Abstract
It is a well-established fact that the tRNA genes in yeast can function as chromatin barrier elements. However, so far there is no experimental evidence that tRNA and other Pol III-transcribed genes exhibit barrier activity in mammals. This study utilizes a recently developed reporter gene assay to test a set of Pol III-transcribed genes and gene clusters with variable promoter and intergenic regions for their ability to prevent heterochromatin-mediated reporter gene silencing in mouse cells. The results show that functional copies of mouse tRNA genes are effective barrier elements. The number of tRNA genes as well as their orientation influence barrier function. Furthermore, the DNA sequence composition of intervening and flanking regions affects barrier activity of tRNA genes. Barrier activity was maintained for much longer time when the intervening and flanking regions of tRNA genes were replaced by AT-rich sequences, suggesting a negative role of DNA methylation in the establishment of a functional barrier. Thus, our results suggest that tRNA genes are essential elements in establishment and maintenance of chromatin domain architecture in mammalian cells.
Key words: barrier elements, tRNA genes, Pol III-transcribed genes
Introduction
RNA polymerase III (Pol III) transcribes genes involved in diverse cellular functions, including translation (5S rRNA, tRNA), splicing (snRNAs) and signal recognition (7SL).1 In mammals, through SINE activity, Pol III has also played a major role in genome evolution.2,3 Pol III-transcribed human Alu and murine B2 SINEs can be co-regulated with nearby genes, possibly providing an additional level of control of gene activity.4–6 In yeast, tRNA genes prevent the spread of heterochromatin at the mating type locus and centromere repeats, and orphan B boxes associate with the Pol III subunit TFIIIC to create chromosome-organizing clamps.7–10
The structure of Pol III promoter elements varies with a particular gene type. In type I and II, conserved basal promoter elements (i.e., A, B and C boxes) are located within the transcription unit. A and B boxes are found in tRNA genes and related SINEs; A and C boxes are found in RN5S (5S) genes (see Fig. 1B). The B box interacts with TFIIIC and sets up a platform for association of TFIIIB and Pol III.11 The 11 bp B box is conserved throughout eukaryotes: four nucleotides are invariant and essential, while the remaining residues tolerate limited base changes.12,13 In the tRNA secondary structure, the B box forms a portion of the cloverleaf T-loop domain. Thus, sequence variation in the B box in tRNA genes is restricted by dual functional constraints.
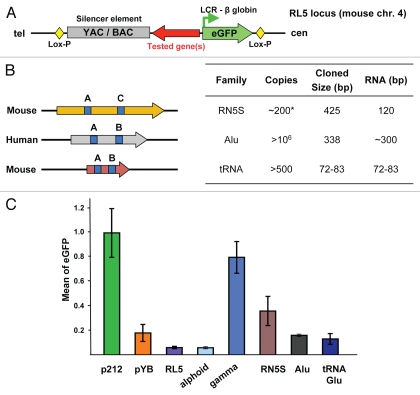
Figure 1.
(A) Schematic diagram of the eGFP reporter gene cassette for targeting a candidate barrier element fragment into the RL5 ectopic site in mouse MEL cells. The red arrow indicates direction of transcription of Pol III-transcribed genes tested for barrier activity. Size of the YAC/BAC silencer element is ∼8 kb, LCR/eGFP transgene is ∼1.4 kb, loxP sequence is 33 bp. The YAC/BAC silencer element is a sequence with a high GC content and 417 CpGs. (B) Structure and genome-wide copy number of interspersed Pol III-transcribed genes incorporated into the cassette shown in (A). Size data refers to that used in constructs in this study. Copy number estimates refer to the mouse haploid genome for RN5S (5S) and to the human genome for Alu and tRNA genes. Estimate does not include pseudogenes. RN5S copy number in clustered tandem arrays is highly variable; hundreds of consensus and 5S-related sequences are interspersed in the human and mouse genome.16,38 A, B and C boxes are essential promoter elements. (C) Mean eGFP fluorescence of cassettes tested for anti-silencing/barrier activity four weeks post-transfection: p212, no silencer element; pYB, with silencer element; a mouse RN5S (5S) gene; a human Alu element; a single mouse tRNA (Glu) gene. RL5 corresponds to fluorescence background of untransfected cells. Human γ- and α-satellite DNAs were used as positive and negative controls for barrier activity.34
Besides basal promoter elements, the factors determining the efficiency of transcription of Pol III genes are not entirely understood. Some SINEs are more transcriptionally-active than others, which may be due to recent expansion. Similarly, tRNA genes in mammalian cells are transcribed with different efficiencies.13–18 Sequences outside of the transcription unit and promoter of tRNA and RN5S (5S) genes appear to influence transcription efficiency despite relatively low levels of sequence conservation in the flanking regions of Pol III-transcribed genes.11 Some Pol III-transcribed genes have upstream TATA boxes that facilitate recruitment of TFIIIB.19
Mammalian genomes carry a large number of interspersed Pol III-dependent genes. For example, the haploid human genome contains > 500 intact tRNA genes, ∼1,000 tRNA pseudogenes, > 1,000 interspersed RN5S (5S) related genes16 and more than a 1,000,000 Alus and MIRs, although most MIRs are degenerated and most Alus are inactive.20–22 By analogy to families of mobile elements that have evolved to perform diverse functions in their host genomes,23–26 it is possible that Pol III-transcribed genes have adopted novel genomic functions in mammalian genomes, one of which may be to help to establish and maintain chromosome domain boundaries by barrier activity.
Barrier elements play an important role in genome function. Without these elements, flanking chromosome domains can induce long-range changes in chromatin structure that can alter gene expression with adverse consequences to the organism.27 The Drosophila Gypsy insulator and the chicken β-globin HS4 fragment (cHS4) are classic barrier elements that include binding sites for Su(Hw) or USF/CTCF, respectively. These binding sites are believed to tether the flanking chromosome region to the nuclear membrane or nuclear matrix.28 Such tethering could sterically restrict access to a chromatin region, thus isolating and protecting the region by a mechanism that may be independent of transcription. It has been also demonstrated that barrier elements result from nucleosome-depletion or nucleosome exclusion because of a bias in nucleotide composition.29 The number, categories and distribution of barrier elements in different genomes is not well-characterized, but given their proposed importance in maintaining chromosome architecture and appropriate transcriptional patterns throughout the genome, it is likely that barrier elements are quite abundant and of variable constitution.30–32 For example, several thousand ETCs (extra TFIIIC sites or orphan B boxes) loci have been identified in the human genome.13,15,16 By analogy to their demonstrated properties in yeast,8–10,33 mammalian ETCs as well as tRNA genes may also act as barrier elements and regulate local chromatin structure.
