Abstract
Objective
To report the tolerance of indinavir combined with ritonavir (IDV/r 800/100mg) twice daily (bid) in sub-Saharan African HIV-infected adults.
Design
Prospective cohort study.
Methods
HAART-naïves patients started zidovudine plus lamivudine plus IDV/r 800/100mg bid. Follow-up included: standardised documentation of morbidity; CD4+ cell count, creatininemia, plasma HIV-1 RNA, and IDV minimal plasma concentration (Cmin) measurements at month-1 (M1), M3 and M6.
Results
70 HIV-1 infected adults (68 women, median CD4 235/mm3) started HAART. At M6, 63% had undetectable viral load, and the median gain in CD4 since baseline was +128/mm3. During the first six months, 21 patients experimented 23 treatment modifications (reduction to IDV/r 400/100mg bid: n=11; switch for efavirenz: n=11; zidovudine replaced by stavudine: n=1), including 22 for digestive intolerance and one for severe anaemia. At M1, M3 and M6, 67, 59 and 48 patients were still receiving IDV/r 800/100 mg bid, of whom 70%, 72% and 60% had IDV Cmin above 5 ng/mL, respectively. In these patients, at M1, M3 and M6, the mean (± SD) IDV Cmin, were 3431 ± 3835ng/ml, 2288 ± 2116ng/ml and 1543 ± 2398ng/ml, respectively. There was no renal insufficiency of any grade, and no symptom of urinary stones.
Conclusion
The IDV/r 800/100mg bid containing regimen led to high IDV Cmin, and high rate of digestive intolerance. There was a surprising lack of nephrological side-effects during the 6 months of follow-up, supporting the hypothesis that nephrological tolerance of IDV might be higher in sub-Saharan African individuals than in American or European ones.
Keywords: Adults - sub-Saharan Africa, Ritonavir - Indinavir - toxicity
Keywords: Adult; Cohort Studies; Cote d'Ivoire; Female; Follow-Up Studies; HIV Infections; blood; drug therapy; virology; HIV Protease Inhibitors; adverse effects; blood; therapeutic use; HIV-1; isolation & purification; HIV-2; isolation & purification; Humans; Indinavir; adverse effects; blood; therapeutic use; Male; RNA, Viral; blood; Retrospective Studies; Time Factors; Treatment Outcome; Viral Load
Introduction
The benefits of pharmacokinetically enhanced protease inhibitor (PI) regimens using ritonavir (RTV) as a booster have long been demonstrated [1,2]. RTV boosting reduces the number of drug intake – thus also reducing the treatment cost -, and diminishes the constraints of food and water requirements, especially for Indinavir (IDV) [3]. RTV-boosted PI (PI/r) regimens are currently rarely employed in sub-Saharan Africa, because RTV soft capsules need to be stored below 25°C. Although World Health Organisation (WHO) experts recognize that PI/r regimens represent valuable alternatives to non nuclesoside reverse transcriptase inhibitors (NNRTI)-based regimens [4,5], no experience of their use has been reported in sub-Saharan Africa so far.
We report here the 6 month-efficacy and tolerance of a highly active antiretroviral treatment (HAART) based on an indinavir/ritonavir (IDV/r) 800/100 mg twice daily (bid) regimen in a cohort of HIV-1 infected patients in Abidjan, the economic capital city of Côte d’Ivoire, West Africa.
Patients and Methods
In December 2002, a randomised trial (Trivacan ANRS 1269) of structured treatment interruptions (STI) was launched in Abidjan [6]. The trial protocol was approved by the ethics committee of the Ministry of Health of Côte d’Ivoire and the institutional review board of the French Agency of Research on AIDS (ANRS, Paris, France). Inclusion criteria in the trial have been previously described [7]. Between inclusion and randomisation in the STI arms, patients received at least 6 months of continuous HAART. We describe here the 6-month outcomes of treatment-naïve patients who started an IDV/r-based regimen at inclusion in the pre-randomisation phase of the trial.
At inclusion, and every month thereafter, patients received 35 days of treatment, i.e. 70 tablets of Duovir® (ZDV 300 mg and 3TC 150, Cipla LTD, Mumbai, India), 140 tablets of Crixivan® (IDV 400 mg, Merk Sharp and Dohme, Haarlem, The Netherlands) and 70 soft capsules of Norvir® (RTV 100 mg, Abbott, Zwolle, The Netherlands). At inclusion, patients were recommended to ingest a minimum of two litters of water per day. They were asked to return to their study centre at day seven, at month-1 (M1) and every month thereafter. During these monthly visits, questionnaires were administered to record adherence to treatment during the previous four days. Patients had free access to the study clinics whenever they had a medical problem. Symptoms were managed following standardized algorithms of treatment [8].
