Abstract
Hepatic fibrogenesis is reduced in the absence of leptin. We hypothesized that leptin protects hepatic stellate cells (HSCs) from apoptosis and tested this in in vitro and in vivo systems. (i) Fas ligand (fas-L)-mediated apoptosis was induced in vitro in activated HSCs in the absence and presence of leptin. (ii) HSC apoptosis was also induced by UV irradiation in the absence and presence of leptin. (iii) Fas-L-mediated apoptosis was induced in vitro in HSCs from db/db mice in the absence and presence of leptin. (iv) Liver fibrosis was induced in wt and db/db mice. (v) Liver fibrosis was induced in wild-type mice with TAA, and mice received additional leptin or a control solution. HSC apoptosis was assessed by TUNEL staining. Western blot for α-SMA was used to determine differences in HSC activation. Results were as follows. (i) Fas-L induced significant apoptosis of HSC, and preincubation with leptin reduced this approximately threefold. (ii) Leptin provided no protection from UV-induced apoptosis. (iii) HSCs from db/db mice were not protected by leptin against fas-L-induced apoptosis. (iv) TAA-induced fibrosis was significantly less in db/db mice compared to wild type. (v) Wild-type mice receiving leptin had less apoptosis and more α-SMA than controls. We conclude that leptin protects HSC from in vitro and in vivo apoptosis. The antiapoptotic effect of leptin requires the long form of the leptin receptor and interacts with the apoptotic pathway proximal to mitochondrial activation.
Keywords: Fibrosis, Leptin, Stellate cells, Apoptosis
Obesity has been identified as a risk factor in the progression of liver fibrosis in a wide range of diseases including hepatitis C infection, alcoholic liver diseases, and NASH [1–3]. With the steady increase in the proportion of obese individuals, obesity may significantly affect the natural history of these conditions in a large number of individuals [4]. It is therefore of great interest to understand the mechanism by which obesity interacts with progression of liver fibrosis. There is a very close relationship between serum levels of leptin and body fat stores in the fed state, and thus leptin may be an important mediator of increased liver fibrosis in obesity [3]. The vital role of leptin in hepatic fibrogenesis was recently identified by a number of groups by demonstrating that mice lacking the hormone leptin (ob/ob mice) are resistant to the induction of liver fibrosis [5–8].
Leptin is a 16-kd molecule with multiple effects on immunity, inflammation, and hematopoiesis in addition to its original description as regulating satiety [9–11]. The leptin receptor has at least six isoforms and isoforms obRa and obRb are expressed widely [12]. The pleiotropic effects of leptin and ubiquitous expression of leptin receptor isoforms mean that the requirement of leptin for the induction of fibrosis may be through a number of cellular pathways [6]. For example, in the original paper, Honda et al. also demonstrated that ob/ob mice did not upregulate hepatic TGF-β in response to thioacetamide. It was hypothesized that this was due to a requirement for leptin for a nonstellate cell population to produce TGF-β, which subsequently is needed for HSC differentiation, and fibrogenesis.
Hepatic stellate cells (HSCs) are central to hepatic fibrogenesis and have been shown to undergo apoptosis by a number of different mechanisms including fas-L-mediated apoptosis [13–15]. This may be due to ligation of fas-L and fas on the same cell and has been proposed as a physiological regulatory mechanism to terminate proliferation of activated HSCs [14]. No previous association has been reported between fas and leptin signaling.
In the current study we show that HSCs possess the extracellular domain of the leptin receptor and, as previously reported, undergo fas-L-induced apoptosis. In addition, we show that leptin at physiological concentrations protects HSC from fas-L-induced apoptosis and that this protection is mediated through the long isoform of the leptin receptor. We also show that this long isoform of the receptor is required to induce chemical hepatic fibrosis. Finally, we show that in vivo treatment of leptin causes substantial survival of activated HSCs in a fibrosis model which is associated with a decrease in HSC apoptosis. These findings suggest that the in vivo requirement for leptin for the induction of liver fibrosis may be due to leptin protecting HSCs from rapid fas-L-mediated apoptosis. Furthermore, leptin antagonists may have a role in removing this protective effect, thus limiting the development of liver fibrosis.
Materials and methods
Hepatic stellate cell isolation
Media (M199) were obtained from Invitrogen (Grand Island, NY) and Cellgro (Herndon, VA), fetal bovine serum (FBS) was from Gemini Biosciences (Woodland, CA), and antibiotics were from Invitrogen. Primary mouse HSCs were isolated from the livers of 6- to 8-week-old C57/B6 (WT) and db/db mice weighing 20–22 and 30–45 g, respectively, purchased from Jackson Laboratories. The method described previously was modified per Liu et al. [16]. Mice were anesthetized and in situ liver perfusion and digestion were performed with pronase E (2.4 mg/ml; Roche Molecular Biochemicals, Chicago, IL) and collagenase B (0.3–0.45 mg/ml; Roche Molecular Biochemicals), and the resulting liver cell suspension was purified by density gradient centrifugation using 9–11% Nycodenz (Sigma). HSCs were plated on uncoated plastic coverslips at a density of 1 × 106 per 1-cm-diameter plate and maintained in M199/10% FBS supplemented with glutamine and antibiotics (penicillin, 100 units/ml; streptomycin, 100 units/ml; gentamicin, 0.1 mg/ml; and fungizone [Invitrogen], 2.5 μg/ml). Cells at day 7 were activated, with more than 95% staining positive for α-smooth muscle actin (SMA).
