Abstract
Atopic dermatitis (AD) is a common inflammatory skin disease caused by multiple genetic and environmental factors. AD is characterized by the local infiltration of T helper type 2 (Th2) cells. Recent clinical studies have shown important roles of the Th2 chemokines, CCL22 and CCL17 in the pathogenesis of AD. To investigate whether polymorphisms of the CCL22 gene affect the susceptibility to AD, we conducted association studies and functional studies of the related variants. We first resequenced the CCL22 gene and found a total of 39 SNPs. We selected seven tag SNPs in the CCL22 gene, and conducted association studies using two independent Japanese populations (1st population, 916 cases and 1,032 controls; 2nd population 1,034 cases and 1,004 controls). After the association results were combined by inverse variance method, we observed a significant association at rs4359426 (meta-analysis, combined P = 9.6×10−6; OR, 0.74; 95% CI, 0.65–0.85). Functional analysis revealed that the risk allele of rs4359426 contributed to higher expression levels of CCL22 mRNA. We further examined the allelic differences in the binding of nuclear proteins by electrophoretic mobility shift assay. The signal intensity of the DNA-protein complex derived from the G allele of rs223821, which was in absolute LD with rs4359426, was higher than that from the A allele. Although further functional analyses are needed, it is likely that related variants play a role in susceptibility to AD in a gain-of-function manner. Our findings provide a new insight into the etiology and pathogenesis of AD.
Introduction
Atopic dermatitis (AD) is a pruritic and chronically relapsing inflammatory skin disease involving disturbed skin barrier functions, cutaneous inflammatory hypersensitivity and defects in the antimicrobial immune defense with a strong genetic background [1]. Predominant infiltration of Th2 cells is a hallmark of acute atopic AD skin lesions [2]. Most patients with AD have peripheral blood eosinophilia and increased serum IgE levels, which are reflected in an increased frequency of peripheral blood skin-homing Th2 cells producing IL-4, IL-5 and IL-13 [1]. C-C motif chemokine 22 (CCL22) and CCL17 are high-affinity ligands for CC-chemokine receptor 4 (CCR4) and induce selective migration of Th2 cells [3]. CCL22 plays a crucial role in controlling the trafficking of Th2 cells into sites of allergic inflammation and is considered to be involved in the pathology of AD [4]. Keratinocytes from patients with AD highly express thymic stromal lymphopoietin (TSLP), and CCL22 is produced by TSLP-treated dendritic cells [5]. CCL22 is upregulated in lesional atopic dermatitis skin compared with healthy skin [6], and keratinocytes in the epidermal layer of AD skin express CCL17 and CCL22 [7]. Serum levels of CCL22 in AD patients are significantly higher than those found in normal controls [8], and the levels correlate positively with disease severity in AD patients [9]. Strong positive correlations between the levels of CCL17, CCL22, and total IgE in serum of patients with AD and SCORing Atopic Dermatitis (SCORAD) have also been reported [10]. Another study reported that overproduction of IgE induced CCL22 secretion from basophils, which are essential for IgE-mediated chronic allergic dermatitis [11]. These findings prompted us to conduct an association and functional study to test whether genetic variations of CCL22 contribute to AD susceptibility.
Several association studies using genetic variants of genes CCL17 and CCR4 in the CCR4 pathway have been conducted to discover genetic components in the pathogenesis of atopic dermatitis [12], [13]. A promoter polymorphism of CCL17, -431C>T, increases the promoter activity and the 431T allele influences higher serum levels of CCL17 [12], but genetic variants in the CCL17 gene are not associated with susceptibility to AD. A recent study also reported that C1014T polymorphism in the CCR4 gene was not associated with AD [13]. However, those studies were performed with small sample sizes and without replication studies. Genetic study of the CCL22 gene has not been conducted.
In this study, we focused on the CCL22 gene, resequenced the gene regions including all exons and introns, and carried out linkage disequilibrium mapping. We performed an association study using two independent populations and functional analyses of the related variants.