At inclusion, M3 and M6, blood cell count (MaxM® coulter, Beckman, Paris, France), CD4 count (True Count® technique on FACScan®, Becton Dickinson, Aalst-Erembodegem, Belgium), plasma HIV-1 RNA (real-time PCR on Taq Man technology Abi Prism 7000, Applied Biosystems, limit of detection at 300 copies/mL) [9], serum liver enzymes and creatininemia were measured. Additional samples were collected at M1, M3 and M6 for plasma and serum depository (storage in a −80°C deep-freezer). Patients were asked not to take their morning drugs before month-1, month-3 and month-6 blood sample collections. Blood collections were thus performed early in the morning, in patients whose last drug intake was on the previous evening.
In August 2005, all deep-freezed stored samples were sent to France for IDV and ZDV plasma concentration measurements by means of high performance liquid chromatography coupled with UV-detection (respective LOQ 5 and 10 ng/mL with previously described methods) [10,11]. Patients with a ZDV plasma concentration above 10 ng/mL were suspected to have taken their treatment within the preceding eight hours. Consequently, their IDV plasma concentrations were considered as non-minimal concentrations (non-Cmin). For all other patients, the IDV concentration was classified as minimal concentration (Cmin). The value of 10 ng/mL eight hours after the last drug intake was chosen according to the pharmacokinetic characteristics of ZDV in HIV-infected patients with normal renal function [12].
Results
From December 2002 to August 2003, 70 HIV-1 infected or HIV-1+2 infected patients started ZDV-3TC-IDV/r. Baseline and follow-up characteristics of patients are shown in table 1.
Table 1.
Baseline and follow-up characteristics
| Baseline | ||
| Male/Female, n (%) | 2/68 | (3/97%) |
| Age (years), median (IQR) | 31 | (29 – 36) |
| Dual VIH 1+2 infected, n (%) | 3 | (4%) |
| WHO clinical stage, n (%) | ||
| 1 | 15 | (21%) |
| 2 | 32 | (46%) |
| 3 | 18 | (26%) |
| 4 | 5 | (7%) |
| CD4 count (/mm3), median (IQR) | 235 | (194 – 313) |
| Plasma HIV RNA (log10/ml), median (IQR) | 4.9 | (4.1–5.4) |
| Haemoglobin level (g/l), median (IQR) | 11.3 | (10.4 – 12) |
| Creatinine clearance (ml/min), median (IQR) | 113.7 | (102–132) |
| Positive serum HBs antigen, n (%) | 7 | (10%) |
| Follow-up | ||
| Cumulative (person-months) | 427 | |
| Per patients (months), median (IQR) | 6.1 | (6.1-6.1) |
| Plasma HIV RNA at M | ||
| log10/ml, median (IQR) | 0.0 | (0.0–3.9) |
| < 300 copies/mL, n (%) | 44 | (63%) |
n: number of patients; %: percentage; IQR: interquartile range; g/l: gramme per litre; /mm3: per cubic millimeter
During the first six months of follow-up, no patient died or was lost to follow-up. From M1 to M6, all percentages of actual/scheduled monthly visits were above 92%. At M1, M2, M3, M4, M5 and M6, respectively 16%, 17%, 19%, 9%, 10% and 7% of the patients declared that they had missed at least one antiretroviral drug intake during the previous four days. The median gain in CD4 since baseline was +124/mm3 (interquartile range [IQR] +49;+218) at M6. At M3 and M6, 45 (64%) and 44 (63%) patients had plasma HIV RNA below 300 copies/mL, respectively.
During the first six months, 21 patients (31%) experimented 23 HAART regimen modifications, including 21 patients with 22 regimen modifications for digestive intolerance (reduction to IDV/r 400/100mg bid: n=10; switch for efavirenz: n=10; reduction to 400/100mg bid followed by switch for efavirenz: n=1) and one patient with a modification for severe anaemia (zidovudine replaced by stavudine). All digestive intolerance events were severe vomiting, including four with admission to the day care hospital for intravenous treatment, two with associated sub-icterus, and one with grade 3 elevation of transaminases. There was no other grade 3 or grade 4 hepatitis.
