Abstract
A clone was isolated that contains the deletion junction region from an individual with a deletion associated with Chinese G gamma + (A gamma delta beta)zero thalassemia. A clone containing the normal DNA corresponding to the 3' breakpoint of this deletion was also isolated. Portions of these two clones were sequenced and compared to the region in the A gamma-globin gene where the 5' breakpoint occurs. This comparison reveals that the breakage and reunion event was nonhomologous and that it probably involved the insertion of 36-41 bases of DNA belonging to the L1 (KpnI) family of repetitive DNA. Genomic mapping revealed that the DNA on the 3' side of this deletion is closely linked in normal DNA to the 3' breakpoints of two different large deletions that are associated with hereditary persistence of fetal hemoglobin (HPFH). We cloned and mapped 35 kbp of normal DNA from this region (greater than 45 kbp downstream of the human beta-globin gene) that contains the 3' breakpoints of the Chinese thalassemia and the two HPFH deletions. An endogenous retrovirus-like element and several other repetitive sequences are located within this region. We show that the Chinese thalassemia deletion is greater than 80 kbp in length and differs in size from the two HPFH deletions by less than 6%. We also show that the Chinese thalassemia deletion is at least 40 kbp larger than several other deletions associated with a very similar phenotype.
Full text
PDF






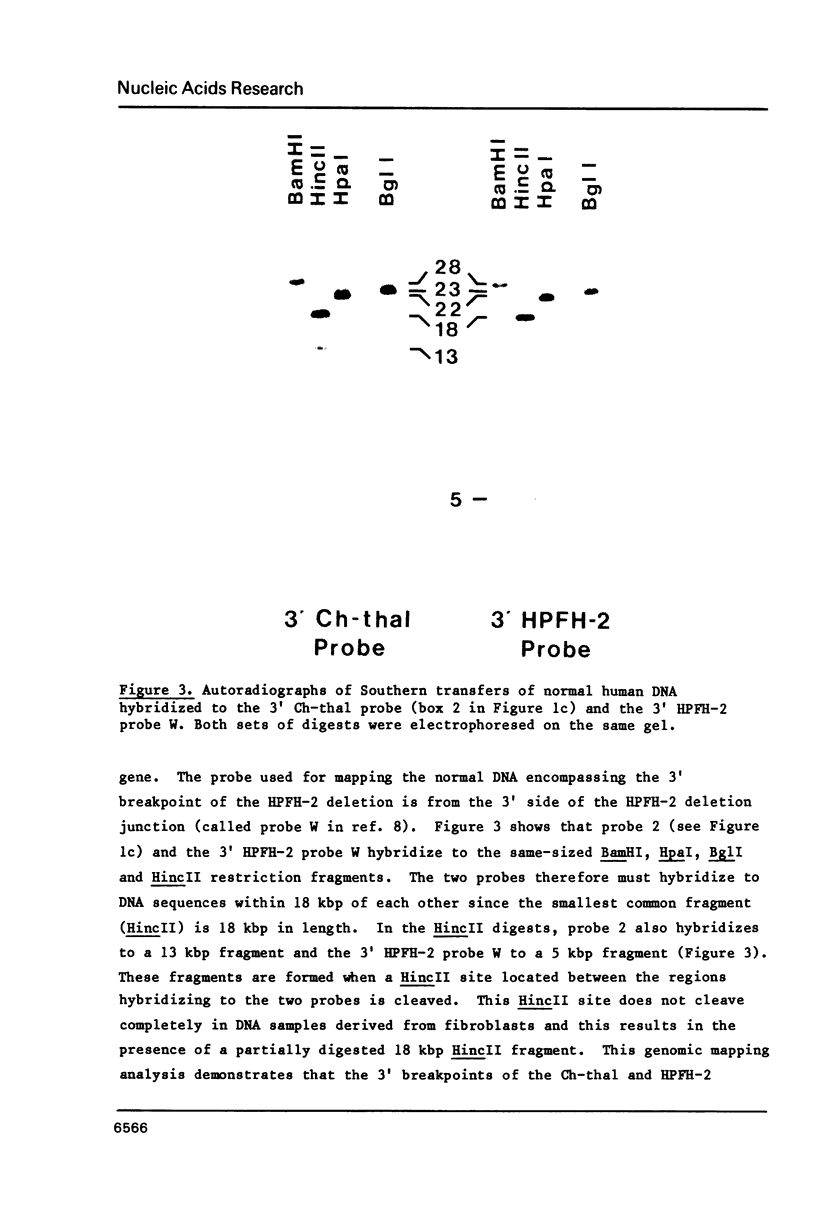
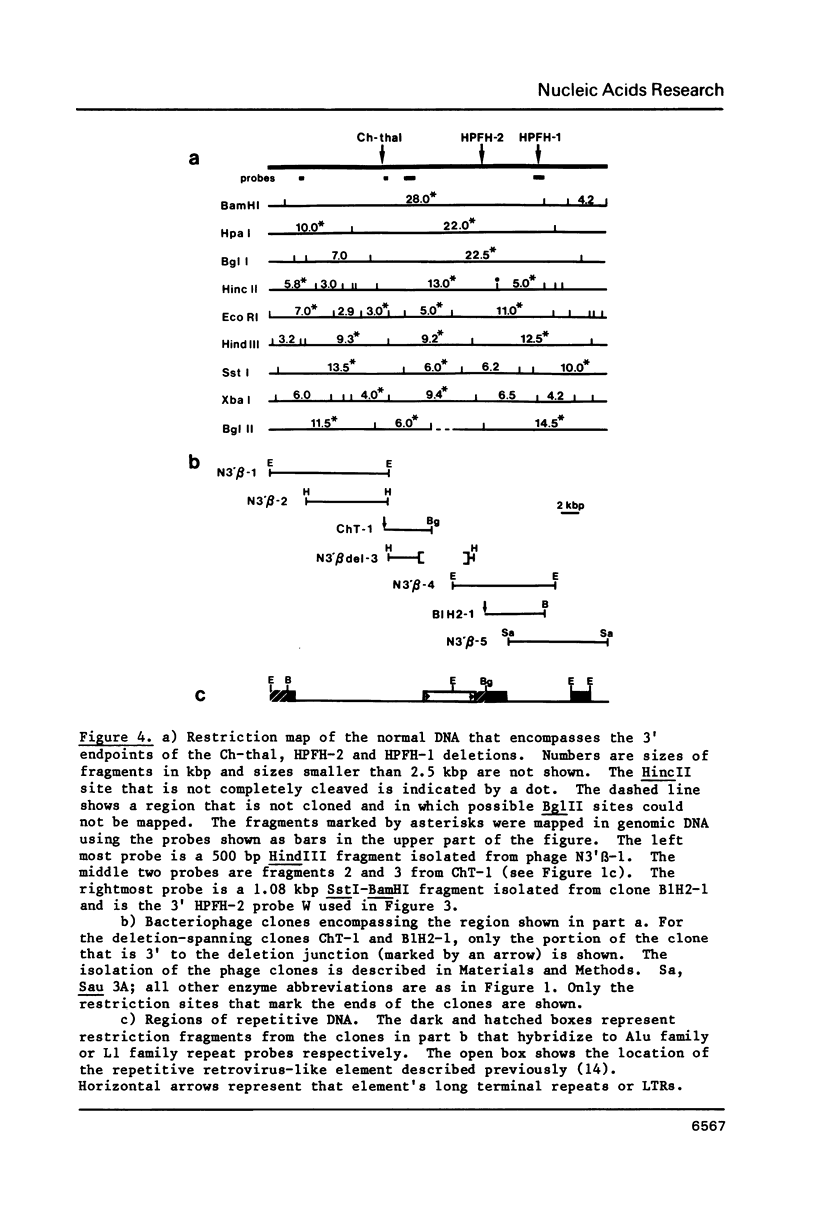
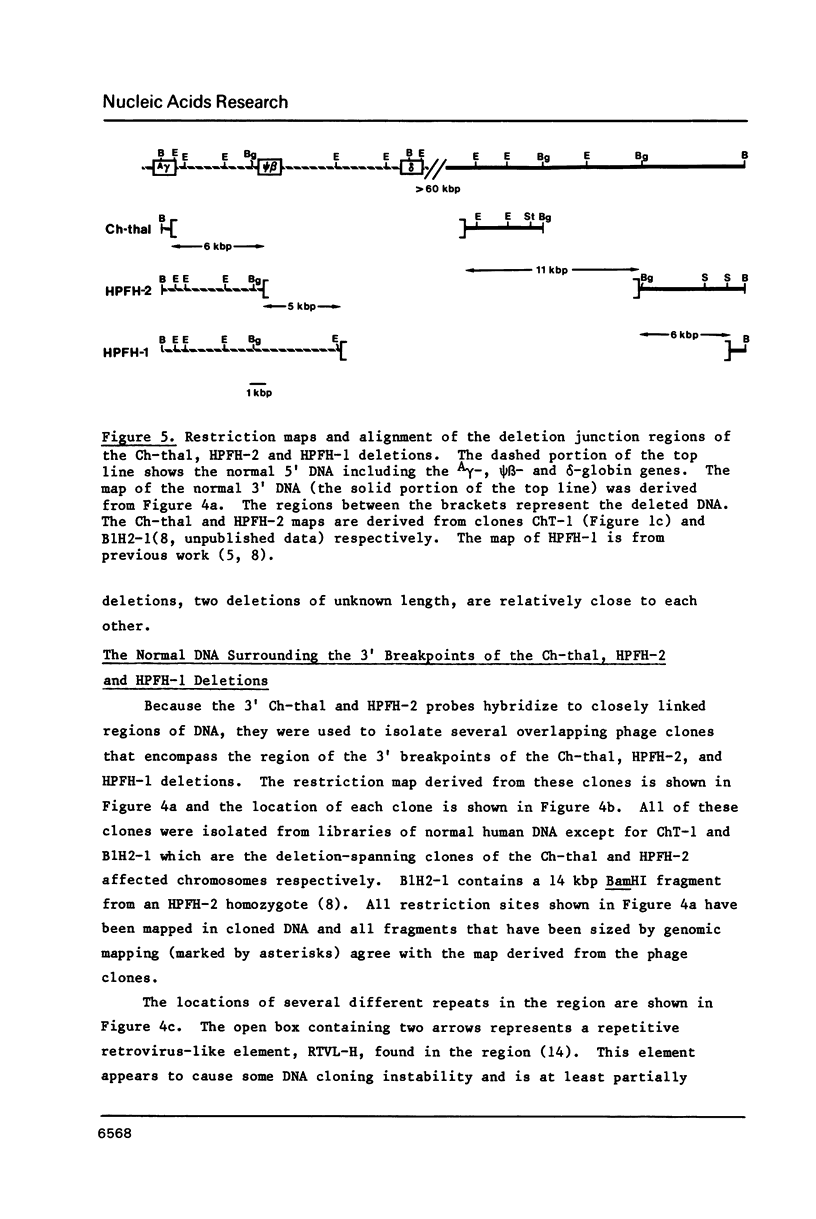







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. A., Kato S., Camerini-Otero R. D. A pattern of partially homologous recombination in mouse L cells. Proc Natl Acad Sci U S A. 1984 Jan;81(1):206–210. doi: 10.1073/pnas.81.1.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Flavell R. A. Physical mapping of the globin gene deletion in hereditary persistence of foetal haemoglobin (HPFH). Nucleic Acids Res. 1980 Apr 11;8(7):1521–1534. doi: 10.1093/nar/8.