Soluble fas-L, ultraviolet irradiation, and leptin
Apoptosis was induced by super fas-L (Apotech, Epalinges, Switzerland; used at 5–15 ng/ml) and cycloheximide (Sigma; used at 10 μg/ml) [17, 18]. Apoptosis via activation of the mitochondrial pathway was induced by ultraviolet (UV) irradiation at a dose of 30 J/m2. Recombinant murine leptin was purchased from Research Diagnostic Inc. (USA), dissolved in sodium citrate titrated to physiological pH with Tris, and used at concentrations ranging from 1 to 100 nM.
Stellate cell apoptosis
Leptin or control PBS was added to day 7 HSCs. Twenty-four hours after adding leptin, soluble fas ligand and cycloheximide were added. At 18 hr the HSCs were fixed with paraformaldehyde (4%) and apoptosis evaluated by DAPI (2 μmol; Vector, Burlingame, CA) or TUNEL (ApopTag, Temecula, CA) staining using a Zeiss LSM 510 with a krypton/argon laser. Chromatin condensation on DAPI staining was used to identify apoptotic cells.
UV irradiation apoptosis was induced after HSC activation at day 7. Six hours after irradiation, HSCs were fixed with 4% paraformaldehyde and apoptosis was evaluated with DAPI (2 μmol). All experiments were repeated at least six times.
α-smooth muscle actin immunofluorescence
Purity of HSC preparations was confirmed by α-SMA labeling. HSCs were incubated with 0.1% Triton, then washed with PBS. The cells were then incubated with unconjugated anti-α SMA-mouse monoclonal antibody (Sigma) at a 1:400 dilution for 1 hr. Fluorescent secondary conjugation was performed with Alexa Fluor 594 donkey anti-mouse IgG at a 1:400 dilution (Molecular Probes, Eugene, OR). Immunofluorescence was determined by direct microscopy. Staining with mouse IgG and secondary antibody alone was performed as a control.
Leptin receptor immunofluorescence
The presence of the leptin receptor on the HSCs was confirmed by immunofluorescence. HSCs were washed with PBS and placed in 50 ml of ice-cold fixation buffer for 20 min at − 4°C. The cells were then washed with saponin buffer and placed in PBS/2% FBS for 10 min, followed by another wash with saponin. Primary incubation with the polyclonal goat anti-mouse leptin receptor antibody (Research Diagnostic Inc.) was done at a 1:100 dilution for 30 min, then the mixture was washed with saponin. The HSCs were then labeled with donkey anti-goat IgG antibody (Molecular Probes) at a 1:400 dilution for 30 min for secondary conjugation. A final wash with saponin wash buffer was done. Fluorescent microscopy was performed to detect leptin receptor immunofluoroscence. Staining with mouse IgG and secondary antibody alone was performed as a control.
In vivo studies
Male WT mice and db/db mice were purchased from Jackson Labaratory (Bar Harbor, ME), were kept at the Yale Animal Facility, and received care under the institutional guidelines. Both WT and db/db mice were separated randomly at 6–8 weeks of age to receive 100 μl of sterile water only or thioacetamide (TAA; 200 mg/kg) dissolved in 100 μl of sterile water intraperitoneally three times a week for 6 weeks. At 6 weeks, mice were sacrificed by ketamine anesthesia followed by exsanguination via the inferior vena cava. Liver tissue was collected and stored in liquid nitrogen for protein analysis. The remaining liver was fixed with 4% paraformaldehyde for histology, then embedded in paraffin and Sirius red, and α-SMA staining was performed.
To test the role of supplementing leptin and its effect on in vivo HSC apoptosis, wild-type (WT) mice were treated with TAA or control as outlined above. During the sixth week of TAA treatment, mice were treated with leptin (1 μg/g) or control PBS for 3 consecutive days prior to sacrifice. Leptin at 1 μg/g was chosen because this dose has been shown to replete leptin-deficient ob/ob mice to a degree sufficient to induce liver fibrosis after treatment with TAA [19]. Mice were then sacrificed and tissue was collected for protein analysis and histology as outlined above.
α-SMA western blot protocol
Cells from liver tissue specimens were lysed with a Cell Lysis Buffer (Cell Signaling, Beverly, MA) diluted in water and PMSF. After agitation in ice for 30 min, this suspension was centrifuged and the supernatant was collected for analysis. The concentration of protein was determined by the Bradford assay. A company-based kit was used to run western blot assays (Invitrogen, Carlsbad, CA). A protein concentration of 25 μg/ul was made in LDS buffer and reducing agent was provided by the manufacturer. The suspension was heated for 10 min at 70°C. The protein samples were electophoresed using 4–12% gels in MES buffer at 150 V for 1 hr. The protein samples were transferred from the gel to a polyvinylidene di-fluoride (PVDF) membrane following treatment with 100% methanol for 1 min and the transfer buffer provided by the manufacturer at 20 V for 30 min. Successful protein transfer was confirmed byusing Ponceau s solution (Sigma). The PVDF membrane was treated with blocking solution (5% dry milk and 0.05% Tween 20 in PBS) for 1 hr. The membrane was then incubated with unconjugated anti-α SMA-mouse monoclonal antibody (Sigma) at 1:1000 dilution in blocking solution overnight at 4°C. The membrane was then washed with blocking solution three times, for 20 min each. Next the membrane was incubated with a secondary anti-mouse horseradish peroxidase IgG (Amersham, NJ) for 1 hr at room temperature, then washed with blocking solution. The membrane was then treated with a chemiluminescent kit (Pierce, Rockford, IL). The membranes were exposed to X-ray film (Kodak, Rochester, NY) for visualization. For the in vivo leptin experiments α-SMA western blot signal was quantified using Scion imaging software (NIH) and expressed as a ratio of the β-actin signal.
