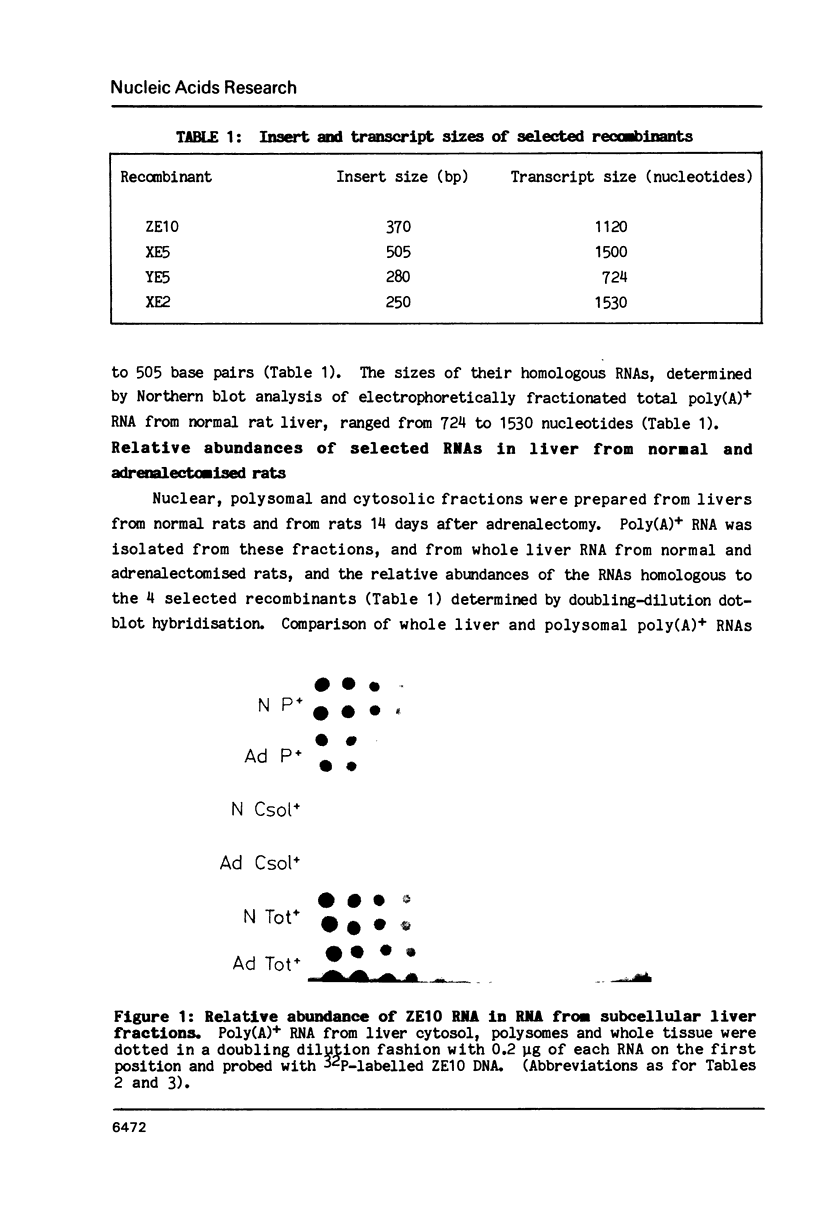
Abstract
We have investigated the mechanisms whereby glucocorticoids control the expression of specific genes in the livers of adult male rats. Construction and differential screening of a cDNA library representing normal rat liver polysomal poly(A)+ RNAs allowed selection of probes for hormonally regulated genes. The mechanism of this regulation was analysed by studying the changes in relative abundance of the RNA sequences homologous to four selected recombinants in RNA from subcellular fractions of liver, and comparing them with that of albumin mRNA. The relative abundance of these four RNA sequences increased to varying degrees in the nucleus, whilst that of three of them was concomitantly depleted in polysomal RNA when circulating levels of glucocorticoids were negligible, i.e. 14 days after adrenalectomy. One of the sequences was identified as alpha 2U-globulin mRNA. Within 2 hours of injecting Dexamethasone (a synthetic glucocorticoid) into rats that had been adrenalectomised 14 days previously, the relative abundances of alpha 2U-globulin RNA in nuclear and polysomal RNA returned to those found in normal rat liver. The data indicate that reduced glucocorticoid levels lead to sequence specific retention of RNA in the nucleus and that the RNA retained is released to the cytoplasm following glucocorticoid injection. Our results provide an example, for the first time, of glucocorticoid regulation of gene expression at the post-transcriptional level of nucleo-cytoplasmic transport.
