Abstract
Small cell lung carcinoma (SCLC) is a neuroendocrine subtype of lung cancer that affects more than 200,000 people worldwide every year with a very high mortality rate. Here, we used a mouse genetics approach to characterize the cell of origin for SCLC; in this mouse model, tumors are initiated by the deletion of the Rb and p53 tumor suppressor genes in the lung epithelium of adult mice. We found that mouse SCLCs often arise in the lung epithelium, where neuroendocrine cells are located, and that the majority of early lesions were composed of proliferating neuroendocrine cells. In addition, mice in which Rb and p53 are deleted in a variety of non-neuroendocrine lung epithelial cells did not develop SCLC. These data indicate that SCLC likely arises from neuroendocrine cells in the lung.
Key words: Rb, p53, SCLC, cell of origin, cancer, lung, neuroendocrine
Introduction
Lung cancer is the leading cause of cancer deaths in the world. In the United States, ∼160,000 patients die from lung cancer every year, more than the combined deaths from colon, breast, bladder and pancreas cancers. The vast majority (80–85%) of lung cancers are non-small cell lung cancer (NSCLC); the remaining 15% of cases show properties of neuroendocrine cells, and most of these tumors are SCLC.1 Approximately 200,000 people die from SCLC every year worldwide. The overall 5-year survival rate for lung cancer is 15%;2 for SCLC alone, it is often much lower.3,4
The distinction between lung cancer subtypes is paramount, because treatment efficacy may differ significantly between these subtypes. However, this distinction is not always evident, as a significant numbers of lung cancers display mixed characteristics, with various number of cells expressing variable levels of neuroendocrine, alveolar and bronchiolar markers.5,6 These observations raise multiple questions, including the cell(s) of origin and the genetic factors that may influence lung cancer evolution. One way to address the question of the heterogeneity of human lug cancers that is invisible at the histopathological level is to perform expression profiling analysis to identify molecular similarities and differences between tumors.7 However, SCLCs are rarely surgically removed, hampering the molecular analysis of a large number of human samples.
Detecting lung cancer at earlier stages would allow for a better efficiency of available treatments, which raises the important point of understanding the early stages of lung cancer, including identifying the cell of origin of the different lung cancer subtypes. This goal is challenging to achieve in human patients, because lung cancers are often detected late and because of the complex genetic and environmental diversity of these patients.
Spontaneous lung tumors in mice are similar in their histopathology and molecular traits to human lung cancer.8 Mouse models for human lung cancers can thus serve as an in vivo system to investigate the mechanisms of lung tumorigenesis. Berns and colleagues have described a mouse model of SCLC9 based on the fact that tumor cells in more than 90% of human SCLCs are mutant for both the p53 and Rb tumor suppressor genes.10 In this model, adenoviral particles expressing the Cre recombinase (Ad-Cre) were injected into the trachea of Rblox/lox; p53lox/lox mice to delete both genes in a subset of lung epithelial cells. Nearly all of these Rb/p53 double mutant mice (and none of the single mutant mice) develop SCLCs; in addition, these tumors express markers of neuroendocrine cells and have the capacity to metastasize.9
The lung epithelium is hypothesized to contain several distinct stem cell and progenitor cell populations that maintain the numerous types of differentiated lung cell populations.11–14 Mucous, basal, ciliated, non-ciliated (Clara and serous) and neuroendocrine cells (NECs) line the conducting airways of the lung. Alveolar type II (AT2) and type I (AT1) epithelial cells line the alveolar space and secrete surfactants and perform gas exchange, respectively. A population of presumptive distal adult lung epithelial stem cells (BASCs, bronchioalveolar stem cells) was also recently identified at the bronchioalveolar duct junction (BADJ).15 Previous work shows that NSCLC may originate from BASCs.15 Different subtypes of lung cancer may arise from distinct lung cell populations (stem cells or differentiated cells), but it is also conceivable that different tumors may grow from the same cell of origin depending on the combination of genetic alterations and under the influence of the tumor microenvironment.16
The cell of origin of SCLC has not been formally identified, although, because SCLCs express neuroendocrine markers, they are commonly thought to arise from NECs or neuroendocrine progenitors (NEPs).17 Here, we sought to use a mouse genetics approach to further investigate the cellular mechanisms of cancer initiation in the lungs. Our experiments indicate that SCLC does not arise from loss of Rb/p53 in distal lung epithelial cells and support the hypothesis that cells in the neuroendocrine lineage give rise to SCLC.
Results
Induction of small neuroendocrine lesions and SCLC following Ad-Cre infection in Rb/p53 and Rb/p53/p130 conditional mutant mice.