Analysis of microscopy data
For the in vitro HSC culturing experiments, HSCs were counted from 10 high-power fields (HPFs). DAPI staining was used to identify all stellate cells. Chromatin condensation on DAPI staining or TUNEL positivity was used to identify apoptotic cells. A range of 400–800 cells was counted per treatment group in each experiment.
For in vivo experiments the established protocol of Iredale et al. was used and the total numbers of TUNEL-positive and α-SMA-positive cells per HPF were counted [14].
Members of our team blinded to the identity of the study groups (A.M. and W.H.) performed the microscopy and quantification. The percentages of DAPI/TUNEL-positive cells among the total stellate cells were then compared among the different groups. For experiments evaluating differences in fibrosis, the following staging protocol was used: Stage 0, no fibrosis; Stage I, mild perivenular fibrosis; Stage II, perivenular fibrosis extending beyond the limiting plate; Stage III, bridging fibrosis; and Stage IV, cirrhosis.
Statistical analysis
Mean percentages with standard deviation and standard error of measurements were used for statistical analysis. Statistical significance was defined as a P value < 0.05 determined by the two-tailed unpaired t-test.
Results
α-SMA and leptin receptor immunofluorescence
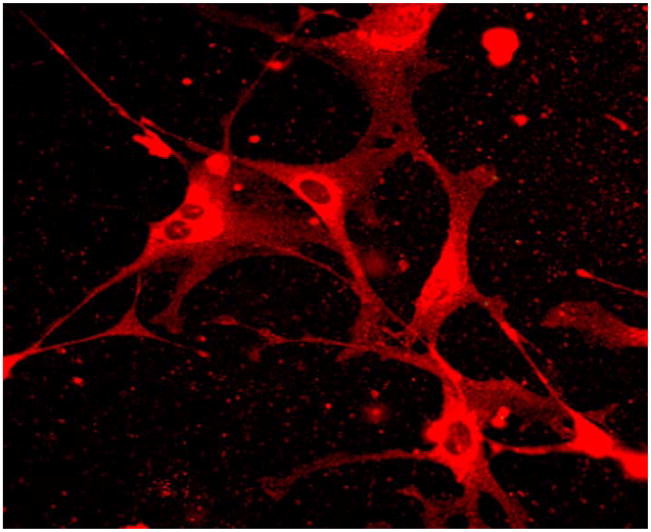
We confirmed the presence of activated stellate cells in our isolations with α-smooth actin labeling. Approximately 3 million to 5 million HSCs were isolated per mouse and a high degree of HSC purity (>95%) was confirmed. Immunofluorescence to the leptin receptor was used to confirm the presence of the leptin receptor on activated HSCs as shown in Fig. 1.
Fig. 1.
Leptin receptor immunofluorescence. Resting stellate cells were isolated and plated on plastic coverslips. After 7 days, stellate cells demonstrated morphological signs of activation. Leptin receptor immunofluorescence demonstrates expression of leptin receptor by activated stellate cells
Leptin protects activated HSCs from fas-L-mediated apoptosis
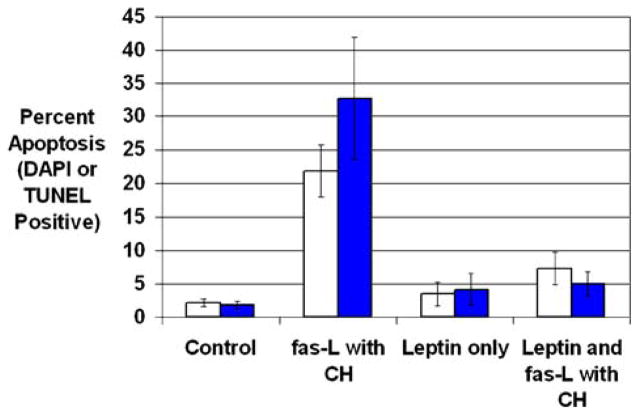
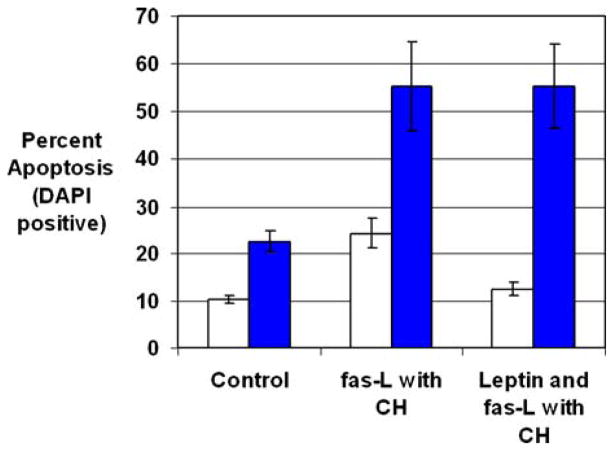
Coculture with fas-L and cyclohexamide (CH) induced significantly greater apoptosis of HSCs than control media (Fig. 2) (control, 2.2 ± 0.6% [SE], vs. fas-L and CH, 21.8 ± 3.9%; P < 0.05). Leptin preincubation in the absence of fas-L and CH did not induce HSC apoptosis over that in control media. Leptin preincubation prior to incubation with fas-L and CH resulted in significantly less HSC apoptosis compared with the fas-L and CH group (leptin and fas ligand with CH, 7.3 ± 2.4%, vs. fas ligand with CH, 21.8 ± 3.9%; P < 0.05) (Fig. 3).
