Abstract
Background
In most cells glucocorticoid receptors (GR) reside predominately in the cytoplasm. Upon hormone binding, the GR translocates into the nucleus, where the hormone-activated GR-complex regulates the transcription of GR-responsive genes. Serine/threonine protein phosphatase type 5 (PP5) associates with the GR-heat-shock protein-90 complex, and the suppression of PP5 expression with ISIS 15534 stimulates the activity of GR-responsive reporter plasmids, without affecting the binding of hormone to the GR.
Results
To further characterize the mechanism by which PP5 affects GR-induced gene expression, we employed immunofluorescence microscopy to track the movement of a GR-green fluorescent fusion protein (GR-GFP) that retained hormone binding, nuclear translocation activity and specific DNA binding activity, but is incapable of transactivation. In the absence of glucocorticoids, GR-GFP localized mainly in the cytoplasm. Treatment with dexamethasone results in the efficient translocation of GR-GFPs into the nucleus. The nuclear accumulation of GR-GFP, without the addition of glucocorticoids, was also observed when the expression of PP5 was suppressed by treatment with ISIS 15534. In contrast, ISIS 15534 treatment had no apparent effect on calcium induced nuclear translocation of NFAT-GFP.
Conclusion
These studies suggest that PP5 participates in the regulation of glucocorticoid receptor nucleocytoplasmic shuttling, and that the GR-induced transcriptional activity observed when the expression of PP5 is suppressed by treatment with ISIS 15534 results from the nuclear accumulation of GR in a form that is capable of binding DNA yet still requires agonist to elicit maximal transcriptional activation.
Background
Glucocorticoids influence a wide spectrum of cellular functions through their action on soluble intracellular receptors. In most cells, unliganded glucocorticoid receptors (GR) reside predominately in the cytoplasm, where they exist as a heteromeric complex comprised minimally of GR, 90-kDa and 70-kDa heat shock proteins (hsp90 and hsp70). Other proteins (i.e. p60/Hop, p23, hsp40, FKBP52, and FKBP51) have been implicated in the assembly/stabilization of the GR-hsp90-hsp70-complex in a form that has high affinity for agonist [for review, see Ref. 1, 2, 3]. Upon agonist binding, the complex undergoes a transformation, and the ligand bound GR translocates into to the nucleus in a manner that is determined by a nuclear localization sequence (NLS) contained in the receptor [4]. There the GR acts as a ligand-activated transcriptional stimulator or repressor of primary response genes by binding to glucocorticoid hormone-responsive elements (GRE) contained in the promoter regions of steroid-responsive genes and either facilitating or repressing the formation of an active transcriptional complex.
Although little is known about the molecular machinery that regulates steroid receptor movement through the cytoplasm and into the nucleus, several studies suggest that movement is influenced by reversible phosphorylation. Evidence for this originated from the studies of Qi et al. [5, 6], which revealed that the hormone insensitivity produced by cellular transformation with v-mos (a serine/threonine protein kinase that acts as an oncogene) results from both a decrease in the nuclear retention of liganded receptor and a decrease in the reutilization of GR protein that cycles back into the cytoplasm. Subsequently, DeFranco et al. [7] reported that treatment with okadaic acid, a potent ser/thr protein phosphatase inhibitor, also results in inefficient nuclear retention of agonist-bound GR and the cytoplasmic "trapping" of GR in a form that is unable to "recycle". Recent studies with okadaic acid suggest phosphorylation alters the high affinity binding of GR to hsp-90, and that an intact cytoskeleton is required for ligand-activated GR translocation through the cytoplasm to the nucleus [8].
The ability of okadaic acid to influence the intracellular partitioning of GR suggests that an okadaic acid sensitive ser/thr protein phosphatase (PPase) participates in the regulation of GR movement. In vitro, okadaic acid acts as a potent inhibitor of serine/threonine protein phosphatases type 1 (PP1) and 2A (PP2A) [9, 10]. Accordingly, many of the effects produced by the treatment of cells with okadaic acid have been attributed to the inhibition of these two enzymes. However, due to toxicity and solubility constraints, in living cells it is difficult to distinguish the actions of PP2A from those of PP1 using okadaic acid. Furthermore, in humans, it is now clear that there are four isoforms of PP1 [PP1α, PP1δ, PP1γ1 and PP1γ2 [11, 12, 13], two isoforms of PP2A (PP2A α and PP2Aβ [14, 15]) and four structurally related phosphatases, PP4 [16], PP5 [17, 18], PP6 [19] and PP7 [20]. Although detailed dose-response studies have not been reported for native PP5, PP6 and PP7, studies with PP4 [21] and recombinant PP5 [18] indicate they are also sensitive to okadaic acid. Like calcineurin (PP2B) and PP2C, PP7 is apparently insensitive to inhibition by okadaic acid [20].
Recent studies indicate that PP5 associates with the GR-hsp90 complex [22, 23] suggesting that PP5 may influence the actions of GRs. However, studying the cellular roles of PP5 has proven difficult, in part, because no physiological substrates for PP5 have been identified. In addition, in crude cell homogenates PP5 resides predominately in an inactive state that represents <1% of the measurable PPase activity. To characterize the cellular roles of PP5 we have, therefore, developed chimeric antisense 2'-O-(2-methoxy) ethylphosphothioate oligonucleotides capable of inhibiting the expression of human PP5 at nanomolar concentrations. Because the lead compound targeting PP5 (ISIS 15534) acts via RNAase H mediated degradation, studies with ISIS 15534 do not allow us to assess how rapid changes in PP5 activity affect cellular functions (Northern analysis indicate that it takes ~ 6 for the mRNA degradation to occur and, due to the half-life of the preexisting protein, it takes ~ 24 hours for the protein levels to fall [24]. Nonetheless, ISIS 15534 potently inhibits the expression of PP5 in cultured cells for ~ 48-72 hours (IC50 of <75 nm), which affords a ~ 24-48 hour window in which the expression of PP5 is essentially ablated [24, 25]. More importantly, because ISIS 15534 has no effect on the structurally related PPases [24], it can be employed to specifically inhibit the actions of PP5 by suppressing PP5 protein levels in cultured human cells.