Recent studies demonstrated apparent overlap in the chromosomal distribution of Pol III- and Pol II-transcribed genes in human cells, suggesting functional interplay between the Pol II and Pol III transcriptional machineries.13,15,16,18 Strikingly, transcriptionally active Pol III genes and Pol II promoter regions share similar epigenetic signatures that are not present in transcriptionally-inactive Pol III genes. This suggests that Pol II occupancy may enable Pol III occupancy, or, conversely, active transcription by Pol III promotes a chromatin environment that is permissive for Pol II recruitment. In other words, as suggested above and previously described in yeast, Pol III genes may act as barrier elements that prevent heterochromatin spreading and maintain an open, transcriptionally permissive chromatin structure in mammalian cells.
Herein, we ask whether tRNA genes can function as barrier elements in the mouse genome. For this purpose, we exploited a recently developed reporter gene assay for a barrier element function in mouse MEL cells34 and tested a series of Pol III-transcribed genes and tRNA gene clusters with variable promoter structures and intergenic regions for ability to prevent heterochromatinmediated reporter gene silencing at the ectopic RL5 site.35,36 The results are consistent with the hypothesis that mammalian tRNA genes may protect a local chromatin structure against a distal silencing activity, as has been observed previously in yeast.
Results
An experimental system for studying barrier activity of Pol III-transcribed genes.
A previously described experimental system for targeting transgene cassettes to the RL5 site in murine erthryoid leukemia (MEL) cells35,36 was adapted by us to detect barrier activity of mammalian genomic DNA repeats.34 Using this system, DNA fragments with potential barrier activity were inserted between an eGFP reporter gene and a heterochromatin-forming bacterial/yeast artificial chromosome vector sequence (YAC/BAC) with silencing activity (Fig. 1A). The reporter gene in this cassette is under control of the human β-globin locus control region (LCR) and the β-globin promoter, which, in the absence of a silencer element, achieves and maintains a constitutive high-level transcriptional activity of the eGFP reporter at the RL5 ectopic site for many months in culture (p212, Fig. 1C). However, when the cassette carries the upstream silencer element, the reporter gene is strongly and stably repressed (pYB, Fig. 1C), and the repressed state is associated with accumulation of H3K9me3.34 The silencer element in this cassette has several regions of high CpG density; these regions and/or cryptic transcription may nucleate heterochromatin and induce gene silencing.
In our initial study, three Pol III-transcribed genes were cloned into the transgene cassette and inserted into RL5: a complete, single mouse tRNA(Glu) gene derived from a cluster on chr1:173.0 Mb; a consensus-matched mouse RN5S (5S) gene and a recently active human Alu repeat (Yb lineage) (Fig. 1B). Because the promoter elements of these genes are identical with the Pol III promoter consensus sequences,37–39 all three genes are expected to be transcriptionally competent. The genes were placed in the same orientation at RL5. The gene-containing constructs, positive (p212) and negative (pYB) control constructs (i.e., with and without a silencer element) were transfected into MEL cells, and gancyclovir-resistant clones were selected. Correct insertions into the same RL5 ectopic site were confirmed by PCR using specific primers as previously described in reference 34. Reporter gene activity was quantified by measuring eGFP fluorescence. As positive and negative controls for barrier activity, γ- and α-satellite DNAs were inserted between the silencer element and eGFP.34 At four weeks post-transfection, reporter gene activity was moderately increased when the cassette carried the RN5S gene (Fig. 1C). However, the effect of RN5S was not so strong as for γ-satellite DNA. A single tRNA gene and an Alu element have relatively weak, if any, barrier activity in this context (Fig. 1C). However, this does not exclude that tRNA genes in mouse cells have barrier activity, because not all single tRNA genes function as barrier elements in yeast.7 While anti-silencing activity of the RN5S gene deserves further analysis, due to recent findings that RN5S genes may function as origins of replication (Larionov V, unpublished data), and that origins of replication protect trans-gene silencing,40 in this work we have focused on the tRNA genes.
Protection of a reporter gene by multiple tRNA genes.
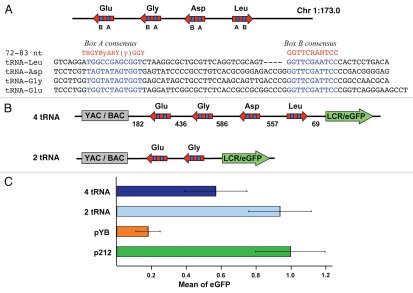
In yeast, tDNA becomes a robust insulator when part of a cluster.7 Therefore, cassettes with two and four tRNA genes were constructed and tested at RL5 to determine whether such repeats have a stronger barrier activity. The cluster of tRNA genes coding for Leu, Asp, Gly and Glu was PCR-amplified from murine chr1:173.0 Mb locus as a 2 kb fragment and cloned into pYB vector (Fig. 2A and B). These four genes contain the internal promoter consensus A and B boxes and are predicted to be competent for transcription and for forming a cloverleaf secondary structure in the tRNA gene product.37,41 The individual tRNA genes in this cluster are separated by 400–500 bp of intervening sequence (IVS) and the PCR product includes 182 and 69 bp of left- and right-flanking endogenous DNA, respectively (4 tRNA, Fig. 2B). A two copies derivative of this cassette was also constructed that carries only the Gly and Glu tRNA genes (2 tRNA, Fig. 2B). A eGFP reporter gene assay indicated that the four and two copies tRNA gene clusters conferred significantly higher expression than the single tRNA(Glu) gene after four weeks in culture (Fig. 2C). A weaker performance of the four copies cluster, Leu + Asp + Gly + Glu, in de-repressing the eGFP reporter gene could be explained by the transcriptional interference between the tRNA(Leu) gene and the eGFP transgene.42 This tRNA gene, present in the four but not in the two gene cluster, is transcribed toward the LCR, opposite to the other three tRNA genes (Fig. 2B).
Figure 2.
(A) Description of the 2 kb tRNA cluster from murine chr 1:173.0 Mb locus. Three identical copies of this cluster are present in the mouse genome. B and A boxes in each tRNA is highlighted in blue. Consensus A and B boxes are highlighted in red. Red arrows show relative transcriptional orientation of tRNA genes in the mouse genome. There are 222 CpG dinucleotides in the 2 kb fragment (GC = 68%), 22 of them localized in tRNAs. (B) Black lines between or outside of four and two tRNA genes indicate native intervening sequence, IVS or end endogenous genomic flanking DNA (in bp). The red arrows indicate direction of transcription of tRNA genes toward eGFP transgene. (C) Mean eGFP fluorescence of cassettes tested for anti-silencing/barrier activity four weeks post-transfection. pYB with silencer element, no gene inserted; p212, no silencer element; a two tRNA genes cluster (2 tRNA); a four tRNA genes cluster (4 tRNA).