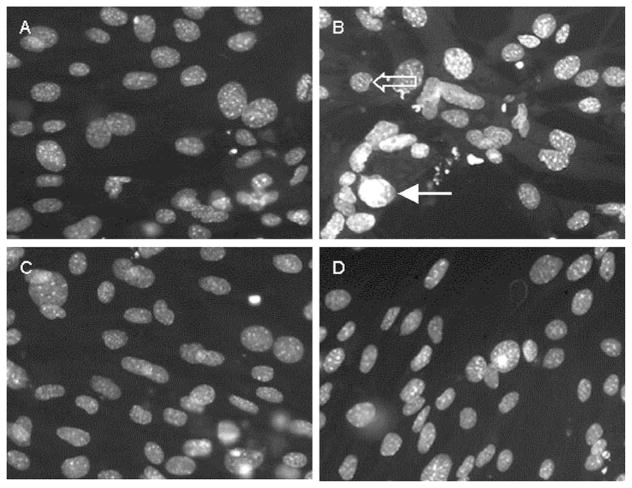
Fig. 2.
Leptin preincubation decreases fas ligand- and cyclohexamide-induced stellate cell apoptosis. Stellate cells were isolated from wild-type mice and plated on plastic coverslips for activation. After 7 days, stellate cells demonstrated morphological signs of activation. Stellate cells were incubated with control medium (A and B) or 100 nM leptin (C and D) for 24 hr. Soluble fas ligand (5–15 ng/ml) and cyclohexamide (10 μg/ml) were added to half the wells (B and D). Eighteen hours after adding fas ligand, cells were fixed with 4% paraformaldehyde, and stained with DAPI. Chromatin condensation (filled arrow) was used to identify apoptotic stellate cells. A nonapoptotic stellate cell is shown (open arrow)
Fig. 3.
Leptin preincubation decreases fas ligand- and cyclohexamide-induced stellate cell apoptosis. Stellate cells were isolated from wild-type mice and plated on plastic coverslips for activation. After 7 days, stellate cells demonstrated morphological signs of activation. Stellate cells were incubated with control medium or 100 nM leptin for 24 hr. Soluble fas ligand (5–15 ng/ml) and cyclohexamide (10 μg/ml) were added to half the wells. Cells were fixed with 4% paraformaldehyde and apoptosis was detected using two methods: DAPI and TUNEL. (A) Chromatin condensation was used to identify apoptotic stellate cells by DAPI (open bars), and apoptotic stellate cells were calculated as a percentage of total stellate cells. (B) Enhanced HSC nuclear staining by the TUNEL assay was used to identify apoptosis and calculated as a percentage of total HSCs (filled bars). Results are shown as mean percentages of apoptotic cells ± SE
To confirm this finding, we used TUNEL as an alternate method of detecting HSC apoptosis. As expected, there was increased HSC apoptosis detected by TUNEL with fas-L and CH compared with the control HSC group (Fig. 3) (control, 1.8 ± 0.6%, vs. fas-L and CH, 32.7 ± 9.2%; P < 0.05). Similar to the DAPI results, leptin was able to protect stellate cells from fas-mediated apoptosis, and its preincubation resulted in an approximately sixfold decrease in TUNEL signal (leptin with fas-L and CH, 5.0 ± 1.8%, vs. fas-L and CH, 32.7 ± 9.2%; P < 0.05).
Leptin does not protect HSCs from the mitochondrial pathway of apoptosis
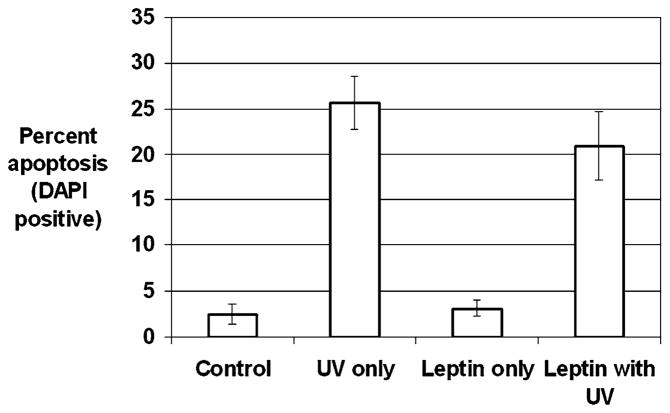
We next determined whether leptin protected HSCs from apoptosis mediated by the mitochondrial pathway. To induce HSC apoptosis by the mitochondrial pathway, UV irradiation was used with an experimental design similar to that used for the fas-L experiments. There was a 10-fold increase in HSC apoptosis with UV irradiation (control, 2.5 ± 1.1%, vs. UV alone, 25.7 ± 2.9; P < 0.05). Leptin preincubation provided HSCs no protection from UV-induced apoptosis (UV alone, 25.7 ± 2.9%, vs. leptin with UV, 20.9 ± 3.8%; P = 0.32) (Fig. 4). This finding suggests that leptin’s protective effect against apoptosis is proximal to the mitochondrial activation.