Full text
PDF




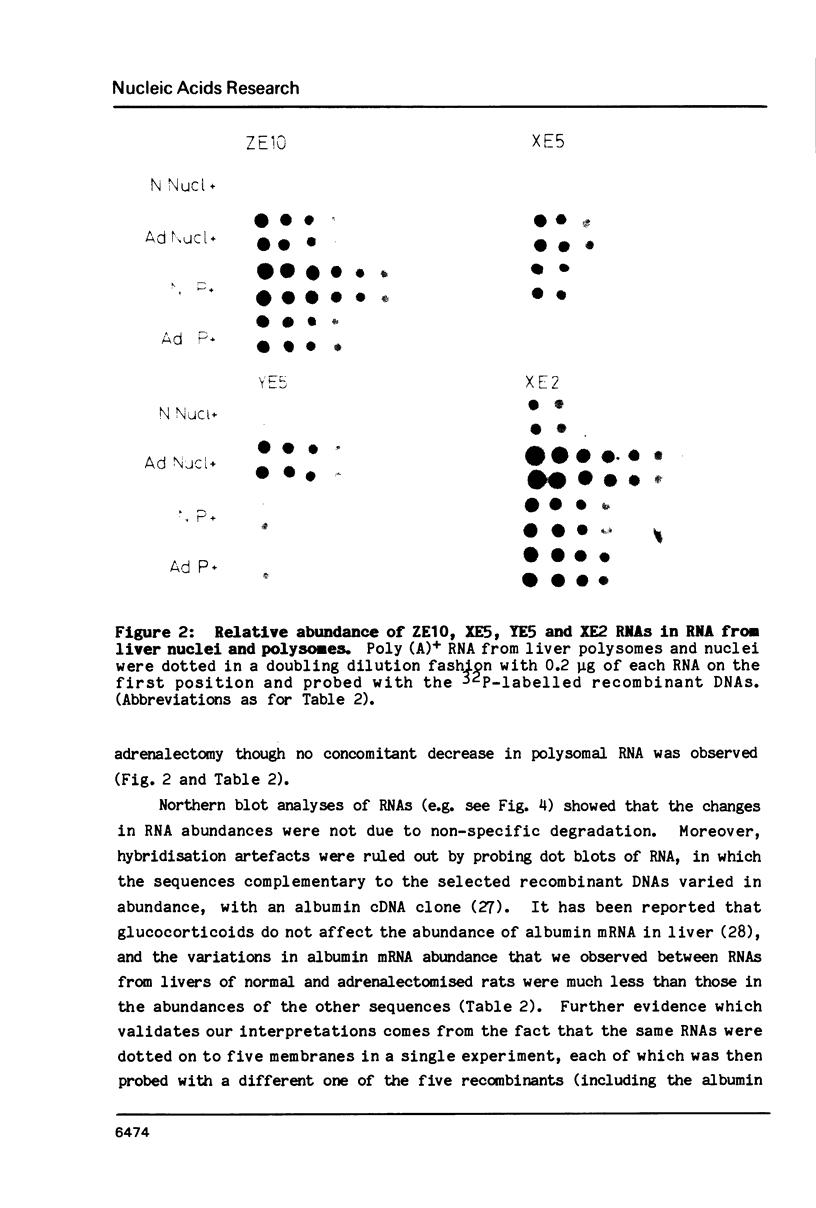

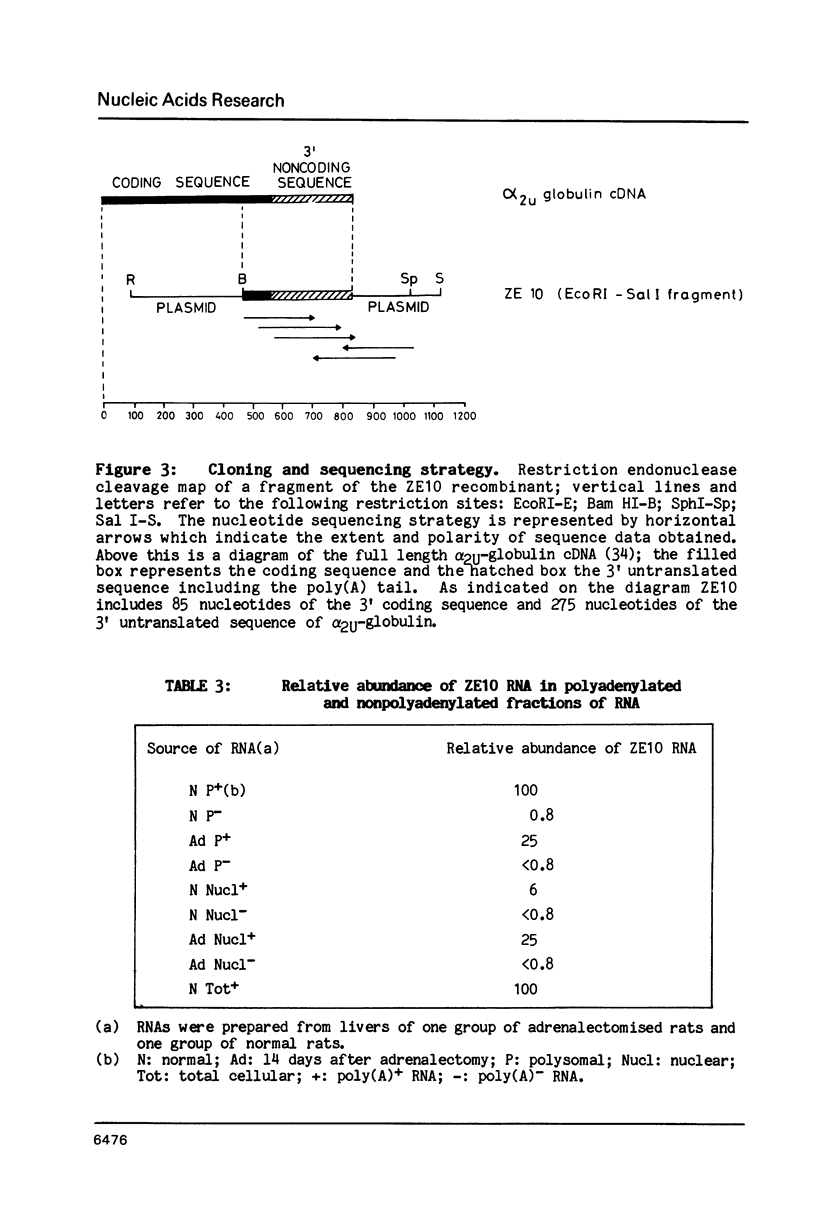






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auffray C., Rougeon F. Purification of mouse immunoglobulin heavy-chain messenger RNAs from total myeloma tumor RNA. Eur J Biochem. 1980 Jun;107(2):303–314. doi: 10.1111/j.1432-1033.1980.tb06030.x. [DOI] [PubMed] [Google Scholar]
- Aviv H., Leder P. Purification of biologically active globin messenger RNA by chromatography on oligothymidylic acid-cellulose. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1408–1412. doi: 10.1073/pnas.69.6.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajwa W., Birnie G. D., Knowler J. T. Changes induced in rat liver polysomal polyadenylated RNAs by depletion of circulating glucocorticoids. Nucleic Acids Res. 1982 Jun 11;10(11):3541–3560. doi: 10.1093/nar/10.11.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnie G. D., MacPhail E., Young B. D., Getz M. J., Paul J. The diversity of the messenger RNA population in growing Friend cells. Cell Differ. 1974 Nov;3(4):221–232. doi: 10.1016/0045-6039(74)90005-0. [DOI] [PubMed] [Google Scholar]
- Brock M. L., Shapiro D. J. Estrogen stabilizes vitellogenin mRNA against cytoplasmic degradation. Cell. 1983 Aug;34(1):207–214. doi: 10.1016/0092-8674(83)90151-4. [DOI] [PubMed] [Google Scholar]
- Brown A., Colen A. H., Fisher H. F. Effect of ammonia on the glutamate dehydrogenase catalyzed oxidative deamination of L-glutamate. The steady state. Biochemistry. 1979 Dec 25;18(26):5924–5928. doi: 10.1021/bi00593a025. [DOI] [PubMed] [Google Scholar]
- Capetanaki Y. G., Flytzanis C. N., Alonso A. Repression of the albumin gene in Novikoff hepatoma cells. Mol Cell Biol. 1982 Mar;2(3):258–266. doi: 10.1128/mcb.2.3.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clissold P. M., Bishop J. O. Molecular cloning of cDNA sequences transcribed from mouse liver endoplasmic reticulum poly(A)mRNA. Gene. 1981 Nov;15(2-3):225–235. doi: 10.1016/0378-1119(81)90132-3. [DOI] [PubMed] [Google Scholar]