To assess the role of PP5 in the regulation of GR-mediated events, binding studies were conducted with [3H]dexamethasone before and after ISIS 15534-mediated suppression of PP5 expression. These studies revealed that the suppression of PP5 expression had no apparent effect on dexamethasone binding, suggesting that PP5 does not affect the formation of the high-affinity ligand binding complex or hormone binding to the GR [25]. In contrast, mobility gel-shift analysis revealed that treatment with ISIS 15534 produces a marked increase in the association of GR with GRE-containing DNA, and transient transfection studies employing a GR-responsive reporter plasmid revealed that the suppression of PP5 expression activates GR-dependent transcription in the absence of hormone [25]. When A549 cells were treated with ISIS 15534 and then dexamethasone, the effect was additive, with maximal dexamethasone induced luciferase activity ~ 10 times greater than the maximal dexamethasone-induced response attainable in the presence of PP5 [25]. Together, these studies indicate that PP5 acts as a suppressor of GR-induced transcription.
To further characterize the mechanism by which PP5 affects GR function, in the present study we employed a GR-GFP fusion protein and fluorescent microscopy to follow the movement of GR in cells treated with dexamethasone and ISIS 15534. These studies indicate that PP5 mediated suppression of GR-function arises from the ability of PP5 to suppress the nuclear accumulation of GRs.
Results
Inhibition of PP5 by okadaic acid
Okadaic acid is widely employed to inhibit PPase activity in eukaryotic cells, and the observed effects are often attributed to the inhibition of PP1 and PP2A. However, as seen in Figure 1, the dephosphorylation of phosphohistone by the catalytic subunit of purified PP5 from bovine brain is also potently inhibited by okadaic acid. Okadaic acid inhibits the activity of PP5 in a dose-dependent manner, having an IC50 of 5.58 +/- 0.41 nM. Under identical assay conditions, okadaic acid is a more potent inhibitor of PP2A (IC50~ 0.05 nM) and weaker inhibitor of PP1 (IC50~ 45 nM). However, the activity of PP5 against histone (865 +/- 31 nMoles Pi/min/mg protein) is less than that of PP2A. Therefore, whereas assays conducted with PP1 and PP2A were conducted with a concentration of enzyme that is diluted below the titration endpoint (defined as the concentration of enzyme after which further dilution no longer affects the IC50; [26]) because the activity of PP5 upon further dilution was below that necessary for accurate quantification, we could not establish a clear titration endpoint with PP5. Based on titration studies with microcystin-LR to estimate the actual amount of PP5 in the reaction, an estimate of the Ki for okadaic acid is 4.07 +/- 0.16 nM. Still, it should be kept in mind that the true Ki may be slightly lower. Nonetheless, it is clear that PP5 is sensitive to inhibition by okadaic acid at the concentrations that also affects the activity of PP1 and PP2A. Therefore, when okadaic acid is employed to treat cells at a concentration of >1-5 nM, the activity of PP5 is also affected.
Figure 1.
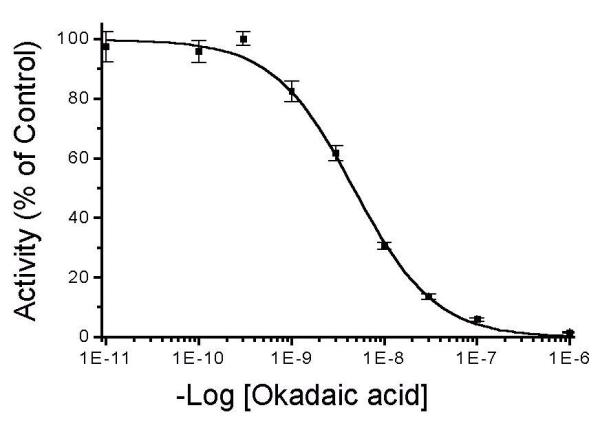
Inhibition of PP5 by okadaic acid. The purified catalytic subunit of PP5 was assayed, using [32P]labeled phosphohistone as a substrate as described in Materials and Methods. The data is expressed as % of controls, with control activity being 4.2 ± 0.25 μmole/min/mg protein. Okadaic acid was mixed with the enzymes for 10 min at 23°C prior to the initiation of the reaction with the addition of substrate. Inhibition assays contained ~ 200 pM PP5. The data represent the mean SD (n = 4).