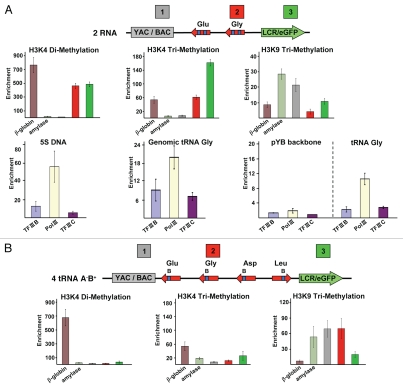
The presence of three identical copies of the Leu + Asp + Gly + Glu cluster in the mouse genome makes it impossible to distinguish tRNA transcripts from the cassette at RL5 from those derived from tRNA genomic loci. We examined histone modification profiles and occupancy of the tDNA cassette by transcription machinery at RL5 by a chromatin immunoprecipitation (ChIP) assay. To distinguish the tDNA cassette at RL5 from the endogenous (genomic) tDNA clusters, a special pair of primers was used for ChIP analysis, one of which was designed from a vector sequence present in the cassette (Table S1). At the tRNA(Gly) gene sequence, the histones have strongly enriched signals for H3K4me2 and H3K4me3 (Fig. 3A; probe 2). Similar enrichments for H3K4me2 and H3K4me3, markers for transcriptionally active chromatin state and open chromatin, were observed in the eGFP transgene (Fig. 3A; probe 3). H3K9me3, a marker for silent chromatin, was associated with the vector YAC/BAC DNA sequence (Fig. 3A; probe 1). This is in agreement with spreading of heterochromatin from the vector DNA.34 These results suggest that tRNA genes allow a transcriptionally permissive chromatin conformation in adjacent transgene sequences by arresting the spreading of transcriptionally inactive chromatin from the vector sequence. Moreover, the observed pattern of histone modifications is very similar to that described for active tRNA genes,15 suggesting that tRNA genes in the 2 tRNA construct are also transcribed. This hypothesis was checked in more direct experiments. Figure 3A summarizes ChIP data for cells carrying the tRNA construct using antibodies to TFIIIB (Brf1), TFIIIC or RNA Pol III and probes for the tRNA(Gly), the pYB vector sequence (negative control) or an endogenous chromosomal copy of the same tRNA gene (positive control) or endogenous 5S DNA (positive control). ChIP data demonstrate a significant enrichment of RNA Pol III and lower but detectable enrichment of TFIIIC and TFIIIB in close proximity to the tRNA(Gly) gene, indicating occupancy with Pol III machinery at RL5. (A relatively low enrichment with mouse chromatin compared with that observed with human chromatin43 is due to the fact that these antibodies were raised against human Pol III). To summarize, our results demonstrate that the tRNA gene cluster that recruits the Pol III holoenzyme complex functions as a barrier element.
Figure 3.
ChIP analysis of tRNA gene cassettes inserted into the RL5 locus. (A) ChIP analysis of lysine 4-dimethylated and trimethylated histone H3 (H3K4me2 and H3K4me3) and lysine 9-trimethylated histone H3 (H3K9me3) with the cassette carrying two tRNA genes. Positions of primers for the cassettes are as follows: (1) YAC/BAC vector backbone; (2) the tRNA(Gly) gene; (3) eGFP coding region. ChIP analysis was performed using antibodies for Brf1/TFIIIB, TFIIIC and RNA Pol III with the cassette carrying two tRNA genes. The endogenous genomic 5S RNA genes as well as genomic copies of the tRNA Gly gene on chromosome 1 that can be amplified using a specific pair of primers (Table S1) were included as positive controls. (B) ChIP analysis of H3K4me2, H3K4me3 and H3K9me3 with the cassette carrying a cluster of 4 tRNA genes with A boxes deleted. Sequences of all primers as well as for two control loci, the murine β-major globin locus and the murine amylase locus, are presented in Table S1.
tRnA genes with AT-rich intervening sequences (IVS) provide prolonged barrier activity.
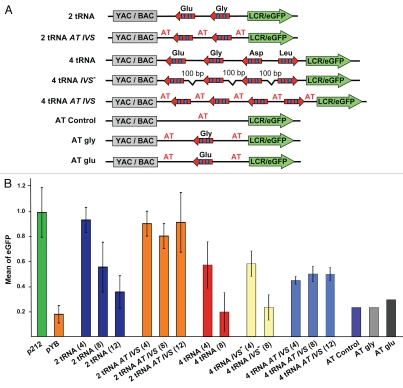
In the endogenous four tRNA gene cluster, the tRNA coding and promoter regions comprise only ∼15% of the total 2 kb fragment. The remaining 85% of the cloned mouse DNA segment is GC-rich, with 68% GC content and >200 CpG dinucleotides lying within the three intergenic (1,580 total bp) and two flanking DNA regions (251 total bp) (Fig. 2B). When the intergenic DNA was reduced to three segments of approximately 100 bp, barrier efficiency was not reduced, with eGFP expression level similar to the entire four tRNA genes cassette (4 tRNA vs. 4 tRNA IVS-, Fig. 4A and B). These results show that the barrier activity does not depend on the IVS length.
Figure 4.
Dependence of tRNA barrier activity on length and content of flanking and intergenic DNA. (A) Schematic of the constructs: 2 tRNA; 4 tRNA; 4 tRNA IVS (Four tRNA genes with deletion of 80% of native IVS, leaving a residual 100 bp GC-rich DNA; 4 tRNA AT-IVS and 2 tRNA AT-IVS (with switching out GC-rich intervening (IVS) and flanking sequences for AT-rich DNA in 4 tRNA and 2 tRNA constructs). The same AT-rich DNA was used for all AT variants. The AT control construct is made up of AT IVS variants, AT-2, AT-1, AT-R (∼300 bp) present in AT intervening sequences and flanks of the constructs: 4 tRNA AT-IVS, 2 tRNA AT-IVS, AT glu and AT gly. AT gly and AT glu constructs represent a single tRNA gene with AT-rich flanking sequences. (B) eGFP fluorescence of cells carrying cassettes with two or four tRNA genes without and with reduced intervening sequences as well as with AT-rich intervening sequences or flanks only was measured after four, eight and 12 weeks post-transfection. eGFP fluorescence of cells carrying single tRNA genes with AT flanks was also analyzed. p212 (after eight weeks post-transfection) was used as a positive control (no silencer element). pYB (after four weeks post-transfection) was used as a negative control (no barrier element).
The entire four tRNA genes cluster protects eGFP reporter gene expression against silencing, but the barrier effect was mostly eliminated by 8 weeks post-transfection (4 tRNA, Fig. 4B). Similar results were observed for the two tRNA genes construct with a longer time frame (2 tRNA, Fig. 4B) and for the 4 tRNA IVS- construct, which lacks most but not all of the GC-rich intergenic/flanking DNA (4 tRNA IVS-, Fig. 4b). A plausible explanation may be that the large number of CpG sites in the IVS sequences facilitate heterochromatization and silencing of the reporter gene, and that the same process silences the reporter gene even when the IVS is truncated to ∼100 bp. Therefore, the cassette was constructed so that the intergenic and flanking sequences in the four tRNA genes cluster were completely substituted with approximately 100 bp AT-rich/CpG-poor DNA (4 tRNA AT-IVS, Fig. 4A and Table S1). As a result, the number of CpG dinucleotides decreased to 28. Reporter gene assays showed that this construct and the intact four tRNA genes cluster protected eGFP expression to a similar extent at 4 weeks post-transfection (4 tRNA vs. 4 tRNA AT-IVS, Fig. 4B). However, protection by the 4tRNA AT-IVS construct was maintained at the same level for at least 12 weeks and did not gradually decrease over time as observed for the constructs with a native IVS (Fig. 4b). Importantly, a construct containing just ∼300 bp of AT-rich sequence without tRNA sequence completely failed to protect eGFP expression from silencing (AT control, Fig. 4A and B); therefore, AT-rich sequences have no intrinsic short- or long-term barrier activity in and of themselves in this assay. A stable eGFP expression was observed at an even higher level, close to the unsilenced level of the p212-positive control even after 12 weeks post-transfecion, when native IVS sequences were substituted by AT-rich IVS in the two tRNA genes construct (2 tRNA AT-IVS, Fig. 4A and B and Table S1).