- Crampton J. M., Davies K. E., Knapp T. F. The occurrence of families of repetitive sequences in a library of cloned cDNA from human lymphocytes. Nucleic Acids Res. 1981 Aug 11;9(15):3821–3834. doi: 10.1093/nar/9.15.3821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danesch U., Hashimoto S., Renkawitz R., Schütz G. Transcriptional regulation of the tryptophan oxygenase gene in rat liver by glucocorticoids. J Biol Chem. 1983 Apr 25;258(8):4750–4753. [PubMed] [Google Scholar]
- Diamond D. J., Goodman H. M. Regulation of growth hormone messenger RNA synthesis by dexamethasone and triiodothyronine. Transcriptional rate and mRNA stability changes in pituitary tumor cells. J Mol Biol. 1985 Jan 5;181(1):41–62. doi: 10.1016/0022-2836(85)90323-7. [DOI] [PubMed] [Google Scholar]
- Dolan K. P., Unterman R., McLaughlin M., Nakhasi H. L., Lynch K. R., Feigelson P. The structure and expression of very closely related members of the alpha 2u globulin gene family. J Biol Chem. 1982 Nov 25;257(22):13527–13534. [PubMed] [Google Scholar]
- FEIGELSON P., FEIGELSON M. Studies on the mechanism of regulation by cortisone of the metabolism of liver purine and ribonucleic acid. J Biol Chem. 1963 Mar;238:1073–1077. [PubMed] [Google Scholar]
- Graham S. V., Tindle R. W., Birnie G. D. Variation in myc gene amplification and expression in sublines of HL60 cells. Leuk Res. 1985;9(2):239–247. doi: 10.1016/0145-2126(85)90086-4. [DOI] [PubMed] [Google Scholar]
- Guyette W. A., Matusik R. J., Rosen J. M. Prolactin-mediated transcriptional and post-transcriptional control of casein gene expression. Cell. 1979 Aug;17(4):1013–1023. doi: 10.1016/0092-8674(79)90340-4. [DOI] [PubMed] [Google Scholar]
- Jacobs H., Birnie G. D. Isolation and purification of rat hepatoma nuclei active in the transport of messenger RNA in vitro. Eur J Biochem. 1982 Jan;121(3):597–607. doi: 10.1111/j.1432-1033.1982.tb05828.x. [DOI] [PubMed] [Google Scholar]
- Kurtz D. T. Rat alpha 2u globulin is encoded by a multigene family. J Mol Appl Genet. 1981;1(1):29–38. [PubMed] [Google Scholar]