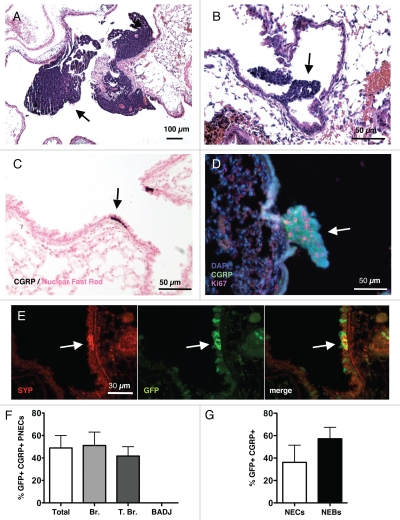
Deletion of Rb and p53 in the lung epithelium of Rblox/lox; p53loxlox mice results in the development of lung tumors 9–12 mo after intranasal instillation of adenoviral particle expressing the Cre recombinase (Ad-Cre); the additional loss of the Rb-related gene p130 enhances tumor initiation and progression.18Rb/p53 and Rb/p53/p130 mutant tumors are often localized in the main airways (Fig. 1A and B and data not shown), where neuroendocrine cells are also present (Fig. 1C). In addition, the analysis of mutant mice 3–6 mo after Cre-mediated deletion of the tumor suppressor genes showed the presence of small lesions that are likely to be the precursor lesions for SCLC, because they express neuroendocrine markers such as Calcitoningene related protein (CGRP) and Synaptophysin (SYP), and because they are comprised of proliferating cells positive for the Ki67 marker (Fig. 1D and data not shown). Furthermore, using Ad-GFP, we found that the adenovirus could infect both isolated neuroendocrine cells (NECs) as well as cells in neuroendocrine bodies (NEBs, composed of several NECs) (Fig. 1E). Overall, ∼40% of CGRP+ cells were GFP+ under these conditions, including CGRP+ cells present in bronchioles and terminal bronchioles (Fig. 1F). Notably, CGRP+ cells were not detected at the BADJ. When CGRP+ GFP+ cells were scored based on their presence as isolated NECs or in NEBs, the frequency of neuroendocrine cell infection was similar (Fig. 1G). Thus, nasal instillation of Ad-Cre in Rblox/lox;p53loxlox mice and Rblox/lox; p130loxlox;p53loxlox mice resulted in the efficient infection of neuroendocrine cells followed by the development of small lesions and fully developed tumors, both of which grew in areas of the lung where neuroendocrine cells are normally found.
Figure 1.
Induction of neuroendocrine lesions and SCLC in the lungs of mice. Hematoxylin and Eosin (H&E) staining of lungs sections from Rb/p53 mutant mice infected with Ad-Cre and aged for 9–12 mo (A) or 3–6 mo (B). Arrows indicate large tumors and small lesions, respectively. (C) Immunostaining for the neuroendocrine marker CGRP (dark brown, arrow) in a section from wild-type mouse lungs counterstained with Nuclear Fast Red. (D) Immunofluorescence analysis of CGRP (green) and Ki67 (red) expression in the lungs of Rb/p53 mutant mice infected with Ad-Cre and aged for 3 mo. DAPI (blue) stains DNA. (E) Immunofluorescence analysis of Synaptophysin (SYP) and GFP expression on a lung section from a mouse infected with Ad-GFP. (F) Quantification of CGRP/GFP double-positive cells in sections from Rb/p53 mutant lungs. (G) Quantification of GFP expression in isolated neuroendocrine cells (NECs) and neuroendocrine bodies (NEBs, defined by clusters of NECs). All pictures are representative of multiple mice. Data for (F and G) come from the analysis of 55 pulmonary neuroendocrine cells or cell clusters from 16 mutant mice.
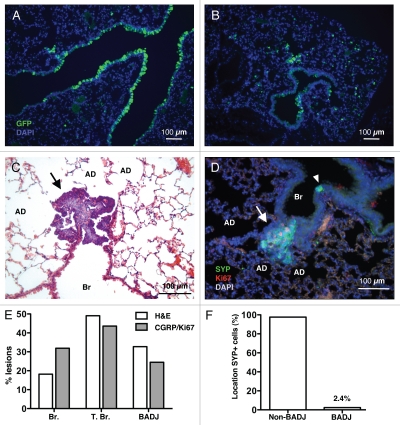
These findings support a model in which SCLC arises from neuroendocrine cells, but did not exclude that other cell types may be at the origin of this cancer. Indeed, upon Ad-GFP infection, many epithelial cell types expressed GFP, including Clara cells, BASCs and AT2 cells (Fig. 2A and B and data not shown). Furthermore, some SCLC lesions grew at the distal ends of the bronchiolar epithelium, including at or close to BADJs (Fig. 2C and D and data not shown). Quantification of small neuroendocrine lesions by histology and by double immunostaining for CGRP+ and Ki67+ cells in Rb/p53 mutant mice showed a distribution across the different lung compartments, including close to BADJs (Fig. 2E). Similar observations were made in Rb/p53/p130 mutant mice (data not shown). In contrast, neuroendocrine cells marked by SYP or CGRP are nearly fully excluded from BADJs (Fig. 2F and data not shown). This observation suggested the possibility that the few neuroendocrine cells present at the BADJ are more likely to initiate SCLC because of the micro-environment, or because they represent different subpopulations of neuroendocrine cells. An alternative possibility is that neuroendocrine tumors originate from a non-neuroendocrine cell type in the terminal bronchioles and/or at the BADJ.
Figure 2.
Early neuroendocrine lesions in various locations along the lung epithelium. Immunostaining for GFP on lung sections from mice infected with Ad-GFP three days before analysis shows that cells lining airways (A) and in the alveolar space (B) express GFP. (C) H&E staining of lung sections from one representative Rb/p53 mutant mouse infected with Ad-Cre and aged for 3 mo. AD, alveolar duct; Br, bronchioles. A typical SCLC lesion with scant cytoplasm and hyperchromatic nuclei (arrow) can be identified at the BADJ. (D) Immunostaining for SYP and Ki67 identifies actively dividing neuroendocrine lesions (arrow). A quiescent NEB is present on the same section (arrowhead). (E) Quantification of the localization of lesions identified by histological criteria in H&E sections (as in C, n = 55 from 16 mice, white bars) and by double staining for SYP and Ki67 (as in D, n = 94 from 25 mice, gray bars). T. Br., terminal bronchioles. (F) Quantification of the localization of SYP+ cells in sections from normal lungs (n = 586 neuroendocrine bodies). Any SYP+ cell within ten cells of the BADJ was counted in the BADJ group.