Results
Polymorphisms of the CCL22 gene and LD mapping
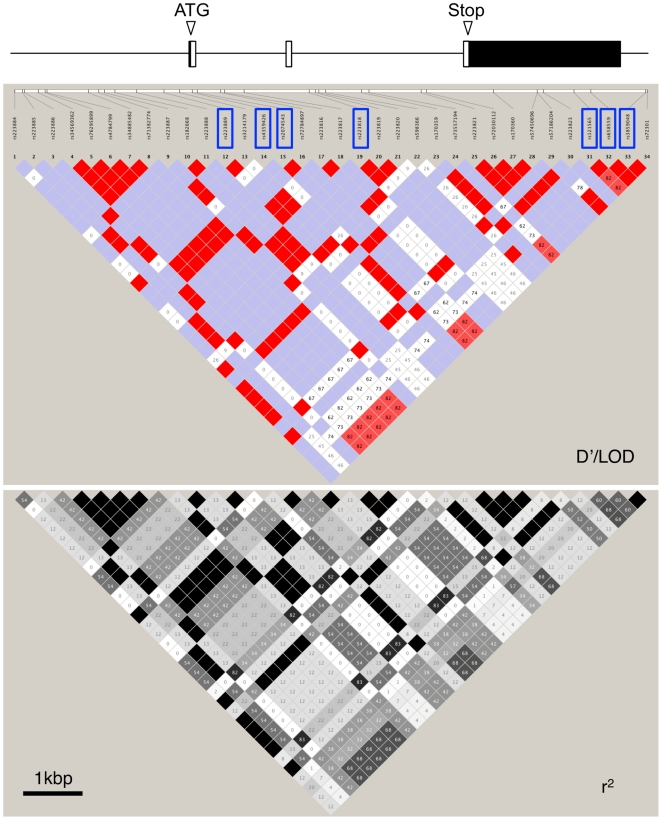
We identified a total of 39 polymorphisms (Table 1). We next performed linkage disequilibrium (LD) mapping and calculated pairwise LD coefficients D′ and r2 among the 34 polymorphisms with MAF>10% using the Haploview 4.2 program (Figure 1). Seven tag SNPs were selected for association studies using tagger in Haploview 4.2, and these polymorphisms captured 34 of the 34 alleles with a mean r2 of 0.990 (r2>0.82). The HapMap JPT database contains genotype data for six SNPs with MAF>10% in the region (data not shown). The SNPs examined in this study covered all six SNPs shown in the HapMap JPT database.
Table 1. Frequencies of polymorphisms of the CCL22 gene.
| SNP* | Location | Amino acid | MAF‡ | NCBI† | ||
| 1 | -3075G/A | 5′-flanking region | - | 0.125 | rs223884 | |
| 2 | -2938G/A | 5′-flanking region | - | 0.208 | rs223885 | |
| 3 | -2903T/A | 5′-flanking region | - | 0.333 | rs223886 | |
| 4 | -2668G/T | 5′-flanking region | - | 0.458 | rs34569362 | |
| 5 | -2550G/C | 5′-flanking region | - | 0.458 | rs76295899 | |
| 6 | -2511G/T | 5′-flanking region | - | 0.458 | rs4784799 | |
| 7 | -2191G/C | 5′-flanking region | - | 0.042 | rs76720124 | |
| 8 | -1795G/A | 5′-flanking region | - | 0.458 | rs34885482 | |
| 9 | -1775G/T | 5′-flanking region | - | 0.083 | rs72784894 | |
| 10 | -1618C/T | 5′-flanking region | - | 0.458 | rs77239447 | |
| 11 | -1515G/T | 5′-flanking region | - | 0.333 | rs223887 | |
| 12 | -1338A/G | 5′-flanking region | - | 0.208 | rs182668 | |
| 13 | -961G/A | 5′-flanking region | - | 0.208 | rs223888 | |
| 14 | -740A/G | 5′-flanking region | - | 0.083 | rs3760071 | |
| 15 | -488T/C | 5′-flanking region | - | 0.333 | rs223889 | § |
| 16 | -215WT/DelG | 5′-flanking region | - | 0.333 | rs3214179 | |
| 17 | 5C/A | exon 1 | Ala2Asp | 0.125 | rs4359426 | § |
| 18 | 88C/A | intron 1 | - | 0.458 | rs2074543 | § |
| 19 | 493T/C | intron 1 | - | 0.458 | rs72784897 | |
| 20 | 559G/A | intron 1 | - | 0.333 | rs223816 | |
| 21 | 902C/T | intron 1 | - | 0.333 | rs223817 | |
| 22 | 2030G/C | intron 2 | - | 0.208 | rs223818 | § |
| 23 | 2134T/C | intron 2 | - | 0.208 | rs223819 | |
| 24 | 2198T/C | intron 2 | - | 0.208 | rs223820 | |
| 25 | 2314G/A | intron 2 | - | 0.292 | rs598366 | |
| 26 | 2936A/G | intron 2 | - | 0.125 | rs170359 | |
| 27 | 3062A/G | intron 2 | - | 0.458 | rs73557194 | |
| 28 | 3766T/A | intron 2 | - | 0.042 | ||
| 29 | 3970G/A | intron 2 | - | 0.125 | rs223821 | |
| 30 | 4064WT/InsAAAAC | intron 2 | - | 0.125 | rs72030112 | |
| 31 | 5222T/C | 3′ UTR | - | 0.125 | rs170360 | |
| 32 | 5978WT/DelT | 3′ UTR | - | 0.125 | rs57450696 | |
| 33 | 5979C/G | 3′ UTR | - | 0.375 | rs57186204 | |
| 34 | 6089T/C | 3′ UTR | - | 0.125 | rs223823 | |
| 35 | 6621A/G | 3′ UTR | - | 0.458 | rs121565 | § |
| 36 | 6910G/A | 3′ UTR | - | 0.417 | rs658559 | § |
| 37 | 7858C/T | 3′-flanking region | - | 0.458 | rs3859048 | § |
| 38 | 7883G/A | 3′-flanking region | - | 0.458 | rs72301 | |
| 39 | 8021G/A | 3′-flanking region | - | 0.042 | rs11865093 |
*Numbering according to the genomic sequence of CCL22 (AC003665). Position 1 is the A of the initiation codon.