No renal insufficiency of any grade and no symptom evocative of uro-nephrological lithiasis were documented. The median creatinine clearance was estimated at 114 ml/min (IQR 103;132) at baseline and 100 ml/min (80;119) at M6 (median difference between baseline and M6: −12 ml/min, IQR −32;+ 0.0).
Table 2 shows the number of patients who: (1) were still receiving IDV/r 800/100 mg bid, (2) had been reduced to IDV/r 400/100mg bid, and (3) had been switched for a non IDV-containing regimen at M1, M3 and M6. Figure 1 describes IDV plasma Cmin and non-Cmin in patients still receiving IDV/r 800/100 mg bid.
Table 2.
number of patients with IDV/r 800/100 and IDV/r 400/100 bid-containing regimens at month-1, month-3 and month-6
| HAART regimen | Number of patients at | ||
|---|---|---|---|
| Month-1 | Month-3 | Month-6 | |
| IDV/r 800/100 bid-containing regimen | 67 | 59 | 49 |
| ZDV plasma concentration unavailable | 5 | 2 | 1 |
| ZDV plasma concentration ≥ 10 ng/ml* | 9 | 11 | 8 |
| ZDV plasma concentration < 10 ng/ml** | 53 | 46 | 40 |
| IDV/r 400/100 bid-containing regimen | 0 | 3 | 10 |
| Non IDV-containing regimen | 3 | 8 | 11 |
Patients with detectable ZDV plasma concentrations were suspected to have taken their antiretroviral drugs within the preceding eight hours. For these patients, the IDV plasma concentration was classified as non-Cmin (see figure 1A)
Patients with undetectable ZDV plasma concentrations were confirmed not to have taken their antiretroviral drugs within the preceding eight hours. For these patients, the IDV plasma concentration was classified as Cmin (see figure 1B).
Figure 1.
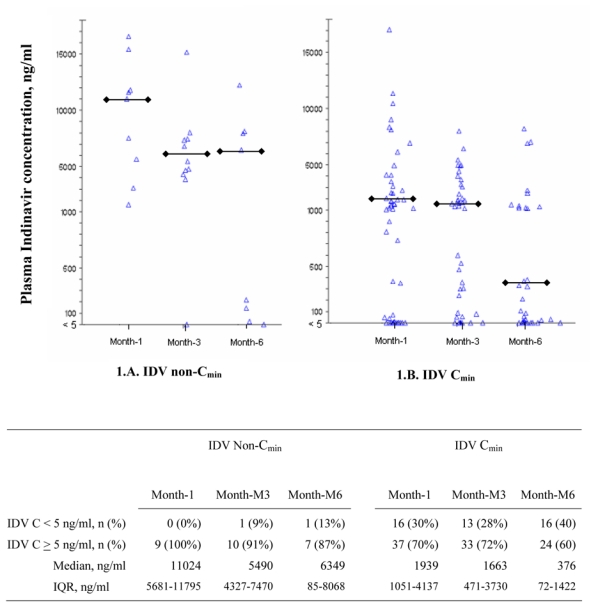
Plasma IDV concentration at M1, M3 and M6 in patients treated with IDV/r (800/100 mg bid)
1A: non-IDV Cmin (patients with ZDV plasma concentration equal or above 10 ng/mL)
1B: IDV Cmin (patients with ZDV plasma concentration below 10 ng/mL)
Each plot represents one IDV concentration measurement
 : median value among patients with IDV concentration ≥ 10 ng/ml
: median value among patients with IDV concentration ≥ 10 ng/ml
C: concentration; IQR: interquartile range
Discussion
Within our cohort study of sub-Saharan African adults starting a IDV/r 800/100 mg bid containing regimen, the percentage of patients with an undetectable viral load at month-6 and the mean gain in CD4 after 6 months of follow-up were in the lower bracket of those previously described in industrialized countries [13–15]. Meanwhile: (i) the percentage of patients with severe digestive intolerance leading to IDV/r dosage reduction or to IDV/r replacement by another drug reached 30%; (ii) the mean IDV concentration in patients still on the 800/100 mg bid dosage decreased within time, suggesting that a significant proportion of patients who were not asked by the medical team to reduce the IDV/r dosage to 400/100 mg bid probably decided by themselves to interrupt the treatment; (iii) IDV Cmin were in the upper bracket of those previously reported in patients receiving the 800/100 mg bid regimen [3,14,16,17]; (iv) In spite of these high concentrations, there was no episode of uro-nephrological side-effects.