Rb/p53 mutant mice do not develop SCLC when Cre expression is driven by the CCSP promoter.
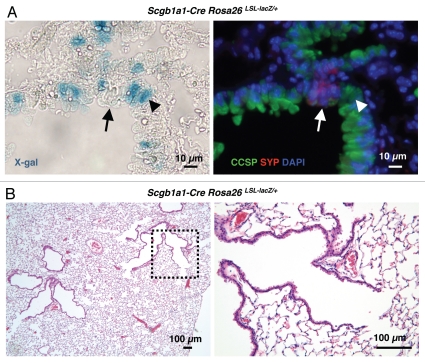
To further characterize the cell type(s) from which SCLC may arise, we sought to express the Cre recombinase in specific lung epithelial cell types in Rb/p53 conditional mutant mice. Because most of the SCLC lesions are found in bronchioles and terminal bronchioles, which are composed in majority of Clara cells, we crossed Rblox/lox;p53loxlox mice to Scgb1a1-Cre mice.19–21Scgb1a1 codes for the Clara cell-specific protein (CCSP, also known as CCA or CC10), which marks Clara cells throughout the distal bronchiolar epithelium as well as BASCs at the BADJ15,22 and Cre expression in the Scgb1a1-Cre strain, has been shown to be limited to the bronchiolar epithelium.19–21 To confirm these previous reports, we crossed Scgb1a1-Cre mice to Rosa26lox-stop-lox-LacZ (Rosa26R) reporter mice, where lacZ expression is induced after Cre-mediated recombination,23 and detected lacZ activity by X-gal staining in the bronchiolar epithelium but not in neuroendocrine cells, as expected (Fig. 3A). While crossing Scgb1a1-Cre mice to mice carrying an inducible oncogenic allele of K-Ras resulted in the efficient development of lung adenocarcinoma,21 we found that none of 12 Scgb1a1-Cre Rblox/lox;p53loxlox mice with constitutive expression of Cre in Clara cells examined between 31 and 47 weeks after birth developed tumors (Fig. 3B). Thus, lung cells expressing CCSP are not prone to initiate SCLC following the inactivation of Rb and p53.
Figure 3.
Constitutive deletion of Rb and p53 in CCSP-expressing cells. (A) Representative X-gal staining of lung sections from an Scgb1a1-Cre Rosa26R mouse (left). The section was also immunostained for CCSP and SYP expression to identify Clara cells (arrowhead) and pulmonary neuroendocrine cells (arrow), respectively (right). (B) H&E staining of a representative lung section from an Scgb1a1-Cre Rb/p53 mutant mouse. The dotted area in the left part is magnified in the right part. No tumors or small lesions are visible.
Cre expression in lung epithelial cells driven by the SP-C promoter does not initiate SCLC in Rb/p53 mutant mice.
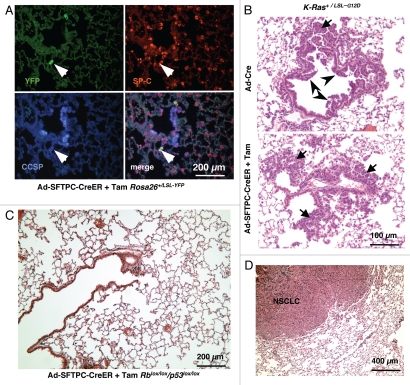
While we never observed any isolated neuroendocrine lesions only in alveoli (data not shown), the growth of lesions at BADJs raised the possibility that BASCs or alveolar cells close to the terminal bronchioles might initiate SCLC lesions growing toward the bronchiolar space. To test this possibility, we first generated an adenoviral vector in which the expression of the CreER recombinase is driven by a 3.7 kb fragment of the SFTPC promoter.24 SFTPC codes for human surfactant protein C (SP-C), which marks AT2 cells as well as BASCs.15,25 To verify the specificity of Cre expression in this Ad-SFTPC-CreER construct, we performed intranasal instillation of this virus in Rosa26LSL-YFP/+ reporter mice.26 We found YFP expression only in SP-C+ alveolar cells and only in the lungs of mice treated with Tam (Fig. 4A and data not shown). We next compared Ad-SFTPC-CreER to Ad-Cre (where Cre expression is driven by the broadly-expressed CMV promoter) in K-Ras+/LSL-G12D conditional mutant mice, which develop NSCLC after Cre-mediated recombination of a transcriptional stop cassette preventing the activation of oncogenic K-Ras.27 As expected, Ad-Cre-infected K-Ras+/LSL-G12D mice developed both bronchiolar hyperplasia and atypical alveolar hyperplasia (AAH) 6–8 weeks after infection (Fig. 4B, top). In contrast, Ad-SFTPC-CreER-infected K-Ras+/LSL-G12D mice treated with tamoxifen (Tam) to induce Cre activity only developed AAH (Fig. 4B, bottom), further suggesting that this virus allows CreER expression in SP-C-expressing cells and not in Clara cells. Based on these observations, we infected eight Rblox/lox;p53loxlox mice with Ad-SFTPC-CreER and treated these mice with Tam before aging them 9–13 mo. Similarly, five Rblox/lox; p130+/lox;p53loxlox mice and three Rblox/lox; p130lox/lox;p53loxlox mice were infected with Ad-SFTPC-CreER and injected with Tam to activate Cre; these triple mutant mice were aged 6 mo. None of these mice developed any neuroendocrine tumors; furthermore, no signs of hyperplastic neuroendocrine lesions were found in lung sections (Fig. 4C and data not shown). Five of these 16 mice developed single lung tumors that had clear histopathological characteristics of NSCLC (Fig. 4D and data not shown), including expression of SP-C and absence of expression of SYP by immunostaining (data not shown). These observations are consistent with the reported development of rare adenocarcinoma in Rb/p53 mice after administration of Ad-Cre, usually because of partial deletion of Rb.9
Figure 4.