Minor allele frequencies (MAF) in the screening population (N = 12).
NCBI, number from the dbSNP of NCBI (http://www.ncbi.nlm.nih.gov/SNP/).
SNPs were genotyped in this study.
Figure 1. Pairwise linkage disequilibrium between 34 SNPs.
LD was measured by D′/LOD (upper) and r2 (lower) estimated using the Haploview 4.2 program (http://www.broad.mit.edu/mpg/haploview/). Boxed variants were genotyped in this study.
Association of CCL22 SNPs with susceptibility to atopic dermatitis
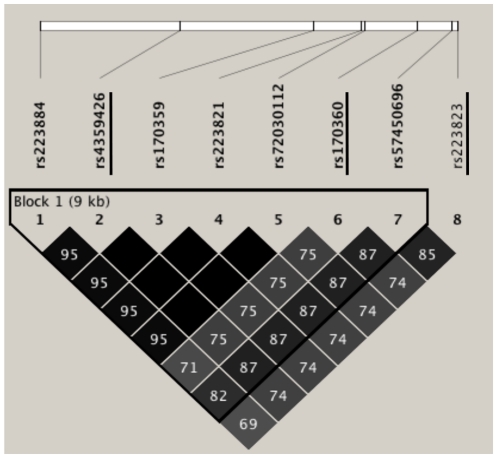
We recruited 916 cases and 1,032 control subjects for the 1st population and 1,034 cases and 1,004 control subjects for the 2nd population, respectively (Table 2). We genotyped seven tag SNPs and all genotype frequencies are shown in Table 3. The rs4359426 (A2D) SNP was associated with AD under an additive genotype model by logistic regression analysis in the first population (P = 0.0072; OR, 0.77; 95% CI, 0.64–0.93) (Table 3). In a replication study, rs4359426 was also associated with AD in the second population (P = 0.00037; OR, 0.71; 95% CI, 0.59–0.86) (Table 3). The direction of association of the SNP was similar in both of the populations. We combined the results using inverse variance method, and observed a significant association at rs4359426 (meta-analysis, P = 0.0000096; OR, 0.74; 95% CI, 0.65–0.85) (Table 3). We next performed further mapping analyses using two genetic variants, rs170360 and rs223823. The two SNPs were selected from among SNPs that were in strong LD (r2>0.87) with rs4359426 (Figure 2). Among the three variants, the strongest association was observed at rs4359426 (Table 4).
Table 2. Clinical characteristics of the subjects.
| Case | Control | |
| 1st population | ||
| Source | The University of TokyoKeio UniversityKyushu UniversityTakao Hospital | Control volunteers |
| Number of samples | 916 | 1,032 |
| Ethnicity | Japanese | Japanese |
| Female | 43.6% | 33.0% |
| Age (mean ± sd) | 30.1±9.5 | 48.5±13.7 |
| 2nd population | ||
| Source | BioBank Japan | University of Tsukuba |
| Number of samples | 1,034 | 1,004 |
| Ethnicity | Japanese | Japanese |
| Female | 43.8% | 54.4% |
| Age (mean ± sd) | 30.8±12.7 | 50.0±9.2 |
Table 3. Genotype counts and case-control association test results of seven tag SNPs.
| Allele | Case | Control | Frequency of allele 2 | ||||||||||
| db SNP ID | 1/2 | 1/1 | 1/2 | 2/2 | N | 1/1 | 1/2 | 2/2 | N | Case | Control | P value | OR (95%CI) |
| 1st population | |||||||||||||
| rs223889 | T/C | 321 | 435 | 151 | 907 | 360 | 502 | 161 | 1023 | 0.406 | 0.403 | 0.82 | - |
| rs4359426 | C/A | 706 | 191 | 12 | 909 | 736 | 269 | 16 | 1021 | 0.118 | 0.147 | 0.0072 | 0.77(0.64–0.93) |
| rs2074543 | G/C | 386 | 404 | 113 | 903 | 447 | 469 | 110 | 1026 | 0.349 | 0.336 | 0.39 | - |
| rs223818 | A/G | 563 | 311 | 39 | 913 | 596 | 369 | 56 | 1021 | 0.213 | 0.236 | 0.093 | - |
| rs121565 | A/G | 294 | 439 | 173 | 906 | 325 | 509 | 195 | 1029 | 0.433 | 0.437 | 0.82 | - |
| rs658559 | G/A | 333 | 434 | 134 | 901 | 374 | 491 | 162 | 1027 | 0.390 | 0.397 | 0.65 | - |
| rs3859048 | C/T | 399 | 410 | 103 | 912 | 466 | 448 | 108 | 1022 | 0.338 | 0.325 | 0.40 | - |
| 2nd population | |||||||||||||
| rs223889 | T/C | 369 | 497 | 163 | 1029 | 364 | 485 | 150 | 999 | 0.400 | 0.393 | 0.65 | - |