Fig. 4.
UV irradiation-induced stellate cell apoptosis detected by DAPI. Stellate cells were isolated from wild-type mice and plated on plastic coverslips for activation. After 7 days, stellate cells demonstrated morphological signs of activation. Stellate cells were incubated with control medium or 100 nM leptin for 24 hr. HSCs were exposed to UV irradiation at a dose of 30 J/m2. Six hours after UV irradiation, HSCs were fixed with 4% paraformaldehyde and stained with DAPI. Chromatin con was used to identify apoptotic stellate cells by DAPI, and apoptotic stellate cells were calculated as a percentage of total stellate cells. Results are shown as -fold increase in apoptosis compared to control baseline ± SE
Leptin’s protective effect against fas-L is mediated by the long isoform of the leptin receptor
The requirement for the long isoform of the leptin receptor in the antiapoptotic effects of leptin was evaluated by comparing the ability of leptin to prevent apoptosis in HSCs from WT and db/db mice. In WT HSCs, the expected fas-Land CH-induced apoptosis occurred and there was protection with leptin. In db/db HSCs there was a significant increase in apoptosis after fas-L and CH compared with control medium, 55.4 ± 9.4% (SE) vs. 22.4 ± 2.1% (P < 0.05) (Fig. 5). The elevated baseline apoptosis in db/db HSCs compared with WT HSCs suggests that leptin and its functional receptor are required for HSC survival under in vitro conditions. Unlike in WT mice, leptin preincubation provided no protection to db/db HSCs treated with fas and CH: db/db fas-L and CH, 55.4 ± 9.4% (SE), vs. db/db fas-L and CH + leptin, 55.3 ± 8.8% (P = 0.84). This finding confirms the requirement for the long isoform of the leptin receptor in leptin’s protective effect on HSCs against fas-L-induced apoptosis.
Fig. 5.
The long isoform of the leptin receptor and leptin’s effect on fas-L-and cyclohexamide-induced HSC apoptosis. HSCs were isolated from wild-type (open bars) and db/db (filled bars) mice and plated on plastic coverslips for activation. After 7 days, HSCs demonstrated morphological signs of activation. Stellate cells were incubated with control medium or 100 nM leptin for 24 hr. Soluble fas-L (10–15 ng/ml) and cycloheximide (10 μg/ml) were used to induce HSC apoptosis. After 18 hr cells were fixed with 4% paraformaldehyde and stained with DAPI. Chromatin condensation was used to identify apoptotic stellate cells, and apoptotic stellate cells were calculated as a percentage of total stellate cells. Results are shown as mean percentages of apoptotic cells ± SE.
The leptin (ob) receptor is required for TAA-induced chemical hepatic fibrosis
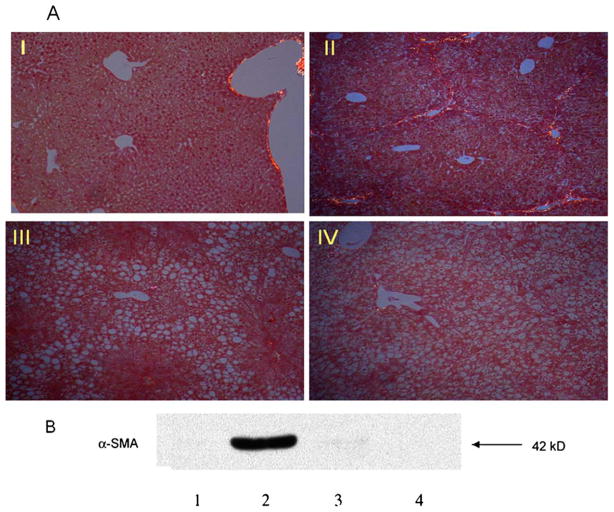
Figure 6A demonstrates that significant fibrosis (Stage III) developed in WT mice treated with TAA (WT-TAA) for 6 weeks. However, in db/db mice treated with TAA (db/db-TAA), there was minimal hepatic fibrosis as shown by Sirius red staining (Stage I). Both WT and db/db control mice showed no evidence of fibrosis.
Fig. 6.
The long isoform of the leptin receptor and TAA-induced hepatic fibrosis. WT and db/db mice were treated with 200 mg/kg TAA or control, intraperitoneally, three times a week for 6 weeks. At 6 weeks mice were sacrificed and liver tissue was collected for protein analysis. Liver tissue was fixed and parrafinized for Sirius red staining. (A) Sirius red staining to detect heptic fibrosis. Group I, WT treated with control; group II, WT treated with TAA; group III; db/db treated with control; group IV, db/db treated with TAA. Fibrosis was staged as follows: Stage 0, no fibrosis; Stage I, mild perivenular fibrosis; Stage II, perivenular fibrosis extending beyond the limiting plate; Stage III, bridging fibrosis; Stage IV, Cirrhosis. (B) Protein expression of α-SMA from liver tissue detected by western blot. Lane 1, WT treated with control; Lane 2, WT treated with TAA; Lane 3, db/db treated with control; Lane 4: db/db treated with TAA
To determine if this difference in hepatic fibrosis between WT and db/db mice is associated with differences in HSC activation, α-SMA protein expression was determined. There was markedly increased expression of α-SMA protein in WT-TAA livers compared with db/db-TAA-treated livers (Fig. 6B). There was minimal expression of α-SMA in control WT and db/db mice.