Deletion of Rb and p53 in SP-C-expressing adult cells using adenovirus. (A) Representative immunofluorescence staining of lung sections from a Rosa26+/LSL-YFP reporter mouse infected with Ad-SFTPC-CreER following activation of Cre by Tam. The arrow points to a cell that is YFP+ and SP-C+ but CCSP− (B) Development of bronchiolar hyperplasia (arrowheads) and atypical alveolar hyperplasia (AAH) (arrows) 6–8 weeks after infection of K-Ras+/LSL-G12D with Ad-Cre (Top). The same mice infected with Ad-SFTPC-CreER and injected with Tam only develop AAH (Bottom). (C) H&E staining of a representative lung section from an Rb/p53 mutant mouse infected with Ad-SFTPC-CreER and injected with Tam and aged over 9 mo. The image is representative of multiple mice. (D) Development of NSCLC in an Rb/p53 mutant mouse infected with Ad-SFTPC-CreER and injected with Tam and aged over 9 mo.
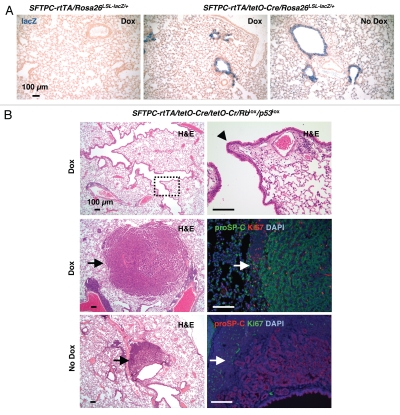
Because activation of Cre in Ad-SFTPC-CreER-infected mice is limited to a subset of alveolar cells, we next crossed Rblox/lox; p53loxlox mice to SFTPC-rtTA/(tetO)7-Cre double transgenic mice.28,29 By crossing these mice to Rosa26R reporter mice, we found that activation of Cre in this system was widespread in alveolar cells, as expected, but also in bronchiolar cells, including in the absence of doxycycline, as previously described in reference 29 (Fig. 5A). However, previous evidence indicates that neuroendocrine cells do not express this transgene or express it at very low levels and rarely.29,30 Even after aging for more than one year, none of the SFTPC-rtTA/(tetO)7-Cre Rblox/lox;p53loxlox mice with (n = 11) and without (n = 6) doxycycline developed SCLC (Fig. 5B). Immunostaining for CGRP and SYP only detected normal NE cells; no increased proliferation was noted in NECs or NEBs (data not shown). We did, however, detect adenocarcinoma development in one SFTPC-rtTA/(tetO)7-Cre Rblox/lox;p53loxlox mouse without doxycycline and three with doxycycline (Fig. 5B); two other mice had undefined extrapulmonary tumors (data not shown). These findings were consistent with the doxycycline-independent recombination we detected in the reporter mice. Together, these experiments indicate that SP-C-expressing cells do not efficiently initiate SCLC upon loss of Rb and p53.
Figure 5.
Induced deletion of Rb and p53 in SP-C-expressing cells. (A) X-gal-stained lung sections from Sftpc-rtTA/tetO-Cre/Rosa26R mice treated with doxycycline or vehicle (Dox or No Dox, respectively). The lungs of Sftpc-rtTA/Rosa26R mice (Dox, far left) were used as a negative control. (B) Representative H&E staining and immunostaining for proSP-C and Ki67 on lung sections from Sftpc-rtTA/tetO-Cre/Rblox/lox/p53lox/lox mice treated with doxycycline or vehicle. The dotted area in the top left part is highlighted in the right part. The arrowhead in the right part indicates normal pulmonary neuroendocrine cell. Tumor cells (arrows) in both Dox and No Dox groups stained positive for proSP-C, a maker of adenocarcinoma as well as alveolar type II cells and Ki67, a marker of proliferation.
Discussion
Several models could explain the neuroendocrine features of SCLC. First, SCLC may arise from mature cells in the neuroendocrine lineage. This is the model supported by our data, since small proliferative lesions arose where neuroendocrine cells are located in the lung epithelium of Rblox/lox;p53loxlox mice after Ad-Cre. This model is also strongly supported by recent evidence from the Berns group using cell type-restricted adenoviral vectors expressing the Cre recombinase.31 In this model, differentiated neuroendocrine cells may re-enter the cell cycle upon loss of the RB and p53 cell cycle inhibitors without losing all their differentiation characteristics. Notably, we have found that mouse tumors passaged several times in allografts using immunodeficient recipient mice tend to lose the expression of CGRP and Synaptophysin (data not shown), supporting a model of slow dedifferentiation with tumor progression. This model is reminiscent of some cases of hepatocellular carcinoma that may arise from mature hepatocytes32 or retinoblastoma that may arise upon dedifferentiation of post-mitotic retinal cells.33,34
An alternative model is that SCLC arises from progenitor cells in the neuroendocrine lineage, cells that may express low levels of differentiation markers and that have an increased natural ability to divide. Loss of RB and p53 in these cells may simply promote proliferation without strongly affecting the differentiation status of the mutant cells. This model highlighting a role for RB and p53 in stem/progenitor cell populations is compatible with other mouse models of human cancers associated with loss of RB and p53 function, including in the blood compartment, in bones, in the retina and in the mammary epithelium.35–39 However, the identity of potential neuroendocrine cell progenitors in adult lung tissue has not yet been established.