| rs4359426 | C/A | 815 | 202 | 12 | 1029 | 722 | 249 | 22 | 993 | 0.110 | 0.148 | 0.00037 | 0.71(0.59–0.86) |
| rs2074543 | G/C | 404 | 484 | 133 | 1021 | 418 | 459 | 120 | 997 | 0.367 | 0.351 | 0.26 | - |
| rs223818 | A/G | 647 | 331 | 42 | 1020 | 585 | 351 | 57 | 993 | 0.203 | 0.234 | 0.019 | 0.84(0.72–0.97) |
| rs121565 | A/G | 317 | 530 | 179 | 1026 | 317 | 500 | 180 | 997 | 0.433 | 0.431 | 0.92 | - |
| rs658559 | G/A | 389 | 486 | 154 | 1029 | 363 | 479 | 148 | 990 | 0.386 | 0.391 | 0.71 | - |
| rs3859048 | C/T | 425 | 484 | 117 | 1026 | 441 | 446 | 113 | 1000 | 0.350 | 0.336 | 0.35 | - |
| Combined | |||||||||||||
| rs223889 | T/C | 690 | 932 | 314 | 1936 | 724 | 987 | 311 | 2022 | 0.403 | 0.398 | 0.63 | - |
| rs4359426 | C/A | 1521 | 393 | 24 | 1938 | 1458 | 518 | 38 | 2014 | 0.114 | 0.147 | 0.0000096 | 0.74(0.65–0.85) |
| rs2074543 | G/C | 790 | 888 | 246 | 1924 | 865 | 928 | 230 | 2023 | 0.359 | 0.343 | 0.16 | - |
| rs223818 | A/G | 1210 | 642 | 81 | 1933 | 1181 | 720 | 113 | 2014 | 0.208 | 0.235 | 0.0044 | 0.86(0.77–0.95) |
| rs121565 | A/G | 611 | 969 | 352 | 1932 | 642 | 1009 | 375 | 2026 | 0.433 | 0.434 | 0.93 | - |
| rs658559 | G/A | 722 | 920 | 288 | 1930 | 737 | 970 | 310 | 2017 | 0.388 | 0.394 | 0.56 | - |
| rs3859048 | C/T | 824 | 894 | 220 | 1938 | 907 | 894 | 221 | 2022 | 0.344 | 0.330 | 0.21 | - |
P values of the two populations were calculated by logistic regression analysis under an additive model. The combined P values were calculated using the inverse variance method. OR, odds ratio; CI, confidence interval; -, not significant.
Figure 2. Pairwise linkage disequilibrium (r2) among eight SNPs in strong LD with rs4359426 in 94 control subjects.
Two tag SNPs, rs170360 and rs223823, were selected for further association study. Underlined SNPs were examined.
Table 4. Genotype counts and case-control association test results for SNPs rs4359426, rs170360 and rs223823.
| Allele | Case | Control | Frequency of allele 2 | ||||||||||
| db SNP ID | 1/2 | 1/1 | 1/2 | 2/2 | N | 1/1 | 1/2 | 2/2 | N | Case | Control | P value | OR (95%CI) |
| 1st population | |||||||||||||
| rs4359426 | C/A | 706 | 191 | 12 | 909 | 736 | 269 | 16 | 1021 | 0.118 | 0.147 | 0.0072 | 0.77(0.64–0.93) |
| rs170360 | T/C | 695 | 199 | 12 | 906 | 734 | 269 | 20 | 1023 | 0.123 | 0.151 | 0.011 | 0.78(0.65–0.95) |
| rs223823 | T/C | 728 | 170 | 11 | 909 | 765 | 252 | 10 | 1027 | 0.106 | 0.132 | 0.0093 | 0.77(0.63–0.94) |
| 2nd population | |||||||||||||
| rs4359426 | C/A | 815 | 202 | 12 | 1029 | 722 | 249 | 22 | 993 | 0.110 | 0.148 | 0.00037 | 0.71(0.59–0.86) |
| rs170360 | T/C | 792 | 220 | 19 | 1031 | 728 | 238 | 26 | 992 | 0.125 | 0.146 | 0.055 | 0.84(0.70–1.00) |
| rs223823 | T/C | 823 | 189 | 8 | 1020 | 780 | 193 | 19 | 992 | 0.100 | 0.116 | 0.11 | 0.85(0.70–1.04) |
| Combined | |||||||||||||
| rs4359426 | C/A | 1521 | 393 | 24 | 1938 | 1458 | 518 | 38 | 2014 | 0.118 | 0.147 | 0.0000096 | 0.74(0.65–0.85) |
| rs170360 | T/C | 1487 | 419 | 31 | 1937 | 1462 | 507 | 46 | 2015 | 0.123 | 0.151 | 0.0017 | 0.81(0.72–0.93) |
| rs223823 | T/C | 1551 | 359 | 19 | 1929 | 1545 | 445 | 29 | 2019 | 0.106 | 0.132 | 0.0030 | 0.81(0.70–0.93) |
P values of the two populations were calculated by logistic regression analysis under an additive model.