Encouraged by the results obtained with 2 tRNA AT-IVS and 4 tRNA AT-IVS constructs, we analyzed a single copy tRNA gene (Glu or Gly) in which the native GC-rich flanking DNA sequences were replaced by AT-rich DNA. These cassettes (AT gly, AT glu) did not protect eGFP expression from silencing (Fig. 4A and B). Therefore, a relatively strong barrier activity of the 2 tRNA cassette, which carries one copy of the tRNA(Gly) and tRNA(Glu) genes, likely reflects a synergistic effect between the two Pol III transcription units, and that a threshold exists before significant barrier activity is detected in this system. Collectively, the data obtained with the tRNA genes constructs carrying synthetic AT-rich IVSs indicate importance of the genomic context for function of tRNA genes as barrier elements and suggest a negative effect of CpG methylation on barrier activity of tRNA genes in mammalian cells.
Barrier activity of orphan B boxes.
tRNA genes are classified as type II Pol III-transcribed genes, because their promoter regions that recruit the Pol III transcription machinery include two highly conserved essential motifs, boxes A and B, which lie within the tRNA coding region. In yeast, the B box appears to directly bind TFIIIC and possess barrier activity when amplified independent of the A box or other gene sequences.9,10,33 Nevertheless, in mouse cells, the four tRNA genes cluster, deleted for the A boxes but retaining the remainder of the coding sequences, including four B boxes and native IVS (4 tRNA A−B+, Fig. 5A), is an ineffective barrier element, resulting in a very low level of eGFP expression close to the level of the negative control construct (pYB vs. 4 tRNA A−B+, Fig. 5B). Thus, in our reporter assay system, box A in the endogenous tRNA gene cluster is essential for effective barrier activity. ChIP analysis did not reveal any enrichment for H3K4me2 and H3K4me3 in the 4 tRNA A−B+ sequence (Fig. 3B). Instead, H3K9me3 was associated with this sequence as well as with the vector sequence (Fig. 3B). ChIP analysis also did not reveal any enrichment of RNA Pol III in close proximity to the tRNA(Gly) gene, indicating that inactivation of the A boxes abolished gene transcription (data not shown).
Figure 5.
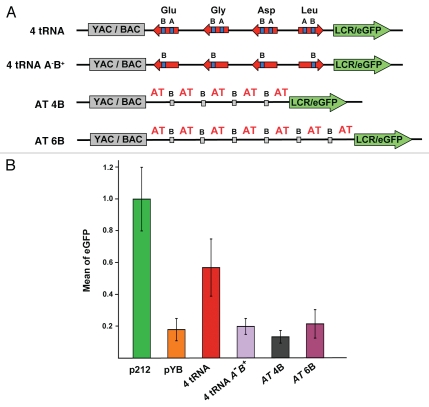
Analysis of tRNA constructs lacking A boxes. (A) Schematic of the constructs with four tRNA genes (4 tRNA); four tRNA genes lacking A boxes (4 tRNA A-B); four or six orphan B boxes within AT-rich DNA sequence (AT 4B, AT 6B). The A box deletion was created by replacement of seven conservative nucleotides in a consensus sequence. (B) eGFP fluorescence of cells carrying the cassettes with different constructs was measured after four or eight weeks post-transfection. p212 was used as a positive control (no silencer element). pYB was used as a negative control (no barrier element).
To investigate if multiple orphan B boxes surrounded by AT-rich flanking DNA have barrier activity in mouse cells, cassettes carrying four or six orphan B boxes were constructed and tested (AT 4B, AT 6B, Fig. 5A). None of them showed significant eGFP protection (Fig. 5B). Thus, clusters of B boxes, even in an AT-rich DNA surrounding, cannot provide barrier activity in our barrier activity assay.
Distribution of tRNA genes near protein-coding genes in the human genome.
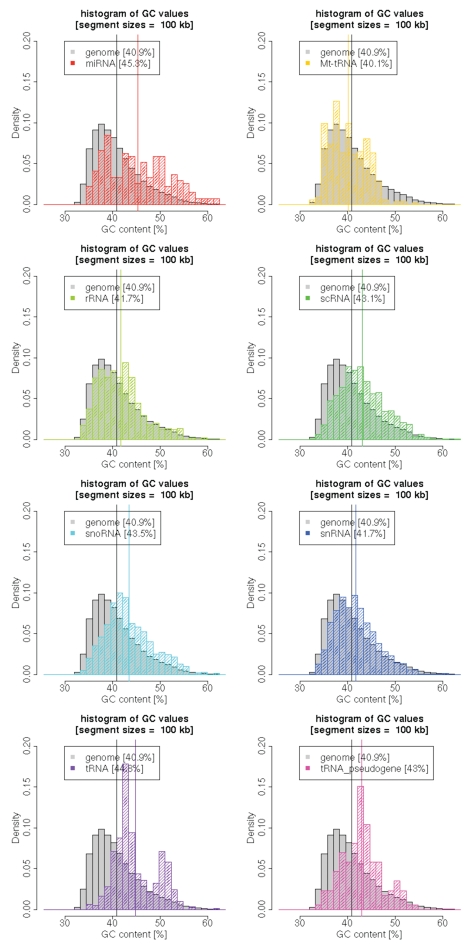
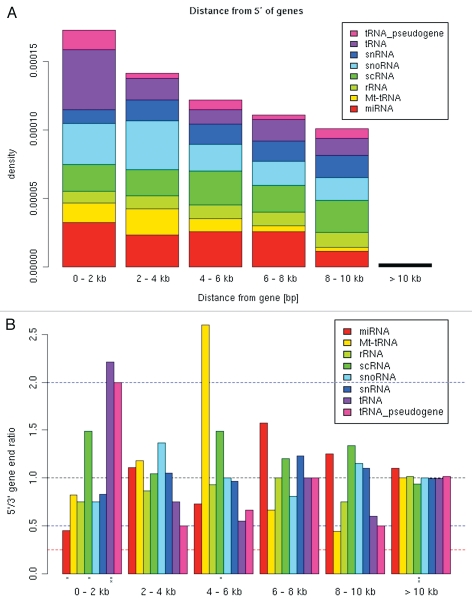
Given the potential of Pol III promoters to influence the expression of nearby Pol II genes, we decided to see whether we could detect any selection of co-occurrence of tRNA genes near protein-coding loci in the human genome, which is annotated much better than the mouse genome. We first found that tRNA genes and pseudogenes are enriched in GC-rich genomic segments (Fig. 6), which are also known to be generich.44,45 A similar trend was also found for micro-RNA genes and small nucleolar RNAs (snoRNAs) but was less pronounced for other RNA gene categories (Fig. 6). Moreover, in agreement with the recently published data,13,15,16,18 tRNA genes are concentrated closer to the 5′ end of genes compared with other RNA gene categories (Fig. 7A). The density of tRNA genes within 2 kb of the transcription start site of protein-coding genes is more than two times higher than in the next 2 kb from the transcription start site. Interestingly, accumulation of tRNA genes is specific to the transcription start sites (TSS) of Pol II genes (5′ end); the tRNA gene density is two-fold lower near 3′ ends of Pol II genes, and the difference is highly significant (Fig. 7B). Note that the 5′/3′ ratio is independent of variable gene density and other biases in the genome.