ISIS 15534 promotes nuclear localization of GR
To determine whether the suppression of PP5 activity influenced the subcellular distribution of GR, plasmids expressing a GR-GFP fusion protein were microinjected into cells that were previously treated with ISIS 15534. In these studies the GR-GFP used was derived from the fusion of GFP to a GR mutant that retained the ability to bind agonist, translocate to the nucleus, and bind DNA, but lacked transactivation activity [27]. When plasmids expressing the GR-GFP were injected into the nuclei of cells and the cells were grown in serum-containing medium, the majority of the expressed GR-GFP localized to the nuclei of the injected cells, as expected due to the presence of glucocorticoids in the serum. Thus, after microinjection, the cells were grown in medium supplemented with serum for 4 to 6 hours and then serum starved overnight to cause the redistribution of GR-GFP. In the absence of serum, GR-GFP localized throughout the cells, with equal intensity in both the nucleus and cytoplasm (Fig. 2A). Upon treatment of these cells with dexamethasone (500 nM for 30 minutes) the GR-GFP efficiently translocated to the nucleus. To assess the role of PP5 in the intracellular partitioning of GR, cells were treated with ISIS 15534 or a mismatched control (ISIS 15521) at a concentration of 500 nM, which essentially ablates PP5 expression in A549 cells after 24 hours [24, 25]. Approximately 24 hours after antisense treatment, the cells were microinjected with pGR(Ala)-GFP and subjected to serum-starvation as described above. In cells treated with the mismatch control prior to microinjection, GR-GFP expression was indistinguishable from that in untreated cells: GR-GFP was distributed evenly throughout the cytoplasm and nucleus (Figure 2A). In contrast, following treatment with ISIS 15534, GR-GFP was localized in the nucleus without dexamethasone treatment. The percentage of cells displaying predominantly nuclear GR-GFP or cytoplasmic GR-GFP from 4 independent experiments were tabulated and are presented in Figure 2B. In control cells in the absence of dexamethasone, 10% of the cells showed nuclear>cytoplasmic localization of GR-GFP. Similarly, 20% of the cells treated with mismatch oligonucleotide displayed greater nuclear than cytoplasmic localization of GR-GFP. By contrast, the percentage of cells showing more nuclear than cytoplasmic distribution of GR-GFP was ~ 93%, ~ 88% and ~ 98% in cells treated with either dexamethasone, ISIS 15534, or ISIS 15534 and dexamethasone, respectively. These results suggest that PP5 plays a role in the nucleo-cytoplasmic partitioning of GR.
Figure 2.
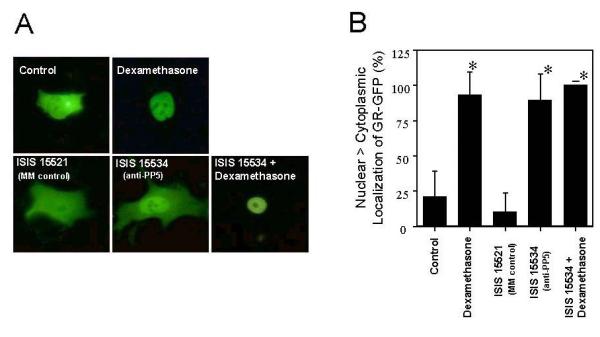
Effects of ISIS 15534 and dexamethasone on the subcellular distribution of GR-GFP.A. Representative subcellular distribution of GR-GFP. A549 cells were grown and treated with ISIS 15521 (a mismatched control antisense oligonucleotide for ISIS 1535), ISIS 15534 (antisense targeting PP5), dexamethasone (500 nM), ISIS 15534 and 500 nM dexamethasone, or vehicle alone (control) as described in Material and Methods. Eighteen hours later, GR-GFP expressing plasmids were microinjected into the nuclei of cells, incubated for 6 hours in serum-containing medium, and then serum-starved for an additional 18 hours. Between 150 and 200 cells were injected for each condition. The cells were either left untreated (control, ISIS 15521, ISIS 15534) or incubated with 500 nM dexamethasone (dexamethasone, ISIS + dexamethasone) for 30 minutes, prior to fixing and observation by fluorescence microscopy. Representative cells from 4 independent experiments are shown. B. Percentage of cells displaying nuclear GR-GFP. Cells from 4 independent experiments were scored for their intracellular distribution of GR-GFP and the mean percentage of cells having greater fluorescence in their nuclei than cytoplasm are shown (± SD). *p ≤ 0.0001 by ANOVA vs untreated or mismatch-treated cells.
Inhibition of PP5 expression does not globally affect nuclear shuttling
The ability of ISIS 15534 treatment to induce the nuclear accumulation of GR-GFP suggests that PP5 influences the subcellular distribution of GR. Alternatively, PP5 could have a more global role, possibly regulating entire nuclear transport pathways. To test the latter, the effect of PP5 suppression on the nucleo-cytoplasmic shuttling of another transcription factor, NFAT, was investigated. In these studies, a stable GFP-NFAT expressing HeLa cell line was employed [38]. As seen in Figure 3, in the absence of any drug, the GFP-NFAT protein is localized entirely within the cytoplasm. Upon treatment with the calcium ionophore ionomycin, GFP-NFAT translocates rapidly to the nucleus. The fusion protein then relocates to the cytoplasm if the drug is washed out of the cells for 6 hours. When the GFP-NFAT HeLa cells were treated with ISIS 15534, the distribution of GFP-NFAT was indistinguishable from untreated cells or cells treated with mismatched control oligonucleotides. Northern analysis confirmed the ability of ISIS 15534 to suppress PP5 expression in Hela cells. Therefore, the results indicate that suppression of PP5 expression does not have a global effect on all nuclear shuttling proteins.
Figure 3.