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botchan M., Stringer J., Mitchison T., Sambrook J. Integration and excision of SV40 DNA from the chromosome of a transformed cell. Cell. 1980 May;20(1):143–152. doi: 10.1016/0092-8674(80)90242-1. [DOI] [PubMed] [Google Scholar]
- Collins F. S., Weissman S. M. The molecular genetics of human hemoglobin. Prog Nucleic Acid Res Mol Biol. 1984;31:315–462. doi: 10.1016/s0079-6603(08)60382-7. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell R. A., Kooter J. M., De Boer E., Little P. F., Williamson R. Analysis of the beta-delta-globin gene loci in normal and Hb Lepore DNA: direct determination of gene linkage and intergene distance. Cell. 1978 Sep;15(1):25–41. doi: 10.1016/0092-8674(78)90080-6. [DOI] [PubMed] [Google Scholar]
- Fritsch E. F., Lawn R. M., Maniatis T. Characterisation of deletions which affect the expression of fetal globin genes in man. Nature. 1979 Jun 14;279(5714):598–603. doi: 10.1038/279598a0. [DOI] [PubMed] [Google Scholar]
- Gerondakis S., Cory S., Adams J. M. Translocation of the myc cellular oncogene to the immunoglobulin heavy chain locus in murine plasmacytomas is an imprecise reciprocal exchange. Cell. 1984 Apr;36(4):973–982. doi: 10.1016/0092-8674(84)90047-3. [DOI] [PubMed] [Google Scholar]
- Grosveld F. G., Lund T., Murray E. J., Mellor A. L., Dahl H. H., Flavell R. A. The construction of cosmid libraries which can be used to transform eukaryotic cells. Nucleic Acids Res. 1982 Nov 11;10(21):6715–6732. doi: 10.1093/nar/10.21.6715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henthorn P. S., Smithies O., Nakatsuji T., Felice A. E., Gardiner M. B., Reese A. L., Huisman T. H. (A gamma delta beta)0-Thalassaemia in Blacks is due to a deletion of 34 kbp of DNA. Br J Haematol. 1985 Feb;59(2):343–356. doi: 10.1111/j.1365-2141.1985.tb02999.x. [DOI] [PubMed] [Google Scholar]
- Hohn B. In vitro packaging of lambda and cosmid DNA. Methods Enzymol. 1979;68:299–309. doi: 10.1016/0076-6879(79)68021-7. [DOI] [PubMed] [Google Scholar]