The combined P values were calculated using the inverse variance method. OR, odds ratio; CI, confidence interval.
Contribution of 5′UTR rs4359426 SNP to mRNA expression levels of CCL22
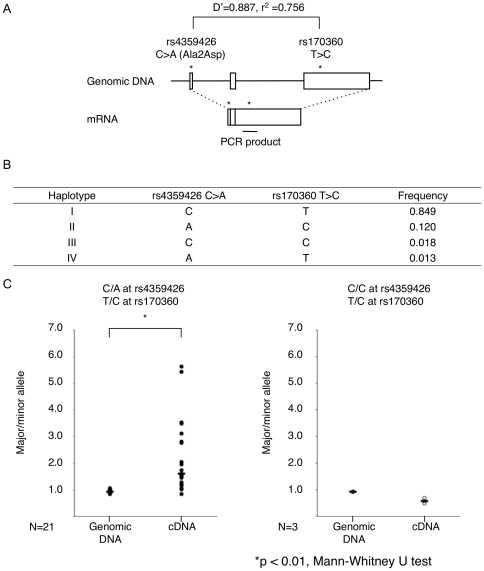
Next, using allele-specific transcript quantification (ASTQ), we evaluated whether the related variants could affect the mRNA expression level in EBV-transformed lymphoblastoid cells. As rs4359426 was located at the 16th nucleotide from the 5′ end of the CCL22 gene (NM_002990.3), we were not able to design primers of the SNP for ASTQ analysis. We therefore designed PCR primers to encompass a SNP in the 3′-UTR of CCL22 (rs170360) that was in strong LD with rs4359426 (Figure 3A). We isolated total RNA from 24 cell lines that were heterozygous with rs170360, and genomic DNA was used as a control for equal biallelic representation. Predicted haplotype frequencies are shown in Figure 3B. The ratio of PCR products was approximately 1.6 for cDNAs and 1.0 for genomic DNA from 21 subjects who were heterozygous for rs4359426 (Figure 3C, left panel); however, such differences were not observed in cells from three subjects who were homozygous for the C allele at rs4359426 (Figure 3C, right panel). These results implied an effect of rs4359426 and/or variants in strong LD with rs4359426 on mRNA expression levels of CCL22. rs4359426 and rs170360 are in absolute LD in the HapMap Caucasian populations. We further examined the expression patterns of rs4359426 and rs170360 using Genevar 3.0.2 dataset, and confirmed that the expression patterns were similar to our findings (data not shown).
Figure 3. Allelic imbalance of gene expression of CCL22 in EBV-transformed cells with heterozygous genotypes.
(A) Genomic structures, locations and LD of the two SNPs. (B) Haplotypes for the two SNPs in the 1st population. (C) The allelic ratio of PCR products from individuals. Heterozygous (left) and homozygous (right) at rs4359426. *Two-tailed P = 0.0000006 by the Mann-Whitney U test.
Transcription factor binding to the rs223821 SNP
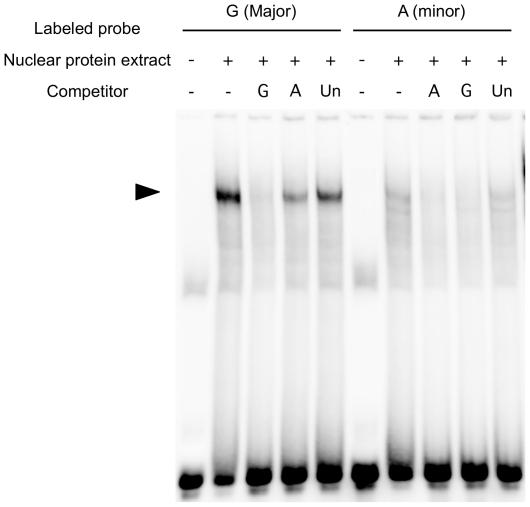
As rs4359426 was in absolute LD with rs170359, rs223821 and rs72030112 (n = 94) (Figure 2), we further examined the allelic differences of these three SNPs in the binding of nuclear proteins by electrophoretic mobility shift assay (EMSA). We could not find any specific binding of nuclear factor(s) to oligonucleotides containing rs170359 and rs72030112. However, we observed that the signal intensity of the DNA-protein complex derived from the G allele of rs223821 was higher than that from the A allele in the presence of THP-1 nuclear extract stimulated with LPS (1 µg/ml) (Figure 4). We confirmed that the complex was diminished by an excess amount of a non-labeled allele-specific competitor probe (Figure 4). This result suggested that an unidentified nuclear factor(s) interacted with the genomic region at intron 2 of CCL22 and the SNP might have an allele-specific effect on expression through varying affinity for a transcription factor.
Figure 4. Electrophoretic mobility shift assays of rs223821.
EMSA was performed using nuclear extracts from THP-1 cells stimulated with LPS (1.0 µg/ml) for 1 hour. DIG-labeled oligonucleotides corresponding to the G allele (lanes 1–5) and A allele (lanes 6–10) were used as probes. Three independent experiments were performed with similar results.