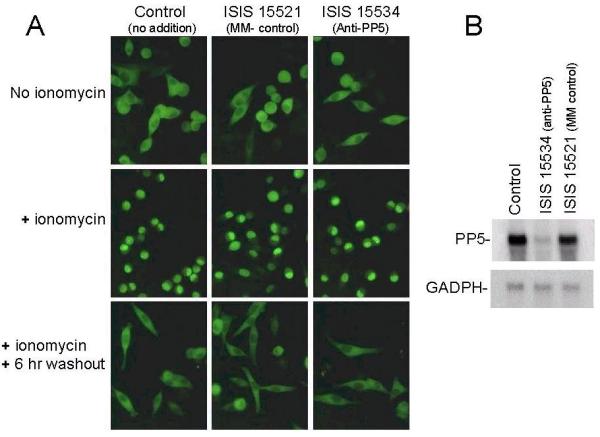
Nucleo-cytoplasmic shuttling of GFP-NFAT.A) GFP-NFAT expressing HeLa cells were grown on glass coverslips and either left untreated (control) or treated with ISIS 15534 or the mismatched control (ISIS 15521) as described in Materials and Methods. Twenty-four hours after oligonucleotide treatment, the cells were either directly fixed with paraformaldehyde (No ionomycin), or ionomycin was added to 1 μM for 30 minutes. After treatment with ionomycin, the cells were either fixed (+ ionomycin) or rinsed in PBS and grown for an additional 6 hours in medium in the absence of drug (washout) before fixing. Representative fields of cells were viewed by fluorescence microscopy and photographed. B). Northern analysis of HeLa cells following treatment with 500 nM ISIS 15534, 500 nM ISIS 155521 or lipofectin alone (control), probing for PP5 and GAPDH confirms that the antisense oligonucleotides are also effective HeLa cells.
Discussion
Like other steroid hormone receptors, GRs are phosphoproteins, and reversible phosphorylation of specific ser/thr residues on the GR or associated proteins has been implicated in the regulation of 1) hormone binding to the cytoplasmic GR-complex, 2) the translocation of the GR between the cytoplasm and the nucleus, 3) the binding of ligand activated GR to consensus GRE in the promoter regions of GR-responsive genes, and 4) the formation of an active transcriptional complex [1, 2, 5, 6, 7, 8, 28, 29, 30, 31]. Many of the kinases that catalyze the phosphorylation of GRs and associated proteins have been identified. However, little is known about the PPases that must also participate in the regulation of GR-mediated signaling networks.
Several recent studies indicate that PP5 participates in the regulation of GR-induced gene expression. First, dexamethasone-induced transcription is markedly enhanced when the expression of PP5 is suppressed by treatment with ISIS 15534 [25]. Second, both co-immunoprecipitation studies and studies with mutant forms of PP5 indicate that PP5 associates with the GR-hsp90 complex [22, 23]. However, binding assays revealed that the suppression of PP5 expression has no apparent effect on [3H]dexamethasone binding [25]. Therefore, PP5 appears to act as a suppressor of GR-induced gene expression via a mechanism that does not alter receptor number or the binding of hormone to the GR. To further characterize the relationship between PP5 and GR, in the present study we employed a GR-GFP fusion protein to track the movement of GRs in A549 cells following treatment with hormones or ISIS 15534. These studies revealed that in the absence of glucocorticoids, GR-GFP localized mainly in the cytoplasm. As expected, treatment with dexamethasone results in the efficient translocation of GR-GFPs into the nucleus. Nuclear accumulation of GR-GFP was also observed when the expression of PP5 was suppressed by treatment with ISIS 15534 after ~ 24 hours. This translocation of GR-GFP occurred without the addition of glucocorticoids and in the presence of serum free media. Thus, in the absence of physiological concentrations of PP5, GRs accumulate in the nucleus of A549 cells. This finding is consistent with data obtained from gel-shift analysis and transfection studies conducted with GR-reporter plasmids, where a decrease in PP5 levels facilitates the association of GR with DNA and produces an increase in GR-transactivation [25]. Together, these studies suggest that the increase in GR-induced transcriptional activity observed when the expression of PP5 is suppressed by treatment with ISIS 15534 results from the nuclear accumulation of GR.
The mechanism by which PP5 suppresses the nuclear accumulation of GR is not clear. The current data is consistent with PP5 acting to suppress the nuclear import of GRs. Alternatively, the data is also consistent with PP5 acting to promote the nuclear export of the GRs. Because the effects of ISIS 15534 treatment are not readily reversible, the washout experiments needed to distinguish between these two possibilities cannot be conducted until a specific and reversible inhibitor of PP5 is developed.
Recent studies indicate that okadaic acid inhibits nuclear transport mediated by import receptors, importin β and transportin, suggesting that an okadaic acid sensitive phosphatase participates in a mechanism that negatively regulates entire nuclear transport pathways [32]. Since PP5 is sensitive to inhibition by okadaic acid, we tested the possibility that PP5 acted at a more global level by assessing the effect of ISIS 15534 on the cellular distribution of NFAT-GFP. In contrast to the findings obtained with GR-GFP, the suppression of PP5 expression had no apparent effect on calcium induced nuclear import or the subsequent export of NFAT-GFP (Figure 3). Therefore, PP5 does not appear to regulate entire nuclear transport pathways.
Conclusions
These studies indicate that PP5 participates in the regulation of GR nucleocytoplasmic shuttling, and that the suppression of PP5 expression results in the nuclear accumulation of GR in the absence of hormone. Therefore, the previously reported increase in GR-induced transcriptional activity that occurs after ISIS 15534 induced suppression of PP5 expression likely results from the nuclear accumulation of GR in a form that is capable of binding DNA yet still requires agonist to elicit maximal transcriptional activation. Still, it is not yet clear if PP5 acts to suppress the nuclear accumulation or to facilitate the nuclear export of GRs. Thus, the precise molecular mechanism by which PP5 suppresses the nuclear accumulation of GR remains to be elucidated.