- Huisman T. H., Schroeder W. A., Efremov G. D., Duma H., Mladenovski B., Hyman C. B., Rachmilewitz E. A., Bouver N., Miller A., Brodie A. The present status of the heterogeneity of fetal hemoglobin in beta-thalassemia: an attempt to unify some observations in thalassemia and related conditions. Ann N Y Acad Sci. 1974;232(0):107–124. doi: 10.1111/j.1749-6632.1974.tb20576.x. [DOI] [PubMed] [Google Scholar]
- Jennings M. W., Jones R. W., Wood W. G., Weatherall D. J. Analysis of an inversion within the human beta globin gene cluster. Nucleic Acids Res. 1985 Apr 25;13(8):2897–2906. doi: 10.1093/nar/13.8.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. W., Old J. M., Trent R. J., Clegg J. B., Weatherall D. J. Major rearrangement in the human beta-globin gene cluster. Nature. 1981 May 7;291(5810):39–44. doi: 10.1038/291039a0. [DOI] [PubMed] [Google Scholar]
- Jones R. W., Old J. M., Trent R. J., Clegg J. B., Weatherall D. J. Restriction mapping of a new deletion responsible for G gamma (delta beta)o thalassemia. Nucleic Acids Res. 1981 Dec 21;9(24):6813–6825. doi: 10.1093/nar/9.24.6813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kioussis D., Vanin E., deLange T., Flavell R. A., Grosveld F. G. Beta-globin gene inactivation by DNA translocation in gamma beta-thalassaemia. Nature. 1983 Dec 15;306(5944):662–666. doi: 10.1038/306662a0. [DOI] [PubMed] [Google Scholar]
- Kutlar A., Gardiner M. B., Headlee M. G., Reese A. L., Cleek M. P., Nagle S., Sukumaran P. K., Huisman T. H. Heterogeneity in the molecular basis of three types of hereditary persistence of fetal hemoglobin and the relative synthesis of the G gamma and A gamma types of gamma chain. Biochem Genet. 1984 Feb;22(1-2):21–35. doi: 10.1007/BF00499284. [DOI] [PubMed] [Google Scholar]
- Loenen W. A., Blattner F. R. Lambda Charon vectors (Ch32, 33, 34 and 35) adapted for DNA cloning in recombination-deficient hosts. Gene. 1983 Dec;26(2-3):171–179. doi: 10.1016/0378-1119(83)90187-7. [DOI] [PubMed] [Google Scholar]
