, Zhao M, Hayashi K, Urata Y, Tanaka N, Fujiwara T, et al. In vivo internal tumor illumination by telomerase-dependent adenoviral GFP for precise surgical navigation. Proc Natl Acad Sci USA. 2009;106:14514–14517. doi: 10.1073/pnas.0906388106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kishimoto H, Urata Y, Tanaka N, Fujiwara T, Hoffman RM. Selective metastatic tumor labeling with green fluorescent protein and killing by systemic administration of telomerase-dependent adenoviruses. Mol Cancer Ther. 2009;8:3001–3008. doi: 10.1158/1535-7163.MCT-09-0556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nguyen QT, Olson ES, Aquilera TA, Jiang T, Scadeng M, Ellies LG, et al. Surgery with molecular fluorescence imaging using activatable cell-penetrating peptides decreases residual cancer and improves survival. Proc Natl Acad Sci USA. 2010;107:4317–4322. doi: 10.1073/pnas.0910261107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McElroy M, Kaushal S, Luiken G, Talamini MA, Moossa AR, Hoffman RM, et al. Imaging of primary and metastatic pancreatic cancer using a fluorophore-conjugated anti-CA19-9 antibody for surgical navigation. World J Surg. 2008;32:1057–1066. doi: 10.1007/s00268-007-9452-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kaushal S, McElroy MK, Luiken GA, Talamini MA, Moossa AR, Hoffman RM, et al. Fluorophoreconjugated anti-CEA antibody for the intraoperative imaging of pancreatic and colorectal cancer. J Gastrointest Surg. 2008;12:1938–1950. doi: 10.1007/s11605-008-0581-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kojima T, Hashimoto Y, Watanabe Y, Kagawa S, Uno F, Kuroda S, et al. A simple biological imaging system for detecting viable human circulating tumor cells. J Clin Invest. 2009;119:3172–3181. doi: 10.1172/JCI38609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahooti S, Porter K, Alpaugh ML, Ye Y, Xiao Y, Jones S, et al. Breast carcinomatous tumoral emboli can result from encircling lymphovasculogenesis rather than lymphovascular invasion. Oncotarget. 2010;1:131–147. doi: 10.18632/oncotarget.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Runck LA, Kramer M, Ciraolo G, Lewis AG, Guasch G. Identification of epithelial label-retaining cells at the transition between the anal canal and the rectum in mice. Cell Cycle. 2010;9:3039–3045. doi: 10.4161/cc.9.15.12437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uchugonova A, Hoffman RM, Weinigel M, Koenig K. Watching stem cells in the skin of living mice noninvasively. Cell Cycle. 2011;10:2017–2020. doi: 10.4161/cc.10.12.15895. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto N, Yang M, Jiang P, Xu M, Tsuchiya H, Tomita K, et al. Real-time imaging of individual fluorescent-protein color-coded metastatic colonies in vivo. Clin Exp Metastasis. 2003;20:633–638. doi: 10.1023/A:1027311230474. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi K, Yang M, Jiang P, Xu M, Yamamoto N, Tsuchiya H, et al. Development of real-time subcellular dynamic multicolor imaging of cancer-cell trafficking in live mice with a variable-magnification whole mouse imaging system. Cancer Res. 2006;66:4208–4214. doi: 10.1158/0008-5472.CAN-05-3927. [DOI] [PubMed] [Google Scholar]