To our knowledge, this is the first time that an experience with IDV/r is reported in sub-Saharan Africa. In industrialized countries, urinary stones have been reported to occur in 18% to 33% of patients on IDV/r 800/100 mg bid [13,14]. IDV nephrological toxicity has been associated with a high urinary pH, a low diuresis, a high concentration of the parent drug or its metabolites in urine, an increasing area under the curve, an IDV Cmin above 500 ng/ml, a low lean body mass, warm weather environments, and the concomitant use of aciclovir [16,18,19–22]. In tropical areas with high temperatures and high levels of humidity, the IDV concentration in urine would have been expected to be higher, thus resulting in higher uro-nephrological toxicity [22, 23]. Furthermore, the urinary pH of HIV-infected adults living in West Africa has been reported to be higher than that of European adults, and this should also result in a higher frequency of IDV related nephrolithiasis in Africa [24]. Finally, most of our patients were women, contrary to most previous studies on IDV/r in industrialized countries. Previous studies demonstrated a higher IDV apparent bioavailability in females than in males or suggested a sex-related metabolism of IDV, but this is not likely to explain the lack of nephrological complications in our study [25,26].
One limitation of our study is the 6-month follow-up, compared with higher durations of exposure to IDV in previous studies in industrialized countries [3,14,16,17]. However, the risk of IDV-associated renal complications has been reported to be higher in patients with IDV treatment < 74 weeks than in those with longer exposure [21], and indinavir crystalluria is frequently observed soon after initiation of therapy [27]. Furthermore, our patients were followed up under cohort conditions, with no patients lost to follow up and with free access to a study clinic at any time. Under these circumstances, the frequency of urinary stones is not likely to have been greatly underestimated. Previous studies showed an increasing risk of urinary stones in Caucasian US patients [28] and a low incidence of IDV nephrological side effects in a population predominantly American with African ancestry [15]. Following these reports, our data support the hypothesis of a lower frequency of IDV-associated urinary stones in sub-Saharan African adults.
In our study, we were unable to assess the efficacy and tolerance of the IDV/r 400/100 mg dosage, because of the reduced number of patients who switched from the IDV/r 800/100 mg bid to the 400/100 mg bid dosage and of the consecutive reduced followed-up time on the 400/100 mg bid dosage. In industrialized countries, IDV/r is now given at the 400/100 mg bid dosage [29,30], provided that plasma concentration measurement-driven dosage adaptations are possible. In most sub-Saharan African areas, therapeutic drug monitoring is not routinely available. Given the low digestive tolerance – leading to low adherence -, and despite of the good nephrological tolerance of the 800/100 mg bid dosage in our study, 400/100 mg bid should probably be the recommended first-line dosage of IDV/r in sub-Saharan Africa. However, this remains to be carefully evaluated in terms of efficacy and tolerance.
Acknowledgments
This study was supported by the French Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS), and the Ivoirian Ministry of Public Health within the collaborative Programme PAC-CI.