A third possibility is that a subset of SCLCs arises from nonneuroendocrine cells that acquire neuroendocrine characteristics during tumorigenesis. Loss of RB function is associated with the development of neuroendocrine tumors in mice, including pituitary, thyroid and adrenal gland tumors.40–42 Evidence suggests that RB inactivation during lung development promotes the expansion of neuroendocrine cells.30 Therefore, it is possible that loss of RB function in non-neuroendocrine lung epithelial cells “reprograms” these cells toward a neuroendocrine fate. In particular, microarrays analysis has suggested that mouse SCLC tumor cells may express SP-C (reviewed in ref. 18). However, our data in mice in which SP-C-expressing and CCSP-expressing cells did not form SCLC indicated that Clara cells, BASCs and AT2 cells, which represent the major non-neuroendocrine cell types in the distal lungs, are unlikely to be the cell lineages of origin in SCLC. Furthermore, the localization of basal cells and other secretory cells in the proximal lung makes it unlikely that SCLC, which is predominant in the distal lung, arises from these lineages. Thus, our data strongly suggest that non-neuroendocrine lung cells are not a frequent cell of origin for SCLC. These data are different from recent data published by the Berns group,31 where SCLC tumors arise from SP-C-expressing cells; in this study, the use of Ad-Cre (and not Ad-CreER) made the adenoviral strategy more efficient (compare Fig. 4A here and Fig. 1 in Sutherland et al.), which likely explains the difference observed.
One explanation for why we also did not observe SCLC in SFTPC-rtTA/(tetO)7-Cre Rblox/lox;p53loxlox mice could be that constitutive deletion of Rb/p53 affects lung cells differently than acute deletion in adult mice, or that the SFTPC-rtTA transgene does not drive Cre expression in a cell type that might serve as a presumptive common progenitor for alveolar cells and neuroendocrine cells.43,44 Yet, the initiation of SCLC from progenitor cells, including progenitors with the ability (normal or induced by loss of Rb/p53) to form cells expressing markers in distinct lung epithelial lineages would help to explain the heterogeneity of lung tumors in certain patients.6,45,46
SCLC is a deadly cancer for which limited therapeutic options currently exist. Our data, together with others' published work,31 support a model in which SCLC arises from the neuroendocrine lineage upon loss of Rb and p53 expression, whereas NSCLC originates from distal lung epithelia after distinct genetic events. Future work to gain a better understanding of the early events during SCLC initiation and development may identify novel ways to detect this type of cancer early, thereby improving the odds that patients will survive their cancer.
Material and Methods
Mice and genotyping.
Conditional mutant Rb mice47 and conditional mutant p53 mice48 were bred to generate double conditional mutant mice for infection with adenoviral particles (see below). In some cases, Rb/p53/p130 triple conditional mutant mice were used, because loss of p130 accelerates tumorigenesis in the context of loss of Rb and p53.18 The same Rb and p53 conditional mutant mice were crossed to to SFTPC-rtTA/(tetO)7-Cre double transgenic mice.28,29 The same conditional allele of p53 and a different conditional allele of Rb48 were bred together and crossed to Scgb1a1-Cre mice.19–21 The two reporter mouse strains used, Rosa26R and Rosa26LSL-YFP/+, have been extensively described in reference 23 and 26. Doxycycline was administered in the drinking water at 1 mg/ml for one month as previously described in references 28 and 29. Tamoxifen (Tam) resuspended in corn oil was injected intraperitoneally daily for five consecutive days at a dose of 2 mg per mouse.49 All mouse experiments were approved by the Children's Hospital Boston Animal Care and Use Committee at Harvard Medical School and by the Administrative Part on Laboratory Animal Care at Stanford, both accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and were performed in accordance with relevant institutional and national guidelines and regulations.
Genomic DNA was prepared using lysis buffer containing Proteinase K (Sigma Aldrich). The primer information and the protocol for genotyping and detecting deletions of Rb and p53 genes were described.18
Adenovirus cloning, preparation and infection.
Ad-Cre and Ad-GFP vectors were purchased from the Vector Development Laboratory (Baylor College of Medicine) and used as described before in reference 18. Details of the cloning strategy for the Ad-SFTPC-CreER vector will be provided upon request. In brief, a CreER cDNA50 was cloned downstream of a 3.7 kb promoter fragment24 and transferred into an adenoviral vector backbone (pAd/PL-DEST™ Gateway® Vector Kit, Invitrogen) before amplification. Viral infections were performed as described in reference 51.
Immunostaining and X-gal staining.