- Mager D. L., Henthorn P. S. Identification of a retrovirus-like repetitive element in human DNA. Proc Natl Acad Sci U S A. 1984 Dec;81(23):7510–7514. doi: 10.1073/pnas.81.23.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann J. R., MacNeish A. S., Bannister D., Clegg J. B., Wood W. G., Weatherall D. J. Delta-beta-thalassaemia in a Chinese family. Br J Haematol. 1972 Oct;23(4):393–402. doi: 10.1111/j.1365-2141.1972.tb07074.x. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Ojwang P. J., Nakatsuji T., Gardiner M. B., Reese A. L., Gilman J. G., Huisman T. H. Gene deletion as the molecular basis for the Kenya-G gamma-HPFH condition. Hemoglobin. 1983;7(2):115–123. doi: 10.3109/03630268309048641. [DOI] [PubMed] [Google Scholar]
- Orkin S. H., Alter B. P., Altay C. Deletion of the A gamma-globin gene in G gamma-delta beta-thalassemia. J Clin Invest. 1979 Sep;64(3):866–869. doi: 10.1172/JCI109535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orkin S. H., Antonarakis S. E., Kazazian H. H., Jr Polymorphism and molecular pathology of the human beta-globin gene. Prog Hematol. 1983;13:49–73. [PubMed] [Google Scholar]
- Piccoli S. P., Caimi P. G., Cole M. D. A conserved sequence at c-myc oncogene chromosomal translocation breakpoints in plasmacytomas. 1984 Jul 26-Aug 1Nature. 310(5975):327–330. doi: 10.1038/310327a0. [DOI] [PubMed] [Google Scholar]
- Rimm D. L., Horness D., Kucera J., Blattner F. R. Construction of coliphage lambda Charon vectors with BamHI cloning sites. Gene. 1980 Dec;12(3-4):301–309. doi: 10.1016/0378-1119(80)90113-4. [DOI] [PubMed] [Google Scholar]
- Rogers J. Molecular biology. CACA sequences - the ends and the means? Nature. 1983 Sep 8;305(5930):101–102. doi: 10.1038/305101a0. [DOI] [PubMed] [Google Scholar]
- Schmid C. W., Jelinek W. R. The Alu family of dispersed repetitive sequences. Science. 1982 Jun 4;216(4550):1065–1070. doi: 10.1126/science.6281889. [DOI] [PubMed] [Google Scholar]