These findings confirm that the long isoform of the leptin receptor is required to induce TAA chemical hepatic fibrosis and that this is associated with enhanced α-SMA expression, suggesting increased HSC activation.
Leptin decreases HSC apoptosis in vivo associated with TAA hepatic fibrosis
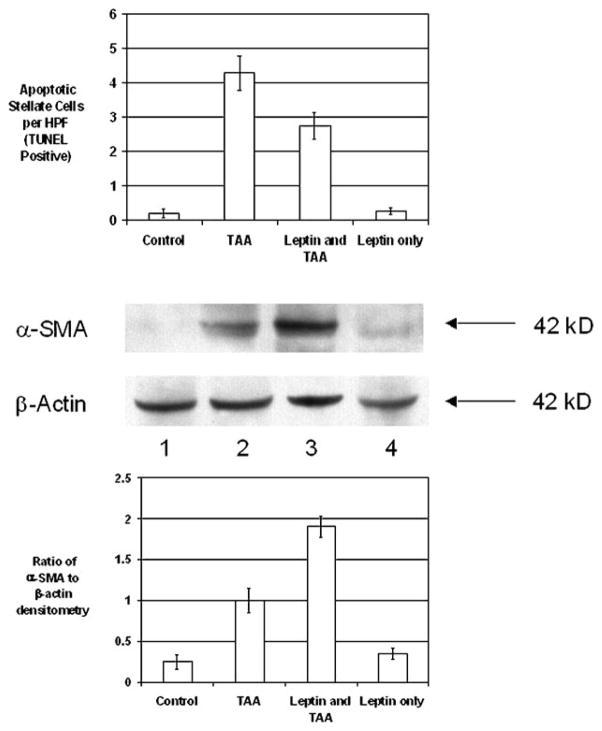
Iredale et al. reported that spontaneous recovery from hepatic fibrosis is accompanied by HSC apoptosis in the liver [20]. We confirm Iredale and coworkers’ findings that HSC TUNEL signal increased in TAA-treated animals (n = 7) compared to control animals (n = 6) (Fig. 7A). We further show that in TAA animals treated with leptin for 3 days prior to sacrifice (n = 6), there was less TUNEL signal than in animals which received TAA only (TAA group, 4.28 ± 0.5 TUNEL-positive cells per HPF, vs. leptin and TAA group, 2.73 ± 0.4 TUNEL-positive cells per HPF; P < 0.05). The control group which did not receive TAA but was given leptin for 3 days prior to sacrifice (n = 6) had very low levels of TUNEL positivity (Fig. 7A).
Fig. 7.
Leptin treatment and in vivo HSC apoptosis after TAA-induced hepatic injury. WT mice were treated with control or TAA (200 mg/kg) for 6 weeks. Three days prior to sacrifice, mice received either 1 μg/g leptin or control daily. After sacrifice, liver tissue was collected for paraffinization, histology, and TUNEL and α-SMA protein analysis. (A) Liver tissue was stained with TUNEL to detect apoptotic nuclei within the liver tissue. Total number of TUNEL-positive cells ± SE was determined per high-power field. (B) Western blot showing α-SMA expression in relation to β-actin expression, as mean ratio ± SE
We next determined if this difference in apoptosis was associated with changes in α-SMA protein expression. In TAA mice treated with leptin, there was markedly increased expression of α-SMA protein compared with that in TAA-treated mice. There was minimal expression of α-SMA in both control groups (Fig. 7B and C).
These findings demonstrate that leptin decreases HSC apoptosis in vivo and this is associated with higher levels of α-SMA expression.
Discussion
The epidemiological studies suggest an association between obesity and hepatic fibrosis in a range of chronic liver diseases including NASH, alcoholic liver disease, and chronic hepatitis C [1, 2, 19]. This has made it vital to understand the obesity-related changes which influence liver fibrosis. Four recent studies have demonstrated that the hormone leptin, which increases proportionally to body fat, is required for liver fibrosis [5–8]. In the absence of leptin (ob/ob mice), a variety of fibrogenic stimuli including thioacetamide, carbon tetrachloride, and a methionine choline-deficient diet failed to induce an increase in collagen I mRNA and, ultimately, liver fibrosis. Leptin has been shown to have multiple direct effects on HSC including enhancing proliferation and increasing collagen I production [6, 8]. Leptin was also recently shown to be able to protect HSC from apoptosis induced by serum depletion or TRAIL [21]. In addition to direct effects on HSC, leptin has a number of effects on adjacent cells, which might enhance HSC function. Primary among these is the leptin-induced enhancement of TGF-β1 production by sinusoidal endothelial cells [7].
We were interested to note that with activation of HSC in vivo there is a significant increase in HSC apoptosis [20]. This high level of HSC apoptosis is maintained during the resolution of fibrosis and provides a mechanism for the decrease in HSC numbers as fibrosis resolves. Based on in vitro studies, apoptosis of HSC is thought to be an active process mediated by fas and fas-L [14]. Membrane levels of fas and fas-L increase as naive HSCs are activated and correlate with increased HSC apoptosis, which can be blocked by fas-antagonist antibodies. In fact fas-mediated apoptosis of HSCs appeared to occur without cell-cell contact, suggesting self-ligation of fas. Our study addresses the question whether fas-induced apoptosis of activated HSCs in vitro can be modulated by a hormone intimately linked with obesity. As we show, there is very significant protection of HSCs from fas-induced apoptosis by physiological concentrations of leptin.