Discussion
CCL22 plays an important role in the recruitment of Th2 cells into the inflammatory lesions of Th2-related diseases such as AD [14]. A recent study reported upregulation of CCL17, CCL18 and CCL22 expression in patients with AD, and suggested that the disease-specific chemokines might recruit specific memory T-cell subsets into the skin [15]. The plasma levels of CCL22 are significantly elevated in AD patients, and the values strongly correlate with disease severity [7], [10]. We identified and replicated the rs4359426 (A2D) variant of CCL22, which was significantly associated with AD. rs4359426 is a non-synonymous SNP and causes an amino acid substitution in the signal peptide-encoding region. We examined the influence of the amino acid substitution on the structure using SIFT (Sorting Intolerant From Tolerant) software, and the substitution at position 2 from Ala to Asp was predicted to be tolerated. In addition, no possible impacts of the amino acid substitution on the structure and function of CCL22 were predicted by PolyPhen-2 (polymorphism phenotyping v2).
Functional analyses of the related variants of CCL22 polymorphisms showed that the susceptible allele of rs4359426 might be involved in higher mRNA expression in ASTQ analysis. We confirmed that the expression patterns from Genevar 3.0.2 dataset were similar to our findings. We also demonstrated that the genomic fragment including the risk allele of rs223821 had much higher binding affinity to the nuclear factor(s). Although it is unclear whether higher mRNA expression is influenced by altering expression enhancer activity or mRNA stability, polymorphisms in the CCL22 gene appear to be a genetic component of the pathologic mechanisms leading to atopic dermatitis, putatively via increased CCL22 mRNA expression.
Genetic studies reveal underlying cellular pathways, and in some cases, point to new therapeutic approaches. A recent study using a humanized model of asthma showed a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation [16]. In the study, administration of a CCR4-blocking antibody abolished airway eosinophilia, goblet cell hyperplasia, IgE synthesis and bronchial hyperreactivity [16]. IL-13 is an important mediator of Th2 immune responses, and there many IL-13-positive cells in AD skin lesions [17]. A recent study has shown that IL-13 induces a significant increase in the expression of CCL22 in human keratinocytes, and blocking of CCL22 in IL-13-stimulated cells results in 70–90% inhibition in migration of CD4+CCR4+ T cells [18]. These findings suggest that targeting the CCL22/CCR4 pathway might be therapeutically efficacious as a new treatment for atopic dermatitis.
The involvement of CCL22 has been reported in several immune-mediated diseases. A recent study has shown by immunohistochemistry that CCL22 is not expressed in normal skin and is markedly expressed in the lesions of atopic dermatitis, allergic contact dermatitis, and psoriasis vulgaris [19]. Another report has shown that CCL22 is present within the synovial membrane in rheumatoid arthritis and osteoarthritis patients and in high amounts in the synovial fluid of patients with rheumatoid arthritis and psoriatic arthritis [20]. To examine whether the functional SNPs found in this study are associated with those diseases will be needed for understanding of the interconnectivity of the molecular mechanisms underlying distinct diseases.
In summary, we found a significant association between susceptibility to AD and polymorphisms affecting CCL22 expression in Japanese populations. Our findings strongly support the important role of CCL22 in AD. Although the effect of the non-synonymous SNP on protein function remains unclear, it is likely that related variants play a role in susceptibility to AD in a gain-of-function manner. Further functional analyses and replication studies in other populations are needed; however, our findings provide insights into the pathophysiology of AD.
Materials and Methods
Subjects
A total of 1,950 case subjects with AD were recruited from several hospitals as described [21]. Case subjects in a second population were obtained from the BioBank Japan [22]. All case subjects were diagnosed according to the criteria of Hanifin and Rajka [23]. A total of 1,032 control volunteers in the first set who had no history of AD were recruited by detailed physicians' interviews. For the second set, a total of 1,004 controls who had never been diagnosed with AD were recruited during their annual health checkup in the University of Tsukuba (Table 2). All individuals were Japanese and gave written informed consent to participate in the study. This research project was approved by the ethics committees at the Institute of Medical Science, the University of Tokyo and the RIKEN Yokohama Institute.
Resequencing of the CCL22 gene and genotyping
We first resequenced the CCL22 region to identify genetic variations using DNA from 12 subjects with AD. We surveyed the gene from 3 kb of the 5′ flanking region to a 1 kb continuous 3′ flanking region of the last exon on the basis of genomic sequences from the NCBI database (NC_000016.9). The PCR product was reacted with BigDye Terminator v3.1 (Applied Biosystems), and sequences were assembled and polymorphisms identified using the SEQUENCHER program (Gene Codes Corporation, Ann Arbor, MI).
Genotyping of the seven SNPs in CCL22 was performed by the TaqMan™ allele-specific amplification (TaqMan-ASA) method (Applied Biosystems) and multiplex-PCR based Invader assay (Third Wave Technologies).