Materials and Methods
Reagents
Tissue culture medium, Lipofectin® and TRIzol® were purchased from Life Technologies (Gaithersburg, MD). DECEprime™ II DNA labeling, and MAXIscript™ in vitro transcription kits were purchased from Ambion Inc. (Austin, TX). [α-32P]dATP and [α-32P]UTP were purchased from Dupont NEN (Boston, MA). Protein kinase A (3':5'-cyclic AMP dependent), phosphorylase b (EC 2.4.1.1), crude histone (type 2AS) and p-nitrophenyl phosphate (PNPP) were obtained from Sigma Chemical Company.
Cell Culture
A549 lung carcinoma cells were obtained from the American Type Tissue Collection. A stable HeLa cell line expressing GFP-NFAT was generously provided by Larry Gerace (The Scripps Research Institute, La Jolla, CA). HeLa and A549 cells were grown in Dulbecco's modified Eagle's medium containing 1 g of glucose/liter (DMEM) and 10 % heat-inactivated FBS. All cell cultures were routinely passed when 90-95% confluent.
Oligonucleotide Synthesis Assay for Oligonucleotide Inhibition of PP5 Expression
2'-O-(2-methoxy)ethylphosphothioate oligonucleotides were synthesized and purified as previously described [33]. To suppress the expression of PP5, the indicated cells were plated in 60 mm dished and cultured in DMEM containing 10% FCS. When the cells were about 70% confluent, they were treated with oligonucleotides as previously described [24, 25]. Briefly, cells were washed with DMEM. A solution (1 ml) of DMEM containing the oligonucleotides at the indicated concentration and 15 μg/ml DOTMA/DOPE (Lipofectin®; GIBCO-BRL) was then added. After incubating the cells at 37°C for 4 hours, the cells were washed and cultured in fresh DMEM containing 10% FCS for 17 hours. The cells were then harvested, and total RNA was extracted with TRIzol Reagent (GIBCO-BRL) according to the methods of the manufacturer. Total RNA (20 μg) was fractionated on 1% agarose gels containing formaldehyde and transferred to DURLON-UV (Stratagene) nylon membranes. Following UV cross-linking, the filters were hybridized with a [32P]probe for human PP5. The human PP5 cDNA probe was generated from the full length coding region of PP5 and [32P]labeled with DECAprime® DNA Labeling Kit (Ambion) according to the manufacturer's protocol. Hybridization was performed in the presence of 50% formamide at 42°C for 16 hours. Following hybridization, the membrane was subjected to two low stringency washes (2 × SSC) at room temperature and then two high stringency washes (0.1 × SSC/0.5% SDS) at 55°C. Hybridization was visualized by autoradiography, and the filters were then stripped and reprobed with a [32P]labeled glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA probe to confirm equal loading. Quantification of hybridization signals was achieved by analysis of the scanned autoradiograms using the NIH Image program (ImagePC).
Preparation of phosphoprotein substrates
Histone (type 2AS from Sigma) was phosphorylated with cAMP-dependent protein kinase (protein kinase A) as described previously [34]. Briefly, 20 mg of histone was incubated with 1 mg of protein kinase A, in a 20 mM Tris-Cl buffer (pH 7.4) containing 1 mCi [32P]ATP (150 μM ATP), 100 μM cAMP, 5 mM DTT, and 5 mM MgCl2 in a final volume of 4 ml. The reaction was allowed to continue for 3.5 to 4 hrs at 30°C and terminated by the addition of 1.3 ml of 100% TCA. The precipitated phosphohistone was collected by centrifugation at 3000 × g for 5 min. The supernatant was discarded, and the pellet was redissolved in 4 ml of 1 M Tris-Cl (pH 8.2). TCA was added to precipitate the phosphohistone, and this precipitation-resuspension wash was repeated 5 times. The pellet produced upon the sixth TCA precipitation was washed 2 times with 4 ml of ethanol:ethyl ether (1:4; v:v) and then 2 additional times with acidified ethanol:ethyl ether (1:4; 0.1 N HCl). The washed histone was allowed to air dry and was then resuspended in 5 mM Tris-Cl (pH 7.4). This procedure yields phosphohistone with a specific activity > 4.5 × 106 CPM/nMole incorporated phosphate.
Determination of phosphatase activity
Phosphatase activity against phosphohistone was determined by the quantification of [32P] liberation from phosphohistone as described previously [34]. Assays, 80 μl total volume, containing 50 mM Tris-HCl, pH 7.4, 0.5 mM DTT, 1 mM EDTA, 100 μM oleic acid (assay buffer) and [32P]phosphoprotein (1-2 μM PO4), were conducted at 37°C as described previously [34] using PP5 purified from bovine brain. The serine/threonine protein phosphatase type 5 (PP5) was purified from bovine brain according to the following procedure: a bovine brain was homogenized in ~ 3 volumes of buffer A (50 mM HEPES pH 7.5 4°C, 1 mM EDTA, 10 mM PMSF and 0.1% 2-mercaptoethanol) and subjected to centrifugation at 15,000 g for 40 min. followed by a 45%-60% ammonium sulfate fractionation of the supernatant. The pellets from the ammonium sulfate fractionation were resuspended in 60 ml of buffer B (20 mM HEPES pH 8.5 4°C, 1 mM EDTA, and 0.1% 2-mercaptoethanol), desalted on a G-25 sephadex column equilibrated with buffer B, then subjected to sequential chromatography on a 5 ml HiTrap Heparin column (Pharmacia), a 5 ml HiTrap Q column (Pharmacia) and a HiTrap SP column (Pharmacia) respectively. The eluate from the SP column was then diluted 5-fold with buffer C (20 mM tris pH 8.5, 1 mM EDTA and 0.1% 2-mercaptoethanol) and subjected to anion-exchange chromatography on a BioScale Q column (2 ml from BioRad). All steps in the purification procedure were performed at 4°C except for the last step which was performed at 23°C. PP5 activity was followed during the purification by assaying for trypsin-stimulated phosphohydrolase activity at 37°C vs. p-nitrophenyl phosphate in 200 μl reactions containing: 25 mM tris pH 8.3, 25 mM MgCl2, 1 mM DTT and 10 mM p-nitrophenyl phosphate.