Apoptosis is mediated by two principal pathways: death receptor, mediated by fas-L and TNF-related ligands; and mitochondrial pathway, mediated by mitochondrial release of cytochrome c [22]. We tested whether leptin’s protective effect against apoptosis was generalized or specific to one of these two pathways. UV irradiation causes mitochondrial release of cytochrome c, which, through a series of caspase cascades, results in caspase 3 activation and cellular apoptosis [23]. We show that leptin has no protective effect on UV-induced apoptosis mediated primarily by the mitochondrial pathway in HSCs. This suggests that leptin’s protective effect on HSCs against apoptosis is mediated through inhibition of apoptotic pathways proximal to mitochondrial activation.
Under in vivo conditions, HSCs interact with various cells that can modulate apoptosis such as hepatocytes and Kupffer cells [24, 25]. Furthermore, leptin has been shown to affect the function of many cells within the liver. To determine if leptin’s antiapoptotic effects are significant in vivo, we applied a previously developed a model of liver fibrosis and HSC apoptosis. Iredale et al. demonstrated that after 4 weeks of TAA treatment, HSCs were undergoing significant apoptosis [20]. We found that treating mice with leptin three days prior to sacrifice after 6 weeks of TAA treatment resulted in a significant decrease in apoptosis and increase in α-SMA. This demonstrates that the in vitro inhibition of HSC apoptosis by leptin also occurs in vivo, and sustained exposure to high levels of leptin may enhance hepatic fibrogenesis by inhibition of HSC apoptosis.
In summary, our study shows that leptin provides protection to stellate cells from fas ligand-mediated apoptosis. Leptin’s protective effect is specific for the death receptor pathway, as it provides no protection to HSCs from the mitochondrial pathway of apoptosis. This protective effect extends to an in vivo environment and elevated levels of leptin could increase hepatic fibrosis by protecting HSCs from apoptosis. We also show that in the absence of a functional long isoform of the leptin receptor, there is decreased hepatic fibrosis and a loss of leptin’s protective effect on HSC apoptosis. These findings place protection of HSCs from apoptosis as central to the requirement of leptin for the development of liver fibrosis.
Acknowledgments
This work was supported by a KO8 award (K08DK002965), the Yale Liver Center (NIH P 30 DK34989), a Yale Liver Center Training Grant (NIH T32 DK07356), and a Pilot Project grant from the Yale Liver Center. There are no competing interests and no sponsors, and approval was obtained for all animal experiments.
Contributor Information
Amir Qamar, Section of Digestive Diseases, Yale University School of Medicine, New Haven, Connecticut 06520, USA.
Shehzad Zafar Sheikh, Section of Digestive Diseases, Yale University School of Medicine, New Haven, Connecticut 06520, USA.
Ali Masud, Section of Immunobiology, Yale University School of Medicine, New Haven, Connecticut 06520, USA. Section of Immunobiology, Yale University School of Medicine, New Haven, Connecticut 06520, USA. Section of Pediatrics, Yale University School of Medicine, New Haven, Connecticut 06520, USA.
Muhammad Nauman Jhandier, Section of Digestive Diseases, Yale University School of Medicine, New Haven, Connecticut 06520, USA.
Irteza Bin Inayat, Section of Digestive Diseases, Yale University School of Medicine, New Haven, Connecticut 06520, USA.
Wyel Hakim, Section of Digestive Diseases, Yale University School of Medicine, New Haven, Connecticut 06520, USA.
Wajahat Zafar Mehal, Email: wajahat.mehal@yale.edu, Section of Digestive Diseases, Yale University School of Medicine, New Haven, Connecticut 06520, USA. Section of Digestive Diseases, Yale University, 333 Cedar Street, 1080 LMP, New Haven, Connecticut 06520-8019, USA.