Allele-specific transcript quantification (ASTQ)
We conducted allelic expression analyses by TaqMan assay using SNP genotyping probes as described [24]. EBV-transformed lymphoblastoid cells were obtained from the Health Science Research Resources Bank of Japan. Genomic DNA was used as a control for equal biallelic representation. The allelic ratio for each cDNA and genomic DNA was measured.
Electrophoresis Mobility Shift Assay
EMSA was performed using nuclear extracts from THP-1 cells stimulated with LPS (1.0 µg/ml) for 1 hour. DIG-labeled oligonucleotides corresponding to the G allele (lanes 1–5) and A allele (lanes 6–10) were used as probes. The oligonucleotide sequences were 5′-ATCGCCTGAACCCGGGAGTTGGAGGTT for the G allele and 5′-ATCGCCTGAACCCAGGAGTTGGAGGTT for the A allele. For competition, a 100-fold excess of unlabeled G or A allele oligonucleotides or unrelated oligonucleotides (Un) (TFIID) was used.
Statistical analysis
We calculated allele frequencies and tested agreement with Hardy-Weinberg equilibrium using a chi-square goodness-of-fit test. We then compared differences in the allele frequencies between case and control subjects by logistic regression analysis under an additive model and calculated odds ratios (ORs) with 95% confidence intervals (CIs). Results for the 1st and 2nd populations were combined by fixed effect inverse-variance method using Genome-Wide Association Meta Analysis (GWAMA, http://www.well.ox.ac.uk/gwama/tutorial.shtml) [25]. We applied Bonferroni corrections, the multiplication of P values by the number of variants investigated. Corrected P values of less than 0.05 were judged to be significant. The expression patterns of SNPs were obtained from Genevar (GENe Expression VARiation) 3.0.2 (Wellcome Trust Sanger Institute). We examined the influence of amino acid substitution on the structure using SIFT software (http://sift.jcvi.org/) and PolyPhen-2 (polymorphism phenotyping v2) (http://genetics.bwh.harvard.edu/pph2/).
Acknowledgments
We thank all the patients for participating in the study as well as the collaborating physicians for collecting samples. We also thank the members of BioBank Japan and the Rotary Club of Osaka-Midosuji District 2660 Rotary International in Japan for supporting our study and M. T. Shimizu, H. Sekiguchi, A. I. Jodo, N. Kawaraichi and the technical staff of the Center for Genomic Medicine for providing technical assistance. We thank K. Barrymore for proof reading this document.
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology and from the Ministry of Health, Labour and Welfare, Japan. This work was conducted as part of the BioBank Japan Project. The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
References
- 1.Fonacier LS, Dreskin SC, Leung DY. Allergic skin diseases. J Allergy Clin Immunol. 2010;125:S138–149. doi: 10.1016/j.jaci.2009.05.039. [DOI] [PubMed] [Google Scholar]
- 2.Fiset PO, Leung DY, Hamid Q. Immunopathology of atopic dermatitis. J Allergy Clin Immunol. 2006;118:287–290. doi: 10.1016/j.jaci.2006.03.046. [DOI] [PubMed] [Google Scholar]
- 3.Andrew DP, Chang MS, McNinch J, Wathen ST, Rihanek M, et al. STCP-1 (MDC) CC chemokine acts specifically on chronically activated Th2 lymphocytes and is produced by monocytes on stimulation with Th2 cytokines IL-4 and IL-13. J Immunol. 1998;161:5027–5038. [PubMed] [Google Scholar]
- 4.Guttman-Yassky E, Lowes MA, Fuentes-Duculan J, Whynot J, Novitskaya I, et al. Major differences in inflammatory dendritic cells and their products distinguish atopic dermatitis from psoriasis. J Allergy Clin Immunol. 2007;119:1210–1217. doi: 10.1016/j.jaci.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 5.Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, et al. Human epithelial cells trigger dendritic cell-mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 6.Guttman-Yassky E, Suárez-Fariñas M, Chiricozzi A, Nograles KE, Shemer A, et al. Broad defects in epidermal cornification in atopic dermatitis identified through genomic analysis. J Allergy Clin Immunol. 2009;124:1235–1244. doi: 10.1016/j.jaci.2009.09.031. [DOI] [PubMed] [Google Scholar]
- 7.Horikawa T, Nakayama T, Hikita I, Yamada H, Fujisawa R, et al. IFN-gamma-inducible expression of thymus and activation-regulated chemokine/CCL17 and macrophage-derived chemokine/CCL22 in epidermal keratinocytes and their roles in atopic dermatitis. Int Immunol. 2002;14:767–773. doi: 10.1093/intimm/dxf044. [DOI] [PubMed] [Google Scholar]