Dephosphorylation reactions were routinely conducted for 10 minutes and initiated with the addition of substrate. The dephosphorylation of substrate was kept to less than 10 % of the total phosphorylated substrate, and the reaction was linear with respect to enzyme concentration and time. [32P]phosphate liberated by the enzymes was extracted as a phosphomolybdate complex and measured according to the methods of Killilea et al. [35]. Inhibition of phosphatase activity by okadaic acid was determined by adding the inhibitors to the enzyme mixture 10 minutes prior to initiating the reaction with the addition of substrate.
GR-GFP expression vectors
A mutant rat glucocorticoid receptor that retained hormone binding, nuclear translocation activity, and specific DNA binding activity, but was incapable of transactivation was fused to a S65T mutant of green fluorescent protein to create pGR(Ala)-GFP. GR(Ala)-GFP expression is driven by the CMV immediate early promoter and enhancer. All manipulations were performed as described, and all constructs were confirmed by sequencing [36]. Protein-free plasmid DNA was purified using Qiagen maxiprep columns (San Diego, CA) and contained predominantly supercoiled plasmid with no contaminating RNA or low molecular weight contaminants.
GFP-GR translocation
Cells were plated on etched coverslips and grown until the cultures were ~ 70 % confluent. The cells were then either treated with oligonucleotides as described above or left untreated. Eighteen hours later, purified protein-free pGR(Ala)-GFP suspended in phosphate-buffered saline (0.25 mg/ml) was microinjected into the nuclei of the cells [37]. Typically, 150-200 cells were injected for each condition. After microinjection the cells were incubated in medium containing 10% FBS for 6 hours at 37°C. The cells were then washed several times with serum-free media and placed in serum deficient media for 14 hours to prevent GFP-GR translocation to the nucleus, prior to treatment with 500 nM dexamethasone (30 min.) or with vehicle alone. The cells were washed 3 times with PBS, fixed by incubation in PBS supplemented with 4 % paraformaldehyde for 10 min and washed again 3 times with PBS before mounting on slides with DABCO/DAPI mounting solution. GR-GFP expression and localization were monitored by fluorescence microscopy. Nuclei were localized by DAPI staining. Images were captured with an Optronics cooled CCD camera and imported into Adobe Photoshop. The experiments were repeated 4 times (6 for control). One-way ANOVA was performed to determine statistical significance of the results using Instat 2.03 (GraphPad Software, San Diego, CA).
GFP-NFAT nuclear shuttling assay
Stably-transfected HeLa cells expressing GFP-NFAT [38] were grown on glass coverslips to approximately 30% confluency and either left untreated or treated with, ISIS 15534, or ISIS 15521 (a mismatched oligonucleotide control for ISIS 15534) in the absence of serum as described above. Four hours later, FBS was added to 10% and the cells were grown for an additional 24 hours. The cells were then treated with 1 μM ionomycin for 30 minutes, after which time they were either fixed immediately or washed extensively with PBS and incubated for 6 hours in medium lacking the drug ("washout"). Control cells were not incubated with ionomycin. GFP-NFAT localization was monitored by fluorescence microscopy, and images were captured with an Optronics cooled CCD camera and imported into Adobe Photoshop.
Acknowledgments
Acknowledgements
We thank Larry Gerace (The Scripps Research Institute, La Jolla, CA) for the generous gift of HeLa-GFP-NFAT cells. Supported in part by grants from National Institutes of Health [CA60750 (REH) and HL59956 (DAD)] and the Swiss National Research Foundation [31.49572.96 (SR)].
Contributor Information
David A Dean, Email: dean@northwestern.edu.
Gudrun Urban, Email: gurban@jaguar1.usouthal.edu.
Ileana V Aragon, Email: largon@jaguar1.usouthal.edu.
Mark Swingle, Email: prospero@zebra.net.
Beth Miller, Email: bmiller@bbl.usouthal.edu.
Sandro Rusconi, Email: Sandro.Rusconi@unifr.ch.
Manuel Bueno, Email: Manuel.Bueno@unifr.ch.
Nicholas M Dean, Email: ndean@isisph.com.
Richard E Honkanen, Email: honkanen@sungcg.usouthal.edu.