Mouse lungs were inflated and fixed with 4% paraformaldehyde in phosphate-buffered saline (PFA/PBS) overnight, then processed for paraffin embedding. Five µm sections were used for immunostaining following rehydration and antigen-retrieval in Trilogy Pretreatment Solution (Cell Marque). The sections were incubated with primary antibodies overnight and then with fluorescent secondary for an hour (1:200, Invitrogen) before mounting in an anti-fade reagent containing DAPI for nuclear counter-staining (Vectashield). Biotin-conjugated secondary antibodies (1:200, Vector Laboratories) were detected via a biotin-peroxidase complex with diaminobenzidine substrate (Vectastain ABC, Vector Laboratories).52 Sections were stained with Hematoxylin and Eosin (H&E) or counterstained in Nuclear Fast Red. The primary antibodies were as follows: Ki67 (1:100, BD Biosciences), Pro-surfactant protein C (proSP-C) (1:100, Dr. Jeff Whitsett, University of Cincinnati), Clara cell secretory protein (CCSP) (1:1,000, Santa Cruz Biotechnology), GFP (1:200, Molecular Probes), Calcitonin gene-related peptide (CGRP) (1:1,000, Sigma) and Synaptophysin (SYP) (1:100, Neomarkers). For X-gal staining, lungs were inflated with 4% PFA for 10 min, washed in PBS with 0.02% NP-40 and incubated in X-gal staining solution overnight. Stained lungs were washed in PBS/0.02% NP-40 and post-fixed in 4% PFA/PBS.
Acknowledgments
We are grateful to Dr. Jeff Whitsett for the SFTPC-rtTA/(tetO)7-Cre mouse lines and the proSP-C antibodies and to Dr. Deming Gou for the gift of the SP-C promoter plasmid. We also thank Dr. Anton Berns for the generous gift of the Rb and p53 conditional mutant mice. We thank the American Lung Association (K.P., J.S.), the Parker B. Francis Fellowship Program (K.P.), the Damon Runyon Cancer Research Foundation (J.S.), the American Cancer Society (Research scholar awards RSG-10-071-TBG for J.S. and RSG-08-082-01-MGO for C.F.K.), the National Institute of Health (C.F.K. RO1 HL090136 and U01 HL100402), the Harvard Stem Cell Institute (D.M.R., C.F.K.), United Against Lung Cancer (K.K.W.) and the American Lung Association (K.K.W.) NCI CA140594 (KKW).
Abbreviations
- SCLC
small cell lung carcinoma
- Rb
retinoblastoma
- Ad
adenovirus
- BASCs
bronchioalveolar stem cells
- BADJ
bronchioalveolar duct junction
- AT2 cells
alveolar type 2 cells
- NEP
neuroendocrine progenitor
- NEC
neuroendocrine cell
- NEB
neuroendocrine body
- CGRP
calcitonin-gene related protein
- SYP
synaptophysin
- CCSP
clara cell specific protein
- SP-C
surfactant protein C
- AAH
atypical alveolar hyperplasia
References
- 1.Beasley MB, Brambilla E, Travis WD. The 2004 World Health Organization classification of lung tumors. Semin Roentgenol. 2005;40:90–97. doi: 10.1053/j.ro.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 2.Travis WD, Travis LB, Devesa SS. Lung cancer. Cancer. 1995;75:191–202. doi: 10.1002/1097-0142(19950101)75:1+<191::AID-CNCR2820751307>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 3.Worden FP, Kalemkerian GP. Therapeutic advances in small cell lung cancer. Expert Opin Investig Drugs. 2000;9:565–579. doi: 10.1517/13543784.9.3.565. [DOI] [PubMed] [Google Scholar]
- 4.Socinski MA, Bogart JA. Limited-stage small-cell lung cancer: the current status of combined-modality therapy. J Clin Oncol. 2007;25:4137–4145. doi: 10.1200/JCO.2007.11.5303. [DOI] [PubMed] [Google Scholar]
- 5.Adelstein DJ, Tomashefski J, Jr, Snow NJ, Horrigan TP, Hines JD. Mixed small cell and non-small cell lung cancer. Chest. 1986;89:699–704. doi: 10.1378/chest.89.5.699. [DOI] [PubMed] [Google Scholar]
- 6.Sørhaug S, Steinshamn S, Haaverstad R, Nordrum IS, Martinsen TC, Waldum HL. Expression of neuroendocrine markers in non-small cell lung cancer. APMIS. 2007;115:152–163. doi: 10.1111/j.1600-0463.2007.apm_542.x. [DOI] [PubMed] [Google Scholar]
- 7.Sweet-Cordero A, Mukherjee S, Subramanian A, You H, Roix JJ, Ladd-Acosta C, et al. An oncogenic KRAS2 expression signature identified by cross-species gene-expression analysis. Nat Genet. 2005;37:48–55. doi: 10.1038/ng1490. [DOI] [PubMed] [Google Scholar]
- 8.Shmidt EN, Nitkin AY. Pathology of Mouse Models of Human Lung Cancer. Comp Med. 2004;54:23–26. [PubMed] [Google Scholar]
- 9.Meuwissen R, Linn SC, Linnoila RI, Zevenhoven J, Mooi WJ, Berns A. Induction of small cell lung cancer by somatic inactivation of both Trp53 and Rb1 in a conditional mouse model. Cancer Cell. 2003;4:181–189. doi: 10.1016/S1535-6108(03)00220-4. [DOI] [PubMed] [Google Scholar]
- 10.Beasley MB, Lantuejoul S, Abbondanzo S, Chu WS, Hasleton PS, Travis WD, et al. The P16/cyclin D1/Rb pathway in neuroendocrine tumors of the lung. Hum Pathol. 2003;34:136–142. doi: 10.1053/hupa.2003.8. [DOI] [PubMed] [Google Scholar]