- Leys E. J., Crouse G. F., Kellems R. E. Dihydrofolate reductase gene expression in cultured mouse cells is regulated by transcript stabilization in the nucleus. J Cell Biol. 1984 Jul;99(1 Pt 1):180–187. doi: 10.1083/jcb.99.1.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masiakowski P., Breathnach R., Bloch J., Gannon F., Krust A., Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982 Dec 20;10(24):7895–7903. doi: 10.1093/nar/10.24.7895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rousseau G. G. Control of gene expression by glucocorticoid hormones. Biochem J. 1984 Nov 15;224(1):1–12. doi: 10.1042/bj2240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer G., Schmid W., Strange C. M., Röwekamp W., Schütz G. Isolation of cDNA clones coding for rat tyrosine aminotransferase. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7205–7208. doi: 10.1073/pnas.79.23.7205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid W., Scherer G., Danesch U., Zentgraf H., Matthias P., Strange C. M., Röwekamp W., Schütz G. Isolation and characterization of the rat tryptophan oxygenase gene. EMBO J. 1982;1(10):1287–1293. doi: 10.1002/j.1460-2075.1982.tb00026.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumm D. E., Webb T. E. Effect of physiological concentrations of insulin and antidiabetic drugs on RNA release from isolated liver nuclei. J Cell Biochem. 1983;23(1-4):223–229. doi: 10.1002/jcb.240230119. [DOI] [PubMed] [Google Scholar]
- Thayer R. E. An improved method for detecting foreign DNA in plasmids of Escherichia coli. Anal Biochem. 1979 Sep 15;98(1):60–63. doi: 10.1016/0003-2697(79)90705-x. [DOI] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannice J. L., Taylor J. M., Ringold G. M. Glucocorticoid-mediated induction of alpha 1-acid glycoprotein: evidence for hormone-regulated RNA processing. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4241–4245. doi: 10.1073/pnas.81.14.4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann L. M., Burns J. H., Birnie G. D. Differences among the polyadenylated RNA sequences of human leucocyte populations: an approach to the objective classification of human leukaemias. EMBO J. 1983;2(1):9–13. doi: 10.1002/j.1460-2075.1983.tb01372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]