References
- Tsai MJ, O'Malley BW. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu Rev Biochem. 1994;63:451–486. doi: 10.1146/annurev.bi.63.070194.002315. [DOI] [PubMed] [Google Scholar]
- Bamberger CM, Schulte HM, Chrousos GP. Molecular determinants of glucocorticoid receptor function and tissue sensitivity to glucocorticoids. Endocr Rev. 1996;17:245–261. doi: 10.1210/edrv-17-3-245. [DOI] [PubMed] [Google Scholar]
- Pratt WB, Toft DO. Steroid receptor interactions with heat shock protein and immunophilin chaperones. Endocr Rev. 1997;18:306–360. doi: 10.1210/edrv.18.3.0303. [DOI] [PubMed] [Google Scholar]
- Picard D, Yamamoto KR. Two signals mediate hormone-dependent nuclear localization of the glucocorticoid receptor. EMBO J. 1987;6:3333–3340. doi: 10.1002/j.1460-2075.1987.tb02654.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qi M, Hamilton BJ, DeFranco DB. v-mos oncoproteins affect the nuclear retention and reutilization of glucocorticoid receptors. Mol Endocrinol. 1989;3:1279–1288. doi: 10.1210/mend-3-8-1279. [DOI] [PubMed] [Google Scholar]
- Qi M, Stasenko LJ, DeFranco DB. Recycling and desensitization of glucocorticoid receptors in v-mos transformed cells depend on the ability of nuclear receptors to modulate gene expression. Mol Endocrinol. 1990;4:455–464. doi: 10.1210/mend-4-3-455. [DOI] [PubMed] [Google Scholar]
- DeFranco DB, Qi M, Borror KC, Garabedian MJ, Brautigan DL. Protein phosphatase types 1 and/or 2A regulate nucleocytoplasmic shuttling of glucocorticoid receptors. Mol Endocrinol. 1991;5:1215–1228. doi: 10.1210/mend-5-9-1215. [DOI] [PubMed] [Google Scholar]
- Galigniana MD, Housley PR, DeFranco DB, Pratt WB. Inhibition of glucocorticoid receptor nucleocytoplasmic shuttling by okadaic acid requires intact cytoskeleton. J Biol Chem. 1999;274:16222–16227. doi: 10.1074/jbc.274.23.16222. [DOI] [PubMed] [Google Scholar]
- Cohen P, Holmes CF, Tsukitani Y. Okadaic acid: a new probe for the study of cellular regulation. Trends Bio Sci. 1990;15:98–102. doi: 10.1016/0968-0004(90)90192-E. [DOI] [PubMed] [Google Scholar]
- Cohen P. Classification of protein-serine/threonine phosphatases: identification and quantitation in cell extracts. Methods Enzymol. 1991;201:389–397. doi: 10.1016/0076-6879(91)01035-z. [DOI] [PubMed] [Google Scholar]
- Song Q, Khanna KK, Lu H, Lavin MF. Cloning and characterization of a human protein phosphatase 1-encoding cDNA. Gene. 1993;129:291–295. doi: 10.1016/0378-1119(93)90282-8. [DOI] [PubMed] [Google Scholar]
- Barker HM, Craig SP, Spurr NK, Cohen PT. Sequence of human protein serine/threonine phosphatase 1 gamma and localization of the gene (PPP1CC) encoding it to chromosome bands 12q24.1-q24.2. Biochim Biophys Acta. 1993;1178:228–233. doi: 10.1016/0167-4889(93)90014-G. [DOI] [PubMed] [Google Scholar]
- Barker HM, Brewis ND, Street AJ, Spurr NK, Cohen PT. Three genes for protein phosphatase 1 map to different human chromosomes: sequence, expression and gene localization of protein serine/threonine phosphatase 1 beta (PPP1CB). BiochemBiophys Acta. 1994;1220:12–218. doi: 10.1016/0167-4889(94)90138-4. [DOI] [PubMed] [Google Scholar]
- Stone R, Hofsteenge J, Hemmings A. Molecular cloning of cDNAs encoding two isoforms of the catalytic subunit of protein phosphatase 2A. Biochemistry. 1987;26:7215–7220. doi: 10.1021/bi00397a003. [DOI] [PubMed] [Google Scholar]
- Arino J, Woon CW, Brautigan DL, Miller TB, Jr, Johnson GL. Human liver phosphatase 2A: cDNA and amino acid sequence of two catalytic subunit isotypes. Proc Natl Acad Sci U S A. 1988;85:4252–4256. doi: 10.1073/pnas.85.12.4252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X, Cheng A, Honkanen RE. Genomic organization of the human PP4 gene encoding a serine/threonine protein phosphatase (PP4) suggests a common ancestry with PP2A. Genomics. 1997;44:336–343. doi: 10.1006/geno.1997.4891. [DOI] [PubMed] [Google Scholar]
- Becker W, Kentrup H, Klumpp S, Schultz JE, Joost HG. Molecular cloning of a protein serine/threonine phosphatase containing a putative regulatory tetratricopeptide repeat domain. J Biol Chem. 1994;269:22586–92. [PubMed] [Google Scholar]
- Chen MX, McPartlin AE, Brown L, Chen YH, Barker HM, Cohen PT. A novel human protein serine/threonine phosphatase, which possesses four tetratricopeptide repeat motifs and localizes to the nucleus. EMBO J. 1994;12:4278–4290. doi: 10.1002/j.1460-2075.1994.tb06748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastians H, Ponstingl H. The novel human protein serine/threonine phosphatase 6 is a functional homologue of budding yeast Sit4p and fission yeast ppe1, which are involved in cell cycle regulation. J Cell Sci. 1996;109:2865–2874. doi: 10.1242/jcs.109.12.2865. [DOI] [PubMed] [Google Scholar]