- 11.Berns A. Stem cells for lung cancer? Cell. 2005;121:811–813. doi: 10.1016/j.cell.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 12.Pitt BR, Ortiz LA. Stem cells in lung biology. Am J Physiol Lung Cell Mol Physiol. 2004;286:621–623. doi: 10.1152/ajplung.00392.2003. [DOI] [PubMed] [Google Scholar]
- 13.Hong KU, Reynolds SD, Watkins S, Fuchs E, Stripp BR. Basal cells are a multipotent progenitor capable of renewing the bronchial epithelium. Am J Pathol. 2004;164:577–588. doi: 10.1016/S0002-9440(10)63147-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reynolds SD, Giangreco A, Power JH, Stripp BR. Neuroepithelial bodies of pulmonary airways serve as a reservoir of progenitor cells capable of epithelial regeneration. Am J Pathol. 2000;156:269–278. doi: 10.1016/S0002-9440(10)64727-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, et al. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–835. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 16.Sutherland KD, Berns A. Cell of origin of lung cancer. Mol Oncol. 2010;4:397–403. doi: 10.1016/j.molonc.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Calbó J, Meuwissen R, van Montfort E, van Tellingen O, Berns A. Genotype-phenotype relationships in a mouse model for human small-cell lung cancer. Cold Spring Harb Symp Quant Biol. 2005;70:225–232. doi: 10.1101/sqb.2005.70.026. [DOI] [PubMed] [Google Scholar]
- 18.Schaffer BE, Park KS, Yiu G, Conklin JF, Lin C, Burkhart DL, et al. Loss of p130 accelerates tumor development in a mouse model for human small-cell lung carcinoma. Cancer Res. 2010;70:3877–3883. doi: 10.1158/0008-5472.CAN-09-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynolds SD, Zemke AC, Giangreco A, Brockway BL, Teisanu RM, Drake JA, et al. Conditional stabilization of beta-catenin expands the pool of lung stem cells. Stem Cells. 2008;26:1337–1346. doi: 10.1634/stemcells.2008-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Simon DM, Arikan MC, Srisuma S, Bhattacharya S, Tsai LW, Ingenito EP, et al. Epithelial cell PPAR[gamma] contributes to normal lung maturation. FASEB J. 2006;20:1507–1509. doi: 10.1096/fj.05-5410fje. [DOI] [PubMed] [Google Scholar]
- 21.Ji H, Houghton AM, Mariani TJ, Perera S, Kim CB, Padera R, et al. K-ras activation generates an inflammatory response in lung tumors. Oncogene. 2006;25:2105–2112. doi: 10.1038/sj.onc.1209237. [DOI] [PubMed] [Google Scholar]
- 22.Chang A, Ramsay P, Zhao B, Park M, Magdaleno S, Reardon MJ, et al. Physiological regulation of uteroglobin/CCSP expression. Ann N Y Acad Sci. 2000;923:181–192. doi: 10.1111/j.1749-6632.2000.tb05529.x. [DOI] [PubMed] [Google Scholar]
- 23.Soriano P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- 24.Gou D, Narasaraju T, Chintagari NR, Jin N, Wang P, Liu L. Gene silencing in alveolar type II cells using cell-specific promoter in vitro and in vivo. Nucleic Acids Res. 2004;32:134. doi: 10.1093/nar/gnh129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Perez-Gil J. Structure of pulmonary surfactant membranes and films: the role of proteins and lipid-protein interactions. Biochim Biophys Acta. 2008;1778:1676–1695. doi: 10.1016/j.bbamem.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 26.Srinivas S, Watanabe T, Lin CS, William CM, Tanabe Y, Jessell TM, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jackson EL, Willis N, Mercer K, Bronson RT, Crowley D, Montoya R, et al. Analysis of lung tumor initiation and progression using conditional expression of oncogenic K-ras. Genes Dev. 2001;15:3243–3248. doi: 10.1101/gad.943001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perl AK, Tichelaar JW, Whitsett JA. Conditional gene expression in the respiratory epithelium of the mouse. Transgenic Res. 2002;11:21–29. doi: 10.1023/A:1013986627504. [DOI] [PubMed] [Google Scholar]
- 29.Perl AK, Wert SE, Nagy A, Lobe CG, Whitsett JA. Early restriction of peripheral and proximal cell lineages during formation of the lung. Proc Natl Acad Sci USA. 2002;99:10482–10487. doi: 10.1073/pnas.152238499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simpson DS, Mason-Richie NA, Gettler CA, Wikenheiser-Brokamp KA. Retinoblastoma Family Proteins Have Distinct Functions in Pulmonary Epithelial Cells In vivo Critical for Suppressing Cell Growth and Tumorigenesis. Cancer Res. 2009 doi: 10.1158/0008-5472.CAN-09-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutherland KD, Proost N, Brouns I, Adriaensen D, Song JY, Berns A. Cell of origin of small cell lung cancer: inactivation of Trp53 and rb1 in distinct cell types of adult mouse lung. Cancer Cell. 2011;19:754–764. doi: 10.1016/j.ccr.2011.04.019. [DOI] [PubMed] [Google Scholar]
- 32.Gu G, Wells JM, Dombkowski D, Preffer F, Aronow B, Melton DA. Global expression analysis of gene regulatory pathways during endocrine pancreatic development. Development. 2004;131:165–179. doi: 10.1242/dev.00921. [DOI] [PubMed] [Google Scholar]