Figure 6.
GC content of 100 kb segments around human RNA genes. We calculated the GC content of from 5′ and 3′ 50 kb-long sequences flanking the RNA genes. The histogram of the GC distribution is compared with the whole genome (gray), calculated as 100 kb long, non-overlapping segments. Vertical bars represent graphical visualization of averages for a given RNA gene group and the whole genome.
Figure 7.
Distribution of Pol II-transcibed genes in the human genome. (A) RNA Pol III gene distance from transcription start of Pol II genes. The density of RNA genes near transcription start sites of RefSeq genes is plotted according to their absolute distance from the starts. (B) Ratio of RNA gene density near 5′ and 3′ ends of Pol II genes. We compared the distribution of RNA genes near 5′ vs. 3′ ends of protein-coding genes within six discrete intervals (0–2 kb, 2–4 kb, 4–6 kb, 6–8 kb, 8–10 kb and >10 kb). Black dashed line indicates 5′/3′ ratio of 1 (random expectation); blue and red lines indicate two-fold and four-fold ratios, respectively. Marks at the bottom indicate statistical significance using the Fisher exact (binomial) test (*p-value < 0.05, ** < 0.01).
Since many, especially older, RNA pseudogenes are often fragmented, we re-analyzed the data by joining closely located RNA genes coding for the same product into one, and the results remained very similar (Fig. S1). Altogether, these results indicate that tRNA genes are enriched in gene-rich regions and are often close to TSS of protein-coding genes.
Discussion
Previous studies in yeast showed that tRNA genes regulate chromatin structure and function as barrier elements.7,9,10,33,46 Herein, we present evidence for a similar phenomenon in the mouse genome, which contains approximately 1,000-times more putative RNA Pol III-transcribed genes and potential orphan B boxes than the yeast genome. Our results show that 2 and 4 tRNA genes clusters exhibit barrier activity when inserted into an ectopic chromosomal site in mouse cells. Using another experimental system, Kamakaka and co-authors came to a similar conclusion that tDNAs function as insulators in human cells (Dr. Kamakaka, personal communication).
A single copy of a tRNA gene did not demonstrate a significant barrier activity in the barrier activity assay used here. This is similar to yeast cells, where often a single copy of the tRNA gene is not able to function as barrier but becomes effective in two or more copies.7 This observation may explain a clustering of tRNA genes in the human genome, specifically in 5′ upstream regions of annotated Pol II TSS.16 A molecular mechanism by which actively transcribed tRNA genes act as barriers for heterochromatin remains to be determined. Based on the studies in yeast, insulation by tRNA genes may require interaction of Pol III factors with chromatin remodeling and histone-modifying enzymes and a spatial organization of these loci involving TFIIIC-mediated tethering to the specific nuclear territory (reviewed in ref. 47). It is quite possible that tDNA insulation in mammals occurs by a similar mechanism. The results of our study also demonstrate that a genomic context strongly influences the relative strength of barrier activity associated with tRNA genes. In particular, inter-genic CpG dinucleotides and/or high GC content DNA consistently suppresses the barrier activity of adjacent tRNA genes. This suggests that the GC-rich IVS sequences of tRNA genes may undergo methylation that negatively affects their potential barrier activity. Consistent with this, the tRNA genes clusters with AT-rich IVS DNA sequences exhibit robust and long-term barrier activity. Though additional experiments are required to confirm that loss of barrier activity is indeed caused by IVS DNA methylation, our findings suggest that a genomic context plays a role in determining which tRNA genes are constitutively active in dividing cells and can function as barriers, and which genes are either irreversibly silenced or conditionally-activated.
In yeast, complete tRNA genes are not required for barrier activity, which can be also observed for tandem clusters of synthetic B boxes.8,9,33 In the barrier activity assay used here, four orphan B boxes in the context of the native high GC-content IVS DNA sequences lacked barrier activity. No sustained barrier activity was observed with synthetic arrays with four or six orphan B boxes either, even in the context of AT-rich IVS DNA sequences. However, these results do not rule out the possibility that clusters with a bigger number of B boxes (that have potentially a higher affinity to TFIIIC) may create strong barriers.
It is tempting to speculate on the role diverse classes of Pol III-transcribed genes may play in modulating local chromatin conformation. Interspersed tRNA-derived SINE elements constitute a major class of Pol III-dependent genes that has active and inactive members. By analogy to active and inactive tRNA genes, it is possible that transcriptionally-competent SINEs and those that are not transcribed but are capable of binding TFIIIC and localized near Pol III-transcribed loci potentially might have barrier activity. Because some nucleotide substitutions in the Pol III consensus binding sites are tolerated,11 in silico identification of functional SINEs is difficult. However, if identified, transcriptionally-competent SINE clusters could be tested for barrier activity using the assay described in this study.
In the Drosophila genome, a small fraction of the putative 14,000 insulator sites identified by ChIP shows cell-type regulation.32 In human cells, the same has been demonstrated for a fraction of tRNA genes.14 If some SINEs are similarly under regulation, a significant source of gene modulation is available for mammalian cells.