- Huang X, Honkanen RE. Molecular cloning, expression, and characterization of a novel human serine/threonine protein phosphatase, PP7, that is homologous to Drosophila retinal degeneration C gene product (rdgC). J Biol Chem. 1998;273:1462–1468. doi: 10.1074/jbc.273.3.1462. [DOI] [PubMed] [Google Scholar]
- Hastie CJ, Cohen PT. Purification of protein phosphatase 4 catalytic subunit: inhibition by the antitumor drug fostriecin and other tumor suppressors and promoters. FEBS Lett. 1998;431:357–361. doi: 10.1016/S0014-5793(98)00775-3. [DOI] [PubMed] [Google Scholar]
- Russell LC, Whitt SR, Chen MS, Chinkers M. Identification of conserved residues required for the binding of a tetratricopeptide repeat domain to heat shock protein 90. J Biol Chem. 1999;274:20060–3. doi: 10.1074/jbc.274.29.20060. [DOI] [PubMed] [Google Scholar]
- Silverstein AM, Galigniana MD, Chen MS, Owens-Grillo JK, Chinkers M, Pratt WB. Protein phosphatase 5 is a major component of glucocorticoid receptor.hsp90 complexes with properties of an FK506-binding immunophilin. J Biol Chem. 1997;272:16224–16230. doi: 10.1074/jbc.272.26.16224. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Dean NM, Honkanen RE. Serine/threonine protein phosphatase type 5 acts upstream of p53 to regulate the induction of p21(WAF1/Cip1) and mediate growth arrest. J Biol Chem. 1998;273:12250–12258. doi: 10.1074/jbc.273.20.12250. [DOI] [PubMed] [Google Scholar]
- Zuo Z, Urban G, Scammell JG, Dean NM, McLean TK, Aragon I, Honkanen RE. Ser/Thr protein phosphatase type 5 (PP5) is a negative regulator of glucocorticoid receptor-mediated growth arrest. Biochemistry. 1999;38:8849–57. doi: 10.1021/bi990842e. [DOI] [PubMed] [Google Scholar]
- Walsh AH, Cheng A, Honkanen RE. Fostriecin, an antitumor antibiotic with inhibitory activity against serine/threonine protein phosphatases types 1 (PP1) and 2A (PP2A), is highly selective for PP2A. FEBS Lett. 1997;416:230–4. doi: 10.1016/S0014-5793(97)01210-6. [DOI] [PubMed] [Google Scholar]
- Lanz RB, Wieland S, Hug M, Rusconi S. A transcriptional repressor obtained by alternative translation of a trinucleotide repeat. Nucleic Acids Res. 1995;23:138–145. doi: 10.1093/nar/23.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwan IJ, Wright AP, Gustafsson JA. Mechanism of gene expression by the glucocorticoid receptor: role of protein-protein interactions. BioEssays. 1997;19:153–160. doi: 10.1002/bies.950190210. [DOI] [PubMed] [Google Scholar]
- Maiyar AC, Huang AJ, Phu PT, Cha HH, Firestone GL. p53 stimulates promoter activity of the sgk serum/glucocorticoid-inducible serine/threonine protein kinase gene in rodent mammary epithelial cells. J Biol Chem. 1996;271:12414–22. doi: 10.1074/jbc.271.21.12414. [DOI] [PubMed] [Google Scholar]
- Rogatsky I, Waase CLM, Garabedian MJ. Phosphorylation and inhibition of rat glucocorticoid receptor transcriptional activation by glycogen synthase kinase-3 (GSK-3). Species-specific differences between human and rat glucocorticoid receptor signaling as revealed through GSK-3 phosphorylation. J Biol Chem. 1998;273:14315–14321. doi: 10.1074/jbc.273.23.14315. [DOI] [PubMed] [Google Scholar]
- Krstic MD, Rogatsky I, Yamamoto KR, Garabedian MJ. Mitogen-activated and cyclin-dependent protein kinases selectively and differentially modulate transcriptional enhancement by the glucocorticoid receptor. Mol Cell Biol. 1997;17:3947–3954. doi: 10.1128/mcb.17.7.3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehlenback RH, Gerace L. Phosphorylation of the nuclear transport machinery down-regulates nuclear protein import in vitro. J Biol Chem. 2000;275:17848–56. doi: 10.1074/jbc.M001455200. [DOI] [PubMed] [Google Scholar]
- Cheng A, Dean NM, Honkanen RE. Serine/threonine protein phosphatase type 1gamma1 is required for the completion of cytokinesis in human A549 lung carcinoma cells. J Biol Chem. 2000;275:1846–1854. doi: 10.1074/jbc.275.3.1846. [DOI] [PubMed] [Google Scholar]
- Huang X, Swingle MR, Honkanen RE. Photoreceptor serine/threonine protein phosphatase type 7: cloning, expression, and functional analysis. Methods Enzymol. 2000;315:579–93. doi: 10.1016/s0076-6879(00)15869-0. [DOI] [PubMed] [Google Scholar]
- Killilea SD, Aylward JH, Mellgren RL, Lee EY. Purification and properties of bovine myocardial phosphorylase phosphatase (protein phosphatase C). Arch Biochem Biophys. 1978;191:638–646. doi: 10.1016/0003-9861(78)90402-2. [DOI] [PubMed] [Google Scholar]
- Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current protocols in molecular biology New York: John Wiley & Sons; 1994.
- Dean DA. Import of plasmid DNA into the nucleus is sequence specific. Exp Cell Res. 1997;230:293–302. doi: 10.1006/excr.1996.3427. [DOI] [PubMed] [Google Scholar]
- Kehlenbach RH, Dickmanns A, Gerace L. Nucleocytoplasmic shuttling factors including Ran and CRM1 mediate nuclear export of NFAT In vitro. J Cell Biol. 1998;141:863–74. doi: 10.1083/jcb.141.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]