References
- 1.Saah AJ, Winchell GA, Nessly ML, Seniuk MA, Rhodes RR, Deutsch PJ. Pharmacokinetic profile and tolerability of indinavir-ritonavir combinations in healthy volunteers. Antimicrob Agents Chemother. 2001;45:2710–5. doi: 10.1128/AAC.45.10.2710-2715.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bucher HC, Bichsel M, Taffe P, et al. Ritonavir plus saquinavir versus single protease inhibitor therapy in protease inhibitor-naive HIV-infected patients: the Swiss HIV Cohort Study. HIV Med. 2002;3:247–53. doi: 10.1046/j.1468-1293.2002.00113.x. [DOI] [PubMed] [Google Scholar]
- 3.Aarnoutse RE, Wasmuth JC, Fatkenheuer G, et al. Administration of indinavir and low-dose ritonavir (800/100 mg twice daily) with food reduces nephrotoxic peak plasma levels of indinavir. Antivir Ther. 2003;8:309–14. [PubMed] [Google Scholar]
- 4.Hirsch MS, Brun-Vezinet F, Clotet B, et al. Antiretroviral drug resistance testing in adults infected with human immunodeficiency virus type 1: 2003 recommendations of an International AIDS Society-USA Panel. Clin Infect Dis. 2003;37:113–28. doi: 10.1086/375597. [DOI] [PubMed] [Google Scholar]
- 5.Condra JH, Holder DJ, Schleif WA, et al. Genetic correlates of in vivo viral resistance to indinavir, a human immunodeficiency virus type 1 protease inhibitor. J Virol. 1996;70:8270–6. doi: 10.1128/jvi.70.12.8270-8276.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Danel C, Moh R, Minga A, et al. Failure of a CD4-guided structured antiretroviral treatment interruption strategy in HIV-infected adults: a randomized trial in West Africa (Trivacan ANRS1269 Trial) Lancet. 2006 doi: 10.1016/S0140-6736(06)68887-9. in press. [DOI] [PubMed] [Google Scholar]
- 7.Moh R, Danel C, Sorho S, et al. Haematological changes in adults receiving a zidovudine-containing HAART regimen in combination with cotrimoxazole in Cote d’Ivoire. Antivir Ther. 2005;10:615–24. doi: 10.1177/135965350501000510. [DOI] [PubMed] [Google Scholar]
- 8.Anglaret X, Dakoury-Dogbo N, Bonard D, et al. Causes and empirical treatment of fever in HIV-infected adult outpatients, Abidjan, Cote d’Ivoire. AIDS. 2002;16:909–18. doi: 10.1097/00002030-200204120-00011. [DOI] [PubMed] [Google Scholar]
- 9.Rouet F, Ekouevi D, Chaix M, et al. Real-Time PCR Test for Human Immunodeficiency Virus Type-1 Infection in an African Resource-Limited Setting. J Clin Microbiol. 2005;43:2709–17. doi: 10.1128/JCM.43.6.2709-2717.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Good SS, Reynolds DJ, de Miranda P. Simultaneous quantification of zidovudine and its glucuronide in serum by high-performance liquid chromatography. J Chromatogr. 1988;431:123–33. doi: 10.1016/s0378-4347(00)83075-3. [DOI] [PubMed] [Google Scholar]
- 11.Woolf E, Au T, Haddix H, Matuszewski B. Determination of L-735 524, an human immunodeficiency virus protease inhibitor, in human plasma and urine via high-performance liquid chromatography with column switching. J Chromatogr A. 1995;692:45–52. doi: 10.1016/0021-9673(94)00608-c. [DOI] [PubMed] [Google Scholar]
- 12.Drew RH, Weller S, Gallis HA, Walmer KA, Bartlett JA, Blum MR. Bioequivalence assessment of zidovudine (Retrovir) syrup, solution, and capsule formulations in patients infected with human immunodeficiency virus. Antimicrob Agents Chemother. 1989;33:1801–3. doi: 10.1128/aac.33.10.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Voigt E, Wickesberg A, Wasmuth JC, et al. First-line ritonavir/indinavir 100/800 mg twice daily plus nucleoside reverse transcriptase inhibitors in a German multicentre study: 48-week results. HIV Med. 2002;3:277–82. doi: 10.1046/j.1468-1293.2002.00123.x. [DOI] [PubMed] [Google Scholar]
- 14.Konopnicki D, De Wit S, Poll B, Crommentuyn K, Huitema A, Clumeck N. Indinavir/ritonavir-based therapy in HIV-1-infected antiretroviral therapy-naive patients: comparison of 800/100 mg and 400/100 mg twice daily. HIV Med. 2005;6:1–6. doi: 10.1111/j.1468-1293.2005.00255.x. [DOI] [PubMed] [Google Scholar]