References
- 1.Henriksen JH, Holst JJ, Moller S, Brinch K, Bendtsen F. Increased circulating leptin in alcoholic cirrhosis: relation to release and disposal. Hepatology. 1999;29(6):1818–1824. doi: 10.1002/hep.510290601. [DOI] [PubMed] [Google Scholar]
- 2.Romero-Gomez M, Castellano-Megias VM, Grande L, et al. Serum leptin levels correlate with hepatic steatosis in chronic hepatitis C. Am J Gastroenterol. 2003;98(5):1135–1141. doi: 10.1111/j.1572-0241.2003.07450.x. [DOI] [PubMed] [Google Scholar]
- 3.Shimuzu H, Shimomura Y, Hayashi R, Ohtani K, Sato N, Futawatari T, Mori M. Serum leptin concentration is associated with total body fat mass but not abdominal fat distribution. Int J Obese Relat Metab Disord. 1997;21(7):536–541. doi: 10.1038/sj.ijo.0800437. [DOI] [PubMed] [Google Scholar]
- 4.Sanyal AJ. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 5.Honda H, Ikejima K, Hirose M, et al. Leptin is required for fibrogenic responses induced by thioacetamide in the murine liver. Hepatology. 2002;36(1):12–21. doi: 10.1053/jhep.2002.33684. [DOI] [PubMed] [Google Scholar]
- 6.Ikejima K, Takei Y, Honda H, et al. Leptin-receptor mediated signaling regulates hepatic fibrogenesis and remodeling of extracellular matrix in the rat. Gastroenterology. 2002;122(5):1399–1410. doi: 10.1053/gast.2002.32995. [DOI] [PubMed] [Google Scholar]
- 7.Sakaida I, Jinhua S, Uchida K, Terai S, Okita K. Leptin receptor-deficient Zucker (fa/fa) rat retards the development of pig serum-induced liver fibrosis with Kupffer cell dysfunction. Life Sci. 2003;73(19):2491–2501. doi: 10.1016/s0024-3205(03)00653-2. [DOI] [PubMed] [Google Scholar]
- 8.Saxena NK, Ikeda K, Rockey DC, Friedman SL, Anania FA. Leptin in hepatic fibrosis: evidence for increased collagen production in stellate cells and lean littermates of ob/ob mice. Hepatology. 2002;35(4):762–771. doi: 10.1053/jhep.2002.32029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Anania FA. Leptin, liver, and obese mice—fibrosis in the fat lane. Hepatology. 2002;36(1):246–248. doi: 10.1053/jhep.2002.34359. [DOI] [PubMed] [Google Scholar]
- 10.Loffreda S, Yang SQ, Lin HZ, et al. Leptin regulates proinflammatory immune responses. FASEB J. 1998;12(1):57–65. [PubMed] [Google Scholar]
- 11.Sanchez-Margalet V, Martin-Romero C, Santos-Alvarez J, Goberna R, Najib S, Gonzalez-Yanes C. Role of leptin as an immunomodulator of blood mononuclear cells: mechanisms of action. Clin Exp Immunol. 2003;133(1):11–19. doi: 10.1046/j.1365-2249.2003.02190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sweeney G. Leptin signaling. Cell Signal. 2002;14(8):655–663. doi: 10.1016/s0898-6568(02)00006-2. [DOI] [PubMed] [Google Scholar]
- 13.Canbay A, Friedman S, Gores G. Apoptosis: The nexus of liver injury and fibrosis. Hepatology. 2004;39(2):273–278. doi: 10.1002/hep.20051. [DOI] [PubMed] [Google Scholar]
- 14.Saile B, Knittel T, Matthes N, Schott P, Ramadori G. CD95/CD95L-mediated apoptosis of the hepatic stellate cell. A mechanism terminating uncontrolled hepatic stellate cell proliferation during hepatic tissue repair. Am J Pathol. 1997;151(5):1265–1272. [PMC free article] [PubMed] [Google Scholar]
- 15.Yoon JH, Gores GJ. Death receptor-mediated apoptosis and the liver. J Hepatol. 2002;37(3):400–410. doi: 10.1016/s0168-8278(02)00209-x. [DOI] [PubMed] [Google Scholar]
- 16.Liu C, Gaca MD, Swenson ES, Vellucci VF, Reiss M, Wells RG. Smads 2 and 3 are differentially activated by transforming growth factor-beta (TGF-beta) in quiescent and activated hepatic stellate cells. Constitutive nuclear localization of Smads in activated cells is TGF-beta-independent. J Biol Chem. 2003;278(13):11721–11728. doi: 10.1074/jbc.M207728200. [DOI] [PubMed] [Google Scholar]
- 17.Leblanc H, Lawrence D, Varfolomeev E, et al. Tumor-cell resistance to death-receptor induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med. 2002;8(3):274–281. doi: 10.1038/nm0302-274. [DOI] [PubMed] [Google Scholar]
- 18.Lu G, Janjic BM, Janjic J, Whiteside TL, Storkus WJ, Vujanovic NL. Innate direct anti-cancer effector function of human immature dendritic cells. II. Role of TNF, lymphotoxin-alpha (1) beta (2), fas ligand and TNF-related apoptosis inducing ligand. J Immunol. 2002;168(4):1831–1839. doi: 10.4049/jimmunol.168.4.1831. [DOI] [PubMed] [Google Scholar]
- 19.Clark JM, Brancati FL, Diehl AM. Nonalcoholic fatty liver disease. Gastroenterology. 2002;122(6):1649–1657. doi: 10.1053/gast.2002.33573. [DOI] [PubMed] [Google Scholar]
- 20.Iredale JP, Benyon RC, Pickering J, et al. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saxena NK, Titus MA, Ding X, Floyd J, Srinivasan S, Sitaraman SV, Anania FA. Leptin as a novel profibrogenic cytokine in hepatic stellate cells: mitogenesis and inhibition of apoptosis mediated by extracellular regulated kinase (Erk) and Akt phosphorylation. FASEB J. 2004;18(13):1612–1614. doi: 10.1096/fj.04-1847fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407(6805):770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- 23.Kulms D, Schwarz T. Molecular mechanisms of UV-induced apoptosis. Photodermatol Photoimmunol Photomed. 2000;16(5):195–201. doi: 10.1034/j.1600-0781.2000.160501.x. [DOI] [PubMed] [Google Scholar]
- 24.Canbay A, Taimr P, Torok N, Higuchi H, Freidman S, Gores GJ. Apoptotic body engulfment by a human stellate cell line is profibrogenic. Lab Invest. 2003;83(5):655–663. doi: 10.1097/01.lab.0000069036.63405.5c. [DOI] [PubMed] [Google Scholar]
- 25.Dodig M, Mullen KD. New mechanism of killing activated hepatic stellate cells. Hepatology. 2003;38(4):1051–1053. doi: 10.1002/hep.1840380432. [DOI] [PubMed] [Google Scholar]