- Slightom J. L., Blechl A. E., Smithies O. Human fetal G gamma- and A gamma-globin genes: complete nucleotide sequences suggest that DNA can be exchanged between these duplicated genes. Cell. 1980 Oct;21(3):627–638. doi: 10.1016/0092-8674(80)90426-2. [DOI] [PubMed] [Google Scholar]
- Spritz R. A., Orkin S. H. Duplication followed by deletion accounts for the structure of an Indian deletion beta (0)-thalassemia gene. Nucleic Acids Res. 1982 Dec 20;10(24):8025–8029. doi: 10.1093/nar/10.24.8025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trent R. J., Jones R. W., Clegg J. B., Weatherall D. J., Davidson R., Wood W. G. (A gamma delta beta) thalassaemia: similarity of phenotype in four different molecular defects, including one newly described. Br J Haematol. 1984 Jun;57(2):279–289. doi: 10.1111/j.1365-2141.1984.tb02897.x. [DOI] [PubMed] [Google Scholar]
- Tuan D., Feingold E., Newman M., Weissman S. M., Forget B. G. Different 3' end points of deletions causing delta beta-thalassemia and hereditary persistence of fetal hemoglobin: implications for the control of gamma-globin gene expression in man. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6937–6941. doi: 10.1073/pnas.80.22.6937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan D., Murnane M. J., deRiel J. L., Forget B. G. Heterogeneity in the molecular basis of hereditary persistence of fetal haemoglobin. Nature. 1980 May 29;285(5763):335–337. doi: 10.1038/285335a0. [DOI] [PubMed] [Google Scholar]
- Van der Ploeg L. H., Konings A., Oort M., Roos D., Bernini L., Flavell R. A. gamma-beta-Thalassaemia studies showing that deletion of the gamma- and delta-genes influences beta-globin gene expression in man. Nature. 1980 Feb 14;283(5748):637–642. doi: 10.1038/283637a0. [DOI] [PubMed] [Google Scholar]
- Vanin E. F., Henthorn P. S., Kioussis D., Grosveld F., Smithies O. Unexpected relationships between four large deletions in the human beta-globin gene cluster. Cell. 1983 Dec;35(3 Pt 2):701–709. doi: 10.1016/0092-8674(83)90103-4. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]