Our data on computational analysis of distribution of Pol III-driven genes in the human genome are in agreement with experimental results on Pol lII occupancy in the genome.13,15–18 A considerable fraction of Pol III-occupied loci in the human genome is localized at the 5′ region of protein-coding genes, with a tendency to be concentrated within 2 kb of the transcription start site. While we cannot exclude that Pol II transcription may enable Pol III occupancy, these results support the hypothesis that Pol III-transcribed genes contribute to a local chromatin architecture permissive for transcription by Pol II. At the same time, a genome-scale analysis revealed a complex link between Pol III occupancy and gene expression. Not all genes with Pol III occupancy are transcribed.18 Also, a significant fraction of Pol III-occupied loci in the human genome is localized in the promoter regions of housekeeping genes with a high CpG content16 that may undergo methylation, leading to inactivation of the barrier element. This indicates that the Pol III-occupied loci, along with their flanking regions, represent complex regulatory elements. These regulatory elements can be distant from a particular gene, e.g., the LCR of the β-globin locus, and can be associated with transcription factors and/or insulator proteins that provide a balanced gene regulation. For example, co-occurrence of Pol III-occupied loci and a known insulator protein, CTCF, has been described in human genome-wide ChIP assays.13 Besides structuring DNA looping, CTCF protects DNA from methylation. Therefore, its binding to Pol III-transcribed loci may provide a prolonged barrier activity.
This study demonstrates that tRNA genes in an AT-rich context can be engineered to protect specific regions in a mouse chromosome from epigenetic silencing, a result that has important implications for gene expression experiments in mammalian cells. Previous studies showed that cHS4,48 human replicators,40 and γ-satellite DNA34 can also protect a cloned transgene from silencing. However, sometimes these elements do not provide a stable barrier activity over long periods of cell growth in culture49 or may be cell-type dependent.34 Therefore, new barrier elements need to be identified and characterized for achieving a stable transgene expression in mammalian cells. To explore this possibility, synthetic tRNA genes-derived barrier elements as well as other known barrier elements are now being incorporated into a human artificial chromosome (HAC)-based gene delivery vector with a conditional centromere (Larionov V, unpublished data).50,51 Ultimately, we expect to develop a HAC-based system that achieves consistent regulated expression of full-size human genes with minimal risk of uncontrolled epigenetic silencing of Pol II-dependent transcription units.
Materials and Methods
Targeting and constructs.
The targeting method and its modifications were described by Feng and co-authors35,36 and Kim and co-authors.34 The use of inverted loxP sites increases the efficiency of targeting, since excision is prevented and results in recovery of targeted constructs in two orientations. For simplicity, here we refer only to clones in orientation A unless otherwise noted. Correct targeting is determined by PCR across the recombination junctions and results in a single insertion of the cassette into a defined locus at mouse chromosome 4 flanked by the Map17 and Tal1 genes. Recovery of random background insertions is rare due to gancyclovir selection for HyTK loss and lack of positive selection for transfected plasmids.
Queried DNA was inserted into the MluI site in the pYB vector. Orientation of tRNA genes was maintained in all constructs so that transcription was toward the YAC/BAC module, with the exception of the tRNA(Leu) gene as noted above. “A” orientation results are reported here. Owing to an orientation-dependent suppression of expression at the RL5 site,35 “B” orientation eGFP expression was uniformly low. Pol III genes were PCR amplified from total genomic DNA (see Table S1 for primers). Variants inserted to the pYB vector were constructed by PCR from ligations of end-digested PCR products amplified with primers containing restriction enzyme sites added to their 5′ ends. Primer pairs data used for constructing tRNA gene variants are available upon request. An AT-rich sequence was derived from a human chromosome 6 alphoid centromeric repeat lacking the CENP-B box that induces seeding of heterochromatin. The sequence was PCR amplified with specific primers (Table S1). These regions are not known to contain motifs for DNA binding proteins.
Fluorescence quantification.
eGFP fluorescence was measured for ∼1–2 × 104 cells for each targeted cell line or controls on a FacsCalibur machine using CellQuest for acquisition control and FlowJo for analysis.34 A minimum of three cell lines was analyzed for mean GFP fluorescence for each construct at 4 weeks post-transfection or also at 8 and 12 weeks for longer term assays as needed.
Tissue culture.
Murine erythroid leukemia (MEL) suspension cells with the RL5 site were maintained in DMEM (Invitrogen 11965) with 10% FBS (Hyclone). For electroporation, 100 ug of pYB construct and 50 ug of Cre expression plasmid in 100 ul 1/10th T10E1 were added to 700 ul PBS in 0.4 cm cuvettes and were co-electroporated at 230 V and 1,000 uF in a BioRad GenePulser Xcell. Selection for loss of HyTK following Cre-mediated cassette exchange was begun 48 h post-electroporation with 10 uM Gancyclovir and dilution into 96-well plates. Gancyclovirresistant clones were expanded, washed in PBS and resuspended to ∼105 per ml for FACS analysis.
Chromatin immunoprecipitation (ChIP) assay.
ChIP assay was performed using ChIP assay kit (Upstate, New York) according to the manufacturer's instructions. Briefly, 6 × 106 cells were fixed with 1% formaldehyde for 10 min at 37°C. After serial washings, cells were resuspended to 1 × 106 cells per 200 µl of SDS Lysis Buffer and sonicated for 30 sec with subsequent 30 sec intervals for 16 min at 4°C using a Bioruptor (Cosmo Bio). Immunoprecipitated DNA was obtained with antibodies to lysine 4-di- and tri-methylated histone H3 (H3K4me2 and H3K4me3), lysine 9-tri-methylated histone H3 (H3K9me3) (Upstate, New York), antibodies 4289, 1900 and 128 that recognize human TFIIIC, Pol III and Brf1, respectively,52–54 and was quantitated by real-time PCR using the icycler IQ (Bio-Rad). The sequences of primers are listed in Table S1.
Sequence analysis.
We used the human genome NCBI Build 36.1 and hg18 version of the USCS annotation database55 to analyze the distribution of RNA genes in the human genome. Coordinates of RNA genes and pseudogenes were obtained from the “rnaGene” table; protein-coding genes were extracted from the RefSeq annotation tables in the database. We have excluded the category of miscellaneous RNAs from the analysis and split the tRNA category into gene and pseudogenes using information in the “rnaGene” table. To compare distribution of RNA genes and protein-coding genes for each RNA gene in the rnaGene table, we calculated its nucleotide distance from the closest 5′ end of a protein-coding gene (RefSeq). We also calculated RNA gene distances to the closest 3′ end of a gene as a control (which, in some cases, is the same gene as 5′ gene). No distinction was made whether the tRNA gene is inside or outside the protein-coding genes, only absolute distances to 5′ and 3′ ends were considered. After obtaining 5′ and 3′ distances to coding genes, we compared the distribution of RNA genes near 5′ vs. 3′ ends of protein-coding genes within six discrete intervals (0–2 kb, 2–4 kb, 4–6 kb, 6–8 kb and >10 kb). For each of the intervals, we compared the number of tRNA genes within and outside this interval for both 5′ and 3′ distance using the Fisher exact (binomial) test. All plots and statistical tests were obtained in the R statistical package (www.r-project.org).
Acknowledgments
Thanks to Mark Batzer for the Alu-Yb data, general support and discussion. This research was supported by the intramural research program of the NIH, National Cancer Institute, Center for Cancer Research.