- 15.Acosta EP, Wu H, Hammer SM, et al. Comparison of two indinavir/ritonavir regimens in the treatment of HIV-infected individuals. J Acquir Immune Defic Syndr. 2004;37:1358–66. doi: 10.1097/00126334-200411010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Solas C, Basso S, Poizot-Martin I, et al. High indinavir Cmin is associated with higher toxicity in patients on indinavir-ritonavir 800/100 mg twice-daily regimen. J Acquir Immune Defic Syndr. 2002;29:374–7. doi: 10.1097/00126334-200204010-00008. [DOI] [PubMed] [Google Scholar]
- 17.Burger DM, Hugen PW, Aarnoutse RE, et al. A retrospective, cohort-based survey of patients using twice-daily indinavir + ritonavir combinations: pharmacokinetics, safety, and efficacy. J Acquir Immune Defic Syndr. 2001;26:218–24. doi: 10.1097/00042560-200103010-00003. [DOI] [PubMed] [Google Scholar]
- 18.Arnaiz JA, Mallolas J, Podzamczer D, et al. Continued indinavir versus switching to indinavir/ritonavir in HIV-infected patients with suppressed viral load. AIDS. 2003;17:831–40. doi: 10.1097/00002030-200304110-00008. [DOI] [PubMed] [Google Scholar]
- 19.Burger D, Boyd M, Duncombe C, et al. Pharmacokinetics and pharmacodynamics of indinavir with or without low-dose ritonavir in HIV-infected Thai patients. J Antimicrob Chemother. 2003;51:1231–8. doi: 10.1093/jac/dkg198. [DOI] [PubMed] [Google Scholar]
- 20.Duval X, Peytavin G, Albert I, et al. Determination of indinavir and nelfinavir trough plasma concentration efficacy thresholds according to virological response in HIV-infected patients. HIV Med. 2004;5:307–13. doi: 10.1111/j.1468-1293.2004.00226.x. [DOI] [PubMed] [Google Scholar]
- 21.Herman JS, Ives NJ, Nelson M, Gazzard BG, Easterbrook PJ. Incidence and risk factors for the development of indinavir-associated renal complications. J Antimicrob Chemother. 2001;48:355–60. doi: 10.1093/jac/48.3.355. [DOI] [PubMed] [Google Scholar]
- 22.Dieleman JP, Sturkenboom MC, Jambroes M, et al. Risk factors for urological symptoms in a cohort of users of the HIV protease inhibitor indinavir sulfate: the ATHENA cohort. Arch Intern Med. 2002;162:1493–501. doi: 10.1001/archinte.162.13.1493. [DOI] [PubMed] [Google Scholar]
- 23.Balani SK, Woolf EJ, Hoagland VL, et al. Disposition of indinavir, a potent HIV-1 protease inhibitor, after an oral dose in humans. Drug Metab Dispos. 1996;24:1389–94. [PubMed] [Google Scholar]
- 24.Mortier E, Toure S, Seyler C, Bloch M, Anglaret X. Urinary pH in HIV-infected adults in Ivory Coast and in France. AIDS. 2003;17:2003–5. doi: 10.1097/00002030-200309050-00028. [DOI] [PubMed] [Google Scholar]
- 25.Kappelhoff BS, Huitema AD, Sankatsing SU, et al. Population pharmacokinetics of indinavir alone and in combination with ritonavir in HIV-1-infected patients. Br J Clin Pharmacol. 2005;60:276–86. doi: 10.1111/j.1365-2125.2005.02436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lin JH, Chiba M, Chen IW, Nishime JA, Vastag KJ. Sex-dependent pharmacokinetics of indinavir: in vivo and in vitro evidence. Drug Metab Dispos. 1996;24:1298–306. [PubMed] [Google Scholar]
- 27.Gagnon RF, Tecimer SN, Watters AK, Tsoukas CM. Prospective study of urinalysis abnormalities in HIV-positive individuals treated with indinavir. Am J Kidney Dis. 2000;36:507–15. doi: 10.1053/ajkd.2000.9791. [DOI] [PubMed] [Google Scholar]
- 28.Dworkin MS, Wan PT. Indinavir, zidovudine, lamivudine: 3-year follow-up. Ann Intern Med. 2001;134:165. doi: 10.7326/0003-4819-134-2-200101160-00019. [DOI] [PubMed] [Google Scholar]
- 29.Ghosn J, Lamotte C, Ait-Mohand H, et al. Efficacy of a twice-daily antiretroviral regimen containing 100 mg ritonavir/400 mg indinavir in HIV-infected patients. AIDS. 2003;17:209–14. doi: 10.1097/00002030-200301240-00011. [DOI] [PubMed] [Google Scholar]
- 30.Duvivier C, Myrto A, Marcelin AG, et al. Efficacy and safety of ritonavir/indinavir 100/400 mg twice daily in combination with two nucleoside analogues in antiretroviral treatment-naive HIV-infected individuals. Antivir Ther. 2003;8:603–9. [PubMed] [Google Scholar]