- 33.Ajioka I, Martins RA, Bayazitov IT, Donovan S, Johnson DA, Frase S, et al. Differentiated horizontal interneurons clonally expand to form metastatic retinoblastoma in mice. Cell. 2007;131:378–390. doi: 10.1016/j.cell.2007.09.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicolay BN, Bayarmagnai B, Moon NS, Benevolenskaya EV, Frolov MV. Combined inactivation of pRB and hippo pathways induces dedifferentiation in the Drosophila retina. PLoS Genet. 2010;6:1000918. doi: 10.1371/journal.pgen.1000918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Viatour P, Somervaille TC, Venkatasubrahmanyam S, Kogan S, McLaughlin ME, Weissman IL, et al. Hematopoietic stem cell quiescence is maintained by compound contributions of the retinoblastoma gene family. Cell Stem Cell. 2008;3:416–428. doi: 10.1016/j.stem.2008.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Calo E, Quintero-Estades JA, Danielian PS, Nedelcu S, Berman SD, Lees JA. Rb regulates fate choice and lineage commitment in vivo. Nature. 2010;466:1110–1114. doi: 10.1038/nature09264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang Z, Deng T, Jones R, Li H, Herschkowitz JI, Liu JC, et al. Rb deletion in mouse mammary progenitors induces luminal-B or basal-like/EMT tumor subtypes depending on p53 status. J Clin Invest. 2010;120:3296–3309. doi: 10.1172/JCI41490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou Z, Flesken-Nikitin A, Nikitin AY. Prostate cancer associated with p53 and Rb deficiency arises from the stem/progenitor cell-enriched proximal region of prostatic ducts. Cancer Res. 2007;67:5683–5690. doi: 10.1158/0008-5472.CAN-07-0768. [DOI] [PubMed] [Google Scholar]
- 39.Macpherson D. Insights from mouse models into human retinoblastoma. Cell Div. 2008;3:9. doi: 10.1186/1747-1028-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Williams BO, Schmitt EM, Remington L, Bronson RT, Albert DM, Weinberg RA, et al. Extensive contribution of Rb-deficient cells to adult chimeric mice with limited histopathological consequences. EMBO J. 1994;13:4251–4259. doi: 10.1002/j.1460-2075.1994.tb06745.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jacks T, Fazeli A, Schmitt EM, Bronson RT, Goodell MA, Weinberg RA. Effects of an Rb mutation in the mouse. Nature. 1992;359:295–300. doi: 10.1038/359295a0. [DOI] [PubMed] [Google Scholar]
- 42.Wikenheiser-Brokamp KA. Retinoblastoma family proteins: insights gained through genetic manipulation of mice. Cell Mol Life Sci. 2006;63:767–780. doi: 10.1007/s00018-005-5487-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wuenschell CW, Sunday ME, Singh G, Minoo P, Slavkin HC, Warburton D. Embryonic mouse lung epithelial progenitor cells co-express immuno-histochemical markers of diverse mature cell lineages. J Histochem Cytochem. 1996;44:113–123. doi: 10.1177/44.2.8609367. [DOI] [PubMed] [Google Scholar]
- 44.Rawlins EL, Clark CP, Xue Y, Hogan BL. The Id2+ distal tip lung epithelium contains individual multipotent embryonic progenitor cells. Development. 2009;136:3741–3745. doi: 10.1242/dev.037317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brambilla E, Lantuejoul S, Sturm N. Divergent differentiation in neuroendocrine lung tumors. Semin Diagn Pathol. 2000;17:138–148. [PubMed] [Google Scholar]
- 46.Sequist LV, Waltman BA, Dias-Santagata D, Digumarthy S, Turke AB, Fidias P, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3:26. doi: 10.1126/scitranslmed.3002003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sage J, Miller AL, Perez-Mancera PA, Wysocki JM, Jacks T. Acute mutation of retinoblastoma gene function is sufficient for cell cycle re-entry. Nature. 2003;424:223–228. doi: 10.1038/nature01764. [DOI] [PubMed] [Google Scholar]
- 48.Marino S, Vooijs M, van Der Gulden H, Jonkers J, Berns A. Induction of medulloblastomas in p53-null mutant mice by somatic inactivation of Rb in the external granular layer cells of the cerebellum. Genes Dev. 2000;14:994–1004. [PMC free article] [PubMed] [Google Scholar]
- 49.Burkhart DL, Ngai LK, Roake CM, Viatour P, Thangavel C, Ho VM, et al. Regulation of RB transcription in vivo by RB family members. Mol Cell Biol. 2010;30:1729–1745. doi: 10.1128/MCB.00952-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McLaughlin ME, Kruger GM, Slocum KL, Crowley D, Michaud NA, Huang J, et al. The Nf2 tumor suppressor regulates cell-cell adhesion during tissue fusion. Proc Natl Acad Sci USA. 2007;104:3261–3266. doi: 10.1073/pnas.0700044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ho VM, Schaffer BE, Karnezis AN, Park KS, Sage J. The retinoblastoma gene Rb and its family member p130 suppress lung adenocarcinoma induced by oncogenic K-Ras. Oncogene. 2009;28:1393–1399. doi: 10.1038/onc.2008.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.MacPherson D, Sage J, Kim T, Ho D, McLaughlin ME, Jacks T. Cell type-specific effects of Rb deletion in the murine retina. Genes Dev. 2004;18:1681–1694. doi: 10.1101/gad.1203304. [DOI] [PMC free article] [PubMed] [Google Scholar]