Supplementary Material
References
- 1.Dieci G, Fiorino G, Castelnuovo M, Teichmann M, Pagano A. The expanding RNA polymerase III transcriptome. Trends Genet. 2007;23:614–622. doi: 10.1016/j.tig.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Hedges DJ, Batzer MA. From the margins of the genome: mobile elements shape primate evolution. Bioessays. 2005;27:785–794. doi: 10.1002/bies.20268. [DOI] [PubMed] [Google Scholar]
- 3.Kazazian H., Jr Mobile elements: drivers of genome evolution. Science. 2004;303:1626–1632. doi: 10.1126/science.1089670. [DOI] [PubMed] [Google Scholar]
- 4.Lunyak VV, Prefontaine GG, Núñez E, Cramer T, Ju BG, Ohgi KA, et al. Developmentally regulated activation of a SINE B2 repeat as a domain boundary in organogenesis. Science. 2007;317:248–251. doi: 10.1126/science.1140871. [DOI] [PubMed] [Google Scholar]
- 5.Sasaki T, Nishihara H, Hirakawa M, Fujimura K, Tanaka M, Kokubo N, et al. Possible involvement of SINEs in mammalian-specific brain formation. Proc Natl Acad Sci USA. 2008;105:4220–4225. doi: 10.1073/pnas.0709398105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saito Y, Suzuki H, Tsugawa H, Nakagawa I, Matsuzaki J, Kanai Y, et al. Chromatin remodeling at Alu repeats by epigenetic treatment activates silenced microRNA-512-5p with downregulation of Mcl-1 in human gastric cancer cells. Oncogene. 2009;28:2738–2744. doi: 10.1038/onc.2009.140. [DOI] [PubMed] [Google Scholar]
- 7.Donze D, Kamakaka RT. RNA polymerase III and RNA polymerase II promoter complexes are heterochromatin barriers in Saccharomyces cerevisiae. EMBO J. 2001;20:520–531. doi: 10.1093/emboj/20.3.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Scott KC, Merrett SL, Willard HF. A heterochromatin barrier partitions the fission yeast centromere into discrete chromatin domains. Curr Biol. 2006;16:119–129. doi: 10.1016/j.cub.2005.11.065. [DOI] [PubMed] [Google Scholar]
- 9.Noma K, Cam HP, Maraia RJ, Grewal SI. A role for TFIIIC transcription factor complex in genome organization. Cell. 2006;125:859–872. doi: 10.1016/j.cell.2006.04.028. [DOI] [PubMed] [Google Scholar]
- 10.Simms TA, Dugas SL, Gremillion JC, Ibos ME, Dandurand MN, Toliver TT, et al. TFIIIC binding sites function as both heterochromatin barriers and chromatin insulators in Saccharomyces cerevisiae. Eukaryot Cell. 2008;7:2078–2086. doi: 10.1128/EC.00128-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geiduschek EP, Tocchini-Valentini GP. Transcription by RNA polmerase III. Annu Rev Biochem. 1988;57:873–914. doi: 10.1146/annurev.bi.57.070188.004301. [DOI] [PubMed] [Google Scholar]
- 12.Gaëta BA, Sharp SJ, Stewart TS. Saturation mutagenesis of the Drosophila tRNA (Arg) gene B-Box intragenic promoter element: requirements for transcription activation and stable complex formation. Nucleic Acids Res. 1990;18:1541–1548. doi: 10.1093/nar/18.6.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moqtaderi Z, Wang J, Raha D, White RJ, Snyder M, Weng Z, et al. Genomic binding profiles of functionally distinct RNA polymerase III transcription complexes in human cells. Nat Struct Mol Biol. 2010;17:635–640. doi: 10.1038/nsmb.1794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dittmar KA, Goodenbour JM, Pan T. Tissue-specific differences in human transfer RNA expression. PLoS Genet. 2006;2:221. doi: 10.1371/journal.pgen.0020221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barski A, Chepelev I, Liko D, Cuddapah S, Fleming AB, Birch J, et al. Pol II and its associated epigenetic marks are present at Pol III-transcribed noncoding RNA genes. Nat Struct Mol Biol. 2010;17:629–634. doi: 10.1038/nsmb.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oler AJ, Alla RK, Roberts DN, Wong A, Hollenhorst PC, Chandler KJ, et al. Human RNA polymerase III transcriptomes and relationships to Pol II promoter chromatin and enhancer-binding factors. Nat Struct Mol Biol. 2010;17:620–628. doi: 10.1038/nsmb.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canella D, Praz V, Reina JH, Cousin P, Hernandez N. Defining the RNA polymerase III transcriptome: Genome-wide localization of the RNA polymerase III transcription machinery in human cells. Genome Res. 2010;20:710–721. doi: 10.1101/gr.101337.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raha D, Wang Z, Moqtaderi Z, Wu L, Zhong G, Gerstein M, et al. Close association of RNA polymerase II and many transcription factors with Pol III genes. Proc Natl Acad Sci USA. 2010;107:3639–3644. doi: 10.1073/pnas.0911315106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Geiduschek EP, Kassavetis GA. The RNA polymerase III transcription apparatus. J Mol Biol. 2001;310:1–26. doi: 10.1006/jmbi.2001.4732. [DOI] [PubMed] [Google Scholar]
- 20.Schmid CW. Human Alu subfamilies and their methylation revealed by blot hybridization. Nucleic Acids Res. 1991;19:5613–5617. doi: 10.1093/nar/19.20.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu WM, Maraia RJ, Rubin CM, Schmid CW. Alu transcripts: cytoplasmic localisation and regulation by DNA methylation. Nucleic Acids Res. 1994;22:1087–1095. doi: 10.1093/nar/22.6.1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Smit AF, Riggs AD. MIRs are classic, tRNA-derived SINEs that amplified before the mammalian radiation. Nucleic Acids Res. 1995;23:98–102. doi: 10.1093/nar/23.1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eickbush TH. Telomerase and retrotransposons: which came first? Science. 1997;277:911–912. doi: 10.1126/science.277.5328.911. [DOI] [PubMed] [Google Scholar]
- 24.Jordan IK, Rogozin IB, Glazko GV, Koonin EV. Origin of a substantial fraction of human regulatory sequences from transposable elements. Trends Genet. 2003;19:68–72. doi: 10.1016/S0168-9525(02)00006-9. [DOI] [PubMed] [Google Scholar]
- 25.Lippman Z, Gendrel AV, Black M, Vaughn MW, Dedhia N, McCombie WR, et al. Role of transposable elements in heterochromatin and epigenetic control. Nature. 2004;430:471–476. doi: 10.1038/nature02651. [DOI] [PubMed] [Google Scholar]