- 8.Shimada Y, Takehara K, Sato S. Both Th2 and Th1 chemokines (TARC/CCL17, MDC/CCL22, and Mig/CXCL9) are elevated in sera from patients with atopic dermatitis. J Dermatol Sci. 2004;34:201–208. doi: 10.1016/j.jdermsci.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 9.Hashimoto S, Nakamura K, Oyama N, Kaneko F, Tsunemi Y, et al. Macrophage-derived chemokine (MDC)/CCL22 produced by monocyte derived dendritic cells reflects the disease activity in patients with atopic dermatitis. J Dermatol Sci. 2006;44:93–99. doi: 10.1016/j.jdermsci.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Jahnz-Rozyk K, Targowski T, Paluchowska E, Owczarek W, Kucharczyk A. Serum thymus and activation-regulated chemokine, macrophage-derived chemokine and eotaxin as markers of severity of atopic dermatitis. Allergy. 2005;60:685–688. doi: 10.1111/j.1398-9995.2005.00774.x. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe M, Satoh T, Yamamoto Y, Kanai Y, Karasuyama H, et al. Overproduction of IgE induces macrophage-derived chemokine (CCL22) secretion from basophils. J Immunol. 2008;181:5653–5659. doi: 10.4049/jimmunol.181.8.5653. [DOI] [PubMed] [Google Scholar]
- 12.Sekiya T, Tsunemi Y, Miyamasu M, Ohta K, Morita A, et al. Variations in the human Th2-specific chemokine TARC gene. Immunogenetics. 2003;54:742–745. doi: 10.1007/s00251-002-0520-2. [DOI] [PubMed] [Google Scholar]
- 13.Tsunemi Y, Sekiya T, Saeki H, Hirai K, Ohta K, et al. Lack of association of CCR4 single nucleotide polymorphism with atopic dermatitis in Japanese patients. Acta Derm Venereol. 2004;84:187–190. doi: 10.1080/00015550410025859. [DOI] [PubMed] [Google Scholar]
- 14.Yamashita U, Kuroda E. Regulation of macrophage-derived chemokine (MDC, CCL22) production. Crit Rev Immunol. 2002;22:105–114. [PubMed] [Google Scholar]
- 15.Fujita H, Shemer A, Suárez-Fariñas M, Johnson-Huang LM, Tintle S, et al. Lesional dendritic cells in patients with chronic atopic dermatitis and psoriasis exhibit parallel ability to activate T-cell subsets. J Allergy Clin Immunol. 2011 doi: 10.1016/j.jaci.2011.05.016. In press. [DOI] [PubMed] [Google Scholar]
- 16.Perros F, Hoogsteden HC, Coyle AJ, Lambrecht BN, Hammad H. Blockade of CCR4 in a humanized model of asthma reveals a critical role for DC-derived CCL17 and CCL22 in attracting Th2 cells and inducing airway inflammation. Allergy. 2009;64:995–1002. doi: 10.1111/j.1398-9995.2009.02095.x. [DOI] [PubMed] [Google Scholar]
- 17.Hamid Q, Naseer T, Minshall EM, Song YL, Boguniewicz M, et al. In vivo expression of IL-12 and IL-13 in atopic dermatitis. J Allergy Clin Immunol. 1996;98:225–231. doi: 10.1016/s0091-6749(96)70246-4. [DOI] [PubMed] [Google Scholar]
- 18.Purwar R, Werfel T, Wittmann M. IL-13-stimulated human keratinocytes preferentially attract CD4+CCR4+ T cells: possible role in atopic dermatitis. J Invest Dermatol. 2006;126:1043–1051. doi: 10.1038/sj.jid.5700085. [DOI] [PubMed] [Google Scholar]
- 19.Vulcano M, Albanesi C, Stoppacciaro A, Bagnati R, D'Amico G, et al. Dendritic cells as a major source of macrophage-derived chemokine/CCL22 in vitro and in vivo. Eur J Immunol. 2001;31:812–822. doi: 10.1002/1521-4141(200103)31:3<812::aid-immu812>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 20.Flytlie HA, Hvid M, Lindgreen E, Kofod-Olsen E, Petersen EL, et al. Expression of MDC/CCL22 and its receptor CCR4 in rheumatoid arthritis, psoriatic arthritis and osteoarthritis. Cytokine. 2010;49:24–29. doi: 10.1016/j.cyto.2009.10.005. [DOI] [PubMed] [Google Scholar]
- 21.Shimizu M, Matsuda A, Yanagisawa K, Hirota T, Akahoshi M, et al. Functional SNPs in the distal promoter of the ST2 gene are associated with atopic dermatitis. Hum Mol Genet. 2005;14:2919–2927. doi: 10.1093/hmg/ddi323. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura Y. The BioBank Japan Project. Clin Adv Hematol Oncol. 2007;5:696–697. [PubMed] [Google Scholar]
- 23.Hanifin JM, Rajka G. Diagnostic features of atopic dermatitis. Acta Derm Venereol. 1980;92:44–47. [Google Scholar]
- 24.Onouchi Y, Ozaki K, Buns JC, Shimizu C, Hamada H, et al. Common variants in CASP3 confer susceptibility to Kawasaki disease. Hum Mol Genet. 2010;19:2898–2906. doi: 10.1093/hmg/ddq176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mägi R, Morris AP. GWAMA: software for genome-wide association meta-analysis. BMC Bioinformatics. 2010;11:288. doi: 10.1186/1471-2105-11-288. [DOI] [PMC free article] [PubMed] [Google Scholar]