- 26.Smalheiser NR, Torvik VI. Mammalian microR-NAs derived from genomic repeats. Trends Genet. 2005;21:322–326. doi: 10.1016/j.tig.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Gilbert MK, Tan YY, Hart CM. The Drosophila boundary element-associated factors BEAF-32A and BEAF-32B affect chromatin structure. Genetics. 2006;173:1365–1375. doi: 10.1534/genetics.106.056002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dorman ER, Bushey AM, Corces VG. The role of insulator elements in large-scale chromatin structure in interphase. Semin Cell Dev Biol. 2007;18:682–690. doi: 10.1016/j.semcdb.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bi X, Yu Q, Sandmeier JJ, Zou Y. Formation of boundaries of transcriptionally silent chromatin by nucleosome-excluding structures. Mol Cell Biol. 2004;24:2118–2131. doi: 10.1128/MCB.24.5.2118-31.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xie X, Mikkelsen TS, Gnirke A, Lindblad-Toh K, Kellis M, Lander ES. Systematic discovery of regulatory motifs in conserved regions of the human genome, including thousands of CTCF insulator sites. Proc Natl Acad Sci USA. 2007;104:7145–7150. doi: 10.1073/pnas.0701811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim TH, Abdullaev ZK, Smith AD, Ching KA, Loukinov DI, Green RD, et al. Analysis of the vertebrate insulator protein CTCF-binding sites in the human genome. Cell. 2007;128:1231–1245. doi: 10.1016/j.cell.2006.12.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nègre N, Brown CD, Shah PK, Kheradpou P, Morrison CA, Henikoff JG, et al. A comprehensive map of insulator elements for the Drosophila genome. PLoS Genet. 2010;6:1000814. doi: 10.1371/journal.pgen.1000814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Valenzuela L, Dhillon N, Kamakaka RT. Transcription independent insulation at TFIIIC-dependent insulators. Genetics. 2009;183:131–148. doi: 10.1534/genetics.109.106203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JH, Ebersole T, Kouprina N, Noskov VN, Ohzeki J, Masumoto H, et al. Human gamma-satellite DNA maintains open chromatin structure and protects a transgene from epigenetic silencing. Genome Res. 2009;19:533–544. doi: 10.1101/gr.086496.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng YQ, Seibler J, Alami R, Eisen A, Westerman KA, Leboulch P, et al. Site-specific chromosomal integration in mammalian cells: highly efficient CRE recombinase-mediated cassette exchange. J Mol Biol. 1999;292:779–785. doi: 10.1006/jmbi.1999.3113. [DOI] [PubMed] [Google Scholar]
- 36.Feng YQ, Lorincz MC, Fiering S, Greally JM, Bouhassira EE. Position effects are influenced by the orientation of a transgene with respect to flanking chromatin. Mol Cell Biol. 2001;21:298–309. doi: 10.1128/MCB.21.1.298309.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Looney JE, Harding JD. Structure and evolution of a mouse tRNA gene cluster encoding tRNAAsp, tRNAGly and tRNAGlu and an unlinked, solitary gene encoding tRNAAsp. Nucleic Acids Res. 1983;11:8761–8775. doi: 10.1093/nar/11.24.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hallenberg C, Nederby Nielsen J, Frederiksen S. Characterization of 5S rRNA genes from mouse. Gene. 1994;142:291–295. doi: 10.1016/0378-1119(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 39.Carter AB, Salem AH, Hedges DJ, Keegan CN, Kimball B, Walker JA, et al. Genome-wide analysis of the human Alu Yb-lineage. Hum Genomics. 2004;1:167–178. doi: 10.1186/1479-7364-1-3-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fu H, Wang L, Lin CM, Singhania S, Bouhassira EE, Aladjem MI. Preventing gene silencing with human replicators. Nat Biotechnol. 2006;24:572–576. doi: 10.1038/nbt1202. [DOI] [PubMed] [Google Scholar]
- 41.Ross BM, Looney JE, Harding JD. Nucleotide sequence of a mouse tRNA Leu gene. Nucleic Acids Res. 1986;14:5567–5572. doi: 10.1093/nar/14.13.5567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Eszterhas SK, Bouhassira EE, Martin DI, Fiering S. Transcriptional interference by independently regulated genes occurs in any relative arrangement of the genes and is influenced by chromosomal integration position. Mol Cell Biol. 2002;22:469–479. doi: 10.1128/MCB.22.2.469-79.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kenneth NS, Marshall L, White RJ. Recruitment of RNA polymerase III in vivo. Nucleic Acids Res. 2008;36:3757–3764. doi: 10.1093/nar/gkn272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bernardi G, Olofsson B, Filipski J, Zerial M, Salinas J, Cuny G, et al. The mosaic genome of warm-blooded vertebrates. Science. 1985;228:953–958. doi: 10.1126/science.4001930. [DOI] [PubMed] [Google Scholar]
- 45.Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, et al. International Human Genome Sequencing Consortium. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. doi: 10.1038/35057062. [DOI] [PubMed] [Google Scholar]
- 46.Iwasaki O, Tanaka A, Tanizawa H, Grewal SI, Noma K. Centromeric localization of dispersed Pol III genes in fission yeast. Mol Biol Cell. 2010;21:254–265. doi: 10.1091/mbc.E09-09-0790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kirkland JG, Kamakaka RT. tRNA insulator function: insight into inheritance of transcription states? Epigenetics. 2010;5:96–99. doi: 10.4161/epi.5.2.10775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gaszner M, Felsenfeld G. Insulators: exploiting transcriptional and epigenetic mechanisms. Nat Rev Genet. 2006;7:703–713. doi: 10.1038/nrg1925. [DOI] [PubMed] [Google Scholar]
- 49.Jakobsson J, Rosenqvist N, Thompson L, Barraud P, Lundberg C. Dynamics of transgene expression in a neural stem cell line transduced with lentiviral vectors incorporating the cHS4 insulator. Exp Cell Res. 2004;298:611–623. doi: 10.1016/j.yexcr.2004.04.037. [DOI] [PubMed] [Google Scholar]
- 50.Nakano M, Cardinale S, Noskov VN, Gassman R, Vagnarelli P, Kandels-Lewis S, et al. Inactivation of a human kinetochore by specific targeting of chromatin modifiers. Dev Cell. 2008;14:507–522. doi: 10.1016/j.devcel.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chi Y, Kim JH, Kazuki Y, Hoshiya H, Takiguchi M, Hayashi M, et al. Human artificial chromosome with a conditional centromere for gene delivery and gene expression. DNA Res. 2011;18:1–16. doi: 10.1093/dnares/dsq031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fairley JA, Scott PH, White RJ. TFIIIB is phosphorylated, disrupted and selectively released from tRNA promoters during mitosis in vivo. EMBO J. 2003;22:5841–5850. doi: 10.1093/emboj/cdg544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cairns CA, White RJ. p53 is a general repressor of RNA polymerase III transcription. EMBO J. 1998;17:3112–3123. doi: 10.1093/emboj/17.11.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Athineos D, Marshall L, White RJ. Regulation of TFIIIB during F9 cell differentiation. BMC Mol Biol. 2010;11:21. doi: 10.1186/1471-2199-11-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rhead B, Karolchik D, Kuhn RM, Hinrichs AS, Zweig AS, Fujita PA, et al. The UCSC Genome Browser database: update 2010. Nucleic Acids Res. 2010;38:613–619. doi: 10.1093/nar/gkp939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.